Abstract
Purpose
Serum anti-Mullerian hormone (AMH) levels estimate ovarian reserve. The purpose of this study was to identify a minimum serum AMH level that correlates with acceptable clinical pregnancy rate (CPR) in women with severe diminished ovarian reserve (DOR) undergoing in vitro fertilization (IVF).
Methods(s)
A historical cohort of severe DOR participants (age ≥35) with day 3 FSH of >10 ng/mL were included (n = 120). Participants were categorized into 3 groups: AMH <0.2 (Group 1, n = 38), AMH = 0.2-0.79 (Group 2, n = 57) and AMH ≥ 0.8 (Group 3, n = 25) ng/mL. The main outcome was CPR. The number of retrieved and mature oocytes, transferred embryos, spontaneous abortion (SAB) and live birth (LB) rates were also evaluated.
Result(s)
Among the three groups, there was no difference in day 3 FSH and estradiol, total gonadotropins dose used per cycle, or LB. Participants in Group 1 were two years older than those in Group 2 and had significantly higher BMI than those in Groups 2 and 3. The three groups significantly differed in AFC (Group 1< Group 2< Group 3; p = 0.001) and cycle cancellation rate (Group 1> Group 2> Group 3; p = 0.006), and had a trend toward significance in SAB rate (Group 1> Group 2> Group 3; p = 0.06). Group 3 had significantly more retrieved and mature oocytes than Groups 1 or 2. Group 2 and 3 had significantly higher CPR per cycle start compared to Group 1. Although Group 2 had significantly fewer oocytes retrieved and mature oocytes than Group 3, CPR per cycle start for both groups was not different. ROC curve indicated that the point of maximal inflection between lower and higher CPR represents an AMH value of 0.2 ng/mL.
Conclusion(s)
AMH of 0.2 ng/mL appears to be a meaningful threshold for predicting CPR in women with severe DOR at our practice. This information can be crucial during the pre-cycle counseling of these women.
Keywords: Anti-Mullerian hormone, Diminished ovarian reserve, Clinical pregnancy, In vitro fertilization
Introduction
Anti-Mullerian hormone (AMH) is a protein produced by granulosa cells in the ovaries and is secreted into the bloodstream [1]. AMH plays a role in the regulation of follicular recruitment and oocyte development within the ovary [2]. In women, the highest value of AMH is attained after puberty and subsequently decrease with age, likely reflecting the age-related decline in ovarian reserve [1]. Serum AMH levels estimate ovarian reserve and the proximity of menopause [3]. Although we and others [4–12] have recently shown that serum AMH is affected by several factors including obesity, serum leptin and serum vitamin D; AMH remains one of the most reliable markers of ovarian reserve and is commonly used in assisted reproductive technology (ART) as a predictor of ovarian response in ovarian stimulation protocols in women with normal or diminished ovarian reserve (DOR) [13–15]. A low or undetectable AMH is usually observed in women with severe DOR [14, 16, 17].
Determining a minimum serum AMH cutoff to counsel women with severe DOR regarding their chances of achieving a clinical pregnancy is challenging due to the statistically low number of successful clinical pregnancy (CPR) and live birth (LB) rates in this patient population. It has been suggested that AMH <0.8–1.0 ng/mL is a poor predictor of ovarian response and likely CPR in all women with normal ovarian reserve [16, 18, 19] and an AMH <0.2 ng/mL has been suggested as a poor predictor of CPR in women with DOR [15]. The AMH level which can predict a relatively good CPR among severe DOR patients has not yet been established. We therefore aimed to identify an AMH level that discriminates between good and poor CPR in women with severe DOR.
