Abstract
Purpose
Shoulder arthroplasty is one of the options for the treatment of complex proximal humeral fractures. The purpose of this study was to assess the clinical and radiographic results of the trabecular metal shoulder prosthesis in the treatment of complex proximal humeral fractures.
Methods
Fifty-one consecutive patients with complex proximal humeral fractures who underwent primary shoulder arthroplasties with the trabecular metal™ prosthesis were enrolled in this study. At the final follow-up appointment, 42 of the patients (82.4 % of the total patients enrolled) were available for both clinical and radiographic evaluation. There were 28 women and 14 men with a mean age of 65.4 ± 10.7 years. The dominant arm was involved in 30 of the cases. According to Neer’s classification, there were seven three-part fractures, 27 four-part fractures and eight head-splitting fractures. Additionally, there were 37 hemiarthroplasties and five total shoulder arthroplasties.
Results
After a mean follow-up of 37.0 ± 8.4 months (range 24–52 months), the average ranges of motion were: 38.6 ± 15.0° for external rotation, L3 level for internal rotation and 132.3 ± 36.0° for forward elevation. The mean American Shoulder and Elbow Surgeons, visual analogue scale and University of California, Los Angeles scores were 82.1 ± 14.1, 0.4 ± 1.1 and 28.8 ± 5.1, respectively. The post-operative radiographs exhibited an anatomically attached greater tuberosity in 39 of the 42 shoulders. Of the three patients with greater tuberosity complications, as displayed by their radiographs, two were observed with malpositioned tuberosities, while the other greater tuberosity was resorbed. Proximal migration of the prosthesis was observed in all three shoulders with greater tuberosity complications and in two shoulders with an anatomically attached greater tuberosity. No neurovascular injury, infection or prosthetic loosening was identified during the final follow-up appointments.
Conclusions
Satisfactory results can be expected with the trabecular metal shoulder prosthesis for the treatment of complex proximal humeral fractures. The post-operative radiographs demonstrated an anatomically healed greater tuberosity in 93 % of the patients at a minimum follow-up time of two years.
Keywords: Proximal humeral fracture, Shoulder arthroplasty, Shoulder reconstruction, Trabecular metal
Introduction
Proximal humeral fractures are common injuries that account for 4–8 % of all fractures. Of these fractures, 80–85 % are non-displaced or minimally displaced and thus can be successfully treated conservatively. Displaced fractures should be managed surgically to obtain satisfying results [1]; however, the optimal treatment choice for complex proximal humeral fractures remains controversial. Shoulder arthroplasty is a treatment option for many types of fractures, including comminuted three- and four-part fractures, anatomical neck fractures and head-splitting fractures that occur in the elderly population [2, 3]. Trabecular metal (TM) prostheses have been widely applied in arthroplastic surgeries and have the advantage of facilitating bone ingrowth by the tantalum porous layers around the components. Reports concerning the treatment of proximal humeral fractures with TM prostheses, however, are rare. The purpose of this study was to evaluate the clinical and radiographic results of primary shoulder arthroplasties for the treatment of complex proximal humeral fractures with the TM prosthesis.
Materials and methods
A consecutive series of patients was selected for this study on the basis of the following inclusion criteria: (1) patients with complex proximal humeral fractures who underwent primary shoulder arthroplasty with a TM prosthesis, (2) fresh injuries (less than three weeks from injury to surgery) and (3) patients with a minimum follow-up of two years. The exclusion criteria were (1) patients with a history of previous operations on the affected shoulder; (2) delayed fractures, including malunion or nonunion fractures; (3) open fractures and (4) concomitant neurovascular injuries.
Between September 2008 and February 2011, 51 consecutive patients who met the inclusion criteria and were treated by primary shoulder arthroplasty with tantalum TM prostheses (Zimmer, Warsaw, IN, USA) and included in this study.
