Abstract
Purpose
The shape and size of the suprascapular notch (SSN) is one of the most important risk factors in suprascapular nerve entrapment. The aim of the study was to perform a morphological study of SSN variations.
Methods
A total of 616 computer tomography scans of scapulae were retrospectively analysed in 308 patients. The examination focused on the suprascapular region. The type of suprascapular notch was determined by using a classification based on three geometrical measurements: maximal depth (MD), superior (STD) and middle (MTD) transverse diameters.
Results
In the scans, five types of SSN were noted. In type I (24.18 %) maximal depth was greater than superior transverse diameter. Type II (1.95 %) has equal MD, STD and MTD. In type III (56.16 %) the superior transverse diameter was greater than the maximal depth. Scapulae with bony foramen were classified as type IV (4.72 %). In type V a discrete notch (12.99 %) was found. Additionally, types I and III were divided into three subtypes: A, B and C. The frequency of type I and IV was lower in females than in males, but type III was more common in females than males. Distribution of other types of SSN in both groups was similar.
Conclusion
Knowledge of the anatomical variations of the suprascapular notch described in this study should be helpful in endoscopic and open procedures of the suprascapular region and also may increase the safety of operative decompression of the suprascapular nerve.
Keywords: Suprascapular notch, Anatomical variations, Decompression of the suprascapular nerve, Suprascapular nerve entrapment
Introduction
The suprascapular notch (SSN) is a variable depression on the superior border of the scapula. Through this structure, the suprascapular nerve and vein traverse the upper border of the scapula under the superior transverse scapular ligament (STSL) [1]. The SSN is the most important point along the course of the suprascapular nerve (SN) because this region is the main site of injury and compression of the SN [2, 3]. This pathology was first described by Andre Thomas in 1936 [4]. Suprascapular nerve entrapment is characterised by chronic, poorly localised pain in the posterior or/and lateral region of the shoulder, which may radiate down the arm or up into the neck, weakness of abduction and increased external rotation of the arm, with atrophy of the supra- and infraspinatus muscles [2, 3, 5–8]. Males are approximately three to four times more likely to suffer from suprascapular nerve entrapment than females [2, 5–8]. This pathology is also important from a demographic point of view because it mainly occurs in patients under 35 years of age [2, 7–9].
The shape and size of the suprascapular notch is the most important factor in the aetiopathology of supracapular nerve entrapment [10, 11]. A narrow SSN may predispose a patient to this neuropathy [12]. Also, a V-shaped notch is more likely to be associated with nerve entrapment [8]. Therefore, knowledge of the morphology of the suprascapular region, especially SSN shape and STSL variations, is particularly important in various techniques associated with arthroscopic SN decompression [13–15]. In our opinion this study is the most comprehensive description of suprascapular notch morphology. It is also based on the specific geometrical parameters of the SSN which describe almost all variations of these structures.
The aim of the study was a thorough examination of the sexual dimorphism of the suprascapular notch based on new imaging techniques. To our knowledge, no large-scale radiological study of the suprascapular notch variations described in this study has been reported previously.
Materials and methods
A total of 616 computer tomography scans of shoulders were retrospectively analysed in 308 randomised patients who were being investigated as part of a standard CT chest protocol between May 2008 and April 2012 for disease of the lungs or cardiovascular system. The research project and all procedures were approved by the Bioethics Commission of the Medical University. Dual-phase helical CT was performed with a 32-row MDCT scanner (Toshiba Aquilion 32; Toshiba Medical System, Japan). All shoulders were analysed with post-processing tools; multiplaner reconstruction (MPR) and maximum intensity projection (MIP) images were obtained along the sagittal and coronal planes, as well as coronal curved MIP images, and three-dimensional volume rendering. The criteria of exclusion were scapula fracture or tumour and previous scapular surgery.
