Abstract
Purpose
Assess fertility preservation (FP) measures chosen by patients newly diagnosed with malignancy and their outcomes.
Methods
Reproductive-age patients referred for FP underwent counseling and elected cryopreservation vs. no treatment. Outcome measures included ovarian stimulation, FP choice, oocytes/zygotes retrieved/cryopreserved and pregnancy outcome.
Results
From 2005 to 2012, 136 patients were counseled with 124 electing treatment: 83 oocyte-only, 21 oocyte + zygote and 20 zygote-only cryopreservation. Age, partnership and financial status factored into FP choice. Treatment was completed in 12 ± 2 days with 14 ± 11 metaphase-II oocytes harvested and cryopreserved/cycle. Eight patients returned to attempt pregnancy; three succeeded.
Conclusions
Our data demonstrate that oocyte and/or zygote banking are feasible FP options for women with malignancy; given the choice, the majority elected oocyte cryopreservation, highlighting desire for reproductive autonomy. Continued growth and research, combined with interdisciplinary communication, will ensure that appropriate candidates are offered FP and the potential for future parenthood, an important quality-of-life marker for survivors.
Keywords: Cancer survivor, Fertility preservation, Oocyte cryopreservation, Embryo cryopreservation, Quality-of-life
Introduction
Malignancy in the reproductive-age female is a major health concern. In 2012, an estimated 790,740 women were diagnosed with cancer; approximately 9 % before age 45 [36]. Fortunately, due to improvements in early detection and treatment, female cancer death rates decreased by approximately 14 % between 1990 and 2008 [14]. Improved survival has resulted in a focus on survivorship issues, specifically those thought to improve quality of life [31], with the opportunity for family after cure at the forefront [33, 35]. Currently available data for most tumors suggest that post-treatment pregnancy does not necessarily impose an increased risk with regard to cancer progression or obstetrical/neonatal outcomes [3, 16, 17], making fertility preservation (FP) an attractive option to a newly-diagnosed patient.
Opportunities for parenthood after cancer have historically often only been available through adoption or the use of donor gametes. Innovations in ART, particularly in the field of FP (i.e. cryopreservation methods), have expanded these options to include those allowing the use of one’s own gametes. Although embryo cryopreservation has been considered the standard of care, technologies behind oocyte and tissue cryopreservation are moving these latter options into the mainstream arena with the experimental designation recently lifted from oocyte cryopreservation (OC) by the ASRM in the setting of anticipated gonadotoxic treatments [29]. Recent advances in technology have demonstrated success rates comparable to fresh IVF [5, 21, 32]. In addition, laboratory evaluation of oocytes retrieved in the setting of cancer appears comparable to those harvested for non-malignant indications [26, 40]. OC offers reproductive-age patients several advantages including: eliminating the need for donor sperm, minimizing the ethical, personal and religious constraints associated with embryo freezing and importantly, providing reproductive autonomy. Many patients presenting for FP today are not in a personal relationship conducive to childrearing. The cryopreservation of oocytes rather than embryos may provide an advantage when offering FP to a single, newly-diagnosed cancer patient and may be appropriate even in the setting of a partner. Here, we report our experience in offering oocyte (OC) and/or 2-pronuclear (i.e. zygote; ZC) cryopreservation to reproductive-age cancer patients.
Materials and methods
One year after establishing successful OC techniques, The New York University Fertility Center (NYUFC) initiated a FP program for women with cancer. This report includes all patients who presented to a single reproductive endocrinologist between April 2005 and February 2012 (n = 136). FP counseling was provided in compliance with ASRM guidelines [28]; patients offered and electing to proceed with treatment were consented for treatment (including disposition of oocytes and/or zygotes in the case of non-usage or patient demise). Institutional Review Board approval (#S12-00764) was obtained to report cycle outcomes.
Despite an initial desire to preserve fertility, 12 patients were either discouraged from doing so or elected not to proceed as a result of the following factors: advanced (≥43 y) or premenarchal age, clinical condition (terminal disease and or cardiac/respiratory compromise), parity (already had “enough” children), medical risk-adversity, or lack of financial/insurance resources. Therefore, outcomes are reported for those women electing OC (n = 83), ZC (n = 20), or a combination of OC + ZC (n = 21). Nine patients cycled twice for a total of 133 FP treatment cycles.
