Abstract
Purpose
The aim of this study is to determine whether vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) could increase the survival of xenografted human ovarian tissue in an experimental rabbit model.
Methods
Fresh human ovarian tissue was xenotransplanted into the back muscle of 25 castrated female New Zealand rabbits for 6 weeks with the immunosuppression of FTY720 (2 mg/kg/d). Rabbits were randomly divided into five experimental groups: (A) graft and host treatment with VEGF (50 ng/ml); (B) graft and host treatment with bFGF (100 ng/ml); (C) graft and host treatment with VEGF(50 ng/ml) + bFGF (100 ng/ml); (D) graft and host treatment with normal saline; (E) control group, no treatment. 4 weeks after transplantation, human menopausal gonadotropin (HMG) 10 IU was administered every second day in group A, group B, group C and group D for 2 weeks. Graft survival was assessed by graft recovery rate, histological analysis, immunohistochemical staining for CD31 and Ki-67expression, TUNEL assay.
Results
After 6 weeks of grafting, the number of CD31-positive stained cells increased significantly in group A, group B and group C compared to the control group. All groups showed strong Ki-67 immunostaining in ovarian stroma. Only one rabbit in group C retained the grafts’ follicles. Grafting resulted in relative lower fibrosis in group A and group C compared to the control group. Apoptosis was significantly lower in group C compared to the control group.
Conclusions
Fresh human ovarian cortex grafted into the back muscle of rabbit can sustain part of ovarian tissue function with the immunosuppression of FTY720, although follicle number diminishes significantly after grafting. The administration of VEGF and bFGF, especially the combination of them, may trigger angiogenesis, reduce apoptosis and fibrosis, increase survival in transplanted human ovarian tissue.
Keywords: Ovarian preservation, Xenotransplantation, VEGF, bFGF, Angiogenesis, Rabbit
Introduction
Advances in cancer diagnosis and treatment have greatly enhanced life expectancy of young women who suffer from malignant diseases. But radical operation, chemo- and radiotherapy often result in massive destruction of the ovarian reserve, losing both endocrine and reproductive functions [1]. For these patients, ensuring their reproductive capacity after oncologic treatment has become a major concern. Furthermore, because of delaying childbearing, there is a higher probability of encountering nonparous patients with a malignancy requiring treatment that is potentially sterilizing. Although some fertility conservation options are currently available, the most appropriate choice of these methods depends on the individual’s status.
Apart from fertility-sparing surgery and surgical ovarian transposition, embryo cryopreservation is the most established option for preserving fertility [2]. But it is only an option for patients who have a partner or are willing to accept fertilization by donor sperm; it also requires ovarian stimulation for follicular development and necessitates a delay of the chemotherapy initiation. Cryopreservation of oocytes does not require a partner, but the problem of unfertilized mature oocytes being damaged during the freezing or thawing process still needs to be improved [3]. Ovarian tissue cryopreservation and transplantation emerges as a promising option for preserving future reproductive potential. Ovarian tissue cryopreservation requires neither a sperm donor nor ovarian stimulation and thus can be performed immediately after cancer diagnosis. Although still experimental, ovarian cortex cryopreservation coupled with autologous transplantation has been thoroughly studied, the live births after autologous transplantation of fresh or frozen/thawed ovarian tissue are over 30 [4–6]. However, the cellular and molecular injury with the freezing and thawing process and the retransmission of malignant cells in the reimplantation of ovarian tissue [7] are the main concerns during the ovarian tissue cryopreservation and autotransplantation.
Human ovarian xenografting is a useful model to determine the potential of ovarian transplantation. It may reduce the risk of transmission of cancer cells back into body without delaying the start of cancer therapy and avoid the possible exposure to high hormonal level when patients undergo ovarian stimulation. These factors make ovarian xenotransplantation an attractive and promising assisted reproductive technology when specific safety and ethical issues are addressed in the future.
For successful freezing or transplantation, very small fragments (on the order of 1–2 mm) of the ovarian cortex are requested [4]. The small grafts, without the distinct arterial feeding blood vessels and surgical reanastomosis, are acutely dependent on the neovascularization process. Thus, implants are exposed to ischemic damage in the post-transplantation period until new vasculature develops. In mice, initial perfusion of the ovarian autograft is observed 3 days post-transplantation [8]. In rat, functional vessels within the ovarian xenografts have been detected by MRI and histology from day 7 and on [9]. In humans, hypoxic period is identified during the first 5 days after transplantation followed by gradual oxygenation of the ovarian transplant over the next 5 days [10]. Revascularization process is completed associated with massive follicle loss and limits the longevity and success of ovarian transplants [10]. Ischemic injury during autograft processes induces the depletion of 60–95 % of the follicular reserve, including the loss of virtually the entire population of growing follicles [11]. It has proved that more follicles die from ischaemia during transplantation than from freeze–thaw injury during cryopreservation [12]. Therefore, there is a great need to enhance neoangiogenesis after ovarian transplantation.
The regulation of ovarian angiogenesis is a complex process that involves multiple vasoactive and angiogenic factors [13]. Vascular endothelial growth factor (VEGF) is an angiogenic factor with known effects on the immigration, proliferation, and differentiation of endothelial cells, formation of immature veins, and vein permeability [14]. Basic fibroblast growth factors (bFGF) is one of the FGF family, and it plays important roles in various developmental processes, such as stimulating endothelial cells migration and mitosis, maintaining granulosa cell viability during follicular development. In ovary, VEGF and bFGF are expressed by granulosa and theca cells, as well as the corpus luteum and may be involved in the follicular development [15, 16]. bFGF also has been shown to protect cells, including cultured granulosa cells from undergoing cell death [17]. Nevertheless, the functions of the two angiogenic factors in the ovarian xenotransplantation have not yet been fully elucidated.
Considering the fact that early initiation of angiogenesis is a crucial factor for the maintenance of transplanted ovarian tissues, angiogenic factors need to be present in effective amounts at transplanted position, for the critical time immediately after transplantation in order to induce neovascularization and therefore prevent ischaemic hypoxia [18].
