Abstract
Multiple sclerosis (MS) relapses impose a substantial clinical and economic burden. Teriflunomide is a new oral disease-modifying therapy approved for the treatment of relapsing MS. We evaluated the effects of teriflunomide treatment on relapse-related neurological sequelae and healthcare resource use in a post hoc analysis of the Phase III TEMSO study. Confirmed relapses associated with neurological sequelae [defined by an increase in Expanded Disability Status Scale/Functional System (sequelae-EDSS/FS) ≥30 days post relapse or by the investigator (sequelae-investigator)] were analyzed in the modified intention-to-treat population (n = 1086). Relapses requiring hospitalization or intravenous (IV) corticosteroids, all hospitalizations, emergency medical facility visits (EMFV), and hospitalized nights for relapse were also assessed. Annualized rates were derived using a Poisson model with treatment, baseline EDSS strata, and region as covariates. Risks of sequelae and hospitalization per relapse were calculated as percentages and groups were compared with a χ2 test. Compared with placebo, teriflunomide reduced annualized rates of relapses with sequelae-EDSS/FS [7 mg by 32 % (p = 0.0019); 14 mg by 36 % (p = 0.0011)] and sequelae-investigator [25 % (p = 0.071); 53 % (p < 0.0001)], relapses leading to hospitalization [36 % (p = 0.015); 59 % (p < 0.0001)], and relapses requiring IV corticosteroids [29 % (p = 0.001); 34 % (p = 0.0003)]. Teriflunomide-treated patients spent fewer nights in hospital for relapse (p < 0.01). Teriflunomide 14 mg also decreased annualized rates of all hospitalizations (p = 0.01) and EMFV (p = 0.004). The impact of teriflunomide on relapse-related neurological sequelae and relapses requiring healthcare resources may translate into reduced healthcare costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00415-013-6979-y) contains supplementary material, which is available to authorized users.
Keywords: Clinical trial, Economics, Multiple sclerosis, Outcome assessment (Health Care), Teriflunomide
Introduction
Multiple sclerosis (MS) relapses are variable in nature, typically last from a week to a month, vary in severity, and significantly impact patients, their families, and their caregivers [4, 12, 16]. Severe relapses (e.g., those requiring hospitalization) are associated with a substantial economic burden [9, 10, 18]. Although periods of remission usually follow a relapse (especially during the early phase of MS), symptoms often do not completely resolve, with residual neurological deficits persisting in up to 57 % of patients, which contributes further to disability progression [7]. Variability in recovery also means that patients often require different levels of care [6, 9, 15].
Teriflunomide is a new once-daily oral disease-modifying therapy recently approved for the treatment of relapsing forms of MS (RMS) [1]. The Phase III TEMSO (TEriflunomide Multiple Sclerosis Oral) trial showed that both doses of teriflunomide (7 and 14 mg) reduced the annualized relapse rate by over 31 % (p < 0.001 vs. placebo); disability progression confirmed for 12 weeks (14 mg only) and magnetic resonance imaging (MRI) parameters of disease burden and activity were also significantly reduced with evidence of a dose effect [11, 17]. Teriflunomide was well tolerated with a well-characterized safety profile, with similar incidences of adverse events (AEs), serious AEs (SAEs), and AEs leading to drug discontinuation in the two teriflunomide groups and the placebo group. The most frequent AEs with teriflunomide (incidence ≥10 % and ≥2 % greater than placebo) were alanine aminotransferase increases, alopecia (hair thinning), diarrhea, influenza, nausea, and paresthesia [1].
This post hoc analysis of TEMSO evaluated the effects of teriflunomide on a range of relapse outcomes and healthcare resource use.
Methods
Study design
The TEMSO trial (NCT00134563) was a 2-year, multinational, multicenter, randomized, double-blind, placebo-controlled, parallel-group study designed to evaluate the efficacy and safety of teriflunomide in reducing the frequency of relapses and delaying accumulation of physical disability in patients with RMS. Detailed methodology of the TEMSO study has been published previously [11, 17]. Briefly, eligible patients were aged 18–55 years, met McDonald criteria for MS [8], and exhibited a relapsing clinical course, with or without progression. For enrollment, patients were required to be ambulatory [Kurtzke’s Expanded Disability Status Scale (EDSS) [5] score ≤5.5], with at least two clinical relapses within the previous two years, or at least one during the preceding year, but with no relapses within 60 days of randomization. Patients were stratified by baseline EDSS score (≤3.5 vs. >3.5) and were randomized 1:1:1 to receive either placebo, teriflunomide 7 or 14 mg, once daily for 108 weeks. The primary study objective was to determine the efficacy of teriflunomide in reducing the annualized relapse rate. Secondary study objectives included determining the efficacy of teriflunomide on delaying the progression of the disease over the duration of the study (key secondary objective), MRI parameters (principally total lesion volume), and patient-reported fatigue (by the Fatigue Impact Scale).