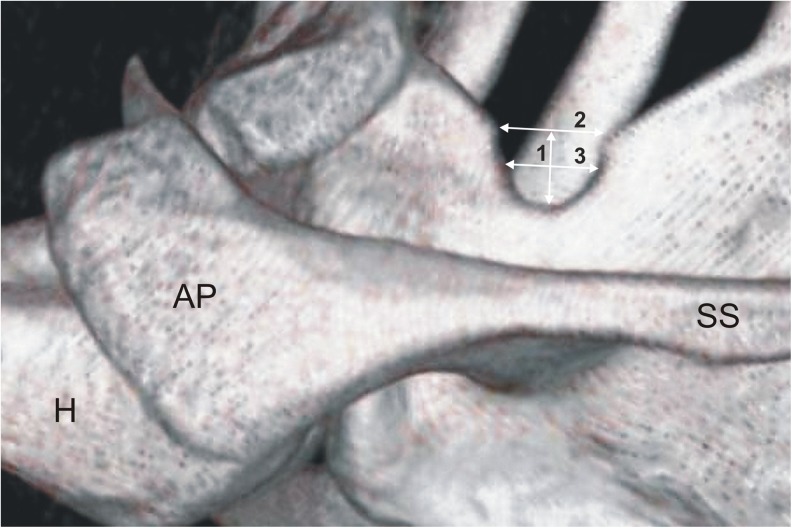
The suprascapular regions of all 616 CT scans of the scapulae were analysed. The type of suprascapular notch was determined using the classification system described by Polguj et al. [16]. This classification system is based on the shape of the inferior border of the incisure as well as the comparison of the three geometrical measurements. These SSN dimensions were defined as follows (Fig. 1) [16]:
The maximal depth (MD)—the maximum value of the longitudinal measurements taken in the vertical plane from an imaginary line between the superior corners of the notch to the deepest point of the suprascapular notch
The superior transverse diameter (STD)—the maximum value of the horizontal measurements taken in the horizontal plane between the corners of the SSN on the superior border of the scapula
The middle transverse diameter (MTD)—the value of the horizontal measurements taken in the horizontal plane between the opposite walls of the SSN at a midpoint of the MD and perpendicular to it
Fig. 1.
Three-dimensional volume rendering (VR) MDCT of the scapula demonstrating measurements of the suprascapular notch: 1—maximal depth, 2—superior transverse diameter, 3—middle transverse diameter