Typically, severe combined immune deficiency (SCID) mice are used as xenograft recipients owing to their T- and B-cell deficiency, which allow survival of the transplanted grafts without a rejection reaction. But due to the immunodeficiency, strict aseptic microscopic operation and costly feeding conditions are needed. So we hypothesized the large mammal – rabbit as recipient. Because it is feasible to do the surgical procedure on rabbit without special instruments, and monitor easily and repeatedly through noninvasive techniques such as blood samples testing, cervical-vaginal cytology, ultrasounds and et al. Value can as well be found in investigating the rabbit as xenograft recipient with the development of anti-xenograft immune rejection technology.
The aim of this study is to investigate if the transplantation effect of human ovarian tissues after xenografting into rabbit can be improved by host and graft treatments with VEGF, bFGF, or both.
Materials and methods
Experimental animals
Nonpregnant adult female New Zealand rabbits (8–12 months old, weight 2.5 ± 0.2 kg, N = 25) were housed in individual cages in an efficiency particulate air-filtered positive pressure room, at constant room temperature (24 °C). Animals had free access to standard rabbit chow and water under 12 h light: 12 h darkness conditions. Rabbits were provided by the Experimental Animal Center of Zhejiang University. This study was approved by Experimental Animal Ethics Committee of Zhejiang University (Hangzhou, China).
Animal bilateral oophorectomy
Rabbits underwent bilateral oophorectomy at laboratory of Experimental Animal Center of Zhejiang University (Hangzhou, China), anesthetized by 1 ml/kg pentobarbital sodium (intravenous (i.v.)). The abdominal region was shaved and cleaned, sterilized with povidon-iodine solution, then placed with surgical dressings. After infiltrating the lower abdomen with lidocaine, medical laparotomy was then performed to isolate and remove the ovary. After ensuring adequate hemostasis, the abdominal cavity was closed in layers. All animals received penicillin 120 mg one time per day for 3 days after wounding.
Human ovarian tissue preparation
Nine patients undergoing surgery for gynecologic disease, aged 30 to 35 years (mean age ± standard deviation (SD), 32.9 ± 1.96 years) were included in this study, following informed consent and approval of Women’s Hospital, School of Medicine, Zhejiang University ethical committee. The patients’ characteristics, including age and disease status when available, are listed in Table 1. Ovarian tissue was collected in a sterile tissue culture dish and immediately transported to the laboratory in Leibovitz L-15 medium (Sigma, St Louis, MO, USA) containing 500 IU/ml penicillin G and 500 IU/ml streptomycin at 4 °C and minced into 2.0 × 2.0 × 1.0 mm before transplantation. One slice from every ovarian sample was fixed in Bouin’s solution immediately after ovarian dissection for evaluating pregrafting tissue condition and follicular density following histological preparation.
Table 1.
Transplantation data
| Patient(no.) | Histology | Age(y) | rabbits(n) | Groups |
|---|---|---|---|---|
| 1 | cervical carcinoma | 31 | 2 | A,C |
| 2 | endometrial cancer | 31 | 3 | A,C,E |
| 3 | cervical carcinoma | 35 | 3 | B,D,E |
| 4 | cervical carcinoma | 34 | 3 | A,B,D |
| 5 | cervical carcinoma | 32 | 3 | C,D,E |
| 6 | cervical carcinoma | 33 | 3 | B,C,D |
| 7 | endometriosis | 35 | 3 | B,C,E |
| 8 | cervical carcinoma | 30 | 3 | A,D,E |
| 9 | endometriosis | 35 | 2 | A,B |
Human fresh ovarian tissue xenotransplantation
Ovariectomized rabbits received 1 ml FTY720 (Novartis, Switzerland; 2 mg/kg/d diluted in distilled water) intramuscularly 3 days before transplantation. FTY720 is a potent immunosuppressive agent which interferes with the induction of a marked decrease in the number of peripheral blood lymphocytes [19]. To provide adequate local concentration of angiogenic factor immediately after xenografting, fresh ovarian slices were divided randomly into five groups and incubated at 37 °C, 5 % CO2 humidified incubator for 1 h in the handling medium containing different drugs: (A) human recombinant VEGF (50 ng/ml, PEPROTECH, USA), (B) human recombinant bFGF (100 ng/ml, PEPROTECH, USA), (C) VEGF (50 ng/ml) + bFGF(100 ng/ml) or (D) normal saline, (E) control tissues receiving nothing.
Recipient rabbits had been randomly divided into five above groups before transplantation. On transplantation day, operators picked rabbits randomly in corresponding group (Table 1), anesthetized as described previously. Back region was shaved and cleaned, sterilized with povidon-iodine solution, and placed sterile drapes. 3 cm incision was made respectively at the two sides of the skin near the buttocks, paralled with the dorsal midline. Each side made 6 incisions in muscle, ovarian tissue fragments were picked randomly and inserted into the incisions, then sutured with 4–0 nylon sutures. Finally, skin incisions were closed with 3–0 nylon sutures. All procedures were performed under aseptic conditions. After transplantation, the rabbits need hypodermic injection with corresponding drugs:
-
(A)
Pretransplantation grafts treatment with human recombinant VEGF: rabbits accepted hypodermic injection with 1 ml human recombinant VEGF (50 ng/ml) at transplant site 7 days after transplantation (n = 5).
-
(B)
Pretransplantation grafts treatment with human recombinant bFGF: rabbits accepted hypodermic injection with 1 ml human recombinant bFGF (100 ng/ml) at transplant site 7 days after transplantation (n = 5).
-
(C)
Pretransplantation grafts treatment by VEGF + bFGF: rabbits accepted hypodermic injection with 1 ml VEGF (50 ng/ml) + bFGF (100 ng/ml) at transplant site 7 days after transplantation (n = 5).
-
(D)
Pretransplantation grafts treatment by normal saline: rabbits accepted hypodermic injection with equivalent normal saline at transplant site 7 days after transplantation (n = 5).
