Abstract
Purpose
The clinical use of closed-suction drainage, which aims to reduce postoperative wound haematomas and infection, is common. This study was performed to determine whether closed-suction drainage is safe and effective in promoting wound healing and reducing blood loss and other complications compared with no-drainage in total hip arthroplasty.
Methods
The literature search was based on PubMed, the Cochrane Library, MEDLINE, and EMBASE. The data were evaluated using the generic evaluation tool designed by the Cochrane Bone, Joint and Muscle Trauma Group, and then analysed using RevMan 5.0. Twenty randomised controlled trials involving 3,186 patients were included in our analysis.
Results
The results of our meta-analysis indicate that closed-suction drainage reduces the requirement for dressing reinforcement, but increases the rate of homologous blood transfusion. No significant difference was observed in the incidence of infection, blood loss, changes in haemoglobin and haematocrit, functional assessment, or other complications when the drainage group was compared with the no-drainage group.
Conclusions
Our results of the comparison between closed-suction drainage and no drainage in THA have indicated that the routine use of closed-suction drainage for elective total hip arthroplasty may be of more harm than benefit.
Keywords: Closed-suction drainage, Total hip arthroplasty, Blood loss, Transfusion, Meta-analysis
Introduction
Open drainage and closed-suction drainage are two of the most common and direct methods to promote drainage of liquid from wounds or cavities in surgical procedures. Compared with the open drainage, closed-suction drainage has more powerful suction from the drainage bottle to enhance the negative pressure. Our meta-analysis is applied purely to the use of closed-suction drainage of surgical wounds in total hip arthroplasty (THA).
Closed-suction drainage has been widely used in many orthopaedic surgical procedures, including THA, based on the theory of effectively decreasing haematoma formation, which is theoretically associated with decreasing postoperative pain and limb swelling, accelerating wound healing, and prevention of infection [1, 2]. However, some authors have advocated that not using drainage would have more benefits in THA [3–22], because closed drainage leads to blood loss after THA by eliminating the tamponade effect and potentially allows retrograde infection.
To our knowledge, the true effect of closed-suction drainage in THA has not been clearly clarified, with the exception of a mixed meta-analysis that included various surgical procedures before March 2006 [23]. However, no valuable advantages have been observed by using closed-suction drainage. Therefore, we postulated that there is no difference in outcomes of wound infection, wound healing, homologous transfusion, haemoglobin and hematocrit levels, wound haematoma, limb swelling or pain levels between closed-suction drainage of the wound and no drainage after total hip arthroplasty. Consequently, we conducted a meta-analysis of randomised controlled trials (RCTs) comparing the outcomes of closed-suction drainage with no drainage after THA to provide a powerful and rational conclusion regarding the use of drainage after THA.
Materials and methods
We followed the methodological guidelines outlined by the Cochrane Collaboration (Oxford, UK) to conduct this meta-analysis [24]. The findings were reported according to the standard described in the ‘Preferred reporting items for systematic reviews and meta-analyses’ statement in the review [25].
Inclusion criteria
This meta-analysis evaluated RCTs in English, comparing closed-suction drainage with no drainage in terms of postoperative efficacy and safety. Studies involving other types of surgery were excluded, including total hip surface replacement, hemiarthroplasty, fracture fixation, and others. Articles were excluded if they did not include a no-drainage control group. Animal and cadaver studies were also excluded. The participants were adults who had undergone THA, regardless of the type or size of prosthesis or bone cement used.
Search strategy
Two of the authors completed the search of electronic databases, including MEDLINE, PubMed, EMBASE, and the Cochrane Library, for trials of closed-suction drainage and THA published before December 2012. Articles not published in English were excluded. During the electronic database search, the following Medical Subject Headings (MeSH) terms were used: ‘suction’, ‘drainage’, ‘drain’, ‘hip arthroplasty’, ‘hip replacement’, and ‘joint replacement’. All terms were also used in all possible combinations to search the databases. We also searched the reference lists of related reviews and original articles identified for any relevant trials including clinical trials and RCTs involving adult humans.
Data extraction
To select suitable references, two authors independently scanned the titles and abstracts for appropriate articles. First, the EndNote X6 software was used to remove duplicates. When there was uncertainty about any of the above vital information, the full article was retrieved for further scrutiny, or the authors of individual trials were contacted directly to provide further information when necessary. Each RCT received a score of 0 to 24 depending on ‘The Cochrane Bone, Joint and Muscle Trauma Group’ (Table 1). Data was extracted from the included studies by two reviewers independently. If there was any disagreement, a consensus was reached through discussion by a review team. If controversy remained, disagreements were resolved by consultation with the senior reviewer, W.L.
Table 1.