Standard protocol approvals, registrations, and patient consents
The TEMSO trial was performed in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by central and local ethics committees and each site’s institutional review board; patients gave written informed consent prior to the study.
Study evaluations
This post hoc analysis evaluated the effect of teriflunomide treatment on outcomes from protocol-defined confirmed relapses occurring between treatment randomization and treatment discontinuation in the TEMSO study. Suspected relapses were defined as the appearance of a new clinical sign/symptom, or clinical worsening of a previous sign/symptom that persisted for a minimum of 24 h in the absence of fever. Patients were required to visit the investigational site within seven days following onset of a suspected relapse. Confirmed relapses required either an increase in EDSS score of ≥0.5 points (or ≥1.0 point when EDSS = 0 at the previous assessment), or a 1-point change in the Functional System (FS) in at least two systems or a 2-point change in one system (excluding bowel/bladder and cerebral) from the last EDSS/FS assessment. Suspected and confirmed relapses could be treated with intravenous (IV) corticosteroids according to the investigator’s judgment; the preferred regimen was methylprednisolone sodium succinate 1 g once daily for 3–5 days.
The following relapse outcomes were evaluated: relapses with sequelae, relapses associated with increased healthcare resource use (i.e., hospitalization or requiring IV corticosteroids), and relapse intensity subjectively rated as mild, moderate, or severe by the investigator at the initial relapse assessment.
Relapses with sequelae were defined in two ways: objectively by confirmed changes in EDSS/FS (sequelae-EDSS/FS) or subjectively by the investigator (sequelae-investigator). The objective definition utilized an increase of EDSS/FS as defined for a confirmed relapse in the TEMSO primary analysis (see above), but at a different time point. Thus, any increase of EDSS/FS was derived using the last assessment before the start of the relapse and the assessment done at least 30 days post relapse. As sequelae may persist beyond 30 days, the derivation was also performed using assessments carried out at least 60, 90, 120, 150, and 180 days post relapse. In cases where two successive relapses occurred without any EDSS/FS assessment performed between, the first relapse was excluded from the analysis. Sequelae subjectively determined by the investigator were defined as relapses that resulted in incomplete neurological recovery at the end of a relapse as reported by the treating neurologist in the relapse section of the Case Report Form (CRF), where outcomes were defined as either recovered with sequelae (including worsened intensity, ongoing, or unknown) or without sequelae. No further specific recommendations were provided to investigators to assess relapse recovery and/or the presence or absence of sequelae.
The impact of teriflunomide treatment on healthcare resource consumption was also evaluated. These assessments included confirmed relapses for which hospitalization was required, confirmed relapses requiring IV corticosteroids, length of stay in hospital due to a confirmed relapse, all hospitalizations, and any emergency medical facility visits (EMFV; a visit to a medical facility/hospital for emergency care not resulting in admission). The ‘all hospitalization’ outcome documented hospitalizations recorded either from the adverse event form or the relapse form and was included to evaluate the treatment effect of teriflunomide on all hospitalizations observed in the study.
Statistical analyses
Consistent with the primary analysis of TEMSO, all post hoc analyses described herein were performed on the modified intention-to-treat population (i.e., all patients randomized and exposed to study medication for at least one day; n = 1086) according to the treatment group as randomized. All analyses on relapse outcomes were conducted on protocol-defined relapses. The number and percentage of patients free of relapse were summarized for each relapse outcome. Adjusted annualized rates were also calculated for all relapse outcomes, all hospitalizations, and EMFV using a Poisson regression model with robust error variance, which accommodates potential over-dispersed data appropriately. The total number of the outcome of interest was defined as the response variable; treatment, EDSS strata at baseline, and region were covariates; and log-transformed standardized study treatment duration was defined as an offset variable. Risks of sequelae, hospitalization, and requirement for IV corticosteroids per relapse were calculated as raw percentages observed over the course of the study; treatment groups were compared with a χ2 test versus placebo. The number of nights spent in hospital for relapse and EMFV over the study period was summarized per year. Among all relapses, raw percentages of relapses rated mild or moderate/severe intensity as per investigator judgment were presented in each treatment group and compared versus placebo using a χ2 test.
