Abstract
Purpose
To improve human primordial follicle culture.
Methods
Thin or thick ovarian slices were cultured on alginate scaffolds or in PEG-fibrinogen hydrogels with or without bpV (pic), which prevents the conversion of phosphatidylinositol-trisphosphate (PIP3) to phosphatidylinositol-bisphosphate (PIP2) or 740Y-P which converts PIP2 to PIP3. Follicular growth was evaluated by follicular counts, Ki67 immunohistochemistry, and 17β-estradiol (E2) levels.
Results
BpV (pic) had a destructive effect on cultured follicles. Thawed-uncultured samples had more primordial follicles than samples cultured in basic medium and fewer developing follicles than samples cultured in PEG-fibrinogen hydrogels with 740Y-P. There were more atretic follicles in samples cultured on alginate scaffolds than in PEG-fibrinogen hydrogels, and in samples cultured in PEG-fibrinogen hydrogels with 740Y-P than in PEG-fibrinogen hydrogels with basic medium. Ki67 staining was higher in PEG-fibrinogen hydrogels than on alginate scaffolds. E2 levels were higher in thick than in thin slices.
Conclusions
PEG-fibrinogen hydrogels appear to have an advantage over alginate scaffolds for culturing human primordial follicles. Folliculogenesis is not increased in the presence of substances that enhance PIP3 production or with thin rather than thick sectioning.
Keywords: Alginate scaffolds, 17β-estradiol (E2), Human primordial follicles, Ki67, Polyethylene glycol (PEG)-fibrinogen hydrogels, PIP3
Introduction
With the increase in cancer survival rates among young women of reproductive age, problems of radiation- and chemotherapy-related ovarian failure have come to the forefront [1]. The options for fertility preservation in this setting are currently limited. Cryopreservation of ovarian cortical tissue containing immature primordial follicles followed by autotransplantation [1, 2] has so far resulted in livebirths [2, 3], but it carries a risk of reintroducing the malignancy [1, 4, 5]. This risk could be potentially eliminated by embryo transfer after in vitro fertilization of oocytes from primordial follicles matured in culture [4, 6–9]. Culturing primordial follicles in whole ovarian tissue slices (organ culture) would retain the interactions of the follicles with the surrounding stroma cells. Indeed, studies using this method promoted mammalian (including human) activation of primordial follicles to secondary stages [4, 7–9]. Its main draw-back, however, is that the follicles cannot be monitored during growth. Moreover, thin, flat slices (as opposed to thick ones) were shown to accelerate follicular maturation [6], and the signals that prompt the initial activation of the primordial follicles are still uncertain [4].
The PTEN gene, encodes a negative regulator-lipid phosphatase enzyme that converts phosphatidylinositol-triphosphate (PIP3) to phosphatidylinositol-biphosphate (PIP2) [10, 11]. PTEN regulates the primary oocyte effector, forkhead transcription factor (Foxo) 3 [12]. The balance between PTEN and phosphatidylinositide 3 (PI3) kinase influences PIP3 levels and thereby apparently affects oocyte growth and maintenance [12]. Therefore, in attempts to develop a better culture system, researches have incubated mouse and human primordial follicles with the PTEN inhibitors bpV (pic), bpV (HOpic), and 740Y-P, the Akt-activating phosphopeptide, in order to increase PIP3 levels. The results in terms of primordial follicular activation are promising [10, 13]. At the same time advances in biomaterials have made it possible to develop culturing systems that mimic the physiological environment and generate an extracellular matrix-like milieu adjacent to the tissue [14, 15]. Soluble fibrinogen can be combined with polyethylene glycol (PEG), a highly hydrophilic, easily functionalized, non-toxic, biologically inert compound, useful for modifying proteins/glycoproteins [16]. The PEG-fibrinogen hydrogel network provides successful structural control to various cultured cell types, and prevents material biodegradation [17].
The alginate scaffold is also biocompatible, mechanically stable, and hydrophilic, and has been applied successfully for efficient culture transport [9]. We previously showed that organ culture for human ovarian primordial follicles yielded better results on alginate scaffolds than on Matrigel [9].
The aim of the present study was to compare the effectivenss of PEG-fibrinogen hydrogel [17] with alginate scaffold [9] using thin-flat [6] and thick [8, 9] ovarian slices for the culture of human primordial follicles. In addition, we sought to determine if adding substances that enhance PIP3 production in the ovarian slices increases follicular activation and development [10, 13]. As the availability of human tissue for research is scarce, we conducted a multifactorial analysis on the same samples. To improve the assessment of follicular development in organ culture, we applied both morphological and endocrinological methods.
Materials and methods
Sample sources and retrieval
The study was performed on samples of frozen-thawed ovarian tissue obtained from 15 girls and women aged 5–27 years (mean ± SD = 17 ± 7 years) who underwent gynecological laparoscopy for ovarian cryopreservation before chemotherapy [9] or for removal of ovarian cysts (Table 1). The Ethics Committee of Rabin Medical Center approved the study protocol, and informed consent was received from every adult patient or parents of minors.
Table 1.