Surgical techniques
All the operations were performed by a senior surgeon (C.Y.J.) who used the Zimmer TM Humeral Stem (Zimmer, Warsaw, IN, USA). A deltopectoral approach was used. The cephalic vein was preserved and retracted either laterally or medially. Sutures were placed through the bone-tendon junction of the greater and lesser tuberosity fragments for subsequent reduction. For patients with fracture-dislocations, great care was taken when moving the humeral head from a dislocated position to avoid neurovascular injury. The retroversion angle was set to 15–20°. All stems were cemented distal to the proximal TM part. The glenoid was checked intraoperatively, and any signs of degenerative changes necessitated a total shoulder arthroplasty. The cancellous bone graft harvested from the humeral head was placed between the diaphysis and the tuberosity fragments to facilitate a bony union. The tuberosity fragments were then attached to the TM part of the stem by a heavy preset (no. 5), and non-absorbable sutures were then used in a cerclage fashion. A titanium cable (Cable-Ready, Zimmer, Warsaw, IN, USA) was placed around the tuberosity fragments in the same cerclage fashion and was subsequently secured for stable fixation between the tuberosity fragments and the TM parts. Biceps tenodesis was carried out in all cases.
Post-operative management
The arm was placed in a neutral brace, which allowed for slight external rotation, for six weeks after surgery. Passive exercises of the hand, wrist and elbow began on the first post-operative day, depending on the patient’s tolerance. Passive range of motion exercises of the shoulder were started three weeks after the surgery. The immobilisation brace was removed six weeks after the surgery, and active range of motion exercises were commenced following confirmation that the initial healing of the tuberosities had occurred, by assessment of post-operative radiographs. Resisted exercises were not allowed until three months following the surgery.
Clinical evaluation
The patients were asked for a follow-up interview at three weeks, six weeks, three months, six months and every full year after the operation until the last follow-up. The ranges of motion, the American Shoulder and Elbow Surgeons (ASES) score, the University of California, Los Angeles (UCLA) score and the visual analogue scale (VAS) score for pain assessment were calculated for the clinical evaluation of the shoulder at the final follow-up.
Radiographic evaluation
The post-operative radiographs were obtained immediately after surgery with the patients’ arms in a neutral rotation to determine the initial position of the reconstructed tuberosities. At each follow-up, all patients had a standardised series of radiographs, including anteroposterior (AP) views with the arm in three rotations (neutral, external and internal) as well as lateral and axillary views.
According to Boileau et al. [4], the greater tuberosity was considered well-positioned in the vertical plane when its summit was between five and ten millimetres below the line, which was tangent to the top of the prosthetic head and perpendicular to the axis of the stem. Otherwise, the greater tuberosity was considered to be vertically malpositioned. If the greater tuberosity was not visible on the post-operative AP view in the neutral rotation but was observed to be posteriorly migrated from the axillary view or in the internal rotation on the AP view, the greater tuberosity was considered to be horizontally malpositioned. Greater tuberosity resorption was confirmed by the disappearance of the greater tuberosity from any view of the post-operative radiographs. Both tuberosity malposition and resorption were considered radiographic complications of the greater tuberosity. Proximal migration of the prosthesis was evaluated by the measurement of the acromiohumeral distance from the AP view with the arm in the neutral rotation. A distance of less than seven millimetres indicated proximal migration of the humerus [5].
Results
After a minimum follow-up time of two years, 42 patients (82.4 %) were available for both clinical and radiographic evaluations. There were 14 men and 28 women with an average age of 65.4 ± 10.7 years (range 45–85 years). The dominant arm was involved in 30 cases. The mean time from injury to surgery was 10.2 ± 4.9 days. According to Neer’s classification, there were seven three-part fractures (four dislocations), 27 four-part fractures (21 dislocations) and eight head-splitting fractures. Based on the Orthopaedic Trauma Association (OTA) classification [6], there were three B2 fractures, four B3 fractures, nine C2 fractures and 26 C3 fractures. In addition, 37 patients were treated with hemiarthroplasties, and the other five were treated with total shoulder arthroplasties due to pre-existing arthritic wear of the glenoid.