Results
Baseline demographics
Baseline demographics and disease characteristics of the TEMSO study population have been described previously [11]. No significant differences were observed across the three treatment groups.
Effects of teriflunomide treatment on relapse outcomes
Relapses with sequelae: sequelae-EDSS/FS
The annualized rate of relapse with sequelae, defined by EDSS/FS increase at 30 days post relapse, was lower in both teriflunomide groups than in the placebo group (placebo 0.271; teriflunomide 7 mg 0.185; teriflunomide 14 mg 0.173) [Fig. 1a (i)]. Both doses of teriflunomide reduced the annualized rate of relapse with sequelae-EDSS/FS compared with placebo: 7 mg by 32 % (p = 0.0019) and 14 mg by 36 % (p = 0.0011) [Fig. 1a (i)]. The significant treatment effects of both doses of teriflunomide observed on relapse-related sequelae at 30 days post relapse were maintained on sequelae assessed up to 180 days post relapse in the teriflunomide 14 mg group (Fig. 2). In addition, significantly more patients remained free from relapses with sequelae-EDSS/FS in both teriflunomide groups compared with placebo (Table 1). There were no significant differences between the two teriflunomide groups either in terms of the annualized rate of relapse with sequelae-EDSS/FS or in the number of patients remaining free from such relapses [Fig. 1a (i); Table 1]. There were also no significant differences between any of the treatment groups in the risk of sequelae-EDSS/FS per relapse [Fig. 1a (ii)].
Fig. 1.
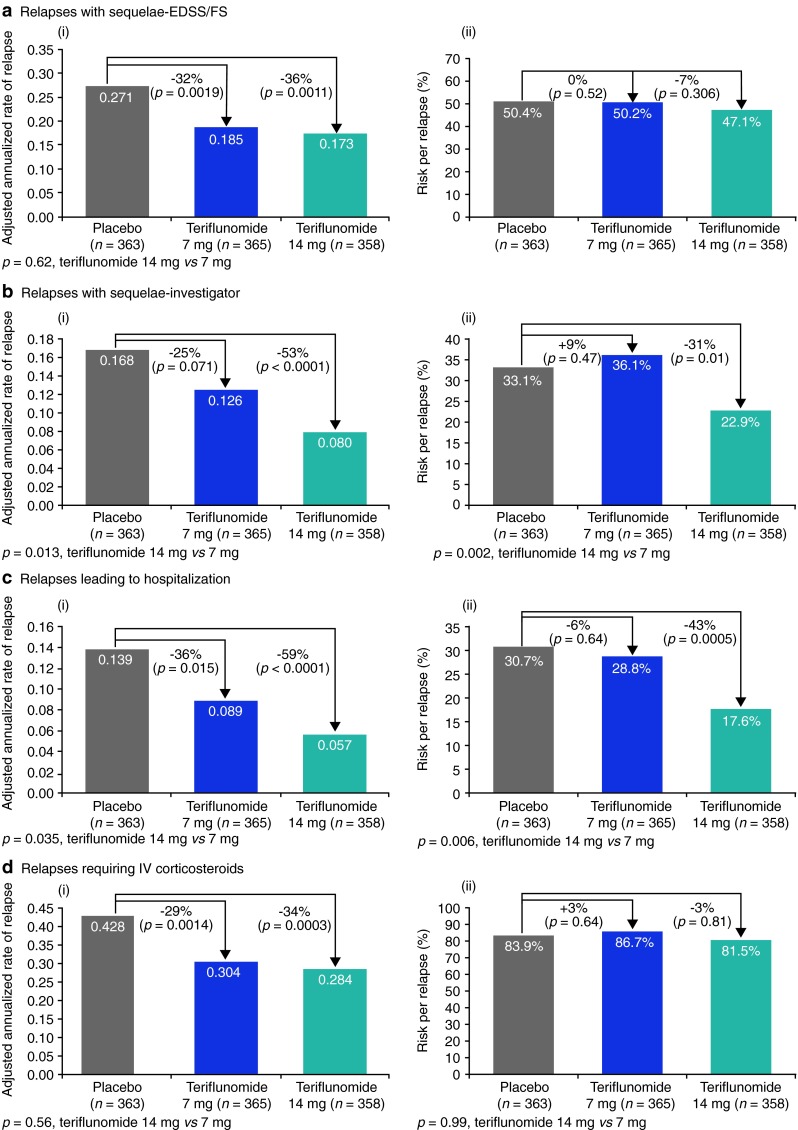
Adjusted annualized rates (i) and risk per relapse (ii) for each outcome analyzed: a relapses with sequelae-EDSS/FS; b relapses with sequelae-investigator; c relapses leading to hospitalization; d relapses requiring IV corticosteroids. Relative change: a positive sign shows a relative increase and a negative sign shows a relative decrease. Adjusted annualized rates were derived using a Poisson model with the total number of the outcome of interest as the response variable and treatment, Expanded Disability Status Scale (EDSS) strata at baseline and region as covariates, and log-transformed standardized study treatment duration as an offset variable. Relapses with sequelae: incomplete neurological recovery, defined by an increase of EDSS or Functional System (FS) 30 days post relapse, assessed every 30 days from 30 to 180 days after relapse or incomplete neurological recovery as assessed by the investigator at the end of relapse. Missing data regarding intravenous (IV) corticosteroid use were reported in 12.6 % of relapses
Fig. 2.