Materials and methods
All DOR patients (age ≥35) defined by a maximal current or historic day 3 FSH of >10 mIU/mL who had available AMH levels and underwent IVF/ICSI cycles at Montefiore’s Institute for Reproductive Medicine and Heath between January 2008 and June 2013 were included in this study (n = 120). Patient and cycle characteristics including: 1) age and BMI of the female partner; 2) maximal current or historic day 3 FSH level and day 3 estradiol (E2); 3) antral follicle count (AFC); 3) total dose (IU’s) of gonadotropin used per cycle; 4) cycle cancellation rate; 5) use of assisted hatching; and 6) type of ART (IVF or ICSI) were evaluated. Cycle determinants included: 1) number of oocytes retrieved; 2) number of mature oocytes; 3) number of transferred embryos; 4) CPR (defined as intrauterine gestational sac on transvaginal ultrasound); and 5) spontaneous abortion (SAB) and LB rates. The severe DOR participants were categorized into three groups: AMH <0.2 ng/mL (Group 1, n = 38), AMH between 0.2 and 0.79 ng/mL (Group 2, n = 57) and AMH ≥0.8 ng/mL (Group 3, n = 25). The 0.2 and 0.8 ng/mL cutoffs were chosen based on previously published data [15–17, 19]. The study was approved by the institutional review board of Montefiore Medical Center at Albert Einstein College of Medicine.
Prevention of premature LH surge was achieved with GnRH agonists (started in the mid-luteal phase of the previous cycle) or antagonists (added daily, starting when the leading follicle reached a diameter of 14 mm or serum E2 reached 400 pg/ml). GnRH agonist microdose flare was also commonly used in our participants. Follicular growth was stimulated with injectable gonadotropins and was monitored with transvaginal ultrasounds and serum E2 levels. Human chorionic gonadotropin (hCG) injection was administered when at least 2 follicles had a mean diameter of greater than 17 mm. Oocyte retrieval was performed 34 h later using transvaginal ultrasound guidance. Fertilization was evaluated 16 to 18 h after insemination. The presence of two pronuclei on day 1 confirmed normal fertilization. All embryo transfers were performed on day 3 post retrieval. Pregnancy was first assessed using serum beta-hCG 14 days after oocyte retrieval and luteal support was provided with 50 mg of daily intramuscular injections of progesterone in oil until the documentation of a fetal cardiac activity.
Serum levels of AMH were measured with use of an ELISA kit (DSL-10-14400; Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer’s recommendations. Samples were run in duplicate by a single operator in the same laboratory without knowledge of group assignment. The lower limit of sensitivity was 0.1 ng/mL, and inter- and intra-assay coefficients of variation were less than 10 %.
Clinical pregnancy rate was the outcome of interest. Live birth and SAB rates were also analyzed. Data were expressed as means ± SD or number (percentages). One-way ANOVA was used for normally distributed data and Kruskal Wallis for skewed data. Chi-square test for categorical data with post hoc analysis was used as appropriate for comparison among the three groups. Sensitivity analysis using Receiver Operating Characteristic (ROC) curve (a graph of the sensitivity vs. 1-specificity) was performed to determine a cutoff level for AMH that predicted a relatively good CPR following IVF/ICSI in severe DOR participants. The area under the ROC curve (AUC) was also determined. P value <0.05 was considered to be statistically significant. Analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX).
Results
Among the three groups, there was no difference in maximum day 3 FSH (current or historical) and day 3 estradiol, use of assisted hatching, total gonadotropin dose used per cycle, or number of embryos transferred (Table 1). In addition to having the diagnosis of severe DOR, there was no difference in other infertility diagnoses (tubal factor, male factor, and endometriosis) and there was no difference in the ovarian stimulation protocols among the three groups (data not shown).
Table 1.