-
(E)
Pretransplantation grafts no treatment: rabbits received nothing as control (n = 5).
After transplantation, rabbits received FTY720 (2 mg/kg) daily. The animals in group A, group B, group C and group D were stimulated with 10 IU of human menopausal gonadotropin (HMG) (Livzon Group, Zhuhai, China) every second day for 2 weeks starting at 4 weeks after transplantation.
Graft retrieval and histological preparation
The animals were euthanized 6 weeks after transplantation. The fresh and the transplanted ovarian tissue specimens were prepared for paraffin embedding, cutting into serial sections of 5 μm. Every fourth section was stained with hematoxylin–eosin. The others were used for immunohistochemical study of CD31 and Ki-67 expression and evaluation of apoptosis using the terminal deoxynucleotidyl transferase (TdT)–mediated dUTP nick-end labeling (TUNEL) assay. Histological analysis was examined blindly by two independent observers under a microscope (LEICA, Wetzlar, Germany).
Measurement of neoangiogenesis and proliferative activity
To study neoangiogenesis, epithelial cells of new blood vessels were stained using anti-human CD31. The samples for CD31 expression were incubated with a monoclonal mouse anti-CD31 human antibody (diluted 1: 350; Abcam, Cambridge, UK). Ki-67 is a nuclear antigen associated with cell proliferation and is present throughout the active cell cycle (late G1, S, G2 and M phases), but absent in resting cells (G0). The sections for Ki-67 staining were incubated with a monoclonal mouse anti-Ki-67 human antibody (diluted 1:400; Dako, Glostrup, Denmark). After incubation with primary antibody, the sections incubated with Dako EnvisionTM Peroxidase, and at last, visualized with 3, 3’-diaminobenzidine tetrahydrochloride (Dako). All slides were counterstained with hematoxylin. Blank control was performed, and the negative control was subjected to the same technique except that the primary antibody was replaced by PBS.
To calculate vascular density, CD31 staining vessels were counting and averaging blood vessels at high magnification (×400) in five different sections in each tissue sample.
Analysis of fibrosis and necrosis
Fibrotic areas were characterized by poor cellularity, as evidenced by a reduced number of cell nuclei and collagen deposits. Necrotic areas were characterized by the presence of pyknotic nuclei in stromal cells and signs of cytolysis, including the presence of cell debris and an empty cytoplasm [20]. Sections were analyzed field by field at a 100 fold magnification. Necrosis and fibrotic areas in the grafts were considered to be signs of reduced ovarian tissue quality. Results were expressed as fibrotic and necrotic areas relative to total section areas.
TUNEL assay
TUNEL staining was carried out on paraffin wax embedded serial sections from fresh tissues (pretransplantation) and grafts. Tissue slices were stained using a commercially available kit (QIA33TDT-FragEL™ kits; Merck KGaA, Darmstadt, Germany) following the manufacturer’s instructions. TUNEL control tissue sections were included in all evaluations, according to the manufacturer’s recommendations. Positively labeled cells were counted at a 400 fold magnification, five different areas of each sample were examined, and the mean number of positively stained cells was determined.
Statistical analysis
Statistical analysis was performed with the SPSS 16 for Windows package (Statistics Package for Social Science, SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to test the hypothesis and the significant difference was considered where P-values were <0.05. The data were expressed as mean ± standard deviation (SD).
Results
Graft recovery and morphologic assay
Two rabbits died 1 week after transplantation during the study, probably due to the surgical infection. A rabbit in group A and one in group C had part dehiscence of the back surgical wound that was resolved by antibiotics and daily wound cleansing. The rest recovered from the wound well.
Xenografts were removed 6 weeks after transplantation. The administration of FTY720 contributed to a prolongation of graft survival in this model. The recovery rate was 93 % for group A (56/60), 83 % for group B (50/60), 88 % for group C (53/60), 87 % for group D (52/60) and 85 % for group E (51/60). Most of the tissues retained their original size, part of them were smaller than pretransplantation. The fragments showed a reddish color, with small visible vessels on the graft surface, and they were attached tightly to the surrounding muscle tissue.
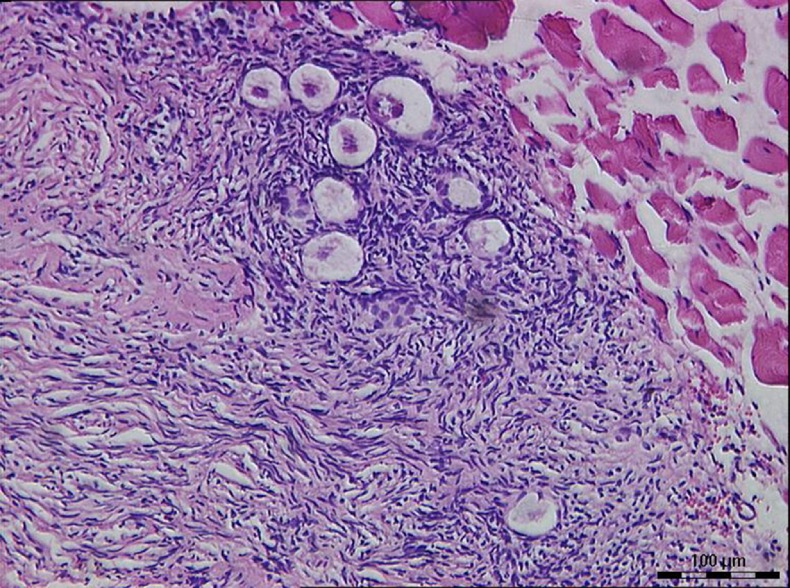
Under microscope, the pretransplantation grafts showed a limited number of follicles, about 0 to 5 follicles in each section at high magnification (×400), most of which were primordial follicles, with no antral follicle. After 6 weeks of xenograft, most of the grafts preserved their original tissue structure; the peripheral tissue of ovarian grafts infiltrated with little neutrophils and macrophages. Only one rabbit retained the follicles of all samples well in group C (from patient 2), most of which were primordial follicles (Fig. 1). Some follicles observed in fibrotic areas, nevertheless, looked healthy.