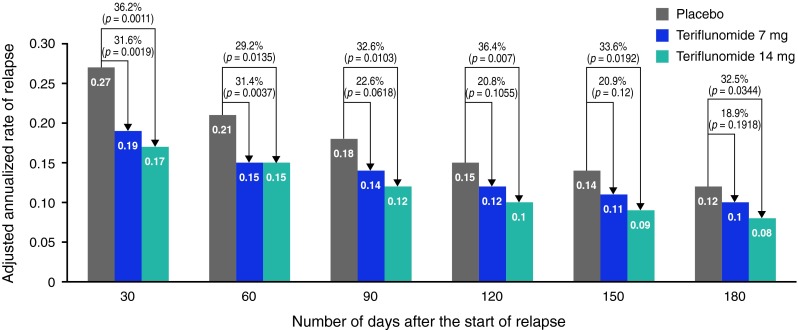
Adjusted annualized rate of relapse with sequelae-EDSS/FS assessed over time. Relapses with sequelae: incomplete neurological recovery, defined by an increase of EDSS Expanded Disability Status Scale or FS Functional System between last assessment before relapse and at least 30 days post relapse, and assessed every 30 days from 30 to 180 days after relapse
Table 1.
Raw percentages of patients free from relapses to relative change versus placebo associated with teriflunomide treatment (TEMSO modified-ITT population)
| Placebo (n = 363) | Teriflunomide 7 mg (n = 365) | Teriflunomide 14 mg (n = 358) | Relative change (%)a | |||
|---|---|---|---|---|---|---|
| 7 mg vs. placebo | 14 mg vs. placebo | 7 mg vs. 14 mg | ||||
| Patients free of relapses with sequelae-EDSS/FS [% (n), 30 days post onset of relapse]b | 64.7 (235) | 72.3 (264) | 77.4 (277) | 12 p = 0.0311 | 20 p = 0.0002 | p = 0.124 |
| Patients free of relapses with sequelae-investigator [% (n), end of relapse]c | 77.4 (281) | 80.0 (292) | 86.9 (311) | 3 p = 0.42 | 12 p = 0.0009 | p = 0.016 |
| Patients free of relapses leading to hospitalization [% (n)] | 80.4 (292) | 86.0 (314) | 90.8 (325) | 7 p = 0.047 | 13 p < 0.0001 | p = 0.049 |
| Patients free of relapses requiring IV corticosteroids [% (n)]d | 55.1 (200) | 62.5 (228) | 66.5 (238) | 13 p = 0.050 | 21 p = 0.002 | p = 0.23 |
ITT intention to treat, IV intravenous, TEMSO TEriflunomide Multiple Sclerosis Oral
aRelative change: a positive number indicates more patients free from relapse compared with placebo
bIncomplete neurological recovery, defined by an increase in the Expanded Disability Status Scale (EDSS) or Functional system (FS) 30 days post relapse
cIncomplete neurological recovery, as assessed by the investigator at the end of relapse
dMissing data regarding intravenous (IV) corticosteroid use was reported in 12.6 % of relapses
Relapses with sequelae: sequelae-investigator
The annualized rate of relapse with sequelae, determined at the end of the relapse by the investigator, was lower in both teriflunomide groups than in the placebo group (placebo 0.168; teriflunomide 7 mg 0.126; teriflunomide 14 mg 0.080) [Fig. 1b (i)]. Although teriflunomide 7 mg was associated with a non-significant reduction of 25 % (p = 0.071 vs. placebo), the 14 mg dose reduced the annualized rate of relapse with sequelae-investigator by 53 % (p < 0.0001 vs. placebo) [Fig. 1b (i)]. Significantly more patients also remained free from relapses with sequelae-investigator in the 14 mg group but not in the 7 mg group compared with placebo (Table 1). Teriflunomide 14 mg had a significantly greater treatment effect than 7 mg on both the annualized rate of relapse with sequelae-investigator (p = 0.013) and the proportion of patients free from such relapses (p = 0.016) [Fig. 1b (i); Table 1, respectively]. Likewise, the risk of sequelae-investigator per relapse was reduced by 31 % with teriflunomide 14 mg (p = 0.010 vs. placebo) [Fig. 1b (ii)], with the 14 mg dose having a more pronounced treatment effect than the 7 mg dose (p = 0.002).