Demographics, cycle characteristics and cycle outcomes of the three groups of women with severe DOR
| Parameter | Group 1 (n = 38) AMH <0.2 ng/mL | Group 2 (n = 57) AMH 0.2-0.79 ng/mL | Group 3 (n = 25) AMH ≥ 0.8 ng/mL | p-value |
|---|---|---|---|---|
| Age (yr) | 41.2 ± 3.3a | 39.3 ± 3.0b | 40.2 ± 2.8 | 0.01a,b |
| BMI (kg/m2) | 32.6 ± 8.4a | 29.9 ± 7.6b | 25.3 ± 3.5c | 0.001a,b,c |
| AFC (#) | 5.4 ± 2.0a | 7.2 ± 2.7b | 8.9 ± 4.1c | 0.001a,b,c |
| Day 3 FSH (IU/l) | 15.8 ± 3.7 | 14.9 ± 6.1 | 13.2 ± 2.1 | 0.11 |
| Day 3 E2 (pg/ml) | 41.3 ± 20.4 | 42.4 ± 21.0 | 39.4 ± 17.3 | 0.83 |
| Gonadotropin dose (IU) | 4677.5 ± 1737.6 | 4666.9 ± 1779.5 | 4369.9 ± 1393.0 | 0.73 |
| Cycle cancellation | 19 (50)a | 18 (32)b | 4 (16)c | 0.006a,b,c |
| ICSI | 16 (42)a | 34 (59) | 17 (68)b | 0.04a,b |
| Assisted hatching | 15 (39) | 23 (40) | 16 (64) | 0.20 |
| # oocytes retrieved | 4.0 ± 2.2a | 4.9 ± 2.2a | 8.9 ± 5.1b | 0.001a,b |
| # mature oocytes | 3.1 ± 2.1a | 3.9 ± 2.2a | 7.1 ± 4.2b | 0.001a,b |
| # embryos transferred | 1.5 ± 1.2 | 1.9 ± 1.2 | 2.4 ± 1.7 | 0.15 |
| CPR per cycle start | 2* (10)a | 17** (35)b | 6*** (29)b | 0.003a,b |
| SAB/CPR | 1 (50) | 5 (29) | 0 (0) | 0.06 |
| Live birth rate/CPR | 0 (0) | 6 (35) | 3 (50) | 0.2 |
Data are expressed as means ± SD or n (%)
Superscripted letters indicates the statistical significance between corresponding groups
BMI body mass index
AFC antral follicle count
FSH follicle stimulating hormone
E2 estradiol
ICSI intra-cytoplasmic sperm injection
CPR clinical pregnancy rate
SAB spontaneous abortion
* 1 ongoing pregnancy
** 6 ongoing pregnancies
*** 2 ongoing pregnancies and 1 ectopic pregnancy
Participants in Group 1 were 2 years older than those in Group 2 (p = 0.01) and had significantly higher BMI than those in Groups 2 and 3 (p = 0.001). As expected, the three groups significantly differed in the number of AFC (Group 1< Group 2< Group 3; p = 0.001) and cycle cancellation rate (Group 1> Group 2> Group 3; p = 0.006). There was a trend toward statistical significance in SAB rate among the three groups (Group 1> Group 2> Group 3; p = 0.06). Group 3 had significantly more oocytes retrieved and more mature oocytes than Groups 1 or 2 (p = 0.001). Groups 2 and 3 showed a significantly higher CPR per cycle start compared to Group 1 (35 % and 29 % vs 10 % respectively, p = 0.003). Interestingly, although Group 2 had significantly fewer oocytes retrieved and mature oocytes than Group 3, CPR per cycle start for the two groups was not different (Table 1). Although there was no difference between LB rates among the three groups, the sample size seemed small, due to the strict inclusion criteria, to detect such a difference.
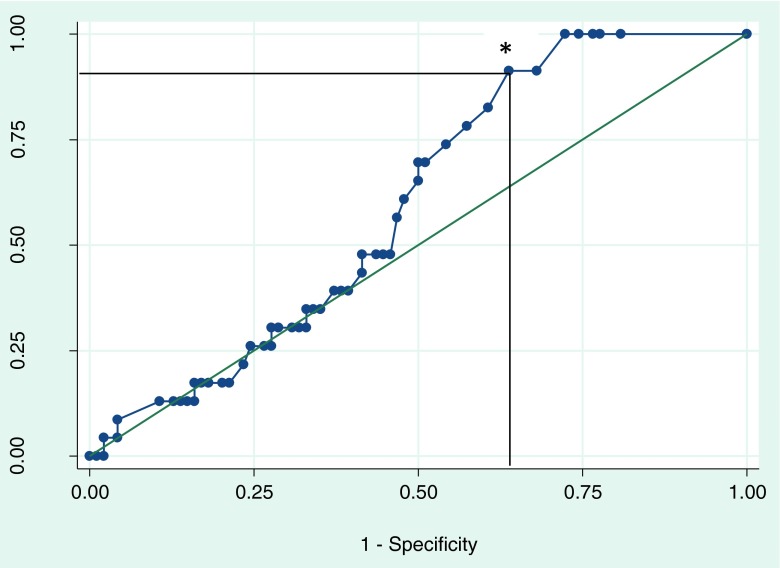
ROC curve (Fig. 1) of AMH indicated that the point of maximal inflection between lower and higher CPR represents an AMH value of 0.2 ng/mL (AUC = 0.6, standard error = 0.06, 95 % confidence interval [0.48– 0.71]) with a 91 % sensitivity and 36 % specificity.