If timely (n = 92), baseline serum estradiol and FSH levels were assessed. Ovarian stimulation included the following regimens: injectable gonadotropins (follitropin beta, Serono Pharmaceuticals, Rockland, MA; Merck, Whitehouse Station, NJ; menotropins, Ferring, Parsippany, NJ) with LH suppression achieved using GnRH antagonist (n = 126; cetrorelix acetate, Serono Pharmaceuticals, Rockland, MA; ganirelix acetate, Merck) or agonist (n = 7; leuprolide acetate, TAP Pharmaceuticals, Lake Forest, IL). Ovulation trigger was achieved with human chorionic gonadotropin (n = 80; hCG, 10,000 units) or GnRH agonist (n = 53; leuprolide acetate, 0.4 cc = 2 mg). The latter was chosen when the peak serum estradiol level was >2500 pg/ml or if the patient was scheduled for chemotherapy or surgery within 2 weeks of oocyte harvest. Additionally, women diagnosed with breast malignancy (estrogen-receptor positive or negative) were offered an aromatase inhibitor (AI; letrozole 5 mg/day; Novartis Pharmaceuticals, East Hanover, NJ) during and/or immediately following ovarian stimulation. This protocol evolved over the study period to include AI administration throughout gonadotropin stimulation, along with the use of a GnRH agonist ovulation trigger, to mitigate overall estradiol response; AIs have been shown to be safe and effective when used in this respect [30].
Thaw/warming cycles performed in cancer patients electing OC, ZC or both are also included in this report. Clinical pregnancy was defined as fetal cardiac activity demonstrated on first-trimester transvaginal ultrasound; ongoing pregnancy was a gestation >14 weeks. Partnership was considered if a patient admitted to a current committed relationship of ≥1y duration. Statistical analysis was performed using chi-square, student T-test or the Mann–Whitney Rank Sum test with significance set at P < 0.05.
Oocyte cryopreservation and thawing/warming
Slow-cooling and vitrification methods were utilized for OC. Oocytes noted to be in metaphase II (MII) when evaluated 1.5 h post-harvest were considered suitable for preservation. OC and thawing/warming methods have been previously reported in detail [12] and are summarized below.
Slow cooling
Oocytes were briefly washed, then equilibrated using 1.5 mol/L propanediol (PROH), placed in loading solution containing 1.5 mol/L PROH plus 0.3 mol/L sucrose, then into cryopreservation straws (Conception Technologies, San Diego, CA). Temperature decrease was achieved using a controlled-rate freezer (Planer Products Limited, Sunbury, UK). At −150 °C, straws were plunged into liquid nitrogen (LN) and transferred to tanks for storage.
For thaw, oocytes were air-warmed, then placed in a 30 °C water bath. Cryoprotectants were removed using stepwise dilutions of PROH and sucrose; surviving oocytes were transferred to PBS and then to fresh media for culture.
Vitrification
Oocytes were washed, equilibrated and then transferred through sequential equilibration solutions containing ethylene glycol (EG) and dimethyl sulfoxide (DMSO) followed by placement in a vitrification solution containing EG, DMSO, and sucrose. Oocytes were then loaded into a CryoTip (Irvine Scientific, Santa Ana, CA) or Cryolock (Bio Diseňo, Bogata, Colombia) cryodevice, which was immediately sealed/capped and plunged into LN.
For warming, cryodevices were first agitated in a 37 °C water bath followed by placement in sequential thawing and equilibration solutions containing decreasing concentrations of sucrose. Oocytes were then washed in sucrose-free media and cultured.
2PN cryopreservation
Zygotes were equilibrated using 1.5 mol/L PROH, then serially transferred through four 1.5 M PROH-containing solutions before being loaded into cryovials (Quinns Advantage® Embryo Freeze Kit; CooperSurgical, Trumball, CT). Temperature was lowered using a Planer controlled-rate freezer with cryovials plunged into LN at −160 °C.