Relapses leading to hospitalization
The annualized rate of relapses leading to hospitalization was lower in both teriflunomide groups than in the placebo group (placebo 0.139; 7 mg 0.089; 14 mg 0.057) [Fig. 1c (i)]. Both doses of teriflunomide reduced the annualized rate of relapses leading to hospitalization compared with placebo: 7 mg by 36 % (p = 0.015) and 14 mg by 59 % (p < 0.0001) [Fig. 1c (i)]. Significantly more patients in both teriflunomide groups than in the placebo group remained free of relapses leading to hospitalization (Table 1). The treatment effects associated with teriflunomide 14 mg were more pronounced than with 7 mg for both the annualized rate of relapses leading to hospitalization (p = 0.035) and the proportion of patients free from such relapses (p = 0.049) [Fig. 1c (i); Table 1]. Teriflunomide 14 mg reduced the risk of hospitalization per relapse by 43 % (p = 0.0005 vs. placebo) [Fig. 1c (ii)], and also had a significantly greater treatment effect than the 7 mg dose (p = 0.006).
Variability exists between countries in hospitalization for MS relapse. However, rates of hospitalization as a routine practice for relapse were similar across the three treatment groups (placebo 78 %; teriflunomide 7 mg 79 %; teriflunomide 14 mg 83 %) while the effects of teriflunomide 14 mg treatment were consistent between individual countries participating in the TEMSO study.
Relapses requiring IV corticosteroids
The annualized rate of relapses requiring IV corticosteroids was lower in both teriflunomide groups than in the placebo group (placebo 0.428; 7 mg 0.304; 14 mg 0.284) [Fig. 1d (i)]. Both doses of teriflunomide reduced the annualized rate of relapses requiring IV corticosteroids compared with placebo: 7 mg by 29 % (p = 0.0014) and 14 mg by 34 % (p = 0.0003) [Fig. 1d (i)]. In addition, both doses of teriflunomide led to more patients remaining free of relapses requiring IV corticosteroids compared with placebo (Table 1). No significant difference was observed between the two teriflunomide doses for either outcome [Fig. 1d (i); Table 1]. IV corticosteroid use during relapse was common and there were no significant between-group differences with regard to the risk of requiring IV corticosteroids per relapse [Fig. 1d (ii)].
Relapse intensity
No significant between-group differences were observed with regard to intensity of relapse, as determined by the investigator at the time of the initial relapse assessment (Table 2).
Table 2.
Intensity of relapse as assessed by investigator at the onset of relapse (TEMSO modified-ITT population)
| Severity of relapse (investigator assessed) | Number of relapses (%) | ||
|---|---|---|---|
| Placebo (n = 335 relapses) | Teriflunomide 7 mg (n = 233 relapses) | Teriflunomide 14 mg (n = 227 relapses) | |
| Mild | 117 (34.9) | 77 (33.0) | 90 (39.6) |
| Moderate/severe | 218 (65.1) | 156 (66.9) | 137 (60.3) |
| p value vs. placebo (χ2 test) | – | 0.6425 | 0.2548 |
ITT intention to treat, TEMSO TEriflunomide Multiple Sclerosis Oral
Effects of teriflunomide treatment on healthcare resource consumption
Over the course of the TEMSO trial, the mean (standard deviation) number of nights spent in hospital for relapse per patient was lower in both teriflunomide groups than in the placebo group [placebo 2.1 (6.5); teriflunomide 7 mg 1.1 (3.5); teriflunomide 14 mg 0.9 (4.1); p < 0.01 for both dose groups vs. placebo]. For 1,000 patients treated for two years, this would translate into 1,000 and 1,200 hospitalized nights for relapse saved with teriflunomide 7 and 14 mg, respectively.