Quality assessment items and possible scores
| A. Was the assigned treatment adequately concealed prior to allocation? |
| 2 = Method did not allow disclosure of assignment |
| 1 = Small but possible chance of disclosure of assignment or unclear |
| 0 = Quasi-randomised or open list/tables |
| B. Were the outcomes of participants who withdrew described and included in the analysis (intention to treat)? |
| 2 = Withdrawals well described and accounted for in analysis |
| 1 = Withdrawals described and analysis not possible |
| 0 = No mention, inadequate mention, or obvious differences and no adjustment |
| C. Were the outcome assessors blinded to treatment status? |
| 2 = Effective action taken to blind assessors |
| 1 = Small or moderate chance of unblinding of assessors |
| 0 = Not mentioned or not possible |
| D. Were the treatment and control group comparable at entry? |
| 2 = Good comparability of groups, or confounding adjusted for in analysis |
| 1 = Confounding small; mentioned but not adjusted for |
| 0 = Large potential for confounding, or not discussed |
| E. Were the participants blind to assignment status after allocation? |
| 2 = Effective action taken to blind participants |
| 1 = Small or moderate chance of unblinding of participants |
| 0 = Not possible, or not mentioned (unless double-blind), or possible but not done |
| F. Were the treatment providers blind to assignment status? |
| 2 = Effective action taken to blind treatment providers |
| 1 = Small or moderate chance of unblinding of treatment providers |
| 0 = Not possible, or not mentioned (unless double-blind), or possible but not done |
| G. Were care programmes, other than the trial options, identical? |
| 2 = Care programmes clearly identical |
| 1 = Clear but trivial differences |
| 0 = Not mentioned or clear and important differences in care programmes |
| H. Were the inclusion and exclusion criteria clearly defined? |
| 2 = Clearly defined, 1 = inadequately defined, 0 = not defined |
| I. Were the interventions clearly defined? |
| 2 = Clearly defined, 1 = inadequately defined, 0 = not defined |
| J. Were the outcome measures used clearly defined? (by outcome) |
| 2 = Clearly defined, 1 = inadequately defined, 0 = not defined |
| K. Were diagnostic tests used in outcome assessment clinically useful? (by outcome) |
| 2 = Optimal, 1 = adequate, 0 = not defined, not adequate |
| L. Was the surveillance active, and of clinically appropriate duration? (by outcome) |
| 2 = Optimal, 1 = adequate, 0 = not defined, not adequate |
Statistical analysis
To perform the meta-analysis, two independent reviewers pooled the data from each study for analysis using the Review Manager software version 5.0 (The Cochrane Collaboration, Oxford, England) and Stata 12.0. Dichotomous data were entered as number of events, while continuous data were entered as means and standard deviations (SDs). Before pooling the data, statistical heterogeneity for each study was assessed. The presence of heterogeneity was explored by a chi-squared test with statistical significance set at a p value of 0.1, and I2 was used to measure the quantity of heterogeneity [26]. The origins of heterogeneity, if present, were analysed according to differences in methodological quality, characteristics of participants, and intervention. Fixed-effects analysis was used to compare trials that did not show heterogeneity, whereas random-effects analysis was used to compare trials that showed heterogeneity. For each study, the odds ratio (OR) and 95 % confidence intervals (CIs) were calculated for dichotomous outcomes, and weighted mean differences (WMD) and 95 % CIs were used for continuous outcomes.
Results
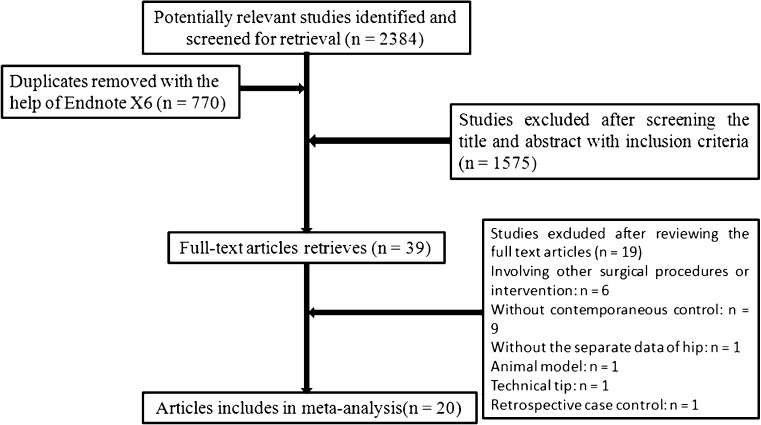
Following the search strategy outlined above, and the inclusion and exclusion criteria, the two independent reviewers located 20 RCTs [3–22] involving 3,186 patients for our analysis (Table 2). The entire process is shown in Fig. 1. The included studies were relatively well designed, and the quality assessment score was high in most of them, with a mode of 20, the highest possible score and a range of 12–20, because double-blind studies were impossible. Osteoarthritis was the most frequent diagnosis, followed by rheumatic arthritis. A summary of outcomes of included studies and the meta-analyses are detailed in two tables (Table 3 and Table 4) and one figure (Fig. 2), respectively.
Table 2.