The measurements of the suprascapular notch were performed using Vitrea 2 system software (Vital Images, Plymouth, MN, USA).
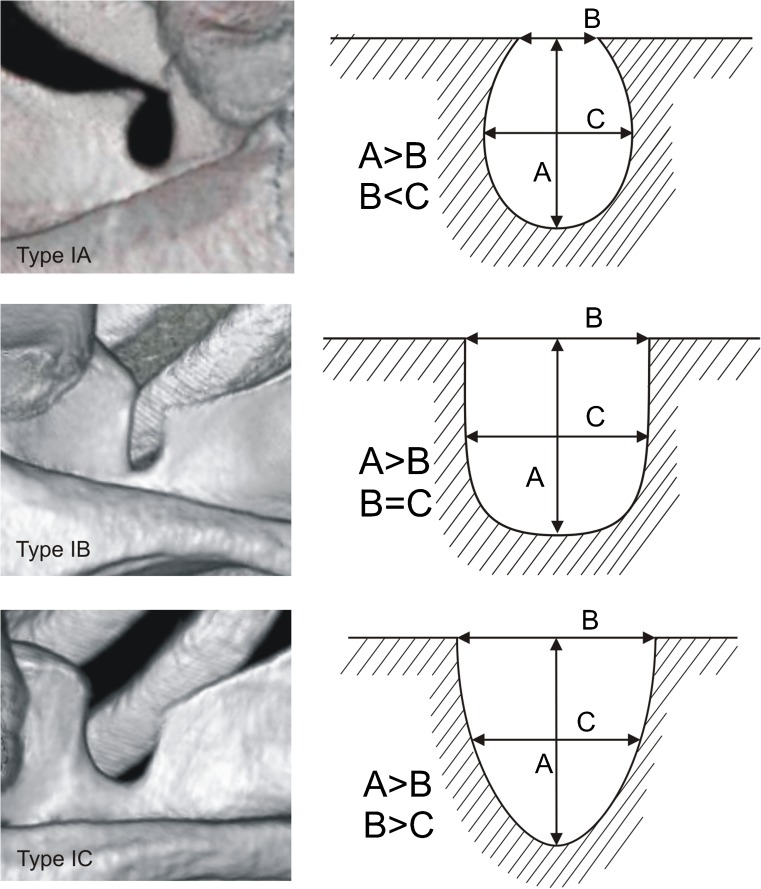
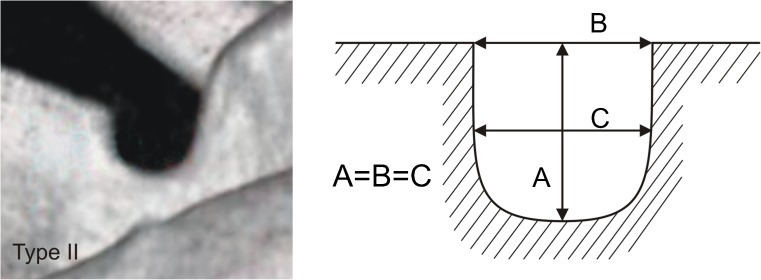
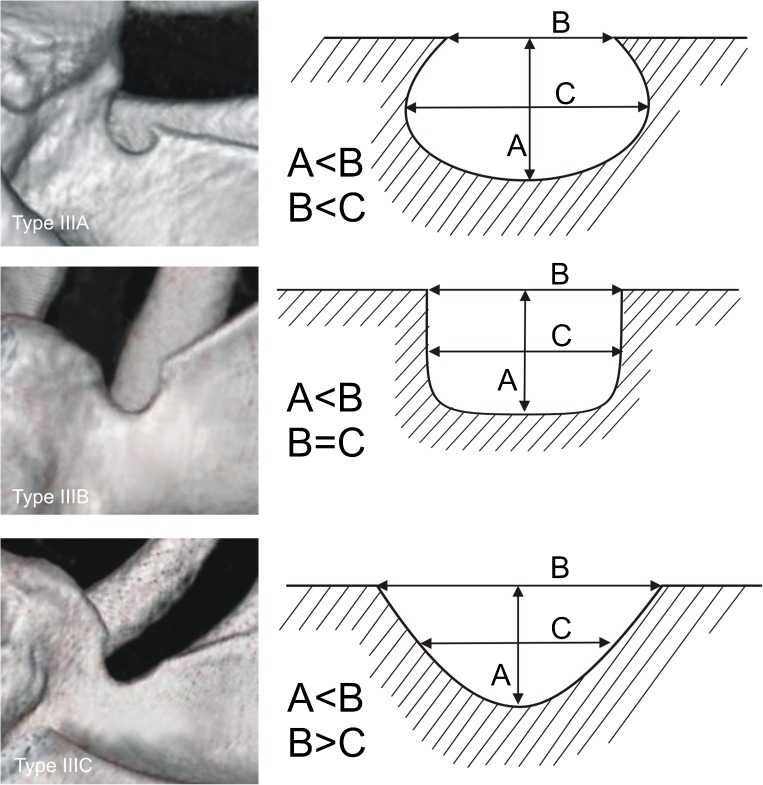
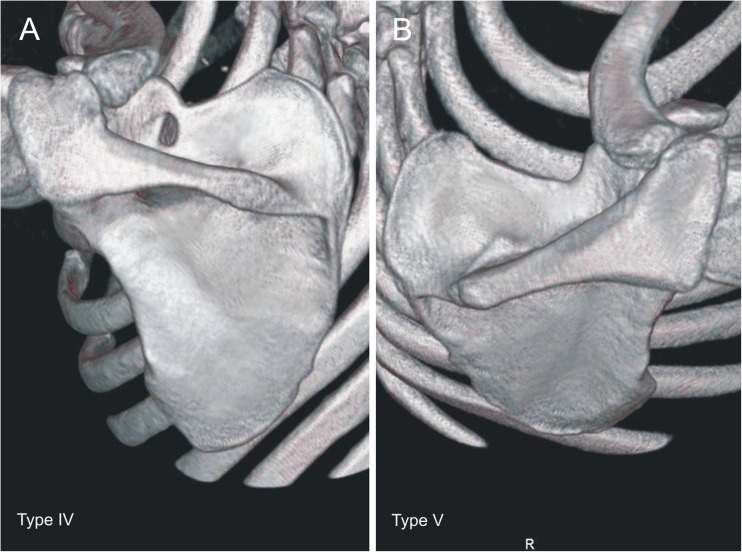
Each of the computer tomography scans of the shoulder area was carefully observed simultaneously by two investigators who classified the scapulae according to a fivefold SSN classification by Polguj et al. [16]. In type I, MD was greater than STD. This type was divided into three subtypes: IA, IB and IC. Subtype IA presented longer MTD than STD, while IC presented shorter MTD than STD and IB presented equal diameters (STD = MTD) (Fig. 2). Type II presented equal MD, STD and MTD (Fig. 3). Type III presented longer STD than MD. As with type I, this type was also divided into three subtypes: IIIA, IIIB and IIIC. In IIIA, MTD was longer than STD. In IIIC, it was the opposite (STD>MTD) and in IIIB these diameters were equal (STD = MTD) (Fig. 4). In type IV, a bony bridge joins the corners of the SSN (Fig. 5a). Type V presents a discrete notch (Fig. 5b).