The annualized rate of all hospitalizations was lower in both teriflunomide groups than in the placebo group (placebo 0.213; teriflunomide 7 mg 0.170; teriflunomide 14 mg 0.140) (Fig. 3a). Teriflunomide 7 mg was associated with a non-significant reduction of 21 % (p = 0.13 vs. placebo) whereas teriflunomide 14 mg reduced the annualized rate of all hospitalizations by 34 % (p = 0.008 vs. placebo) (Fig. 3a). Differences between the two teriflunomide groups were not significant.
Fig. 3.
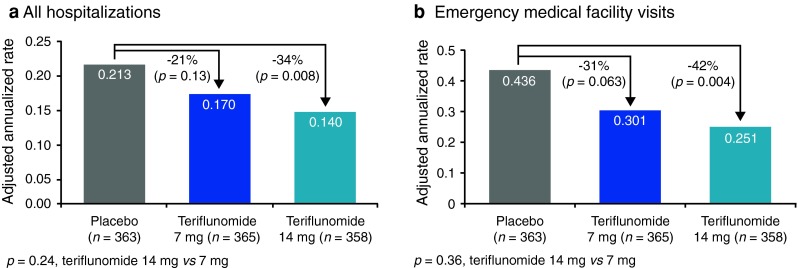
Adjusted annualized rates of a all hospitalizations and b emergency medical facility visits
Teriflunomide 7 mg was associated with a non-significant reduction in the annualized rate of EMFV of 31 % (p = 0.063 vs. placebo), whereas teriflunomide 14 mg significantly reduced this by 42 % (p = 0.004 vs. placebo) (Fig. 3b). Differences between the two teriflunomide groups were not significant.
Discussion
This post hoc analysis of TEMSO evaluated the impact of teriflunomide on relapse-related neurological sequelae, hospitalizations and IV corticosteroid use, and healthcare resource consumption in patients with RMS. Consistent and positive benefits of teriflunomide treatment were observed on a range of relapse outcomes characterized using several definitions. A dose effect of teriflunomide was also noted for a number of the relapse outcomes assessed. In addition, teriflunomide-treated patients had lower rates of healthcare resource use than placebo-treated patients; teriflunomide-treated patients spent significantly fewer nights in hospital for relapse, while those receiving the 14 mg dose had significantly lower annualized rates of all hospitalizations and EMFV. Based on these findings, teriflunomide has the potential to reduce total healthcare resource use and decrease the substantial clinical and economic burden associated with MS relapses.
Randomized clinical trials in MS traditionally evaluate treatment effects in terms of overall impact on relapse frequency. While the challenges of cross-study comparisons are exacerbated by the variable severity and sequelae of relapses and their tendency to decline in frequency over time, meta-analyses suggest that the current first-line injectable therapies [i.e., interferon β-1a/1b (IFNβ-1a/1b) and glatiramer acetate] provide significant but largely similar benefits on relapse frequency [14]. Given the substantial health and economic burdens associated with MS relapses, it is also informative to evaluate the effects of treatment on outcomes from relapse, particularly in terms of neurological sequelae and their impact on overall healthcare resource consumption. However, to the best of our knowledge, such studies are limited. The pivotal placebo-controlled trial of IFNβ-1b showed a twofold reduction in the frequency of relapses rated as moderate or severe compared with placebo, which led to a significant reduction in the number of hospitalizations over the course of the study [3]. The EVIDENCE trial showed that patients treated with high-dose IFNβ-1a (Rebif®; 44 mcg subcutaneously three-times weekly) had a lower rate of IV corticosteroid use for relapses than patients treated with low-dose IFNβ-1a (Avonex®; 30 mcg intramuscularly once weekly), although there were no differences in the occurrence of relapses rated mild, moderate, or severe [13]. More recently, post hoc analyses of the FREEDOMS and TRANSFORMS studies showed that treatment with fingolimod 0.5 mg was associated with fewer severe relapses, reduced IV corticosteroid use/hospitalization for relapse, and a reduction in relapses with incomplete recovery compared with either placebo or Avonex® [2]. This post hoc analysis of the TEMSO data set extends these observations.