Characteristics of included studies
| Trials and year | Region | No. hips (D/ND) | No. of patients | Procedure | Male (%) | Follow-up | Thromboprophylaxis | Antibiotics | QAS |
|---|---|---|---|---|---|---|---|---|---|
| Beer, 1991 | Columbus, USA | 12/12 | 12 | Bilateral | NA | 3 months | Sodium warfarin | NA | 15 |
| Murphy, 1993 | London, UK | 20/20 | 40 | Unilateral | NA | 10 days | NA | NA | 14 |
| Ritter, 1994 | Mooresville, Indiana | 78/62 | 140 | Unilateral | NA | Discharge | Aspirin | NA | 13 |
| O’Brien, 1997 | Belfast, UK | 440/432 | 872 | Unilateral | 42.32 | Discharge | Dextran & mechanical | Cefamandole | 20 |
| Ovadia, 1997 | Tel-Aviv, Israel | 18/12 | 30 | Unilateral | 50 | Discharge | Heparin | NA | 16 |
| Crevoisier, 1998 | Lausanne, Switzerland | 33/33 | 66 | Unilateral | NA | Discharge | Heparin | Cefazolin | 15 |
| Kim, 1998 | Seoul, Korea | 48/48 | 48 | Bilateral | 79.2 | 15 months | NA | NA | 20 |
| Niskanen, 2000 | Lahti, Finland | 27/31 | 58 | Unilateral | 37.9 | 2 months | LMWH | No name | 14 |
| Ravikumar, 2001 | Kent, UK | 12/13 | 23 | Unilateral or Bilateral | 47.8 | 6 weeks | Mechanical | Cefuroxime | 16 |
| Widman, 2002 | Stockholm, Sweden | 10/12 | 22 | Unilateral | 40.9 | NA | Dalteparin | Cloxacillin | 12 |
| Hill, 2003a | Dunfermline, UK | NA | 577 | Unilaterl or bilateral | NA | 6 months | NA | NA | 14 |
| Gonzalez DV, 2004 | New York, USA | 53/51 | 102 | Unilaterl or bilateral | 52.9 | 3 months | Unfractionated Heparin, LMWH or Acetylsalicylic acid | No name | 19 |
| Johansson, 2005 | Linkoping, Sweden | 54/51 | 105 | Unilateral | 50.5 | 2 months | Dalteparin | NA | 18 |
| Walmsley, 2005 | Dunfermline, UK | 282/295 | 552 | Unilaterl or bilateral | 38.6 | 36 months | LMWH | Cefuroxime | 20 |
| Matsuda, 2006 | Tokyo, Japan | 20/20 | 40 | Unilateral | 22.5 | More than 3 months | Mechanical | No name | 20 |
| Dora, 2007 | Zürich, Switzerland | 50/50 | 100 | Unilateral | 45 | 12 months | LMWH | Cephalosporin | 20 |
| Strahovnik, 2010 | Celje, Slovenia | 46/42(24H) 51/42(48H) | 139 | Unilateral | 33.1 | 3 months | LMWH | Cefazolin | 20 |
| Cheung, 2010 | Shropshire, UK | 52/48 | 100 | Unilateral | NA | 12 months | Aspirin & mechanical | Cefuroxime | 18 |
| Kleinert, 2012 | Zurich, Switzerland | 40/40 | 80 | Unilateral | 47.5 | 3 months | LMWH | Cefuroxime | 21 |
| Roth, 2012 | Munich, Germany | 40/40 | 80 | Unilateral | 41.3 | Discharge | LMWH | NA | 17 |
D drainage, ND no drainage, H hour, LMWH low-molecular-weight heparin, QAS quality assessment score, NA not mentioned
a Abstract only
Fig. 1.
Flow chart of included and excluded studies
Table 3.
Detailed summary of included studies
| Trials and year | No. of hips (D/N) | No. of patients, D/N | Mean or mean (SD), D/N | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Reoperation | Blood transfusion | DVT | PE | Wound haematomas | Limb swelling | Reinforcement | Oozing | Total blood loss (mL) | Blood transfusion (U) | Pain (VAS) | ||
| Beer, 1991 | 12/12 | 0/0 | 0/0 | 0/0 | NSD | NSD | |||||||
| Murphy, 1993 | 20/20 | 1/0 | 1455/1134 | ||||||||||
| Ritter, 1994 | 78/62 | 0/0 | 15/13 | 0/0 | 0/0 | 0/0 | 0.59/0.47 | ||||||
| O’Brien, 1997 | 440/432 | 1/2 | 0/1 | NSD | NSD | 48/45 | 1431/784.76 | ||||||
| Ovadia, 1997 | 18/12 | 0/0 | 9/2 | 1/0 | 2/2 | 1.44/1.5 | |||||||