Fig. 2.
Type I of the suprascapular notch. Diameters of SSN: A—maximal depth, B—superior transverse, C—middle transverse
Fig. 3.
Type II suprascapular notch. Diameters of SSN: A—maximal depth, B—superior transverse, C—middle transverse
Fig. 4.
Type III of the suprascapular notch. Diameters of SSN: A—maximal depth, B—superior transverse, C—middle transverse
Fig. 5.
Types IV and V of the suprascapular notch. a Type IV. b Type V
The statistical analysis was performed on Statistica 10 software (StatSoft Polska, Cracow, Poland). Shapiro-Wilk test was used to check whether the distribution was normal or abnormal. The statistical difference between both the suprascapular notch measurements and frequency of types in both sexes were examined using the Mann–Whitney test and chi2 test, respectively (p level of < 0.05 was accepted as statistically significant). The distribution of measurements for the first three types, women and men are presented as mean, standard deviation, median, minimum and maximum (Tables 1 and 2).
Table 1.
Measurements of the suprascapular notch
| Diameter of SSN (mm) | Type I | Type II | Type III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min. | Mean ± SD | Median | Min. | Mean ± SD | Median | Min. | |
| Max. | Max. | Max. | |||||||
| Maximal depth | 10.33 ± 2.74 | 10.1 | 2.5 | 8.0 ± 2.29 | 8.23 | 3.68 | 7.02 ± 2.71 | 6.7 | 2.1 |
| 19.2 | 11.56 | 17.1 | |||||||
| Superior transverse diameter | 7.01 ± 3.19 | 6.85 | 1.6 | 8.02 ± 2.28 | 8.23 | 3.7 | 12.46 ± 3.66 | 12.03 | 4.67 |
| 17.8 | 11.6 | 25.97 | |||||||
| Middle transverse diameter | 7.68 ± 2.34 | 7.44 | 3.15 | 7.98 ± 2.29 | 8.15 | 3.69 | 9.17 ± 2.63 | 8.8 | 3.9 |
| 15.64 | 11.55 | 19.3 | |||||||
SD standard deviation, Min. minimum, Max. maximum, SSN suprascapular notch
Table 2.
Measurements of the suprascapular notch in females and males
| Diameter of SSN (mm) | Female | Male | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min. | Mean ± SD | Median | Min. | |
| Max. | Max. | |||||
| Maximal depth | 6.75 ± 2.23 | 6.73 | 2.1 | 8.31 ± 3.29 | 8.14 | 2.28 |
| 11.08 | 16.4 | |||||
| Superior transverse diameter | 8.74 ± 1.87 | 8.24 | 4.85 | 8.5 ± 3.11 | 8.09 | 3.38 |
| 12.25 | 19.3 | |||||
| Middle transverse diameter | 10.06 ± 3.63 | 9.61 | 2.63 | 10 ± 4.02 | 9.68 | 1.9 |
| 19.3 | 19.84 | |||||
SD standard deviation, Min minimum, Max. maximum, SSN suprascapular notch
Results
Demographic characteristics
Of 308 patients (616 scapulae), 134 were women (268 scapulae) and 174 men (348 scapulae). The mean age (±SD) of the entire group was 62.5 years (±13.9 years). The mean ages divided by sex (±SD) were 62.4 years (±15.1 years) for the men and 62.7 years (±12.5 years) for the women.