This analysis has limitations that are worthy of further discussion. First, any post hoc analysis has inherent limitations; the study was not powered a priori to evaluate the outcomes analyzed herein and therefore, further prospective confirmation is required to support our observations. Second, there is an apparent inconsistency between the positive effects of teriflunomide treatment on certain relapse outcomes and relapse intensity, for which no significant benefit of treatment was observed. However, relapse intensity was assessed by the investigator based on a subjective rating only. For relapses with sequelae, both a subjective assessment based on an investigator rating and a more objective definition were applied which yielded congruent effects. Furthermore, hospitalization for relapse is an objective measure to assess relapse severity/intensity and one in which there is a clear and positive effect of teriflunomide treatment. Although differences exist between countries with regard to the management of relapse, including indications for steroid treatment and the decision to hospitalize for relapse, the fact that countries with varying approaches to relapse management were represented in TEMSO and were evenly distributed across the three treatment groups increase confidence in the validity of this outcome. Finally, while we demonstrate a benefit of teriflunomide treatment in terms of a reduction in healthcare resource use (as demonstrated by positive outcomes on IV corticosteroid use, hospitalizations and EMFV as captured on study CRFs), other factors can also contribute to the consumption of healthcare resources by patients with MS. Further studies are required to determine the effects of teriflunomide treatment on these other contributors to healthcare resource use.
In summary, this post hoc analysis demonstrates that teriflunomide therapy was associated with a reduction in the risk of relapse-related residual neurological deficits, IV corticosteroid use, hospitalization, and EMFV (14 mg dose only). Consequently, teriflunomide may reduce healthcare costs associated with relapses.
Electronic supplementary material
Acknowledgments
This study was supported by Sanofi. Editorial assistance was provided by Scott Chambers, Fishawack Communications Ltd, also funded by Sanofi.
Conflicts of interest
This study was supported by Sanofi.
P.W. O’Connor – Consulting fees and/or research support: Actelion, Bayer, Biogen Idec, BioMS, Cognosci, Daiichi Sankyo, EMD Serono, Genentech, Genmab, Novartis, Roche, sanofi-aventis, Teva, and Warburg Pincus.
F.D. Lublin – Research support: Acorda Therapeutics, Biogen Idec, Novartis, Teva Neuroscience, Genzyme, sanofi-aventis, National Institutes of Health and National MS Society; consulting agreements, advisory board/DSMB membership: Bayer HealthCare, Biogen Idec, EMD Serono, Novartis, Pfizer, Teva Neuroscience, Genmab, Medicinova, Actelion, Allozyne, sanofi-aventis, Acorda, Questcor, Avanir, Roche, Celgene, Abbott, MorphoSys, and Johnson & Johnson; speaker bureau/honorarium agreements: EMD Serono and Teva Neuroscience; stock ownership: Cognition Pharmaceuticals.
J.S. Wolinsky – Consulting agreements/speaker: Astellas, Bayer HealthCare, Celgene, Consortium of MS Clinics, Eli Lilly, Medscape CME, Novartis, PRIME, sanofi-aventis, Serono Symposia International Foundation, Teva, Teva Neurosciences; and the National MS Society; royalties: Millipore (Chemicon International) Corporation; research/contractual support: Clayton Foundation for Research, NIH, and sanofi-aventis.
C. Confavreux – consulting fees: Biogen Dompé, Biogen Idec, Gemacbio, Genzyme Corporation, Hertie Foundation, Novartis, sanofi-aventis, Teva Pharma and UCB Pharma; lecture fees: Bayer Schering, Biogen Idec, Merck Serono, Novartis, sanofi-aventis, and Teva Pharma; research support: Bayer Schering, Biogen Idec, Merck Serono, Novartis, sanofi-aventis, and Teva Pharma; fees for membership of Company Advisory Boards: Biogen Idec, Genzyme, Novartis, sanofi-aventis, Teva Pharma, and UCB Pharma.
G. Comi – consulting fees: Novartis, Teva Pharmaceutical Ind. Ltd, sanofi-aventis, Merck Serono, Actelion, and Bayer Schering; lecture fees: Novartis, Teva Pharmaceutical Ind. Ltd, sanofi-aventis, Merck Serono, Biogen Dompè, Bayer Schering, and Serono Symposia International Foundation.
M.S. Freedman – research/educational grant support: Bayer Healthcare and Genzyme; honoraria/consultation fees: Bayer Healthcare, Biogen Idec, EMD Canada, Novartis, sanofi-aventis, Teva Canada Innovation and is a member of Company Advisory Board/Board of Directors/or other similar group for Bayer Healthcare, Biogen Idec, Merck Serono, Novartis, sanofi-aventis, and Celgene.
T.P. Olsson – consulting fees and/or research support: Biogen Idec, Merck Serono, and sanofi-aventis; participation in scientific advisory boards and/or speaking activities: Merck Serono, Biogen Idec, and sanofi-aventis.