Thaw treatment cycles
Patients scheduled for OC or ZC thaw used either their natural ovulation or artificially prepared endometrium to time embryo replacement. For natural cycles, a 10,000 IU hCG injection was administered when the dominant ovarian follicle reached ≥17 mm; luteal-phase vaginal progesterone supplementation was added the day after hCG injection. If artificially prepared, sequentially increasing doses of oral estradiol (Barr Laboratories, Pomona, NY) were administered until the endometrial diameter reached ≥7 mm; the luteal phase was then supplemented with intramuscular (50 mg/day; Watson Pharmaceuticals, Corona, CA) or intravaginal (100 mg BID; Endometrin, Ferring, Parsippany, NJ) progesterone. In OC cycles, on the day of thaw, a fresh semen sample was obtained (if there was a male partner) or cryopreserved donor sperm was thawed. Semen specimens were processed via isolate (Irvine Scientific) or swim-up techniques and intracytoplasmic sperm injection (ICSI) was performed on all surviving MII oocytes. For ZC cycles, 2-PN zygotes were thawed and cultured until adequate embryo development was noted (most often, the blastocyst stage).
Results
Cycle outcomes
Cycle demographics and outcomes for all FP cycles are shown in Figs. 1, 2 and 3. One-hundred twenty-four patients with a mean age of 31 ± 7y completed treatment. Sixty (48 %) patients were partnered and 5 had at least one child prior to presenting for FP. Malignant diagnoses included 45 gynecologic, 42 breast, 24 hematologic, and 13 other (Fig. 1). In 92 patients, baseline hormone levels were assessed; the mean FSH level was 5 ± 3 IU/L and estradiol, 45 ± 24 pg/ml (no FSH value was >13.5 IU/L). For all patients, FP treatment was completed in 12 ± 2 days with a mean of 18 ± 13 (14 ± 11 MII) oocytes harvested. By diagnosis (Fig. 1), the youngest patients had hematologic malignancies (mean age: 26 ± 5y) and ovarian tumors of low malignant potential (mean age: 28 ± 7y) while the oldest had breast (mean age: 35 ± 5y) and endometrial (mean age: 36 ± 3) cancers. Mean body mass index (23–24 kg/m2) did not differ between the treatment groups. Stimulation as well as oocyte yield and maturity were adequate regardless of diagnosis. With regard to FP options (Fig. 2), 67 % of patients chose OC-only, 16 % ZC-only and 17 % a combination of OC + ZC. The majority (73 %) of patients electing OC-only were single whereas 90 % of those choosing ZC-only were partnered. Of the 21 patients that elected OC + ZC, 20 were partnered. When evaluating outcome by age (Fig. 3), as expected, menstruating teenagers had a robust response to gonadotropin stimulation unless significant ovarian surgery or remote chemotherapy preceded treatment; conversely, older patients had a lesser response. For breast cancer patients, the use of an AI along with gonadotropin stimulation was used in 34 patients resulting in a lower overall peak estradiol rise (826 ± 528 pg/ml) with a similar number (11 ± 9) of MII oocytes retrieved. Adding a GnRH agonist ovulation trigger to this stimulation regimen did not compromise the percent mature oocytes (73 %). In fact, a GnRH-agonist ovulation trigger was used in 53/133 (40 %) treatment cycles overall, resulting in 23 ± 15 oocytes retrieved (17 ± 12 MII; 76 % mature).
Fig. 1.
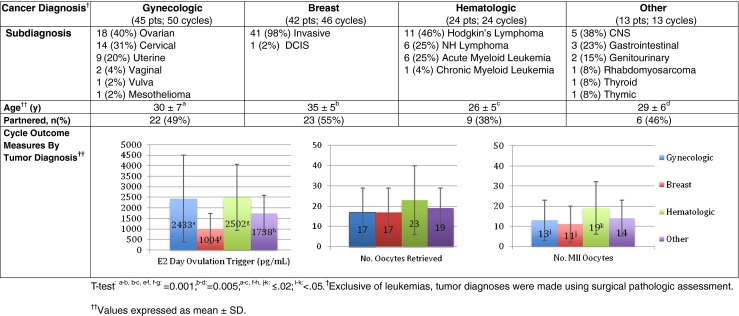
Demographics and outcomes by cancer diagnosis. Five gynecologic and 4 breast cancer patients cycled twice
Fig. 2.