Fig. 1.
Primordial follicles in group C (×200)
Immunohistochemical analysis of neoangiogenesis and proliferative activity
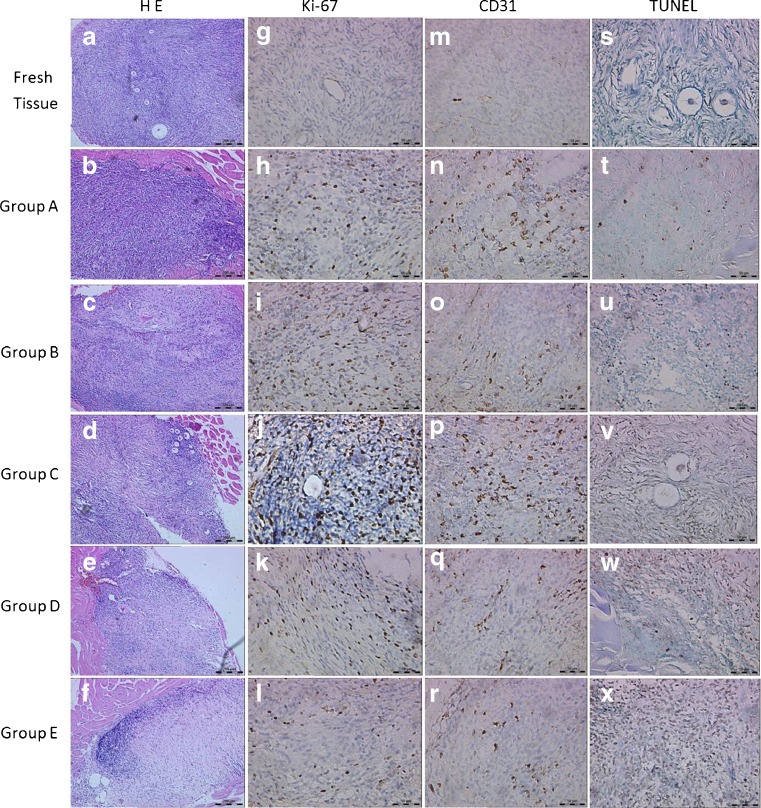
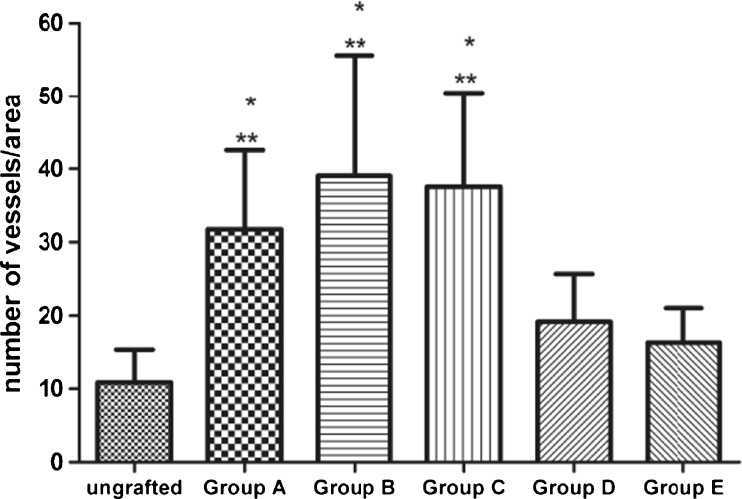
The average number of CD31-positive cells in pregrafted fresh tissues was limited (10.88 ± 4.46, at a 400 fold magnification) and the stain was weak. After transplantation, the number of CD31-positive stained cells increased, higher than the number of pretransplantation, especially in group A (VEGF), group B (bFGF) and group C (VEGF + bFGF) (Figs. 2 and 3). It showed intensive CD31-positive stained cells scattered not only at the interface between the ovarian graft and the rabbit muscle tissue, but also in the graft center (Fig. 2n–p). Furthermore, CD31-positive areas were filled with red blood cells showing functional competency of the newly formed vessels. But they were very low in the fibrosis.
Fig. 2.
Representative photographs of grafted samples and ungrafted fresh tissue. a–f Hematoxylin and eosin stain. Original magnification × 100. g–l Ovarian section stained for Ki-67 expression. Gafted ovarian sections showed the intensive Ki-67 staining cells in the stroma. Original magnification × 400. m–r Ovarian section stained for CD31expression. Note the strong brown staining in the stroma of grafted ovarian section, indicating CD31 expression. Original magnification × 400. s–x Ovarian section stained by TUNEL assay. Original magnification × 400
Fig. 3.
The vascular density was measured in CD31-labeled vessels. Results are presented as mean ± SD. CD31 staining vessels were counted at high-power filed (×400). * P < 0.01, significantly higher than ungrafted tissue, ** P < 0.01, significantly higher than Group E(control group)
Ki-67 mainly stained in granulose cells of follicles and a few stromal cells in pregrafted ovarian tissues. After transplantation, due to the loss of follicles, more Ki-67 positive cells showed in ovarian stroma and stained strong. Part of the primordial follicles recovered from group C showed Ki-67 staining in granulosa cells (Fig. 2j).