| Crevoisier, 1998 | 33/33 | 0/0 | 2/0 | NSD | 2/2 | ||||||||
| Kim, 1998 | 48/48 | 0/0 | 0/0 | 35/44 | NSD | ||||||||
| Niskanen, 2000 | 27/31 | 1/1 | 0/0 | 0/0 | 0/0 | 9/21 | 1/2 | 588/612 | 1.8/1.8 | ||||
| Ravikumar, 2001 | 12-/13 | 1/7 | 1/3 | 2/2 | |||||||||
| Widman, 2002 | 10-/12 | 1/1 | 9/6 | NSD | 4.12(2.9)/1.18(0.92) | ||||||||
| Hill, 2003 | NA | 39/45 | NSD | ||||||||||
| Gonzalez DV, 2004 | 53/51 | 2/0 | 1/0 | 21/18 | 1/1 | 1/0 | 2/0 | NSD | 6/10 | 1.6/1.5 | |||
| Johansson, 2005 | 54/51 | 3/2 | 36/28 | 3/0 | 0/0 | 1695(712)/1510( 656) | 2(2)/1.4(1.5) | ||||||
| Walmsley, 2005 | 282/295 | 19/23 | 1/2 | 3/1 | 2/1 | 0/1 | |||||||
| Matsuda, 2006 | 20/20 | 0/0 | 0/0 | 0/0 | 0/0 | 2/6 | 0/0 | NSD | |||||
| Dora, 2007 | 50/50 | 0/0 | 1/0 | 1/1 | NSD | 2346(1034)/2195(1110) | 2.4(1.6)/2.5(1.9) | 1.9(1.5)/1.8(1.6) (Day 2), 1.8(1.6)/2.0(1.5)(Day 6) |
|||||
| Strahovnik, 2010 | 46/42(24H) 51/42(48H) | 1/0 | 1/0 | 0/0 | 0/0 | 4/20 | 959/1098(24H); 1052/1098(48H) | 2.33/3.235(24H), 3.175/3.235(48H) | |||||
| Cheung, 2010 | 52/48 | 2/0 | 0/0 | 19/6 | 1a | 1.89/1.83 | |||||||
| Kleinert, 2012 | 40/40 | 0/0 | 0/1 | 4/4 | NSD | 1.4(1.9)/2.1(1.7)(Day 1), 1(1.5)/1.4(2.3)(Day 2), 0.7(1.2)/0.7(1.2)(Day 3) |
|||||||
| Roth, 2012 | 40/40 | 0/0 | NSD | 900/900 | 0/0 | 2/1.2(Day1,Rest), 3.8/2.7(Day 1, Stress), 0.6/0.3(Day 4, Rest) 2.2/1.4(Day 4, Stress) |
|||||||
D drainage, ND no drainage, H hour, NSD no significant difference and without detailed data, DVT deep vein thrombosis, PE pulmonary embolism, U unit, VAS visual analogue scale
a One patient had PE without specific group.
Table 4.
Summary of the meta-analyses
| Analysis item | Number of studies | No. of hips (D/N) | Heterogeneity | Analysis model | Statistical method | WMD/OR (95 % CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| I2 | PHet value | |||||||
| Wound infection | 18 | 1346/1272 | 0 % | 0.55 | Fixed-effects | Mantel-Haenszel | 0.85 (0.53, 1.37) | 0.51 |
| Blood loss | 2 | 104/101 | 0 % | 0.89 | Fixed-effects | Mantel-Haenszel | 175.51 (−46.66, 397.68) | 0.12 |
| BN | 9 | 365/336 | 37 % | 0.14 | Fixed-effects | Mantel-Haenszel | 1.68 (1.15, 2.46) | 0.007 |
| BA | 3 | 114/113 | 79 % | 0.009 | Random-effects | Mantel-Haenszel | 0.81 (−0.34,1.96) | 0.17 |
| Wound haematomas | 3 | 383/394 | 39 % | 0.19 | Fixed-effects | Mantel-Haenszel | 0.42 (0.16,1.08) | 0.07 |
| Limb swelling | 3 | 116/94 | 20 % | 0.26 | Fixed-effects | Mantel-Haenszel | 0.43 (0.11,1.77) | 0.24 |
| Reinforcement | 3 | 177/124 | 77 % | 0.01 | Random-effects | Mantel-Haenszel | 0.18 (0.05, 0. 72) | 0.01 |
| Oozing | 4 | 492/483 | 0 % | 0.93 | Fixed-effects | Mantel-Haenszel | 1.02 (0.68, 1.53) | 0.94 |
| Reoperation for wound healing | 11 | 1134/1083 | 0 % | 0.76 | Fixed-effects | Mantel-Haenszel | 0.94 (0.38, 2.31) | 0.89 |
| Thromboembolic complications (DVT) | 9 | 673/614 | 0 % | 0.72 | Fixed-effects | Mantel-Haenszel | 2.44 (0.70, 8.55) | 0.16 |
| Thromboembolic complications (PE) | 9 | 623/564 | 0 % | 0.87 | Fixed-effects | Mantel-Haenszel | 2.38 (0.35, 16.30) | 0.38 |
| Pain | 2 | 80/80 | 0 % | 0.37 | Fixed-effects | Mantel-Haenszel | −0.09 (−0.63, 0.44) | 0.73 |
BN blood transfusion (no. of patients), BA blood transfusion (amount of blood per patient)
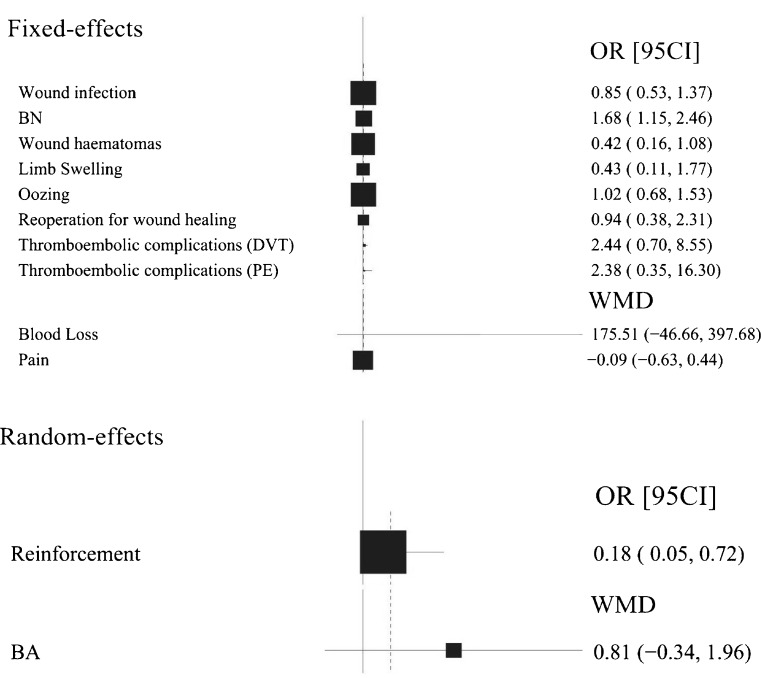
Fig. 2.