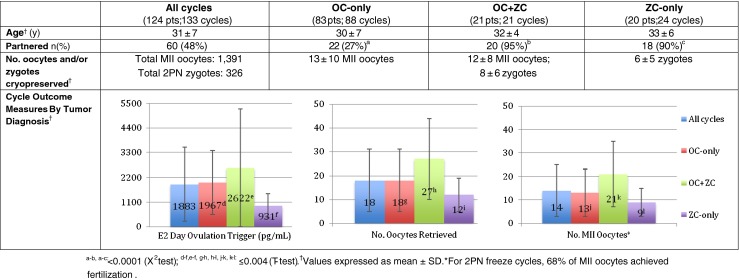
Demographics and outcomes of cycles by FP choice. One-hundred twenty-four patients completed 133 cycles
Fig. 3.
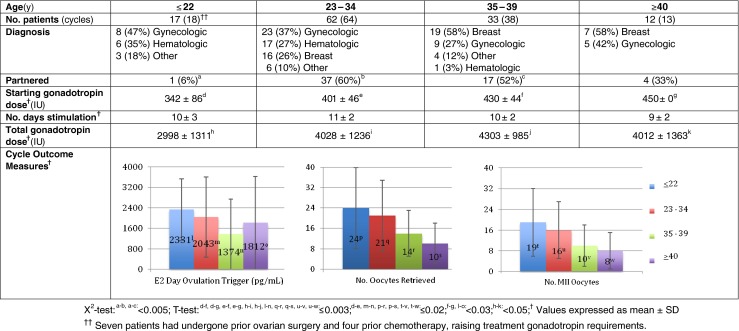
Demographics and outcomes of cycles by patient age at time of cryopreservation. The youngest treated patient was age 15 y. Of ≤22 year-old patients, 6 previously received prior remote chemotherapy and 8 had undergone ovarian surgery before FP treatment; without prior chemotherapy, the starting gonadotropin dosage was generally 225 IU/d administered for 2 days with frequent ovarian monitoring and dose adjustment until the day of ovulation trigger. For 23–34 year olds, 5 had received previous chemotherapy and 8 prior ovarian surgery
Quick-start strategies [23] including pre-treatment with a GnRH antagonist were utilized in 21 patients (mean age 33 ± 6y) with favorable cycle outcomes. In nine of these cycles, patients received a one-time 3 mg dose (preferable, but expensive) while in 21 cycles, a 250 μg dose was administered for 3 consecutive days. Four patients were also co-administered an oral contraceptive pill twice daily for 2–4 days to assist in achieving an optimal stimulation starting point; the latter strategy was avoided in patients with a breast cancer diagnosis. Quick-start cycle outcomes were comparable to those begun on menstrual-cycle day 2: mean number of days of stimulation 11 ± 2; peak estradiol 1349 ± 1216 pg/ml; mean oocytes retrieved 16 ± 8 (13 ± 7 MII). Seventy-seven percent of oocytes (264 MII/345 total) were mature.
Complications
Regarding complications, five patients had unforeseen experiences. Two suffered moderate ovarian hyperstimulation syndrome (OHSS); one was managed conservatively while the other required several emergency-room visits for fluid and pain management during her first round of chemotherapy commencing one day post-oocyte retrieval. One patient required hospitalization with blood transfusion for intra-abdominal bleeding after oocyte retrieval in the setting of a large bladder tumor. Another patient had only 4,000 platelets at the time of oocyte harvest and received platelet transfusion during the procedure; post-operatively, she was prophylactically transferred and maintained in the hospital without incident for 24 h. One additional patient with a large mediastinal mass at initiation of an OC cycle was discontinued on treatment day 3 secondary to compromised respiratory status. No other serious complications occurred.
Thaw cycles
A total of 13 thaws (5 OC + 8 ZC; mean age 34y) have been completed in 8 cancer survivors (Table 1). Three patients had cryopreserved oocytes-only, two zygotes-only and three had both oocytes and zygotes in storage. Five patients completed 2 separate thaw/transfer cycles: one with zygotes, then oocytes; three with zygotes twice; one with oocytes twice. One ZC-thaw only patient achieved two pregnancies. For the 5 OC thaws, 31 oocytes (mean: 6/cycle) underwent warming and ICSI; 15 embryos (mean: 3/cycle) were transferred, resulting in one live birth. For ZC, 25 zygotes (mean: 3/cycle) were thawed with 15 (mean: 2/cycle) transferred culminating in three live births. Thus, overall, the delivery rate per patient was 3/8 (38 %). Notably, the OC success resulted from oocytes previously slow-cooled at age 40.5y and thawed/replaced at age 43y, representing the oldest cancer survivor OC success to date. One additional cancer survivor recently thawed oocytes with the treatment cycle resulting in a viable ongoing twin gestation. Three patients required a gestational surrogate due to hysterectomy; one achieved a live birth. In non-cancer OC thaw cycles (n = 70; mean age: 34 ± 5y; max age 42y), 37 (53 %) women have achieved pregnancy with an overall live birth/ongoing pregnancy rate of 44 %. Similarly, zygote thaws performed in non-cancer patients at our center (n = 43) during the same time period have resulted in a comparable per-patient live-birth rate of 39 %.