Histological analysis of stromal tissue fibrosis and necrosis
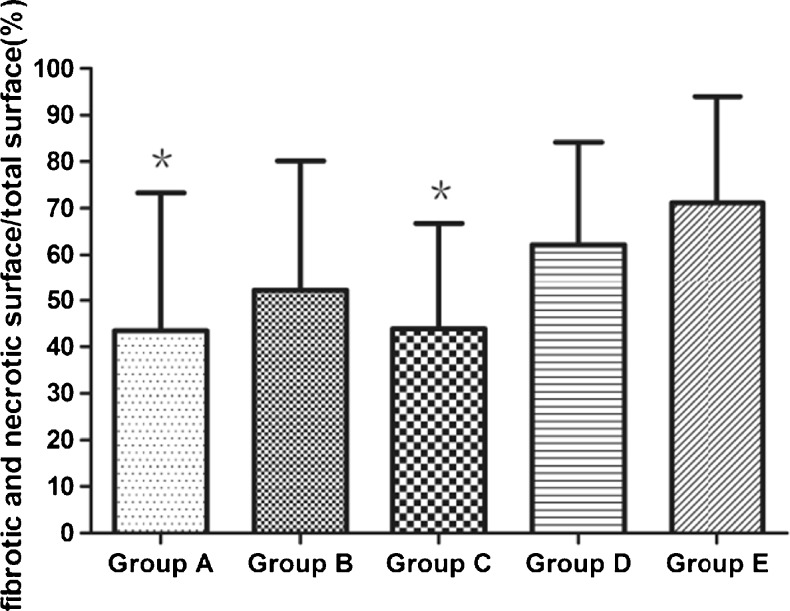
Little fibrosis was detected in pregrafted fresh tissues. After 6 weeks of xenografting, only a few cells showed necrotic features in xenografts, but significant differences in relative fibrotic areas were found according to the different treatment. Relative fibrotic and necrotic areas were significantly less pronounced in group A (VEGF) and group C (VEGF + bFGF) compared with group E (33.69 % ± 24.16 % versus 71.15 % ± 22.86 %, 43.79 % ± 22.97 % versus 71.15 % ± 22.86 %; P < 0.01, respectively), as illustrated in Fig. 4.
Fig. 4.
Relative fibrotic and necrotic area in five groups. Results are presented as mean ± SD. * P < 0.01, significantly lower than Group E (control group)
TUNEL assay
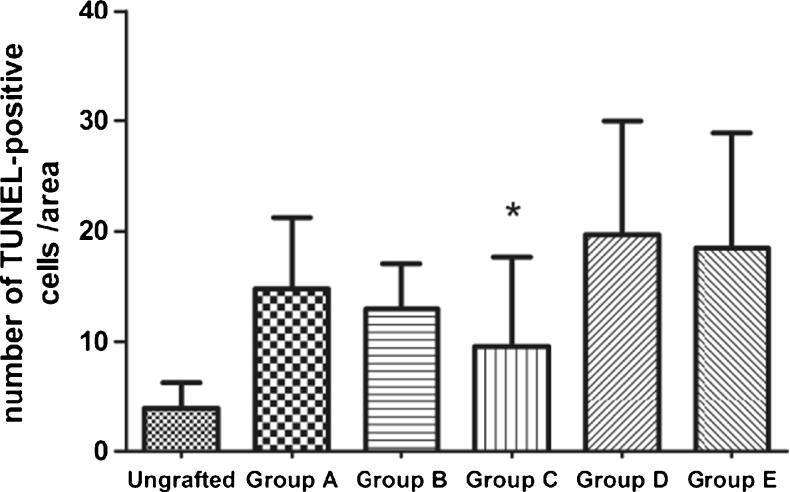
The TUNEL-positive signals were mainly detected in the ovarian stroma. There were few apoptotic cells detected in pregrafted fresh tissues, after transplantation, the number was increased. But the mean values of apoptotic cells in grafts treatment with VEGF + bFGF (Group C) had no statistical differences with that in pregrafted fresh tissues (P > 0.05), and group C also showed lower values than group E (P < 0.05) (Fig. 5). The follicles in group C showed weak staining in the granulosa cells. None of the negative controls showed TUNEL-positive immunoreactivity, while positive signals were consistently observed in the positive controls.
Fig. 5.
Apoptosis expression in ungrafted tissues and grafts. Results are presented as mean ± SD. Positively labeled cells were counted at high-power filed (×400). Only the number of TUNEL-positive cells in group C shows no statistically significant compared with ungrafted one (P > 0.05). * P < 0.05, significantly lower than Group E (control group)
Discussion
Currently, due to its safety and ethical issues, human ovarian tissue xenotransplantation is used in experiment research. Examine the possible presence of malignant cells in ovarian cortex from patients with tumors after xenografting of the ovarian tissue into host [21, 22], analyze follicular developmental potential [23], discuss oocyte quality and ovarian histology and function after cryopreservation [24, 25], investigate the methods improving ovarian function and reducing apoptosis after transplantation [20, 26, 27]. In our study, the ovarian xenotransplantation used pharmacological angiogenic factor in a rabbit model to improve angiogenesis after transplantation.
Graft revascularization after transplantation is still one of the most important problems that lead to substantial follicular loss and tissue fibrosis due to ischemia. Therefore, methods to improve and hasten graft vascularization are greatly needed. No clinical answer has been addressed to improve ovarian graft vascularization, except when the graft was inserted into granulation tissue [28]. Israely et al. [28] reported that implantation of ovarian graft into an angiogenic granulation tissue, created during wound healing, improved graft vascularization and follicular survival. Based on these results, this physiologic phenomenon was successfully applied for autotransplantation in women inducing new blood vessel formation by creating a peritoneal pocket or longitudinal opening of the ovary at the ovarian tissue transplantation sites 1 week before the transplantation [29, 30].
The administration of angiogenic factors has been used in ovarian transplantation animal models [27, 31]. According to previous data, the increased VEGF expression in the grafted ovaries appeared 2 days after transplantation [32], which is also the critical time that the total follicle pool was reduced and the area of the grafted tissue was non-reversibly damaged. In ovarian graft transplantation,VEGF is one of the most important factors for rapid establishment of rich blood supply, which is crucial for the survival of the ovarian follicles. bFGF is known as a growth factor involved in the folliculogenetic process. It has been reported as an initiator of folliculogenesis by inducing primordial follicle development [33] and as a regulator of ovarian angiogenesis. Since cytokines have mostly a paracrine or an autocrine mode of action, we believe that local concentrations play their role in ovarian functions better than blood concentrations. The concentrations of cytokines in the blood may not always contribute significantly to the concentrations of cytokines in the location of ovarian transplantation, especially before establishing the new blood vessels. Therefore we incubated the ovarian tissues with VEGF, bFGF or both before transplantation, and hypodermically injected them at transplant site 7 days after transplantation, for directly and steadily absorption in the critical period of graft vascularization. From day 7 and on, the grafts were perfused by functional vasculature [32].