Forest plot diagram showing a summary of the results of the meta-analysis. BN Blood transfusion (no. of patients), BA Blood transfusion (amount of blood per patient)
Wound infection and reoperation for wound healing
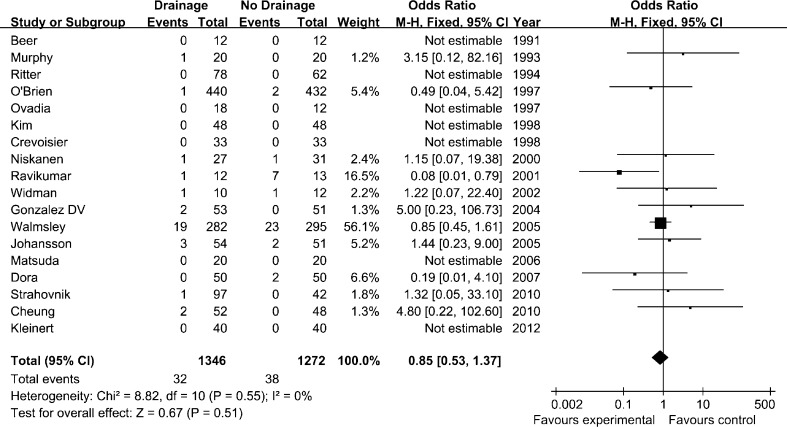
Wound infection was one of the primary criteria in our meta-analysis. Data were pooled for analysis from 18 studies [3–12, 14–20, 22] and there was no evidence of statistical heterogeneity (I2 = 0 %, Phet = 0.55). Meanwhile, the overall rate of reoperation, including joint overhaul, debridement or drainage of a haematoma was also pooled for meta-analysis. However, the difference was not statistically significant either in the rate of wound infection (OR, 0.85 [95 % CI, 0.53–1.37]; p = 0.51, Fig. 3) or the reoperation for wound healing (OR, 0.94 [95 % CI, 0.38–2.31]; p = 0.89) between two groups.
Fig. 3.
Forest plot diagram showing the influence of closed-suction drainage on wound infection. CI confidence interval, SD standard deviation, IV inverse variance, df degrees of freedom, M–H Mantel–Haenszel
Blood loss, transfusion, changes in haemoglobin and hematocrit and thromboembolic complications
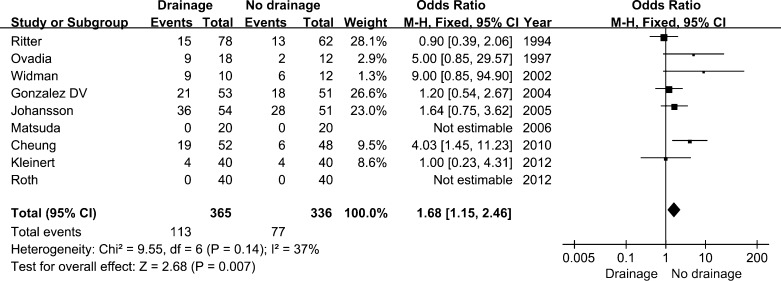
The pooled results from two studies [15, 17] demonstrated that the amount of total blood loss per patient was 175.51 ml more in the drainage group compared with the no drainage group ([95 % CI, −46.66 to 397.68 ml]; p = 0.12). However, there was no significant difference, likely because of the poor data. And no definitive conclusion regarding the effect on blood loss of closed-suction drainage in THA could be drawn. The number of patients who needed homologous transfusion was reported in seven studies [5, 6, 12, 14, 15, 20, 21], and the pooled data was significantly less in the no drainage group who needed transfusion compared with the control group (OR, 1.68 [95 % CI, 1.15–2.46]; p = 0.007) (Fig. 4). The amount of transfusion showed no significant differences between the two groups, depending on the data pooled from three studies [12, 15, 17] (WMD, 0.81 [95 % CI, -0.34–1.96 u ]; p = 0.17). Postoperative haemoglobin was reported by nine of the individual studies [5–7, 11, 15, 16, 18, 20, 22], while five studies [8, 14, 18, 20, 22] reported a change in the haematocrit. However, none reported any significant difference, except Gonzalez Della Valle et al. [14], who reported that patients who received closed-suction drainage had more marked reductions in haematocrit (10.4 vs. 7.4, p = 0.03). There were no differences in the frequency of DVT (OR 2.44 [95 % CI, 0.70–8.55]; p = 0.16) or PE (OR 2.38 [95 % CI, 0.35–16.30]; p = 0.38) between patients in the drainage and no drainage groups after arthroplasty surgery.