Table 1.
OC and ZC thaw cycles performed in cancer survivors
| Thaw type | OC | ZC |
|---|---|---|
| n (cycles) | 5 | 8 |
| Age (y) | 34 ± 6 | 34 ± 5 |
| Diagnosis | Gynecologic, Breast, CNS | Gynecologic, Sarcoma |
| No. thawed | 31 (6/cycle) | 25 (3/cycle) |
| No. embryos transferred | 15 (3/cycle) | 15 (2/cycle) |
| Livebirth Outcome n (%) | 1 (20 %) | 3 (38 %) |
Values expressed as means ± SD
Discussion
Our results demonstrate the potential feasibility of offering FP to patients requiring gonadotoxic therapies. Importantly, as previously shown, FP treatment can be achieved with minimal-to-no delay in cancer treatment [2]. Success relies on an interdisciplinary approach, wherein the importance of referral is recognized not only by oncologists and reproductive endocrinologists, but also by nurses, genetic counselors, social workers, psychologists, and other specialists involved in patient care. In some geographic areas, a FP center may be remote; nonetheless, all reproductive-age cancer patients deserve appropriate counseling including the option to pursue FP at the nearest available facility. The success of such an approach depends on crucial networking between oncologists and fertility specialists. In addition, practitioners interested in performing FP need to become knowledgeable with regard to which cancer treatments have the potential for gonadotoxicity (e.g. alkylating agents, high-dose pelvic radiation) [11, 16, 18, 27, 37, 39]. This is imperative, given that the detrimental effects of cancer treatment on subsequent ART outcomes have been demonstrated [6, 8, 10]. Furthermore, certain gynecologic malignancies (e.g. early stage germ cell, cervical and endometrial tumors) are amenable to fertility-sparing procedures which have the potential to alter options available for appropriately-selected candidates [4, 7, 19, 22, 38]. Together, a strong and up-to-date knowledge base is essential to proper patient counseling.
Inherent to caring for cancer patients is the recognition that this is a different cohort than otherwise healthy, infertile women. Many have systemic comorbid conditions that must be recognized and addressed at the time of FP consultation and treatment. These processes can be generalized for several cancer types or be specific to an individual malignancy. For example, hematologic malignancies may have associated anemia, leukopenia, thrombocytopenia or pancytopenia which may be rapidly worsening. Thus, a complete blood count is recommended at the beginning, during and at the completion of the ovarian stimulation cycle. Concurrent management with the patient’s oncologist assists in addressing such issues, including the need for possible blood-product transfusion or even transfer to a hospital post-oocyte retrieval. Pre-consultation with an anesthesiologist is warranted in patients undergoing oocyte harvest who have increased risks or respiratory disease (e.g. mediastinal or thyroid masses that may compress and thereby cause airway compromise). In addition, patients with more serious co-morbid medical conditions often have subcutaneous ports or established central venous access at the time of FP treatment of which the treating anesthesiologist must be familiar. Lastly, patients diagnosed with genitourinary or gynecologic malignancies may have clinically apparent masses, as well as increased vascularity adjacent to tumor-containing areas. Special care should be taken during oocyte harvest to avoid puncturing these areas.