Suprascapular notch variations
In 616 computer tomography scans of scapulae, all five types of SSN were noted. Among the scapulae examined, type III (56.16 %) (Fig. 4) was the most common and type II (1.95 %) (Fig. 3) was the least. The frequency of type I was 24.18 %. This type was divided into three subtypes: IA (14.12 %), IB (3.08 %) and IC (6.98 %) (Fig. 2). Type III was also divided into three subtypes. The frequency of subtypes IIIA, IIIB and IIIC were 2.92 %, 0.97 % and 52.27 %, respectively (Fig. 4). Type IV (scapulae with bony foramen) was noted in 4.72 % of scapulae (Fig. 5a). The frequency of type V (discrete notch) was 12.99 % (Fig. 5b).
Comparisons between the dimensions for first three types of SSN are summarised in Table 1.
Sexual dimorphism
According to the Mann–Whitney test, only the maximal notch depth (p = 0.00326) was statistically significantly higher in males than in females. Middle transverse diameter and superior transverse diameter were similar in both groups and were statistically insignificant with p = 0.7254 and p = 0.1569, respectively (Table 2).
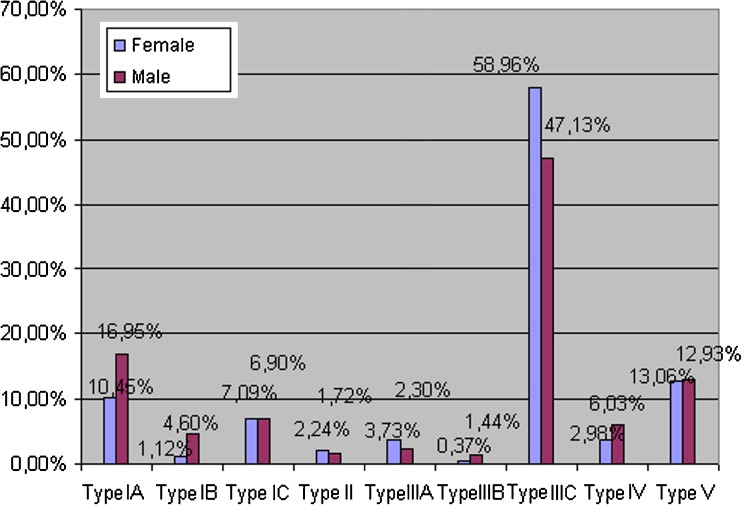
The frequency of subtypes IA and IB was lower in females (10.45 % and 1.12 %) than in males (16.95 % and 4.6 %). According to the chi2 test the difference was statistically significant with p = 0.01621 (type IA) and p = 0.02228 (type IB). Subtypes IIIA and IIIC were more common in females (3.73 % and 58.96 %) than males (2.3 % and 47.13 %). According to the chi2 test the difference was only statistically significantly different for type IIIC (p = 0.00903) but insignificant for type IIIA (p = 0.31741). Type IV (scapulae with bony foramen) was more frequently seen in males (6.03 % vs. 2.98 %). This difference between both sexes was statistically significant according to the chi2 test (p = 0.02415).
The distributions of other types of SSN in the female and male populations were similar. The frequency of types and subtypes in males and females is presented in Fig. 6.
Fig. 6.
Distribution of suprascapular notch types in female and male populations
Discussion
From 1942 to 2011, several classifications of suprascapular notch variations have been proposed in scientific literature [11, 12, 16–22]. However, little use has yet been made of new imaging techniques such as computer tomography or ultrasonography. This study is the first to classify the morphology of SSN based on computer scans of scapulae in a large population.
The two oldest classifications of the type of suprascapular notch were introduced by Hrdricka et al. (1942) [17] and Olivier (1960) [18]. In the first, Hrdricka et al. [17] divide the SSN into five types based on visual observation: shallow (type II), medium (type III) and deep (type IV). In type I, the SSN was absent, and in type V, a complete foramen was formed. In the second classification, Olivier [18] also describes five types of suprascapular notch. In type I, the notch is very small and forms a shallow depression on the superior border of the scapula. Type II is also shallow but more visible. The notches in types III and IV are deep. In type V, a completely ossified STSL forms a bony foramen.
One of the most cited is Rengachary’s classification from 1979 [12], in which the suprascascapular notches are divided into six types. The first type lacks a discrete notch. Their second type is a wide, blunted, V-shaped notch, with its maximum width along the superior border of the scapula. The third type is symmetrical with nearly parallel lateral margins (U-shaped). The fourth type presents a very small V-shaped notch. In the fifth type, the medial part of the STSL is partially ossified, with a U-shaped notch. The sixth type has a completely ossified STSL which forms a bony foramen.