Description of the patients and the allocation to thick and thin slices
| Patient | Age | Disease | Thin/thick slices |
|---|---|---|---|
| 1 | 5 | Neuroblastoma | Thin |
| 2 | 6 | Brain tumor | Thick |
| 3 | 10 | Brain tumor | Thick |
| 4 | 13 | Brain tumor | Thick |
| 5 | 13 | Ewing sarcoma | Thin + thick |
| 6 | 13 | Ewing sarcoma | Thin |
| 7 | 16 | Hodgkin lymphoma | Thin |
| 8 | 17 | Hodgkin lymphoma | Thick |
| 9 | 19 | Ewing sarcoma | Thin + thick |
| 10 | 20 | Ewing sarcoma | Thin + thick |
| 11 | 22 | Acute lymphoblastic leukemia | Thin + thick |
| 12 | 22 | Osteosarcoma | Thin + thick |
| 13 | 23 | Ovarian cyst | Thin |
| 14 | 24 | Hodgkin lymphoma | Thin + thick |
| 15 | 28 | Ovarian cyst | Thin + thick |
All samples were handled in our laboratory within 1 h of surgery [9]. One slice measuring 1–2 mm from each patient was fixed in Bouin’s solution (components purchased from BDH Chemicals Ltd., Poole, England, and Sigma, St. Louis, MO, USA) immediately after ovarian dissection (fresh-uncultured sample), and the remaining ovarian tissue was frozen.
Cryopreservation and thawing of ovarian tissue
Before freezing, the samples were kept on ice with dimethylsulfoxide (DMSO) solution (Sigma). Tissue slices were frozen slowly and gradually with a 1.5 M DMSO in a programmable freezer (Kryo 10; series 10/20, Planer Biomed, Sunbury on Thames, UK), and immediately placed in liquid nitrogen [7–9].
The slices were thawed by washouts with decreasing concentration gradients of DMSO (1.0 M, 0.5 M) and phosphate buffered saline (Biological Industries, Beit Ha’emek, Israel) followed by incubation at 37 °C. One slice of every thawed ovarian sample, similar in size to the fresh-uncultured slices, was fixed in Bouin's solution immediately after thawing (thawed-uncultured sample).
Preparation of PEG-fibrinogen solutions and hydrogels
Concentrated PEG solutions (at least 10 mg/ml) were prepared according to routine procedures and frozen [17]. The solutions were thawed at room temperature, followed by centrifugation for 5 min at 2500 rpm. They were then diluted to 9.9 mg/ml with 0.1 % W/v Irgacure™2959 (Ciba Specialty Chemicals Inc., Basel, Switzerland) and phosphate buffered saline (Biological Industries) to a volume of 1 ml. It is noteworthy that the photo-initiator, Irgacure™2959, is non-toxic to various cell types [18], and at this low concentration creates sufficient photo-polymerization.
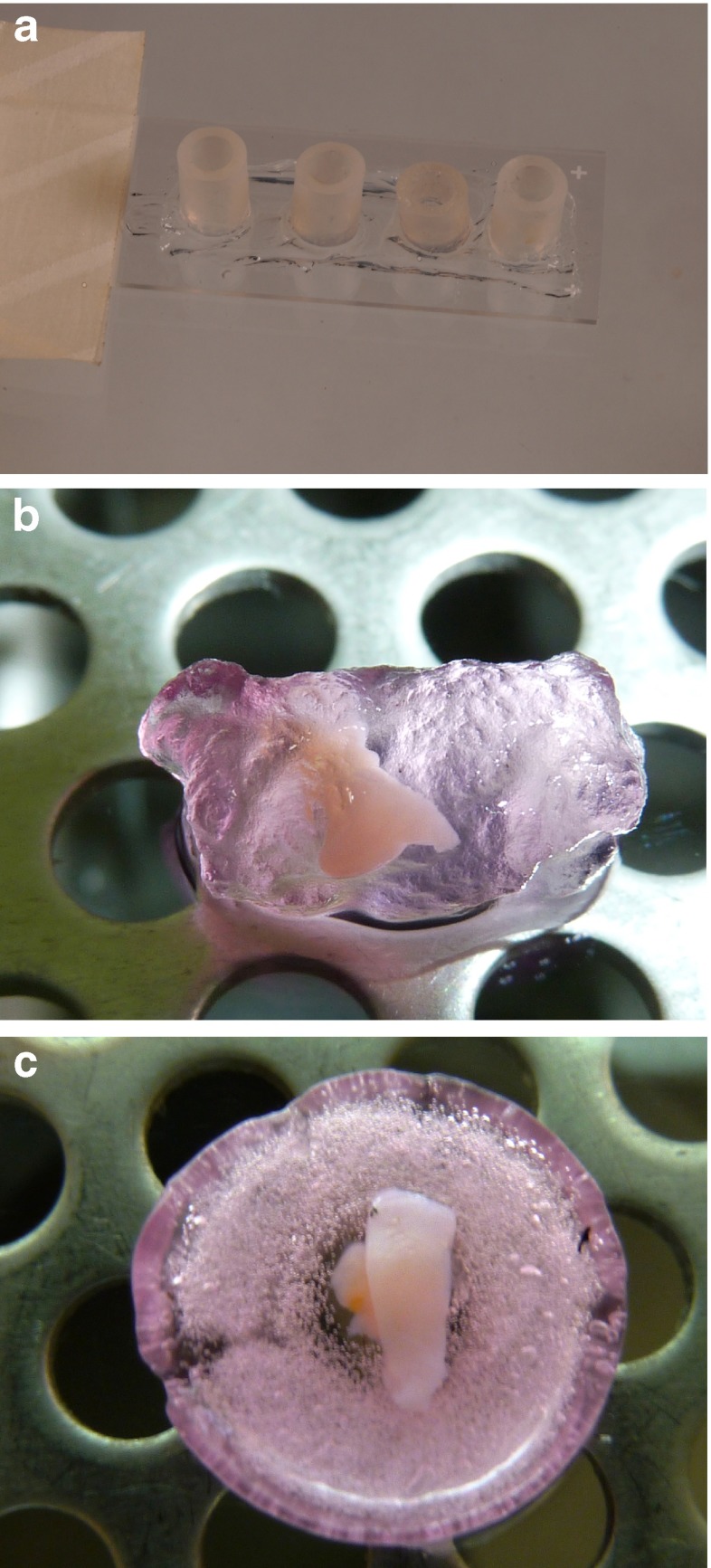
Molds for hydrogel formation were prepared from plastic tubes 0.5 cm in diameter (Avni and Sons, Haifa, Israel) and cut at lengths of 0.5 cm. The tubes were placed on microscope slides (StarFrost, Braunschweig, Germany) smeared with high- vacuum grease (Dow Corning, Wiesbaden, USA) (Fig. 1a) and sterilized under ultraviolet (UV) light in a biological hood for 1 h. The sterile molds were filled with diluted PEG-fibrinogen solution. One or two ovarian slices were placed in each PEG-fibrinogen-containing-mold and the solution was stirred gently with a sterile 21G needle (Shanghai Kindly Enterprise Development Group, Shanghai, China). The mold-containing solutions with the ovarian slices were then photo-polymerized by exposure to UVA light (365 nm, 5 mW/cm2) for 5 min in order to form cross links [17]. Studies have shown that cell survival and viability is not affected by exposure to UVA light for periods of up to 10 min at wavelengths required for hydrogel photo-polymerization [19, 20]. The PEG-fibrinogen hydrogels with the encapsulated ovarian slices (Fig. 1b) were released from the molds by gently pipetting culture medium and transferred into 24-well plates (CELLSTAR, Greiner Bio-One, GmbH, Freickenhausen, Germany) containing 1 ml of culture medium (see Culturing methods).