Although the administration of VEGF and bFGF showed their potential to improve graft and follicular survival in animals, we still need to consider the risk of side effect of those angiogenic factors [31] if the results need to be eventually applied to human autotransplantation. Based on Lund et al. [34] experience with in vivo bFGF and VEGF expression levels in tumor xenografts, bFGF doses higher than 1,000 ng and VEGF higher than 200 ng per chamber were considered unphysiological and therefore irrelevant. In our experiment, the dosage we chose is relatively low and safe.
After 6 weeks of transplantation, stromal tissue fibrosis and necrosis showed differently in five groups. Ovarian stromal fibrosis and necrosis might mainly result from post-transplantation ischemia–reperfusion injury. As stromal cells are recruited to form the follicular theca and may produce paracrine factors essential for folliculogenesis and ovulatory processes, impairment of stromal cells may have consequences for later-stage folliculogenesis and final maturation steps [35]. With the treatment of VEGF and bFGF, the relative fibrotic and necrotic area in group A (VEGF) and group C (VEGF + bFGF) was reduced greatly more than that in group E (control group), but there was no statistical significance between group A and group C. The result was approximatively consistent with the expression of CD31. Endothelial-expressed protein CD31 is a marker of angiogenesis and mediates cell-to-cell adhesion. In group A (VEGF), group B (bFGF) and group C (VEGF + bFGF), CD31-positive stained cells were higher than those in group E, and scattered in the whole graft. But CD31-positive stained cells were very low in the fibrotic area in all groups.
In our study, we observed diffuse CD31 staining cells scattered in non- fibrotic ovarian tissue. According to Friedman et al. [27] study, two patterns of neovascular distribution were identified: a peripheral pattern associated with well-defined borders and more non-atretic follicles; and a diffuse one associated with unclear borders and fewer follicles. They contributed the peripheral pattern to the use of Hyaluronan-rich biological glue containing VEGF-A and vitamin E. It hastened graft adhesion to the tissue, which in turn hastened revascularization of the immediate area surrounding the graft. That may have other explanations. According to Van Eyck et al. [36] study, on days 3 and 5 after ovarian transplantation, human vessels were present throughout the entire graft, vascular structures were preferentially located at the periphery of the graft. On day 10, an increase in human vascular parameters was observed, predominantly in intermediate and central zones of the fragment. In Friedman et al. study, the duration of transplantation was 2 weeks, but in our study, the time was 6 weeks. Our study showed the activity and persistence of human vessels contribute to the revascularization of the grafts.
Ki-67 expression were seen in both folliculars and stromal cells in tissues after transplantation, suggesting not only that the stroma would be able to provide structure and support for developing follicles following grafting, but also that these follicles would be capable of further growth and development. In our study, massive Ki-67-positive cells appeared in the ovarian stroma after grafting. Cell proliferation was evidenced by Ki-67 immunostaining, which is a more sensitive indicator of growth than morphological analysis. It may result from stimulation of cell growth by ischemic and oxidative stress or administration of angiogenic substances after transplantation.
The neovascularization effect was also reflected by TUNEL Assay. With the new microvessels forming and blood infiltrating into grafts, the apoptosis was reduced in most cells. The relatively low apoptosis level in the grafts treated with VEGF and bFGF (group C) implied the better synergetic effect on angiogenesis than single treatment. In this experiment, we should analysis the ovarian function combining apoptosis level with other histological assessments. Although the apoptosis level was no statistical significance between group A (VEGF), group B (bFGF) and group E (control group), the treatment of VEGF or bFGF contributed in saving more ovarian stroma tissue from fibrosis.
VEGF and bFGF stimulate survival, proliferation, migration and differentiation of primary and stable endothelial cells, although the efficiencies of transduction of these responses are dependent on the type of endothelial cell line. Several lines of evidence have indicated VEGF acts as a very potent inducer of in vivo angiogenesis, the activation of VEGF partly influences the induction of bFGF-induced angiogenesis [37]. On the other hand, bFGF may promote angiogenesis directly by affecting the endothelial cells and indirectly through the up-regulation of VEGF in endothelial cells [38]. In addition, bFGF and VEGF have a synergistic effect on the induction of angiogenesis both in vitro and in vivo [39]. In our experiment, the neovascularization effect in group A (VEGF) and group B (bFGF) had no significant difference, but in group C (VEGF + bFGF), the synergistic effect showed its advantage. VEGF and bFGF are the most widely investigated angiogenic molecules to date, but there have been few comparative studies of these two factors. Differences in the mode of growth factor delivery, the dose of growth factor, the type of analysis and animal model may all underlie the differing conclusions. It is important to note that angiogenesis is a complex and multi-step process, many factors, possibly including both VEGF and bFGF, may be required to form a fully functional new network of blood vessels in ovarian transplantation.
In the present study, we presume that the exogenous angiogenic factors supplementation before and after transplantation promoted graft neovascularization. Although VEGF and bFGF augment the neovascularization effect especially under hypoxia, we still have to face the problem of the short half-life of these factors. With the development of tissue engineering [27, 40], the beneficial effect of angiogenic factors on further follicular development or fertility restoration should be confirmed.