In 1998, Ticker et al. [19] distinguished two main types of suprascapular notches: U-shaped and V-shaped. Additionally, the degree of ossification of the superior transverse scapular ligament was evaluated separately.
In 2003, Bayramoglu et al. [20], based on the classification of Rengachary et al. [12], also described two main types: U- and V-shaped notches.
In the next classification from 2007, Natsis et al. [11] distinguished five types of SSN. The first type lacks a discrete notch (8.3 %). The second type presents a notch that is longest in its transverse diameter (41.85 %). The third type presents a notch which is longest in its vertical diameter (41.85 %). In the fourth type, the STSL is calcified and forms a bony foramen (7.3 %). In the fifth type, the scapula has a notch and a bony foramen (0.7 %).
In 2010, Duparc et al. [21] reported that a V-shaped SSN was seen in 36.7 % of shoulders and a U-shaped in 63.3 % of shoulders. In both types, the notch could be more or less open, narrower or wider.
Also in 2010, Iqbal et al. [22], based on the shape, described three types of SSN: U-shaped (13.2 %), V-shaped (20 %) and J-shaped (22 %).
The newest classification was from 2011. Polguj et al. [16] describe a quantitative classification of the SSN based on specific geometrical parameters that clearly distinguish five structural types. It is described in greater detail above in "Materials and methods".
The classifications of Hrdricka et al. [17], Olivier [18], Ticker et al. [19], Bayramoglu et al. [20] and Iqbal et al. [22] are qualitative and not based on specific geometrical parameters. The main reason for using the classification of Polguj [16] in our study is that it is a quantitative classification. It is a simple, clearly-described procedure which requires measurements to be taken. The classification of Polguj [16] is also easy to use and is based on specific geometrical parameters which clearly distinguish each type and subtype of suprascapular notch.
The frequency of type I (“narrow and deep”) was found to be higher in males (28.45 %) than in females (18.66 %). On the other hand type III (“wide and shallow”) was higher in females (63.06 %) than males (50.87 %). This aspect is especially important because males are approximately three to four times more likely to suffer from a suprascapular neuropathy than females [2, 5–8]. It may be partially explained by the “sling effect” proposed in 1979 by Rangrery et al. [12], which assumes that during movements of the arm, the nerve makes only minimal transitional movements and can be pressed against the sharp bony margin of the suprascapular notch by the action of the upper limb. This repeated kinking irritates the nerve and induces microtrauma that can result in suprascapular nerve entrapment [12]. Therefore, hypothetically, when the suprascapular nerve passes by a “narrow and deep” suprascapular notch (type I), it could be more predisposed to injury by the sharp bony walls of this structure.
The second anatomical factor which might explain the higher frequency of suprascapular neuropathy in males than females may be the significantly higher frequency of scapulae with a bony foramen, corresponding to a completely ossified STSL–type IV, in males than females (6.03 % vs. 2.98 %). This appears to be especially important, because such morphological features are estimated to be one of the most important factors of suprascapular neuropathy [3, 11, 12, 16].
In our study, the frequency of scapulae with a bony foramen (type IV) was 4.72 % of all cases, which was higher than that seen by Sinkeet et al. [23] (3 %), Tubbs et al. [24] (3.7 %), Rengachary et al. [12] (4 %) or Wang et al. [25] (4.08 %). However it was lower then that described by Vallois [26] (6.5 %), Natsis et al. [11] (7.3 %) or Bayramoglu et al. [20] (12.5 %). In some populations, scapulae with a bony foramen was found to be very rare, while in others they were found to be common: Alaskan Eskimos (0.3 %) [27] vs. Egyptians 13.6 % [17]. It would be reasonable to suppose that complete ossification of STSL frequency depends on the population. However, in our opinion, an important factor is the mean age of investigated specimens; in our study, the mean age of all patients was 62.5 years, and we assume this to be lower than in the cadaveric study.
Another factor of suprascapular nerve entrapment can be anatomical variations of structures in the suprascapular region, such as a bifid STSL [20, 21] or trifid STSL [19], hypertrophied subscapular muscle [21], a double suprascapular foramen [25] or the co-existence of a notch with a suprascapular foramen [28].