Fig. 1.
Photographs of the culture systems. a Molds for PEG-fibrinogen hydrogel formation. b Ovarian slice encapsulated in a PEG-fibrinogen hydrogel. c Human ovarian slice on alginate scaffold
Alginate scaffold preparation
Alginate scaffolds (Fig. 1c) were prepared from alginate with a high gluconic acid concentration (NovaMatrix FMC Biopolymers, Drammen, Norway) using a freeze-dry technique [9]. The scaffolds were sterilized by exposure to UV light in a biological hood for 1 h.
Culturing methods
Only frozen-thawed samples were used for incubation [7–9], so follicular density could be studied histologically from the fresh-uncultured sample (see “Histological preparation”). This practice eliminated the risk of utilizing poorly populated ovarian tissue. It was supported by earlier studies showing no differences in morphology or in in vitro development between fresh and frozen-thawed human follicles [7–9, 21]. Because the ultimate fertility preservation and restoration technique for cancer patients will require the use of frozen-thawed follicles [4], studies using frozen-thawed ovaries are crucial.
Samples from the same 7 patients were used to obtain both thin slices (width <1 mm) and thick slices (width 1–2 mm, twice the size of the thin slices) (Table 1). In addition, samples from four of the remaining patients were only used for thin slicing, and from the other 4, only for thick slicing (total, 15 samples from 15 patients) (Table 1). Half the samples were placed in PEG-fibrinogen hydrogels in 24-well culture plates (CELLSTAR, Greiner Bio-One) (Fig. 1b) and half were placed on alginate scaffolds (Fig. 1c) in 48-well culture plates (CELLSTAR, Greiner Bio-One). The wells were then filled with one of three culture-medium combinations:
Basic culture medium: alpha minimal essential medium (αMEM) (Biological Industries), human recombinant follicle stimulating hormone (1 IU/ml) (Gonal-F, Serono, Aubonne, Switzerland), 10 % human serum albumin (Irvine Scientific, Santa Ana, CA, USA), insulin, transferrin, and selenium (Sigma) [8].
Basic culture medium supplemented with bpV (pic) (100 μM) [10] (Santa Cruz Biotechnology, Santa Cruz, CA, USA, catalogue number sc-221379).
Basic culture medium supplemented with 740Y-P (500 μg/ml) [10] (Tocris Bioscience, Ellsville, Mo, USA, catalogue number 1983) [10].
Because a normal cellular PIP3/PIP2 balance is essential for many other cellular signals [22], culture medium combinations 2 and 3 were rinsed after 24 h [10, 13] and 48 h, respectively [10], and the samples were further incubated with the basic culture medium.
In the initial studies, samples from seven patients were incubated for 6 and 12 days. More atretic follicles were identified in the second week than the first week (data not included), as noted also in previous studies [6, 9]. Samples from the 8 remaining patients were cultured for only 6 days [6].
Culturing was performed in a standard incubator (95 % air, 5 % CO2) [8]. The culture media were changed every second day, starting (in groups 2 and 3) after the initial bpV (pic) or 740Y-P rinse. Once in 6 days, one slice was removed from culture and fixed immediately in Bouin’s solution (BDH Chemicals, East Yorkshirt, UK, and Sigma), and the spent medium samples were collected for 17β-estradiol (E2) measurement [8, 9].
Histological preparation
All fixed specimens were prepared for paraffin embedding, sectioning, and staining with hematoxylin and eosin [7, 8]. The number of follicles in the uncultured and cultured samples was counted in two different section-levels per sample (50 μm between sections, to avoid counting the same follicle twice), The follicles were classified according to Gougeon [23]: primordial (with a single flat layer of granulosa cells surrounding the oocyte), primary (with a single cuboidal granulosa cell layer surrounding the oocyte) or secondary (with at least two granulosa cell layers and a theca layer surrounding the oocyte). Developing follicles were defined as primary or secondary [7, 8]; atretic follicles were characterized by pyknotic cells, eosinophilia of the ooplasm, and clumping of the chromatin material [23]. Three independent investigators assessed the histological sections (G.L.-S, N.S., R.A.). Unstained sections were placed on OptiPlus positive charged microscope slides (BioGenex Laboratories, San Ramon, CA, USA) for immunohistochemistry assay.