Bilaterally ovariectomized animals have significantly elevated serum LH and FSH concentrations comparing with unmanipulated animals, ovarian graft vascularization in ovariectomized animals was markedly enhanced [41]. Besides, exogenous gonadotropins are also possibly involved in rapid vascular ingrowth and hence improved follicular survival in ovarian transplantation. It was reported that the ovarian transplantation showed a 40- to 60-fold increase in expression of VEGF and TGF beta-1 genes in the grafts, followed by a rapid increase in serum gonadotropin levels [42]. In this experiment, the use of HMG seemingly didn’t improve graft vascularization. The CD31-positive stained cells, TUNEL-positive a cells and fibrotic area in group D had no statistically significant comparing with group E (control group). This effect might be influenced by the timing of the injections relative to the time of grafting. The HMG was used 4 weeks after transplantation, missing the critical time that induced neovascularization and therefore caused fibrosis, which also indicated the importance of using angiogenic factor at the early stage of neovascularization. However, Abir et al. [31] did not observe the improvement of the primordial or growing follicles survival rate in the grafts when started gonadotrophin administration 2 days before and 2 days after grafting, compared with untreated recipients. They suggested that additional exogenous gonadotropin administration is probably unnecessary. So the effect of gonadotrophin is still controversial and its effect on further follicular development or fertility restoration should be confirmed.
It is obvious that the transplantation site has a vital impact on follicle survival by providing sufficient blood supply to the tissue. Soleimani et al. [43] investigated xenotransplantation of human ovarian tissues into mouse back muscle, showed a better histological morphology and survival, and slightly fewer apoptotic follicles. The graft environment may be implicated in the preservation of the stroma, the intramuscular site appears to induce less fibrosis than other sites [20]. In our study, grafts were totally embedded in the muscle tissue, prompt neovascularization led to the rapid integration of the grafts in the muscle tissue during the first few days after grafting, and which was found that mechanical separation of both was difficult in the rabbit that died 1 week after grafting. The intramuscular site also provides a larger manipulation and transplantation field and the back muscle tissue compartment may allow large follicles to develop.
It has been recommended that this graft protocol be limited to patients <35 years of age [44]. Young age is strongly correlated with high follicular density in pretransplantation tissues, but the availability of human ovarian tissue for research, in particular from young patients, is extremely limited. The low numbers of follicles identified in the recovered grafts in our study could indicate accelerated follicular loss after transplantation. However, there might be other explanations. Nowadays, more studies are tending to use the ovarian tissue from patients <30 years [27, 43, 45] for a larger pool of primordial follicles, so the human donors in our experiment were relatively old (all over 30). Based on Soleimani et al. [43] selection criteria, at least five visible follicles in each small ovarian tissue fragment is suitable for grafting. Although the patients we chose were young, we even found no follicle in some fresh control samples unexpectedly. Follicles are distributed unevenly in human ovary, and we might have inadvertently transplanted slices with no or only few follicles [31]. However, on analysis of the results from an endometrial cancer patient (Patient 2), a high number of follicles were identified in the transplanted samples in group C, most of which were primordial follicles. Primordial follicles should be least affected by ischaemia, in a resting state with a lower metabolic activity than large metabolically active follicles, which are more readily succumb to ischaemia [46], and therefore some follicles observed in fibrotic areas looked healthy. The standard of female fertility preservation is determined by the primordial follicle pool size, so it is noteworthy that as long as the pretransplantation samples contain enough follicles, there still has the possibility that follicles could survive well in rabbit model.
In our experiment, we first chose the rabbit as xenograft recipient. The rabbit models have several advantages over mouse models: (1) rabbits are larger than mice, providing greater blood volumes and more tissues to accept more ovarian tissues; (2) it is easier to get blood sampling at any time to test the serum hormone level changes via the ear marginal vein, in order to detect the ovarian endocrine function in vivo; (3) it is easier to perform complex surgical experiments in rabbits; (4) rabbits are more similar to human beings in genetics and physiology than mice, they fill the important position between mouse and large mammal models .
FTY720 is a potent immunosuppressive agent isolated from a culture broth of Isaria sinclairii, a kind of traditional Chinese herbal medicine [47]. A striking feature of FTY720 is an agonist of sphingosine 1 phosphate (S1P) receptors and thereby interferes with the induction of a marked decrease in the number of peripheral blood lymphocytes, especially T-cells. FTY 720 does not impair lymphocyte function, including T-cell activation, but induces the sequestration of circulating mature lymphocytes into the secondary lymphoid tissues and decreases T-cell infiltration into grafted organs [19]. It also inhibits the migration of naïve T cells from thymus into the blood, resulting in a declining supply of naïve T cells to the grafts [48].
In our preliminary experiment, we investigated different dosage of FTY720 used in this ovarian tissue xenotransplantation. We found that FTY720 at 2 mg/kg/d has an efficient immunosuppressive activity with low side effects. In order to reduce immunological rejection and maintain stable plasma concentration of FTY720, we used the drug 3 days before ovarian transplantation and every day after transplantation.
Whether or not FTY720 has an effect on the ovarian function, the report is limited. Zelinski et al. [49] discovered that female macaques given FTY720 by direct intraovarian cannulation for 1 week before ovarian irradiation rapidly resumed menstrual cycles because of maintenance of follicles. Offspring conceived and delivered by radioprotected females developed normally and showed no evidence of genomic instability. FTY720 may preserve ovarian function and fertility in female cancer patients exposed to cytotoxic treatments. In our study, most of the viable grafts showed no apparent changes when inspected. Part of the grafts were smaller than their original size, it might be due to the absorption of ischemic necrosis. The histological study under microscope showed that normal part of ovarian tissues were still similar to their former structure, with scarce lymphocytic infiltration.
In summary, the present study demonstrated that fresh human ovarian cortex grafted into the back muscle of rabbit could sustain part of the ovarian tissue function with the immunosuppression of FTY720, although follicle number diminished significantly after grafting. The administration of VEGF and bFGF, especially the combination of them, may trigger angiogenesis and reduce apoptosis in transplanted human ovarian tissue. The next phase of the study should be the xenotransplantation of cryopreserved human ovarian fragments and a longer transplantation period with gonadotrophin stimulation for more follicles from the ovarian grafts.
Acknowledgments
This research was supported by the Fund of National Science and Technology Support Plan (NO.2012BAI32B04).
Footnotes
Capsule The administration of VEGF and bFGF may trigger angiogenesis, reduce apoptosis and fibrosis, increase survival in human ovarian tissue xenotransplantation.