The initiation of a FP cycle can present logistical issues unique to patients undergoing treatment for medical indications. Depending on the timing of the patient’s last menstrual period and its relation to the initiation of upcoming cancer therapies, strategies may be required to allow for prompt commencement of ovarian stimulation so as not to delay oncologic treatment. As detailed previously by our group [23], several methods are available to initiate ovarian stimulation. In brief, these include the use of progestational agents, GnRH agonists, GnRH antagonists and oral contraceptive pills; often a combination of which can allow for near immediate ovarian stimulation. Which agent(s) are used should be individualized based on a cohort of factors: timing of last menstrual period, urgency for initiation of cancer treatment and the overall health status. Controlled ovarian hyperstimulation often proceeds similarly to standard IVF, albeit with a slightly higher gonadotropin dosage to maximize oocyte yield [23]. The exception is in the young oncofertility patient (i.e. <25y) who is administered a relatively low dose of gonadotropin (~225 IU/day) due to the heightened risk for OHSS. This is supported by our cohort; those patients with hematologic malignancies were overall younger than those patients with other malignancies, and they did have a greater number of MII oocytes retrieved than those with gynecologic or breast disease. Importantly, regardless of diagnosis, even in the setting of a full tumor load (e.g. lymphoma and leukemia), most patients had a good outcome. In addition, we have been successful in stimulating menstruating adolescents (and even one perimenarchal girl yet to experience her first menstrual bleed), albeit we approach this process with great caution and monitor these patients almost daily.
As an alternative to the traditional hCG ovulation trigger, a GnRH agonist is used whenever feasible (notably, avoided in the setting of hypothalamic suppression). This technique causes final maturation of the oocytes followed by rapid and profound luteolysis, decreasing the interval from oocyte harvest to next menses by approximately 1 week [9], particularly important for patients planning to receive chemotherapy immediately as it significantly reduces the incidence of OHSS and allows them to return to their “menstrual baseline” much quicker. Communication between the oncologist and reproductive endocrinologist during ovarian stimulation is imperative as a higher-than-expected ovarian response may mandate delay in cancer treatment or inpatient care during an upcoming round of previously-planned outpatient chemotherapy. Lastly, in patients with hormone-sensitive tumors, consideration should be given to the use of oral AIs administered concurrently with gonadotropins [24]. Although the effect of an abbreviated (two-week) period of elevated estradiol levels on tumor progression/prognosis is probably insignificant, the addition of an AI helps to mitigate the estradiol rise during ovarian stimulation. Our current protocol for patients receiving AI is to begin this medication on treatment cycle “day 2” continuing it until the day of ovulation trigger; a GnRH agonist trigger is also utilized in this setting due to its luteolytic effect.
The preliminary experience at our center demonstrates success in performing FP for medical indications, particularly when compared with standard IVF success rates [41]. At their initial consultation, patients are counseled on the various options available to them and our success rates to date. Of all FP procedures offered at our center, OC affords patients greatest reproductive autonomy. Our thaw data in non-cancer patients attests to the success a program can have using OC and ZC. Certainly, the flexibility and independence inherent in OC is an essential component of FP therapy. Autonomy is clearly important to women as demonstrated by our results; 84 % of patients choose to freeze at least a portion of their oocytes unfertilized despite 48 % of the treated cohort reportedly in a committed relationship. As an example of this phenomenon, the two patients who achieved pregnancy using thawed zygotes both have additional zygotes as well as unfertilized oocytes in storage. Certainly, creating fewer embryos in young patients with an uncertain prognosis mitigates potential future ethical challenges. Furthermore, even for patients in a committed relationship, the option to choose OC ± ZC allows for flexibility in the event that there is a relationship status change following the patient’s cancer treatment.
Thus far, we have not observed any worsening of cancer conditions or antenatal/neonatal complications or morbidity in this patient population, albeit our data set is small with only 8 (and now one additional) cancer survivors returning to attempt pregnancy. However, to date, there have been more than 1,500 live births resulting from OC, with no reported increase in birth anomalies [20, 21, 32, 34]. Similarly, several studies evaluating pregnancy in patients following fertility-sparing treatment for endometrial cancer have found no increased recurrence risk in those patients who undergo ART procedures [13, 15, 25]. This is consistent with survival data of breast patients who underwent FP with gonadotropin stimulation: no difference in relapse-free survival time was observed between those patients who underwent ovarian stimulation and those who did not pursue FP [1]. For the minority of patients in whom fertility-sparing surgery is not an option, they will require a gestational surrogate secondary to previous surgical removal of the cervix and/or uterus or contraindication to pregnancy. In our cohort, three patients who thawed their zygotes/oocytes required a gestational carrier; one achieved a healthy liveborn outcome. Third-party procreation represents an important aspect of counseling that, when appropriate, should be addressed during the initial consultation.