Immunohistochemistry for the proliferating marker Ki67
Ki67 is a cell-cycle-associated nucleoprotein antigen that serves as a proliferation marker during the active cell-cycle phases (G1, S, G2 and mitosis) [24–29]. An increase in Ki67 staining has been reported to correlate directly with activation to cuboidal granulosa cells as well as with follicular growth [26–28].
Ki67 immunohistochemical staining was conducted essentially as described previously for proliferating cell nuclear antigen [7–9]. The sections were incubated for 1 h with a rabbit polyclonal anti-Ki67 antibody (1: 10 and 1: 30, Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-15402). Sections were incubated with a negative control solution that replaced the primary antibody: a normal rabbit IgG antibody (Santa Cruz Biotechnology, sc-2027) diluted to the same concentration as the primary antibody. Thereafter, the samples were incubated with horseradish peroxidase polymer conjugate against mouse and rabbit primary antibodies (SuperPicture HRP, Zymed Laboratories Inc., San Francisco, CA, USA; catalogue number: 879363). Red-brown 3-amino-9-ethylcarbazole staining (Zymed Laboratories) indicated Ki67 expression. We defined a follicle as being positively stained if at least one of its granulosa cells expressed Ki67 [7–9].
E2 accumulation in the culture medium
E2 concentrations were measured by a double antibody radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, CA, USA) [7–9].
Statistical analysis
Data were analyzed by analysis of variance, chi-square test and Fisher’s exact test, as required. P values less than 0.05 were considered statistically significant.
Results
Follicular counts and classification
The distribution of follicles (after 6 days in culture) by type of treatment is presented in Table 2. As our previous experience showed no difference in the growing potential of follicles from adults and girls [8, 9, Abir et al., unpublished data], the results were not differentiated by patient age. For the present analysis, we combined the results for thin and thick samples because a relatively low number of follicles were identified in the thin slices. Figure 2 presents a graphic illustration of the main findings in Table 1. Figure 3 shows histological sections containing follicles at various stages of the experiment.
Table 2.
Follicular counts in samples and E2 secretion after 1 week
| Study condition | Treatment | Primordial follicles | Developing follicles | Atretic follicles | Total | E2 (pg/ml) (mean±SD) |
|---|---|---|---|---|---|---|
| Thawed | – | 161a (74 %)* | 48b (22 %) | 8 (4 %) | 217 | – |
| 12 ± 3** | 4 ± 1 | 1 ± 0.2 | 17 ± 5 | |||
| Matrix | ||||||
| Basic medium | 94 (51 %) | 55 (30 %) | 35 (19 %) | 184 | 251 ± 89c | |
| 5 ± 1 | 3 ± 1 | 2 ± 0.4 | 9 ± 2 | |||
| Alginate Scaffoldd | +bpV (pic) | 0 | 3 (15 %) | 17e (85 %) | 20 | 0.5 ± 0.4 |
| 1 ± 0.3 | 3 ± 2 | 4 ± 2 | ||||
| +740Y-P | 147 (38 %) | 218 (57 %) | 19 (5 %) | 384 | 318 ± 134c | |
| 12 ± 4 | 18 ± 5 | 2 ± 0.5 | 32 ± 9 | |||
| Basic medium | 92 (33 %) | 197 (68 %) | 2 (1 %) | 291 | 174 ± 70c | |
| 7 ± 2 | 14 ± 4 | 0.14 ± 0.04 | 21 ± 6 | |||
| PEG-Fibrinogen | +bpV (pic) | 1 (33 %) | 0 | 2 (67 %) | 3 | 36 ± 35 |
| 0.3 ± 0.2 | 1 ± 0.4 | 1 ± 1 | ||||
| +740Y-P | 83 (55 %) | 56f (37 %) | 11g (7 %) | 150 | 149 ± 38c | |
| 14 ± 6 | 9 ± 4 | 2 ± 1 | 25 ± 10 | |||
*Sum of follicles per treatment group. Percents were calculated from the total number of follicles in the treatment group