Fig. 4.
Forest plot diagram showing the influence of closed-suction drainage on homologous transfusion
Wound haematoma and limb swelling
The outcome of wound haematoma was reported in eight studies [7–9, 12, 14, 16, 17, 20]. However, only two of them [9, 17] showed a significant difference in the reduced number of patients who developed wound haematomas or in the wound haematoma volume in the drainage group compared with the control group; both involved ultrasound examinations. Dora et al. [17] reported a significant difference in the incidence of subfascial haematomas, while Kim et al. [9] reported a significant increase in the large haematomas over ten millimetres in depth. And no definite conclusion could be made that the use of drainage did not significantly reduce the number of patients who developed wound haematomas (OR, 0.42 [95 % CI, 0.16–1.08]; p = 0.07). More studies are needed to confirm our findings. Data on limb swelling were reported in ten studies [3, 5, 7, 12, 14, 17, 19–22]. Two studies [19, 20] supported the conclusion that the use of drainage can reduce the incidence of limb swelling or decrease the volume of the swelling. However, based on the data pooled from three studies [5, 6, 22], no evidence to support the hypothesis that the rate of limb swelling was reduced by using drainage when compared with the placebo group was obtained (OR, 0.43 [95 % CI, 0.11–1.77]; p = 0.24).
Reinforcement of the dressing and oozing from the wound
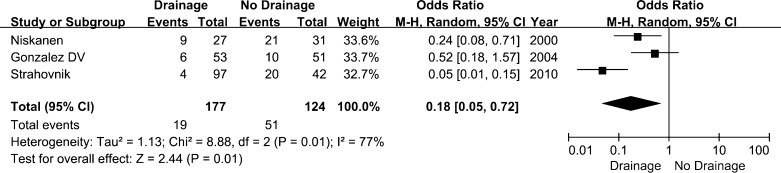
The dressing reinforcement outcome was available in four studies [9, 10, 14, 19]. The OR of dressing reinforcement was reported in three articles [10, 14, 19]. The rate of patients who required reinforcement was significantly lower in the drainage group than in the placebo group (OR, 0.18 [95 % CI, 0.05–0.72]; p = 0.01) (Fig. 5). Four studies [6, 7, 10, 11] reported oozing from the wound. The data of the largest number of subjects included in the studies was pooled for analysis. The rates of oozing from the wound in the drainage and no-drainage groups were 53 of 492 patients and 51 of 483 patients, respectively. The rate of oozing from the wound was not affected by the use of drainage when compared with the placebo group (OR, 1.02 [95 % CI, 0.68–1.53]; p = 0.94).
Fig. 5.
Forest plot diagram showing the influence of closed-suction drainage on reinforcement of the dressing
Functional assessment and pain
The outcome of functional assessment was reported in six studies [8–10, 13, 16, 20]. Four of them [9, 13, 16, 20] reported a significant difference between the two groups following the Harris hip score system. Niskanen et al. [10] reported that the no-drainage group had five more degrees of motion than the drainage group, while Crevoisier et al. [8] reported that the arc of motion had increased on average by six degrees in the drainage group and 11 degrees in the no-drainage group at the time of discharge. As a result, no meaningful conclusion could be drawn from the postoperative drainage data. Eight studies [3, 9, 13, 17, 19, 20, 22] reported data on postoperative pain. And two of them [11, 19] reported that more patients experienced mid-thigh pain in the no-drainage group than in the drainage group, while six [3, 9, 13, 17, 20, 22] reported no significant difference. Based on the data pooled from Dora et al. [17] and Kleinert et al. [20], no significant difference of pain could be found between the groups (WMD, −0.09 [95 % CI, −0.63 to 0.44]; p = 0.73).
Discussion
Since the closed-suction drainage system was first reported in the 19th century [27, 28], it has been used postoperatively for more than 100 years. The original aim of drainage system is to reduce haematoma formation and postoperative oedema, decrease the possibility of infection, and minimise the probability of external contamination of the surgical site. The use of postoperative drainage has been increasingly questioned since the end of the 20th century. A series of RCTs comparing closed-suction drainage and no drainage have been published [3–22]. We extracted these RCTs from the four main medical literature databases to perform a meta-analysis of the efficacy and safety of drainage after hip replacement surgery. One study [13], which was only a conference abstract, was also included in our study to minimise bias against smaller studies that were not published in full. Most of the studies incorporated in our meta-analysis were small and included <100 patients. The postoperative follow-up duration in most of the studies was less than six months, while some followed up only until discharge. All of these differences may affect results and alter the judgments of clinicians. However, despite the differences in clinical trial methodologies, the results of the studies were generally homogeneous.