Our paper has several limitations. First, it is a retrospective review and represents the oncofertility experience of one program (treatment and referral bias). In addition, our center is only beginning to offer ovarian tissue cryopreservation, which may influence the options offered and selected by patients, particularly in the setting of tumors requiring urgent treatment. Regardless, in the literature, success with oocyte and embryo cryopreservation far exceeds that of ovarian tissue freezing for post-pubertal reproductive-age women and therefore should be considered first-line treatment in such patients. The two groups that might benefit from ovarian tissue freezing are patients with a very narrow time window prior to treatment and prepubertal girls who have no other FP option available to them. Despite such limitations, this review offers the largest and most comprehensive report of cancer patients desiring FP treatment (including OC). The reported experience highlights the feasibility of offering such patients these services. FP not only ensures that cancer patients’ reproductive rights are maintained but also that their desire for future parenthood is respected.
Acknowledgments
Dr. Noyes would like to acknowledge Dr. Eleonora Porcu of Bologna, Italy for introducing her to oocyte cryopreservation technology and its application as a fertility preservation measure for cancer patients. She also wishes to thank all the oncologists who entrusted her with the FP care of their patients as well as the entire IVF team at the NYULFC, especially Dr. James Grifo and Patti Labella for their efforts in making the oocyte cryopreservation program a success.
Conflicts of interest
None of the authors has a conflict of interest.
Footnotes
Capsule While undergoing cancer treatment, females considering fertility preservation can often complete these processes concomitantly. Oocyte cryopreservation was most popular, highlighting patient desire for reproductive autonomy.
Contributor Information
Nicole Noyes, Phone: +1-212-2637981, FAX: +1-212-2637853, Email: nnoyes01@gmail.com.
Katherine Melzer, FAX: +1-212-2637853.
M. Elizabeth Fino, FAX: +1-212-2637853.
Jaime M. Knopman, FAX: +1-212-2637853
References
- 1.Azim AA, Costantini-Ferrando M, Oktay M. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 2.Baynosa J, Westphal L, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. 2009;209:603–607. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Byrne J, Rasmussen SA, Steinhorn SC, Connelly RR, Myers MH, Lynch CF, et al. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet. 1998;62:45–52. doi: 10.1086/301677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiva L, Lapuente F, Gonzalez-Cortijo L, Carballo N, Garcia JF, Rojo A, et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111:S101–S104. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 6.Das M, Shehata F, Son WY, Tulandi T, Holzer H. Ovarian reserve and response to IVF and in vitro maturation treatment following chemotherapy. Hum Reprod. 2012;27:2509–2514. doi: 10.1093/humrep/des143. [DOI] [PubMed] [Google Scholar]
- 7.Diaz J, Sonoda Y, Leitao M, Zivanovic O, Vrown C, Chi D, et al. Oncologic outcome of fertility-sparing radical trachelectomy vs. radical hysterectomy for stage 1B1 cervical carcinoma. Gynecol Oncol. 2008;111:255–260. doi: 10.1016/j.ygyno.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Ginsburg ES, Yanushpolsky EH, Jackson KV. In vitro fertilization for cancer patients and survivors. Fertil Steril. 2001;75:705–710. doi: 10.1016/S0015-0282(00)01802-1. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 12.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:609–615. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 13.Han AR, Kwon YS, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Pregnancy outcomes using assisted reproductive technology after fertility-preserving therapy in patients with endometrial adenocarcinoma or atypical complex hyperplasia. Int J Gynecol Cancer. 2009;19:147–151. doi: 10.1111/IGC.0b013e31819960ba. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site, 2012. Website accessed: 1/31/12.