References
- 1.McLaren JF, Bates GW. Fertility preservation in women of reproductive age with cancer. Am J Obstet Gynecol. 2012;207:455–62. doi: 10.1016/j.ajog.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 3.Baka SG, Toth TL, Veeck LL, Jones HJ, Muasher SJ, Lanzendorf SE. Evaluation of the spindle apparatus of in-vitro matured human oocytes following cryopreservation. Hum Reprod. 1995;10:1816–20. doi: 10.1093/oxfordjournals.humrep.a136182. [DOI] [PubMed] [Google Scholar]
- 4.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98:720–5. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Revelli A, Marchino G, Dolfin E, Molinari E, Delle PL, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99:227–30. doi: 10.1016/j.fertnstert.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–14. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 8.Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114:341–6. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- 9.Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI. Magn Reson Med. 2004;52:741–50. doi: 10.1002/mrm.20203. [DOI] [PubMed] [Google Scholar]
- 10.Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–65. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou XH, Wu YJ, Shi J, Xia YX, Zheng SS. Cryopreservation of human ovarian tissue: comparison of novel direct cover vitrification and conventional vitrification. Cryobiology. 2010;60:101–5. doi: 10.1016/j.cryobiol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:883–900. doi: 10.1053/beog.2000.0133. [DOI] [PubMed] [Google Scholar]
- 14.Kaczmarek MM, Schams D, Ziecik AJ. Role of vascular endothelial growth factor in ovarian physiology - an overview. Reprod Biol. 2005;5:111–36. [PubMed] [Google Scholar]
- 15.Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131:254–60. doi: 10.1210/en.131.1.254. [DOI] [PubMed] [Google Scholar]
- 16.Seli E, Zeyneloglu HB, Senturk LM, Bahtiyar OM, Olive DL, Arici A. Basic fibroblast growth factor: peritoneal and follicular fluid levels and its effect on early embryonic development. Fertil Steril. 1998;69:1145–8. doi: 10.1016/S0015-0282(98)00074-0. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds LP, Redmer DA. Expression of the angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in the ovary. J Anim Sci. 1998;76:1671–81. doi: 10.2527/1998.7661671x. [DOI] [PubMed] [Google Scholar]
- 18.Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163:141–6. doi: 10.1016/S0303-7207(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 19.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–44. [PubMed] [Google Scholar]
- 20.Dath C, Van Eyck AS, Dolmans MM, Romeu L, Delle VL, Donnez J, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod. 2010;25:1734–43. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 21.Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’Anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 22.Lotz L, Montag M, van der Ven H, von Wolff M, Mueller A, Hoffmann I, et al. Xenotransplantation of cryopreserved ovarian tissue from patients with ovarian tumors into SCID mice–no evidence of malignant cell contamination. Fertil Steril. 2011;95:2612–4. doi: 10.1016/j.fertnstert.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod. 2010;25:2559–68. doi: 10.1093/humrep/deq192. [DOI] [PubMed] [Google Scholar]
- 24.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–8. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 25.David A, Dolmans MM, Van Langendonckt A, Donnez J, Amorim CA. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil Steril. 2011;95:1241–6. doi: 10.1016/j.fertnstert.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6:e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–82. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 28.Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21:1368–79. doi: 10.1093/humrep/del010. [DOI] [PubMed] [Google Scholar]
- 29.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrow transplantation for sickle cell anaemia: case report. Hum Reprod. 2006;21:183–8. doi: 10.1093/humrep/dei268. [DOI] [PubMed] [Google Scholar]
- 31.Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95:1205–10. doi: 10.1016/j.fertnstert.2010.07.1082. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90:1550–8. doi: 10.1016/j.fertnstert.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–30. doi: 10.1016/S0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 34.Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6:4287–91. [PubMed] [Google Scholar]
- 35.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 36.Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 37.Tille JC, Wood J, Mandriota SJ, Schnell C, Ferrari S, Mestan J, et al. Vascular endothelial growth factor (VEGF) receptor-2 antagonists inhibit VEGF- and basic fibroblast growth factor-induced angiogenesis in vivo and in vitro. J Pharmacol Exp Ther. 2001;299:1073–85. [PubMed] [Google Scholar]
- 38.Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD. Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation. 1995;92:11–4. doi: 10.1161/01.CIR.92.1.11. [DOI] [PubMed] [Google Scholar]
- 39.Mattern J, Koomagi R, Volm M. Coexpression of VEGF and bFGF in human epidermoid lung carcinoma is associated with increased vessel density. Anticancer Res. 1997;17:2249–52. [PubMed] [Google Scholar]
- 40.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laschke MW, Menger MD, Vollmar B. Ovariectomy improves neovascularization and microcirculation of freely transplanted ovarian follicles. J Endocrinol. 2002;172:535–44. doi: 10.1677/joe.0.1720535. [DOI] [PubMed] [Google Scholar]
- 42.Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994;134:1146–54. doi: 10.1210/en.134.3.1146. [DOI] [PubMed] [Google Scholar]
- 43.Soleimani R, Heytens E, Van den Broecke R, Rottiers I, Dhont M, Cuvelier CA, et al. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum Reprod. 2010;25:1458–70. doi: 10.1093/humrep/deq055. [DOI] [PubMed] [Google Scholar]
- 44.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy–a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Amorim CA, David A, Dolmans MM, Camboni A, Donnez J, Van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28:1157–65. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–8. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 47.Fujita T, Hirose R, Yoneta M, Sasaki S, Inoue K, Kiuchi M, et al. Potent immunosuppressants, 2-alkyl-2-aminopropane-1,3-diols. J Med Chem. 1996;39:4451–9. doi: 10.1021/jm960391l. [DOI] [PubMed] [Google Scholar]
- 48.Yagi H, Kamba R, Chiba K, Soga H, Yaguchi K, Nakamura M, et al. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur J Immunol. 2000;30:1435–44. doi: 10.1002/(SICI)1521-4141(200005)30:5<1435::AID-IMMU1435>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Zelinski MB, Murphy MK, Lawson MS, Jurisicova A, Pau KY, Toscano NP, et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95:1440–5. doi: 10.1016/j.fertnstert.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]