Wound infection was one of the first primary outcomes measured in our analysis, and we found no significant difference between the drainage group and the control group. Seventy of 2,618 patients had wound infections, including superficial and deep infections. However, 12 studies reported the use of prophylactic antibiotics, which may have resulted in concealment of the infection outcome as well as loss to follow-up because of the short follow-up time. Incorporation of a limited number in each group means that definitive conclusions cannot be drawn.
A significant advantage for patients managed with no drainage was the reduction of the number of patients who required homologous transfusions. Blood loss was shown to be less in the no-drainage group compared with the drainage group. The data that included the SD, extracted from only two studies, could not represent the true influence of the use of drainage. However, many RCTs, including our study, show the same trend of less total blood loss in the no-drainage group. This may be a consequence of the absence of blood loss into the drain. More blood loss leads to a higher incidence of blood transfusion. Blood transfusion requirements tended to be lower for patients in the no-drainage group. However, a similar conclusion could not be obtained because of the limited number of studies included. Although the volumes of blood loss and homologous blood required were significantly less in patients who were managed with no drainage, these methods failed to show significantly higher levels of postoperative haemoglobin or haematocrit in the pooled data. We attribute this outcome to the influence of transfusion of autologous or allogenic blood.
The only definite advantage for patients managed with drainage was reducing the number of patients who required reinforcement dressings. A substantially greater number of wounds required reinforcement of the dressing in the no-drainage group. This outcome favours the use of drainage because of the reduction in the volume of blood leaking through the wound. Although this may cause some inconvenience and distress to the patient or lead to a requirement for additional nursing care, it may accelerate wound healing. However, no data on the number of days it took for the incision to heal were reported in the studies, including ours.
There were no definite and significant differences or trends in the outcomes of wound haematoma, limb swelling, oozing, reoperation, thromboembolic complications, functional assessment, or pain between the two groups.
The limitations of our study included insufficient sample sizes and data to strengthen the conclusion of our results. Some studies failed to provide sufficient or usable data, which could be pooled for meta-analysis, though we attempted to contact the authors. In addition, the follow-up of patients in some trials was limited. Many patients were followed up only until ten days after surgery or hospital discharge. This may have resulted in underreporting of some results, such as infection, DVT or PE. Furthermore, parameters that could be used to compare closed-suction drainage with no drainage in THA also included length of hospital stay, cost analysis, rehabilitation and acceptability to patients, along with the quantification of some complications, such as wound infection, reinforcement and oozing. However, these factors could not be analysed because of different standards or heterogeneous outcomes in these trials. Moreover, the autotransfusion protocol, allogenic blood transfusion protocol, use of antibiotics, doses or methods of anticoagulation, surgical techniques and treatments for pain probably contributed to the differences observed among studies.
Both our meta-analysis and another study [23] indicate no definite advantage to the use of closed-suction drainage in THA. And this conclusion is consistent with the three years follow-up of a large cohort study performed by Walmsley et al [16]. Two meaningful differences were suggested by our study. The only definite advantage of the use of postoperative drainage demonstrated by our analysis was reduction in the number of patients who require reinforcement of the dressing for incision seepage. However, this is relatively trivial compared with the unique disadvantage of the increased number of patients who require allogenic transfusion. In conclusion, our results of the comparison between closed-suction drainage and no drainage in THA have indicated that the routine use of closed-suction drainage for elective total hip arthroplasty may be of more harm than benefit. However, due to the failure to recognise the importance of adequate reporting of clinical outcomes, more large, blinded and longer follow-up period studies with fully reported results, on this topic are justified in future.
Acknowledgements
We would like to thank Professor Martyn Parker, Dr. Ling-ling Sun and Dr. Tiao Lin for help in this study.