A.E. Miller – research support: Acorda Therapeutics, Biogen Idec, Genentech, Genzyme, sanofi-aventis, Novartis, Roche and Teva; consulting fees: Acorda Therapeutics, Avanir, Biogen Idec, BioMarin, Chelsea Therapeutics, Daiichi-Sankyo, EMD Serono, GlaxoSmithKline, La-Ser, Merck Serono, Novartis Nuron Biotech, ONO, and sanofi-aventis.
C. Dive-Pouletty – employee of Sanofi R&D.
G. Bégo-Le-Bagousse – employee of Sanofi R&D.
L. Kappos – research support: Actelion, Advancell, Allozyne, BaroFold, Bayer HealthCare Pharmaceuticals, Bayer Schering Pharma, Bayhill, Biogen Idec, BioMarin, CLC Behring, Elan, Genmab, Genmark, GeNeuro SA, GlaxoSmithKline, Lilly, Merck Serono, MediciNova, Novartis, Novo Nordisk, Peptimmune, sanofi-aventis, Santhera, Roche, Teva, UCB, and Wyeth, and from the Swiss MS Society, the Swiss National Research Foundation, the European Union, and the Gianni Rubatto, Novartis, and Roche Research Foundations.
Footnotes
For the Teriflunomide Multiple Sclerosis Oral (TEMSO) Trial Group.
References
- 1.Genzyme Corporation US LLC (2012) Aubagio® (teriflunomide) prescribing information
- 2.Haas J, Hartung HP, von Rosenstiel P, Karlsson G, Tang D, Francis G, Kappos L, Cohen J (2011) Effect of fingolimod (FTY720) on severe multiple sclerosis relapses, healthcare utilization and recovery: results from two phase 3 studies, TRANSFORMS and FREEDOMS. Poster P06.049 presented at the 63rd Meeting of the American Academy of Neurology. Honolulu, Hawaii, USA, 9–16 April 2011
- 3.IFNB Multiple Sclerosis Study Group Interferon β-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/WNL.43.4.655. [DOI] [PubMed] [Google Scholar]
- 4.Kalb R. The emotional and psychological impact of multiple sclerosis relapses. J Neurol Sci. 2007;256(Suppl 1):S29–S33. doi: 10.1016/j.jns.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 6.Leone MA, Bonissoni S, Collimedaglia L, Tesser F, Calzoni S, Stecco A, Naldi P, Monaco F. Factors predicting incomplete recovery from relapses in multiple sclerosis: a prospective study. Mult Scler. 2008;14:485–493. doi: 10.1177/1352458507084650. [DOI] [PubMed] [Google Scholar]
- 7.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–1532. doi: 10.1212/01.WNL.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- 8.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 9.Morrow TJ. The costs and consequences of multiple sclerosis relapses: a managed care perspective. J Neurol Sci. 2007;256(Suppl 1):S39–S44. doi: 10.1016/j.jns.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 10.Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28:363–379. doi: 10.2165/11532230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS; TEMSO Trial Group (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
- 12.Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10:54–60. doi: 10.1111/j.1524-4733.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 13.Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, Monaghan E, Li D, Weinshenker B; EVIDENCE Study Group. EVidence of Interferon Dose-response: Europian North American Compartative Efficacy; University of British Columbia MS/MRI Research Group (2002) Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE trial. Neurology 59:1496–1506 [DOI] [PubMed]
- 14.Roskell NS, Zimovetz EA, Rycroft CE, Eckert BJ, Tyas DA. Annualized relapse rate of first-line treatments for multiple sclerosis: a meta-analysis, including indirect comparisons versus fingolimod. Curr Med Res Opin. 2012;28:767–780. doi: 10.1185/03007995.2012.681637. [DOI] [PubMed] [Google Scholar]
- 15.Vercellino M, Romagnolo A, Mattioda A, Masera S, Piacentino C, Merola A, Chio A, Mutani R, Cavalla P. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009;119:126–130. doi: 10.1111/j.1600-0404.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci. 2007;256(Suppl 1):S5–S13. doi: 10.1016/j.jns.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 17.Wolinsky JS, Narayana PA, Nelson F, Datta S, O’Connor P, Confavreux C, Comi G, Kappos L, Olsson TP, Truffinet P, Wang L, Miller A, Freedman MS (2013) Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler [Epub ahead of print]. doi:10.1177/1352458513475723
- 18.Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17(Suppl 5):S139–S145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