Fig. 1.
ROC curve of AMH and clinical pregnancy rates involving IVF cycles in 120 women with severe DOR defined as age ≥35 and day 3 FSH >10 mIU/mL. The star indicates the point of maximal inflection, representing an AMH value of 0.2 ng/mL, AUC = 0.6, standard error = 0.06, 95 % confidence interval (0.48– 0.71) with a 91 % sensitivity and 36 % specificity
Discussion
In this study, we identified an AMH cutoff for acceptable CPR following ART in women with severe DOR. We found that severe DOR patients with a serum AMH level of > 0.2 ng/mL had significantly better CPR than severe DOR patients with an AMH level <0.2 ng/mL. The ROC curve results supported this cutoff. We concluded that, in our practice, an AMH level of 0.2 ng/mL can be considered a relatively good clinical predictor of CPR in women with severe DOR defined as age ≥35 and day 3 FSH >10 mIU/mL.
DOR describes women of reproductive age having regular menses whose fecundity or response to ovarian stimulation is reduced compared with comparably-aged women [20]. Although ovarian reserve tests (such as AFC, day 3 FSH, day 3 inhibin B, AMH, and clomiphene citrate challenge test) have been used and applied in clinical practices, debate continues over the ability of these tests to predict oocyte quality, oocyte quantity, and fecundity [21–23]. The goal of ovarian reserve testing especially in DOR patients is to add more prognostic information to the counseling and planning process in order to help couples choose among treatment options and to have a realistic expectations of fecundity [23]. Experts on ovarian reserve evaluation have long wondered whether a cutoff value for AMH in predicting fertility is adequate [16, 22–24]. Several studies have evaluated an AMH cutoff for predicting CPR and LB rates in women undergoing ART [16, 19, 24]. Gleicher et al. [16] demonstrated using ROC curves that AMH ≥1.06 ng/mL is a predictor of good LB rates in women with DOR. However, in their study, the mean AMH values of their participants ranged from 0.44 to 2.6 ng/mL whereas our participants’ AMH values ranged from 0.1 to 1.43 ng/mL, making our population much more severe in their DOR status.
Although AMH is one of the best markers of ovarian reserve, it has been shown to be altered by environmental factors such as obesity and vitamin deficiencies [6, 8–11]. For instance, we have recently shown that circulating vitamin D level may play a role in predicting serum AMH especially in late reproductive-aged women who are more likely to have DOR than early reproductive-aged women [7]. Additionally, we have shown that not only obesity in the context of DOR but also leptin (elevated concentration in obese women) directly suppressed AMH gene expression in human luteinized granulosa cells [11] indicating a strong relationship between obesity and AMH. Although we did not measure leptin or vitamin D in the present study, these factors and other unknown factors might constitute confounding variables in our severe DOR participants.
We acknowledge the limitations of this study, including its relatively small sample size, the definition of severe DOR and the definition of a “good” CPR. Having a large sample size in this severe DOR population can be challenging since a good number of IVF centers in the USA (personal communication) would not allow such women to enter ART due to a poor success in achieving a pregnancy. We perform approximately 200 IVF cycles at our institution and we had included participants with severe DOR over the last 5 years in order to get a sample size of 120. The purpose of this study was to extend the ART literature pertaining to AMH cutoff values in predicting CPR in women not only with DOR but with severe DOR. DOR has been defined in different ways, including both reduced fecundability (the ability to achieve pregnancy) and poor ovarian response to gonadotropin stimulation; there is no uniformly accepted definition for DOR according to the American Society for Reproductive Medicine [20]. We contentiously used age ≥35 and maximal FSH >10 mIU/mL to diagnose severe DOR. Additionally, the definition of “good” CPR is subject to debate although the percentages reported in this study were relatively acceptable for this patient population.