- 15.Ichinose M, Fujimoto A, Osuga Y, Minaguchi T, Kawana K, Yano T, Kozuma S. The influence of infertility treatment on the prognosis of endometrial cancer and atypical complex endometrial hyperplasia. Int J Gynecol Cancer. 2013;23:288–293. doi: 10.1097/IGC.0b013e31827c18a1. [DOI] [PubMed] [Google Scholar]
- 16.Knopman JM, Papadopoulos E, Grifo J, Fino ME, Noyes N. Surviving childhood and reproductive age malignancy: effects of treatment on fertility, gametes and future parenthood. Lancet Oncol. 2010;11:490–498. doi: 10.1016/S1470-2045(09)70317-1. [DOI] [PubMed] [Google Scholar]
- 17.Li FP, Fine W, Jaffe N, Holmes GE, Holmes FF. Offspring of patients treated for cancer in childhood. J Natl Cancer Inst. 1979;62:1193–1197. [PubMed] [Google Scholar]
- 18.Meirow D. Ovarian injury and modern options to preserve fertility in female cancer patients treated with high dose radio-chemotherapy for hemato-oncological neoplasias and other cancers. Leuk Lymphoma. 1999;33:66–76. doi: 10.3109/10428199909093726. [DOI] [PubMed] [Google Scholar]
- 19.Noyes N, Abu-Rustum N, Ramirez P, Plante M. Options in the management of fertility-related issues after radical trachelectomy in patients with early cervical cancer. Gynecol Oncol. 2009;114:117–120. doi: 10.1016/j.ygyno.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Noyes N, Porcu E, Borini A. With more than 900 babies born, live birth outcomes following oocyte cryopreservation do not appear different from those occurring after conventional IVF. Reprod Biomed Online. 2009;18:769–776. doi: 10.1016/S1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 21.Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryo and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010;94:2078–2082. doi: 10.1016/j.fertnstert.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Noyes N, Knopman J, Long K, Coletta J, Abu-Rustum N. Fertility considerations in the management of gynecologic malignancies. Gynecol Oncol. 2011;120:326–333. doi: 10.1016/j.ygyno.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Noyes N, Knopman J, Melzer K, Fino ME, Friedman B, Westphal L. Oocyte cryopreservation as a fertility preservation measure for cancer patients. Reprod Biomed Online. 2011;23:323–333. doi: 10.1016/j.rbmo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 25.Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, Nam JH. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121:136–142. doi: 10.1097/aog.0b013e31827a0643. [DOI] [PubMed] [Google Scholar]
- 26.Parmegiani L, Cognigni GE, Bernardi S, Cuomo S, Ciampaglia W, Infante FE, Tabarelli de Fatis C, Arnone A, Maccarini AM, Filicori M. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online. 2011;23:505–512. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646–1653. doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Practice Committee of the Society for Assisted Reproductive Technology Practice Committee of the American Society for Reproductive Medicine: Essential elements of informed consent for elective oocyte cryopreservation: a practice committee opinion. Fertil Steril. 2008;90:S134–S135. doi: 10.1016/j.fertnstert.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 29.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–1369. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Reh A, Lu L, Weinerman R, Grifo J, Krey L, Noyes N. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: results of a registry of female cancer patients at 2 years. J Assist Reprod Genet. 2011;28:635–641. doi: 10.1007/s10815-011-9559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh 'versus' vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruddy KJ, Partridge AH. The unique reproductive concerns of young women with breast cancer. Adv Exp Med Biol. 2012;732:77–787. doi: 10.1007/978-94-007-2492-1_6. [DOI] [PubMed] [Google Scholar]
- 34.Rudick B, Opper N, Paulson R, Bendikson K, Chung K. The state of oocyte cryopreservation in the United States. Fertil Steril. 2010;94:2642–2646. doi: 10.1016/j.fertnstert.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 35.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86:530–535. doi: 10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 37.Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late affects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 38.Wang CB, Wang CJ, Huang HJ, et al. Fertility-preserving treatment in young patients with endometrial adenocarcinoma. Cancer. 2002;94:2192–2198. doi: 10.1002/cncr.10435. [DOI] [PubMed] [Google Scholar]
- 39.Waring AB, Wallace WH. Subfertility following treatment for childhood cancer. Hosp Med. 2000;61:550–557. doi: 10.12968/hosp.2000.61.8.1398. [DOI] [PubMed] [Google Scholar]
- 40.Werner M, Reh A, Labella P, Noyes N. Laboratory evaluation in oocyte cryopreservation suggests retrieved oocytes are comparable whether frozen for medical indications, deferred reproduction or oocyte donation. J Assist Reprod Genet. 2010;27:613–617. doi: 10.1007/s10815-010-9455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.www.sart.org (accessed website 6/26/2012).