**Mean±SD per sample
aSignificantly higher primordial follicle count in thawed-untreated samples than after culture with bpV (pic) or basic medium alone, regardless of the matrix (P < 0.05)
bSignificantly higher developing follicle count in thawed-untreated samples than after culture with bpV (pic), regardless of the matrix (P < 0.04)
cSignificantly higher E2 level after culture with basic medium alone (P < 0.03) or with 740Y-P (P < 0.05) than with bpV (pic), regardless of the matrix
dSignificantly higher atretic follicle count on alginate scaffolds than atretic follicles in PEG-fibrinogen hydrogels, regardless of treatment (P < 0.04)
eSignificantly higher atretic follicle count on alginate scaffolds with bpV (pic) than atretic follicles in thawed samples (P < 0.05) and in PEG-fibrinogen hydrogels, regardless of treatment (P < 0.04)
fSignificantly higher developing follicles count in PEG-fibrinogen with 740Y-P than developing follicles in PEG-fibrinogen hydrogels with bpV (pic) (P < 0.05) and in thawed samples (P < 0.03)
gSignificantly higher atretic follicle count in PEG-fibrinogen with 740Y-P than atretic follicles in PEG-fibrinogen hydrogels with basic medium (P < 0.02)
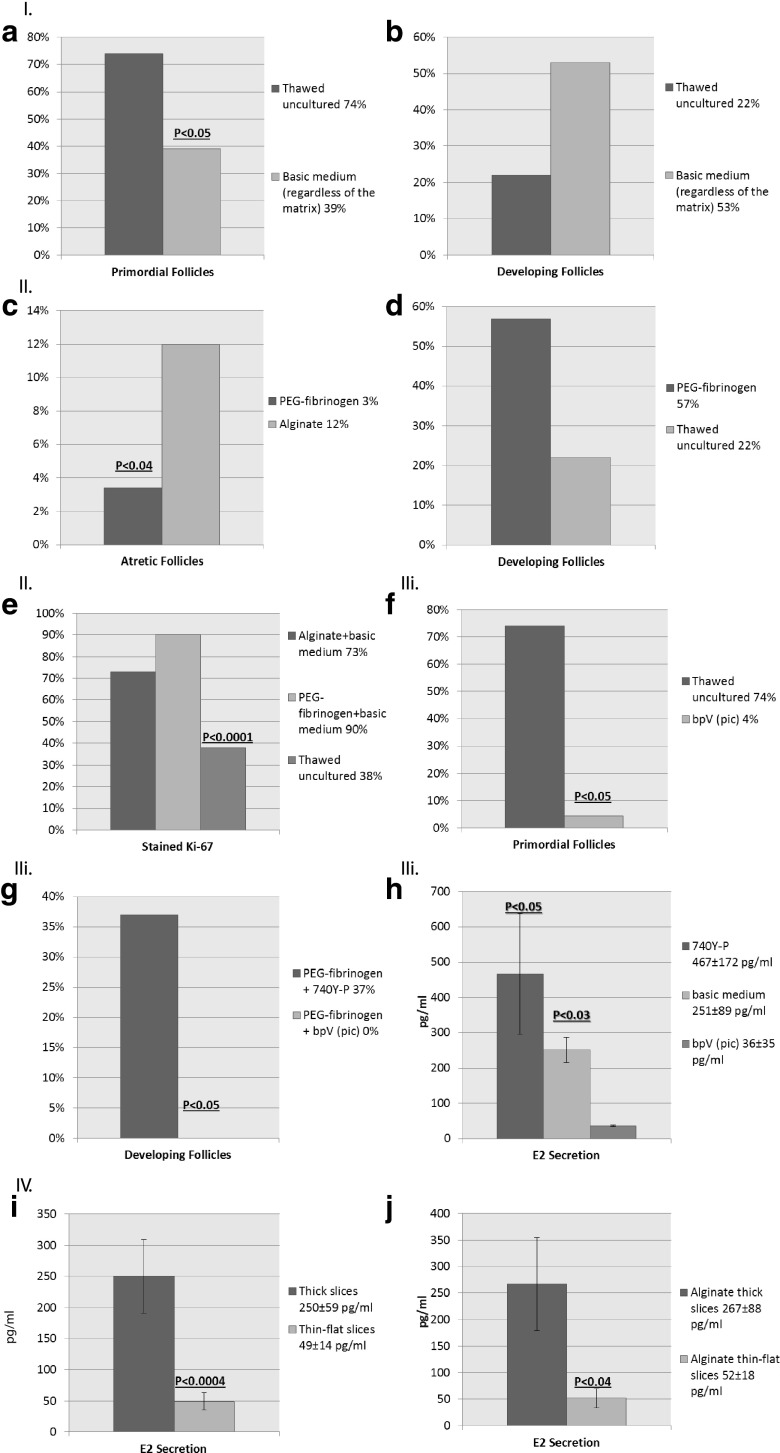
Fig. 2.
Graphs representing main and significant results from Table 1. I. Activation in culture by follicular counts. a Results are in percent of primordial follicles. Primordial follicle count is significantly higher in thawed-untreated samples than after culture with basic medium alone, regardless of the matrix (P < 0.05). b Results are in percent of developing follicles. Developing follicle count is higher in samples cultured with basic medium alone than in thawed-untreated samples, regardless of the matrix (NS). II. PEG-fibrinogen hydrogels versus alginate scaffolds. c Results are in percent of atretic follicles. Atretic follicle count is significantly higher on alginate scaffolds than atretic follicles in PEG-fibrinogen hydrogels, regardless of treatment (P < 0.04). d Results are in percents of developing follicles. Developing follicle count is higher in samples cultured in PEG-fibrinogen hydrogels than in thawed-untreated samples (NS). e Results are in percent of Ki67 positively stained follicles. The proportion of stained follicles is significantly higher on alginate scaffolds or in PEG-fibrinogen hydrogels with basic medium than in thawed-uncultured samples (P < 0.0001). III. Destructive effects of bpV (pic). Results are in percent of primordial follicles. f Primordial follicle count is significantly higher in thawed-untreated samples than in samples cultured with bpV (pic), regardless of the matrix (P < 0.05). (G) Developing follicles count is significantly higher in samples cultured in PEG-fibrinogen hydrogel with 740Y-P than in PEG-fibrinogen hydrogel with bpV (pic) (P < 0.05). h E2 level is significantly higher in samples cultured with 740Y-P (P < 0.05) or with basic medium alone (P < 0.03) than with bpV (pic), regardless of the matrix. IV. Thick versus thin samples. Results are in pg/ml. Values represent mean±standard deviation. i E2 level is significantly higher after culture of thick slices than thin slices (P < 0.0004), regardless of the matrix. j E2 level is significantly higher after culture of thick slices than thin slices on alginate scaffolds (P < 0.04)
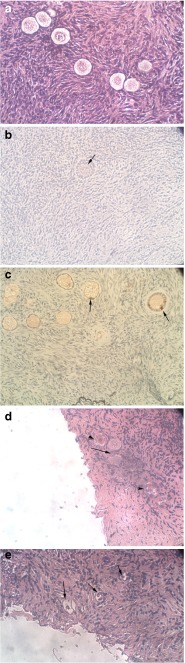
Fig. 3.