In summary, our results indicated that an AMH of ≥0.2 ng/mL appeared to constitute a meaningful threshold for predicting a relatively good CPR (>29 %) in women with severe DOR at our practice. Since pregnancy does occur in severe DOR patients with undetectable AMH (as observed in our study), it is important to emphasize that AMH is not infallible and should not be the sole criteria used to determine whether these severe DOR patients should have access to ART.
Acknowledgments
Conflict of interest
None
Footnotes
Capsule AMH of 0.2 ng/mL appears to be a clinically meaningful cutoff for predicting a relatively “good” clinical pregnancy rate in women with severely diminished ovarian reserve at our practice.
References
- 1.Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–84. doi: 10.1210/en.143.3.1076. [DOI] [PubMed] [Google Scholar]
- 3.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 4.Merhi Z, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB. Relationship of bariatric surgery to Mullerian-inhibiting substance levels. Fertil Steril. 2008;90(1):221–4. doi: 10.1016/j.fertnstert.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 5.Merhi Z. Impact of bariatric surgery on female reproduction. Fertil Steril. 2009;92(5):1501–8. doi: 10.1016/j.fertnstert.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Merhi Z. Weight loss by bariatric surgery and subsequent fertility. Fertil Steril. 2007;87(2):430–2. doi: 10.1016/j.fertnstert.2006.07.1499. [DOI] [PubMed] [Google Scholar]
- 7.Merhi Z, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril. 2012;98(1):228–34. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merhi Z, Zapantis A, Berger D, Jindal S. Insufficient follicular fluid vitamin D levels are associated with anti-mullerian hormone receptor gene upregulation in human luteinized cumulus granulosa cells of small follicles. Endocr Soc 2011.
- 9.Merhi Z, Buyuk E, Israel D, Messerlian G, Eklund B, Chua S Jr.et al. Follicular fluid leptin/adiponectin ratio and age negatively correlate with anti-mullerian hormone gene expression in human luteinized mural granulosa cells. Endocr Soc 2011.
- 10.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–5. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 11.Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S, Jr, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–9. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- 12.Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95(7):2364–8. doi: 10.1016/j.fertnstert.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 13.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–71. doi: 10.1016/S0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakhuda GS. The role of mullerian inhibiting substance in female reproduction. Curr Opin Obstet Gynecol. 2008;20(3):257–64. doi: 10.1097/GCO.0b013e3282fe99f2. [DOI] [PubMed] [Google Scholar]
- 15.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855–64. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94(7):2824–7. doi: 10.1016/j.fertnstert.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 17.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–50. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Buyuk E, Seifer DB, Younger J, Grazi RV, Lieman H. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369–72. doi: 10.1016/j.fertnstert.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 19.Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–65. doi: 10.1093/humrep/den108. [DOI] [PubMed] [Google Scholar]
- 20.Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2012; 98(6):1407–15. [DOI] [PubMed]
- 21.Celik H, Bildircin D, Guven D, Cetinkaya MB, Alper T, Batuoglu AS. Random anti-Mullerian hormone predicts ovarian response in women with high baseline follicle-stimulating hormone levels : anti-Mullerian hormone in poor responders in assisted reproductive treatment. J Assist Reprod Genet. 2012;29(8):797–802. doi: 10.1007/s10815-012-9794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KS, Segars JH. Predicting fertility with antimullerian hormone: is a cutoff value adequate? Fertil Steril. 2012;98(6):1421–2. doi: 10.1016/j.fertnstert.2012.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barad DH, Weghofer A, Gleicher N. Utility of age-specific serum anti-Mullerian hormone concentrations. Reprod Biomed Online. 2011;22(3):284–91. doi: 10.1016/j.rbmo.2010.12.002. [DOI] [PubMed] [Google Scholar]