In 2001, Pecina et al. [29] discovered that another factor of suprascapular nerve entrapment can be a connective tissue band, the ligamentum spinoglenoidale, which may exist in up to 50 % of people. It formed a second fibro-osseous tunnel (spinoglenoid notch) for three or four motor branches of the nerve that supply the infraspinatus muscle.
Our study uses a new quantitative classification of SSN morphology, which, contrary to existing methods, is simple, reproducible, and based on specific geometrical parameters that clearly distinguish each type. As suprascapular nerve entrapment is a rare condition, these features are especially important in clinical practise because it has confirmed its practical viability in multicentre investigations focused on this pathology. To our knowledge, the literature reveals no similar study which describes SSN variation in such a large population.
Conclusion
Our investigation has very precisely described SSN variations in the general population and its diversity between the sexes. The frequency of type I (“narrow and deep”) was higher in males than in females. However, type III (“wide and shallow”) was more common in females than males. Also a significantly higher frequency of scapulae with bony foramena (corresponding to a completely ossified superior traverse scapular ligament) was seen in males than females (6.03 % vs. 2.98 %). Knowledge of suprascapular notch variations may be essential for surgeons performing SN decompression, especially by means of endoscopic techniques.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Moore KL, Dalley AF, Agur AM. Clinical oriented anatomy. 6. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 88–92. [Google Scholar]
- 2.Zehetgruber H, Noske H, Lang T, Wurnig C. Suprascapular nerve entrapment. A meta-analysis. Int Orthop. 2002;26(6):339–343. doi: 10.1007/s00264-002-0392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosk J, Urban M, Rutkowski R. Entrapment of the suprascapular nerve: anatomy, etiology, diagnosis, treatment. Ortop Traumatol Rehabil. 2007;9:68–74. [PubMed] [Google Scholar]
- 4.Pecina M. Who really first described and explained the suprascapular nerve entrapment syndrome. J Bone Joint Surg Am. 2001;83-A(8):1273–1274. [PubMed] [Google Scholar]
- 5.Boykin RE, Friedman DJ, Zimmer ZR, Oaklander AL, Higgins LD, Warner JJ. Suprascapular neuropathy in a shoulder referral practice. J Shoulder Elbow Surg. 2011;20(6):983–988. doi: 10.1016/j.jse.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Vastamaki M, Goransson H. Suprascapular nerve entrapment. Clin Orth Rel Res. 1993;297:135–143. [PubMed] [Google Scholar]
- 7.Inokuchi W, Ogawa K, Horiuchi Y. Magnetic resonance imaging of suprascapular nerve palsy. J Shoulder Elbow Surg. 1998;7(3):223–227. doi: 10.1016/S1058-2746(98)90049-0. [DOI] [PubMed] [Google Scholar]
- 8.Antoniadis G, Richter HP, Rath S, Braun V, Moese G. Suprascapular nerve entrapment: experience with 28 cases. J Neurosurg. 1996;85(6):1020–1025. doi: 10.3171/jns.1996.85.6.1020. [DOI] [PubMed] [Google Scholar]
- 9.Ludig T, Walter F, Chapuis D, Mole D, Roland J, Blum A MR imaging evaluation of suprascapular nerve entrapment. Eur Radiol. 2001;11:216–219. doi: 10.1007/s003300100968. [DOI] [PubMed] [Google Scholar]
- 10.Dunkelgrun M, Iesaka K, Park SS, Kummer FJ, Zuckkerman JD. Interobserver reliability and intraobserver reproducibility in suprascapular notch typing. Bull Hosp Joint Dis. 2003;61:118–122. [PubMed] [Google Scholar]
- 11.Natsis K, Totlis T, Tsikaras P, Appell HJ, Skandalakis P, Koebke J. Proposal for classification of the suprascapular notch: a study on 423 dried scapulas. Clin Anat. 2007;20:135–139. doi: 10.1002/ca.20318. [DOI] [PubMed] [Google Scholar]