Histological and Ki67 immunostaining of uncultured and cultured ovarian samples. a Thawed-uncultured ovarian section from a 22-year-old woman. Note the primordial follicles. Hematoxylin and Eosin staining, original magnification X400. b Section of ovarian sample with Ki67 immunohistochemical staining from a 13-year-old girl cultured in PEG-fibrinogen hydrogel for 1 week. Note the small secondary follicle, with the light red-brown staining indicating Ki67 expression in a portion of the granulosa cells (arrow). Original magnification X400. c Section of ovarian sample with Ki67 immunohistochemical staining from the same patient as in panel (A) cultured in PEG-fibrinogen hydrogel for 1 week. Note the primary and primordial follicles, with the red-brown staining indicating Ki67 expression in a portion of the granulosa cells (arrows). Original magnification X400. d Section of ovarian sample from a 10-year-old girl cultured on alginate scaffold with 740Y-P for 1 week. Note the two primordial-primary follicles (arrow) and the three atretic follicles (arrow heads). Hematoxylin and Eosin staining, original magnification X400. e Section of ovarian sample from the same patient as in panel (B) cultured on alginate scaffold for 1 week with bpV (pic). Note the atretic follicles (arrows). Hematoxylin and Eosin staining, original magnification X400
Several statistically significant findings were noted:
Thawed uncultured samples had more primordial follicles than samples cultured with bpV (pic) or with basic medium alone regardless of the culture matrix (alginate scaffold or PEG-fibrinogen hydrogel) (P < 0.05). In addition, Thawed uncultured samples had more developing follicles than samples cultured with bpV (pic) regardless of the culture matrix (P < 0.04, respectively).
Samples cultured with basic medium alone or with 740Y-P had higher E2 levels than samples cultured with bpV(pic) (P < 0.03 and P < 0.05, respectively), regardless of the matrix.
Samples cultured on alginate scaffold had a higher atretic follicle count than samples cultured in PEG-fibrinogen hydrogel (P < 0.04), regardless of the treatment.
Samples cultured on alginate scaffolds with bpV (pic) had more atretic follicles than thawed uncultured samples (P < 0.05) and samples cultured in PEG-fibrinogen hydrogel (P < 0.04), regardless of the treatment.
Samples cultured in PEG-fibrinogen hydrogel with 740Y-P had higher developing follicle counts than samples cultured in PEG-fibrinogen hydrogel with bpV (pic) (P < 0.05) and thawed uncultured samples (P < 0.03).
Samples cultured in PEG-fibrinogen hydrogel with 740Y-P had a significantly higher atretic follicle count than samples cultured in PEG-fibrinogen hydrogel with basic medium (P < 0.02).
Ki67 staining
The results for Ki67 staining are shown in Figs. 2e and 3b, c. In most cases, Ki67 staining was identified in a portion of the granulosa cells from primordial-primary and secondary follicles, and it was generally weak. More stained follicles were found in samples cultured on alginate scaffold and in PEG-fibrinogen hydrogel with basic medium and with 740Y-P than in thawed uncultured samples (P < 0.0001); in samples cultured in PEG-fibrinogen hydrogel with basic medium alone than on alginate scaffolds with basic medium alone (P < 0.0001); and in samples cultured on alginate scaffold with 740Y-P than on alginate scaffold with basic medium alone (P < 0.0001).
E2 secretion
E2 production was calculated per slice [7–9]. Secretion from the thick and thin slices was adjusted by dividing the values for every thick slice by 2 (Table 2). E2 production was significantly higher with basic medium alone (P < 0.03) and with 740Y-P (P < 0.05) than with bpV (pic), regardless of the matrix. There was significantly more E2 production in thick slices than thin slices, regardless of the treatment or the matrix (250 ± 59 pg/ml and 49 ± 14 pg/ml, respectively, P < 0.0004) and on alginate scaffold than in PEG-fibrinogen hydrogel, regardless of the treatment (267 ± 87 pg/ml and 52 ± 18 pg/ml, respectively, P < 0.04).
Discussion
The morphology-based results of the present study suggest that PEG-fibrinogen hydrogels have an advantage over alginate scaffolds for growing human primordial follicles in organ culture. More developing follicles were identified in PEG-fibrinogen hydrogel (with 740Y-P or with basic medium alone); more Ki67-stained follicles seemed to be detected in PEG-fibrinogen hydrogels; and there were more atretic follicles on alginate scaffolds. The addition of PIP3-inducing agents did not improve follicular development; Indeed bpV (pic) caused follicular deterioration. Use of thin-flat slices did not improve follicular development over thick slices, as indicted by the higher E2 levels in the thin slices.
As different tissue slices were evaluated during organ culture and we could not monitor the same follicle [7–9], we applied several methods of evaluation to ensure accuracy. Presumably because each method was designed to examine a different tissue level, rather than obtaining a direct correlation of the results among the methods, we found that each method provided information on a different aspect of follicular development. The stained sections were used to determine the number and classification (primordial, primary and secondary) of the follicles, reflecting the growth and survival of follicles in culture [4], and the cultured vs. uncultured control samples were used to count the follicles periodically throughout culture and compare the findings among the follicular classes. Thus, a decrease in the number of primordial follicles and a corresponding increase in the number of growing follicles, from primary stages onwards, would signify follicular growth. We used uniform-size samples, and the follicles were counted throughout the field (magnification X100) in 2 to 3 levels per specimen (with at least 50 μm between levels to avoid counting the same follicle twice). Increase in follicular atresia were due to the destructive effect of bpV (pic). There were also more atretic follicles on alginate scaffolds than in PEG-fibrinogen hydrogels.