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Holt BT, Parks NL, Engh GA, Lawrence JM (1997) Comparison of closed-suction drainage and no drainage after primary total knee arthroplasty. Orthopedics 20(12):1121–1124, discussion 1124–1125 [DOI] [PubMed]
- 2.Sundaram RO, Parkinson RW. Closed suction drains do not increase the blood transfusion rates in patients undergoing total knee arthroplasty. Int Orthop. 2007;31(5):613–616. doi: 10.1007/s00264-006-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer KJ, Lombardi AV, Jr, Mallory TH, Vaughn BK. The efficacy of suction drains after routine total joint arthroplasty. J Bone Joint Surg Am. 1991;73(4):584–587. [PubMed] [Google Scholar]
- 4.Murphy JP, Scott JE. The effectiveness of suction drainage in total hip arthroplasty. J R Soc Med. 1993;86(7):388–389. doi: 10.1177/014107689308600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter MA, Keating EM, Faris PM. Closed wound drainage in total hip or total knee replacement. A prospective, randomized study. J Bone Joint Surg Am. 1994;76(1):35–38. doi: 10.2106/00004623-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ovadia D, Luger E, Bickels J, Menachem A, Dekel S. Efficacy of closed wound drainage after total joint arthroplasty. A prospective randomized study. J Arthroplasty. 1997;12(3):317–321. doi: 10.1016/S0883-5403(97)90029-2. [DOI] [PubMed] [Google Scholar]
- 7. O'Brien S, Engela D, James P, Kernohan G, Connolly D, Milligan K, Kettle P, Beverland D (1997) The use of wound drains in total hip replacement surgery. J Orthop Nursing 1:77–83
- 8.Crevoisier XM, Reber P, Noesberger B. Is suction drainage necessary after total joint arthroplasty? A prospective study. Arch Orthop Trauma Surg. 1998;117(3):121–124. doi: 10.1007/s004020050210. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Cho SH, Kim RS. Drainage versus nondrainage in simultaneous bilateral total hip arthroplasties. J Arthroplasty. 1998;13(2):156–161. doi: 10.1016/S0883-5403(98)90093-6. [DOI] [PubMed] [Google Scholar]
- 10.Niskanen RO, Korkala OL, Haapala J, Kuokkanen HO, Kaukonen JP, Salo SA. Drainage is of no use in primary uncomplicated cemented hip and knee arthroplasty for osteoarthritis: a prospective randomized study. J Arthroplasty. 2000;15(5):567–569. doi: 10.1054/arth.2000.6616. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar KJAT, Fordyce MJF, Tuson KWR. Drainage versus nondrainage in total hip arthroplasty. A prospective randomised study. Hip Int. 2001;11:49–53. [Google Scholar]
- 12.Widman J, Jacobsson H, Larsson SA, Isacson J. No effect of drains on the postoperative hematoma volume in hip replacement surgery: a randomized study using scintigraphy. Acta Orthop Scand. 2002;73(6):625–629. doi: 10.1080/000164702321039570. [DOI] [PubMed] [Google Scholar]
- 13.RMF H, I B. (2003) Drain vs no drain in unilateral total hip arthroplasty: a randomised prospective trial [abstract]. J Bone Joint Surg Br 85(Suppl II):104
- 14.Gonzalez Della Valle A, Slullitel G, Vestri R, Comba F, Buttaro M, Piccaluga F. No need for routine closed suction drainage in elective arthroplasty of the hip: a prospective randomized trial in 104 operations. Acta Orthop Scand. 2004;75–1:30–33. doi: 10.1080/00016470410001708050. [DOI] [PubMed] [Google Scholar]
- 15.Johansson T, Engquist M, Pettersson LG, Lisander B. Blood loss after total hip replacement: a prospective randomized study between wound compression and drainage. J Arthroplasty. 2005;20(8):967–971. doi: 10.1016/j.arth.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley PJ, Kelly MB, Hill RM, Brenkel I. A prospective, randomised, controlled trial of the use of drains in total hip arthroplasty. J Bone Joint Surg Br. 2005;87(10):1397–1401. doi: 10.1302/0301-620X.87B10.16221. [DOI] [PubMed] [Google Scholar]
- 17.Dora C, von Campe A, Mengiardi B, Koch P, Vienne P. Simplified wound care and earlier wound recovery without closed suction drainage in elective total hip arthroplasty. A prospective randomized trial in 100 operations. Arch Orthop Trauma Surg. 2007;127(10):919–923. doi: 10.1007/s00402-006-0260-0. [DOI] [PubMed] [Google Scholar]
- 18.Cheung G, Carmont MR, Bing AJF, Kuiper JH, Alcock RJ, Graham NM. No drain, autologous transfusion drain or suction drain? A randomised prospective study in total hip replacement surgery of 168 patients. Acta Orthopaedica Belgica. 2010;76(5):619–627. [PubMed] [Google Scholar]
- 19.Strahovnik A, Fokter SK, Kotnik M. Comparison of drainage techniques on prolonged serous drainage after total hip arthroplasty. J Arthroplasty. 2010;25(2):244–248. doi: 10.1016/j.arth.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Kleinert K, Werner C, Mamisch-Saupe N, Kalberer F, Dora C. Closed suction drainage with or without re-transfusion of filtered shed blood does not offer advantages in primary non-cemented total hip replacement using a direct anterior approach. Arch Orthop Trauma Surg. 2012;132(1):131–136. doi: 10.1007/s00402-011-1387-1. [DOI] [PubMed] [Google Scholar]
- 21.von Roth P, Perka C, Dirschedl K, Mayr HO, Ensthaler L, Preininger B, Hube R. Use of Redon drains in primary total hip arthroplasty has no clinically relevant benefits. Orthopedics. 2012;35(11):e1592–e1595. doi: 10.3928/01477447-20121023-14. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda K, Nakamura S, Wakimoto N, Kobayashi M, Matsushita T. Drainage does not increase anemia after cementless total hip arthroplasty. Clin Orthop Relat Res. 2007;458:101–105. doi: 10.1097/BLO.0b013e31802ea45f. [DOI] [PubMed] [Google Scholar]
- 23.Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT (2008) Cochrane handbook for systematic reviews of interventions, version 5.0.1 [updated September 2008]. The Cochrane Collaboration. Available from www.cochrane-handbook.org. Accessed 31 July 2013
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Snell S. On the suction-operation for cataract. Br Med J. 1876;1–802:595. doi: 10.1136/bmj.1.802.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howe L. On the removal of hard cataract by suction. Trans Am Ophthalmol Soc. 1893;6:594–596. [PMC free article] [PubMed] [Google Scholar]