- 12.Rengachary SS, Burr D, Lucas S, Hassanein KM, Mohn MP, Matzke H. Suprascapular entrapment neuropathy: a clinical, anatomical, and comparative study. Part 2: anatomical study. Neurosurgery. 1979;5:447–451. doi: 10.1227/00006123-197910000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Barwood SA, Burkhart SS, Lo IK. Arthroscopic suprascapular nerve release at the suprascapular notch in a cadaveric model: an anatomic approach. Arthroscopy. 2007;23:221–225. doi: 10.1016/j.arthro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia DN, de Beer JF, van Rooyen KS, du Toit DF. Arthroscopic suprascapular nerve decompression at the suprascapular notch. Arthroscopy. 2006;22:1009–1013. doi: 10.1016/j.arthro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Lafosse L, Tomasi A, Corbett S, Baier G, Willems K, Gobezie R. Arthroscopic release of suprascapular nerve entrapment at the suprascapular notch: technique and preliminary results. Arthroscopy. 2007;23:34–42. doi: 10.1016/j.arthro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Polguj M, Jędrzejewski K, Podgórski M, Topol M. Morphometric study of the suprascapular notch—proposal of classification. Surg Radiol Anat. 2011;33:781–787. doi: 10.1007/s00276-011-0821-y. [DOI] [PubMed] [Google Scholar]
- 17.Hrdicka A. The adult scapula: visual observations. Am J Phys Antropol. 1942;29:73–94. doi: 10.1002/ajpa.1330290107. [DOI] [Google Scholar]
- 18.Olivier G. Pratique anthropologique. Le scapulum. Paris: Vigot Freres; 1960. pp. 194–201. [Google Scholar]
- 19.Ticker JB, Djurasovic M, Strauch RJ, April EW, Pollock RG, Flatow EL, Bigliani LU. The incidence of ganglion cysts and other variations in anatomy along the course of the suprascapular nerve. J Shoulder Elbow Surg. 1998;7(5):472–478. doi: 10.1016/S1058-2746(98)90197-5. [DOI] [PubMed] [Google Scholar]
- 20.Bayramoğlu A, Demiryürek D, Tüccar E, Erbil M, Aldur MM, Tetik O, Doral MN. Variations in anatomy at the suprascapular notch possibly causing suprascapular nerve entrapment: an anatomical study. Knee Surg Sports Traumatol Arthrosc. 2003;11(6):393–398. doi: 10.1007/s00167-003-0378-3. [DOI] [PubMed] [Google Scholar]
- 21.Duparc F, Coquerel D, Ozeel J, Noyon M, Gerometta A, Michot C. Anatomical basis of the suprascapular nerve entrapment, and clinical relevance of the supraspinatus fascia. Surg Radiol Anat. 2010;32(3):277–284. doi: 10.1007/s00276-010-0631-7. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal K, Iqbal R, Khan Anatomical variations in shape of suprascapular notch of scapula. J Morpho Sci. 2010;27(1):1–2. [Google Scholar]
- 23.Sinkeet SR, Awori KO, Odula PO, Ogeng’o JA, Mwachaka PM. The suprascapular notch: its morphology and distance from the glenoid cavity in a Kenyan population. Folia Morphol. 2010;69:241–245. [PubMed] [Google Scholar]
- 24.Tubbs RS, Smyth MD, Salter G, Oakes WJ. Anomalous traversement of the suprascapular artery through the suprascapular notch: a possible mechanism for undiagnosed shoulder pain? Med Sci Monit. 2000;9:116–119. [PubMed] [Google Scholar]
- 25.Wang HJ, Chen C, Wu LP, Pan CQ, Zhang WJ, Li YK. Variable morphology of the suprascapular notch: an investigation and quantitative measurements in Chinese population. Clin Anat. 2011;24(1):47–55. doi: 10.1002/ca.21061. [DOI] [PubMed] [Google Scholar]
- 26.Vallois HV. L’os acromial dans les races humaines. L’ Anthropologie Paris. 1925;35:977–1022. [Google Scholar]
- 27.Hrdicka A. The adult scapula: additional observations and measurements. Am J Phys Antropol. 1942;29:363–415. doi: 10.1002/ajpa.1330290303. [DOI] [Google Scholar]
- 28.Polguj M, Jędrzejewski K, Majos A, Topol M. Coexistence of the suprascapular notch with the suprascapular foramen—rare anatomical variation and a new hypotheses of its formation based on anatomical and radiological studies. Anat Sci Int. 2013;88(3):156–162. doi: 10.1007/s12565-012-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pecina MM, Krmpotic-Nemanic J, Markiewitz AD. Tunnel syndromes. Peripheral nerve compression syndromes. 3. Boca Raton: CRC Press; 2001. pp. 49–55. [Google Scholar]