Various immunocytochemical methods have been developed to evaluate DNA division or granulosa cell proliferation [4, 7–9]. In the present study, Ki67 staining was used to identify granulosa cell activation [24–29]. We detected only weak Ki67 immunostaining, similar to previous studies [8, 9]. It is possible that staining intensity could be increased by substituting the 1 h incubation with the primary antibody with overnight incubation at 4 °C, as described by David et al. [30].
In addition to these morphological tests, the spent media samples can be assessed for hormones such as E2. As E2 is not secreted from unilaminar follicles [4, 7–9], its production indicates the presence [4, 7–9].
As noted above, in the present study, findings of differences in E2 levels supported the destructive effect of bpV(pic) and the lack of advantage in using thin versus thick slicing.
The optimal matrix for culturing whole slices of human ovarian tissue containing primordial follicles is still unknown. Although others reportedly developed primordial follicles in organ culture without a supporting matrix [6], this method was unsuccessful in our laboratory. Matrigel, a supporting extracellular protein-rich-preparation, was found to increase the survival of human primordial follicles [7–9, 21], but it is produced from a mouse sarcoma cell line [31], making its clinical application doubtful. Moreover, alginate scaffolds appear to have a greater stimulatory effect on follicular development than Matrigel scaffolds [9]. The present study suggests that PEG-fibrinogen hydrogels are better than alginate scaffolds for culturing human primordial follicles. We speculate that PEG-fibrinogen provides better mechanical support of the tissue [17], PEG-fibrinogen hydrogels are also advantageous in that growth factors that activate primordial follicles [4, 7, 8] can be supplemented not only to the culture medium [7, 8] but to the PEG-fibrinogen solution before hydrogel formation [32].
So far, hydrogels have been used for culturing isolated preantral follicles from various mammalian species [33], including humans [14]. Although the follicles were successfully cultured to antral stages in alginate hydrogel beads formed under mild conditions, the interpenetrating hydrogel network was improved by combining calcium-dependent alginate with calcium-dependent fibrin [34]. Alginate increases hydrogel-bead density, provides additional? mechanical support and becomes more rigid with follicular expansion [9]. Fibrin is a natural fibrinogen biopolymer; it entraps growth factors and furnishes the hydrogel with mechanical dynamic properties. Its cell-secreted-protease degradation is balanced by the non-degradable alginate. Indeed, fibrin-alginate hydrogel beads were found to enhance the growth of encapsulated mouse follicles and to improve oocyte meiotic competence [33, 34]. Accordingly, it is possible that various compounds may be combined to enhance the encapsulating matrix for ovarian slices.
Our evaluation of the possible role of PIP3-enhancing substances in in vitro follicular development was prompted by previous studies [10, 13] wherein bpV (pic) (mouse and human), bpV (HOpic) (mouse), or 740Y-P (mouse) supplementation of the culture medium promoted primordial follicle activation. Subsequent implantation of these ovaries into murine hosts resulted in follicular growth to the preovulatory stages (mice and human), production of mature oocytes (mice), successful oocyte fertilization, and birth of healthy pups [10, 13]. Moreover, mice with oocyte-specific deletions in PTEN or Foxo3 showed enhanced primordial follicle activation [10, 12, 35].
However, the present results contradict these findings, despite our use of both bpV (pic) and 740Y-P at the same low concentrations and for the same short-term culture periods as Li et al. [10]. Although higher concentrations of these substances might induce more follicular activation, they could also damage other intracellular pathways [22, 35]. Indeed, in our study, bpV (pic) apparently heightened follicular destruction. The discrepancy between our study and that of Li et al. [10] might be due to our purchasing the compound bpV (pic) from another manufacturer, as Calbiochem no longer produces it. In a follow-up study, Adhikari et al. [13] added another PTEN inhibitor, bpV(HOpic), to the culture medium of murine primordial follicles, without testing PIP3 level. Therefore, it seemed unnecessary to test PIP3 levels in the culture medium in the present study. Our results might be improved by cultivating ovarian slices with bpV (HOpic) at the same low concentration (1 μm) used by Adhikari et al. [13], (100-fold lower than the bpV (pic) concentration used previously [10]). Regarding 740Y-P, the positive results reported by Li et al. [10] pertained to murine primordial follicles, whereas we tested human primordial follicles. Although we carefully rinsed off bpV (pic) and 740Y-P, by increasing PIP3 levels in vitro we may have interfered with other important cellular processes in which the PI3K-Akt intracellular signaling pathway is normally involved [22]. At the same time it is conceivable that in the process of ovarian tissue transplantation after the short-term culture period used in the previous studies [10, 13], the in vivo environment corrected or bypassed damage to the PI3K-Akt pathway. This was impossible in our in vitro system. Nonetheless, it is highly unlikely that mature oocytes obtained in mice engrafted with ovarian tissue will be used for clinical fertility restoration in women.
By contrast to Telfer et al. [6] we found that thick ovarian slices promoted better follicular development than thin slices. This difference can be explained by our cutting slices from frozen-thawed rather than fresh specimens. In addition, it is possible that despite numerous attempts, we failed to produce samples that were thin and flat enough or that some tissue-damage occurred during slicing. Results with thin slices might be improved by the use of special tissue-chopper devices [3, 36] rather than manual cutting. It is noteworthy that to the best of our knowledge, no further evidence of benefits of culturing thin slices [6] has been reported to date.
In summary, this study suggests an advantage for PEG-fibrinogen hydrogel for the short-term culture of human primordial follicles. A two-step culturing system might be the optimal strategy [6–9]; i.e., short-term culture of ovarian slices encapsulated in PEG-fibrinogen hydrogels to obtain large secondary follicles, which are then isolated and cultured [6, 37, 38]. So far, however, in vitro follicular growth from primordial stages to mature oocytes has been achieved only in mice [37, 38] with the birth of live pups [38]. Further studies are needed to investigate additional culture matrices and improvements in the culture medium for the growth of human primordial follicles [6, 8, 9].
Acknowledgments
The authors are grateful to Prof. Smadar Cohen from The Avram and Stella Goldstein-Goren Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel for the production of alginate scaffolds in her laboratory. The authors are greatly indebted to Ms. Gloria Ganzach from the Editorial Board of Rabin Medical Center, Beilinson Hospital for the English editing. We are also indebted to our laboratory technician, Ms. Carmela Felz for the histological sectioning. The study was partially funded by a research grant from Tel Aviv University, Leo Minz Fund (to R. A and B. F) and the Israel Cancer Association (to R. A and B. F).
Disclosure summary
RA and BF received grant support from Leo Minz Fund, Tel Aviv University and from the Israel Cancer Association, GL-S, NS, MS, OK, DS, SC and AB-H have nothing to declare.
Footnotes
Galit Lerer-Serfaty and Dr. Nivin Samara contributed equally to this study as first authors.
Capsule
PEG-fibrinogen hydrogels seem to have an advantage over alginate scaffolds for culturing human primordial follicles. Folliculogenesis is not enhanced with the addition of PIP3-inducing substances or use of thin-slice sectioning.
References
- 1.Feigin E, Freud E, Fisch B, Orvieto R, Kravarusic D, Avrahami G, et al. Fertility preservation in female adolescents with malignancies. In: Moorland MT, et al., editors. Cancer in female adolescents. Hauppauge: Nova Science Publishers; 2008. pp. 38–103. [Google Scholar]
- 2.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 3.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Mirco-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1033–97. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]
- 4.Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–98. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- 5.Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25:1708–12. doi: 10.1093/humrep/deq121. [DOI] [PubMed] [Google Scholar]
- 6.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 7.Garor R, Abir R, Erman A, Felz C, Nitke S, Fisch B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91:1967–75. doi: 10.1016/j.fertnstert.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 8.Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96:1246–54. doi: 10.1210/jc.2011-0410. [DOI] [PubMed] [Google Scholar]
- 9.Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S. Alginate scaffold for organ culture of cryopresereved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28:761–9. doi: 10.1007/s10815-011-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Kawarmura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant follicle to generate mature eggs. PNAS. 2010;107:10280–4. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SR, Lee YC. PTEN as a unique promising therapeutic target for occupational asthma. Immunotoxicology. 2008;30:793–814. doi: 10.1080/08923970802285164. [DOI] [PubMed] [Google Scholar]
- 12.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari D, Gorre N, Risal S, Zhao Z, Zhang H, Shen Y, et al. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS One. 2012;7:e39034. doi: 10.1371/journal.pone.0039034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 16.Dikovskey D, Bianco-Peled H, Seliktar D. Investigating the molecular structure and physical properties of PEG-Fibrinogen hydrogels. Adv Eng Mater. 2010;12:B200–9. doi: 10.1002/adem.200980054. [DOI] [Google Scholar]
- 17.Peled E, Boss J, Bejar J, Zinman C, Seliktar D. A novel poly(ethylene glycol)-fibrinogen hydrogel for tibial segmental defect repair in a rat model. J Biomed Mater Res A. 2007;80:874–84. doi: 10.1002/jbm.a.30928. [DOI] [PubMed] [Google Scholar]
- 18.Federovich NE, Oudshoorn MH, Van Geemen D, Hennink WE, Alblas J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–53. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–57. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 20.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpentrating networks. J Biomed Mater Res. 2000;51:164–71. doi: 10.1002/(SICI)1097-4636(200008)51:2<164::AID-JBM4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 21.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–6. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 22.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–86. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 23.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:55–121. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 24.Gerdes J, Lemke H, Baisch J. Cell cycle analysis of cell proliferation associated human nuclear antigen defined by the monoclonal antibody Ki67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 25.Scholzen T, Geredes J. The Ki67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Rivera OE, Varayoud J, Rodrigues HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–12. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 27.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–9. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbri R, Venturoli S, D'Errico A, Iannascoli C, Gabusi E, Valeri B, et al. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–66. doi: 10.1016/S0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 29.Rahmanzadeh R, Hüttmann G, Gerdes J, Scholzen T. Chromophore-assisted light inactivation of pKi67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007;40:422–30. doi: 10.1111/j.1365-2184.2007.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David A, Van Langendonckt A, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27:1088–95. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
- 31.Kleinman HK. Preparation of basement membrane components from EHS tumors. Curr Protoc Cell Biol. 2001;10:10.2.1–10. doi: 10.1002/0471143030.cb1002s00. [DOI] [PubMed] [Google Scholar]
- 32.Seliktar D, Zisch AH, Lutolf MP, Wrana IJL, Hubbell JA. MMP-2 sensitive, VEGF bearing bioactive hydrogels for promotion of vascular healing. Biomed Mater Res A. 2004;68:704–16. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 33.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 36.Kagawa N, Sliber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–77. doi: 10.1016/S1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 37.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–9. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]