After a mean follow-up time of 37.0 ± 8.4 months (range 24–52 months), the average ranges of motion of the patient’s arms were 132.3 ± 36.0° for the forward elevation, 38.6 ± 15.0° for the external rotation and L3 level for the internal rotation. The average VAS pain, ASES and UCLA scores were 0.4 ± 1.1 (0–4), 82.1 ± 14.1 (43–100) and 28.8 ± 5.1 (16–35), respectively.
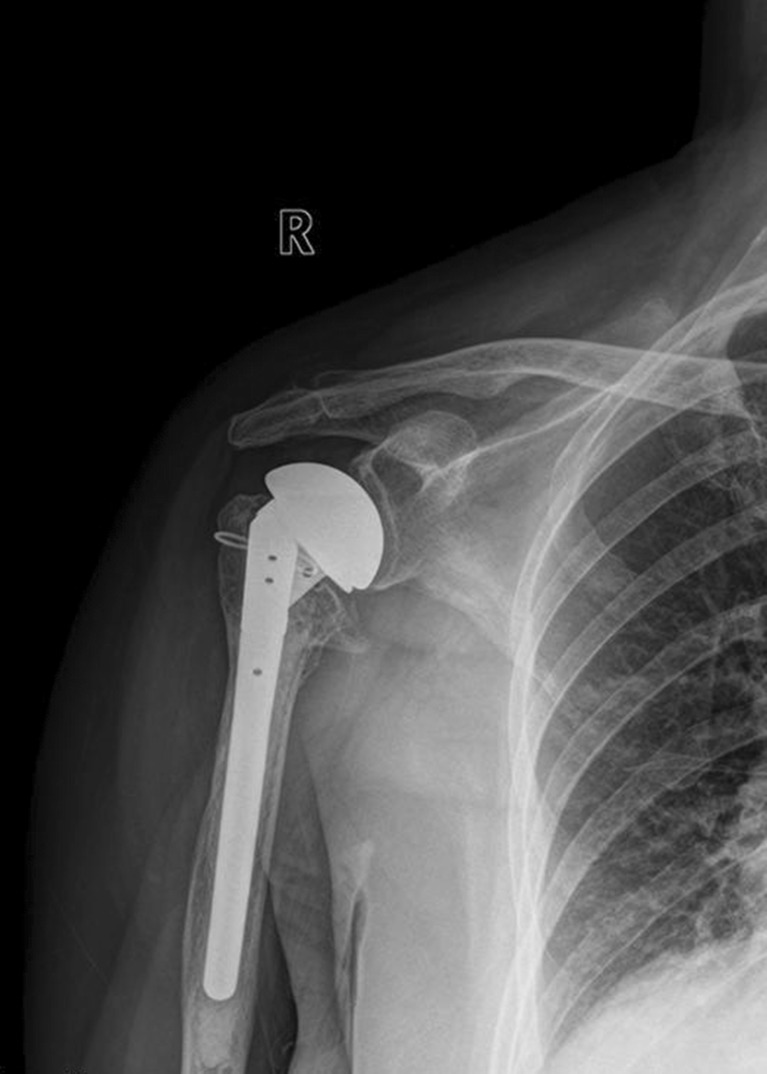
From radiographic evaluations at the patients’ final follow-ups, 39 patients were determined to have greater tuberosities that were well-attached (Fig. 1). Two patients were found to have horizontally malpositioned greater tuberosities. Resorption of the greater tuberosity was detected in one patient. The overall greater tuberosity complication rate, as detected by the radiographic evaluations, was 7 % (3/42). In all three of these patients, the greater tuberosity complications were all found at the post-operative three-month time point.
Fig. 1.
AP view of the right shoulder displaying a well-attached greater tuberosity 4 years after surgery
Complications
No neurovascular injury, infection or prosthetic loosening was identified during the final follow-up appointments. All of the patients with greater tuberosity complications were found to have superior migration of their humeral heads during their final follow-ups. Superior migration of the humeral head was also observed in two patients with well-attached greater tuberosities (Fig. 2).
Fig. 2.
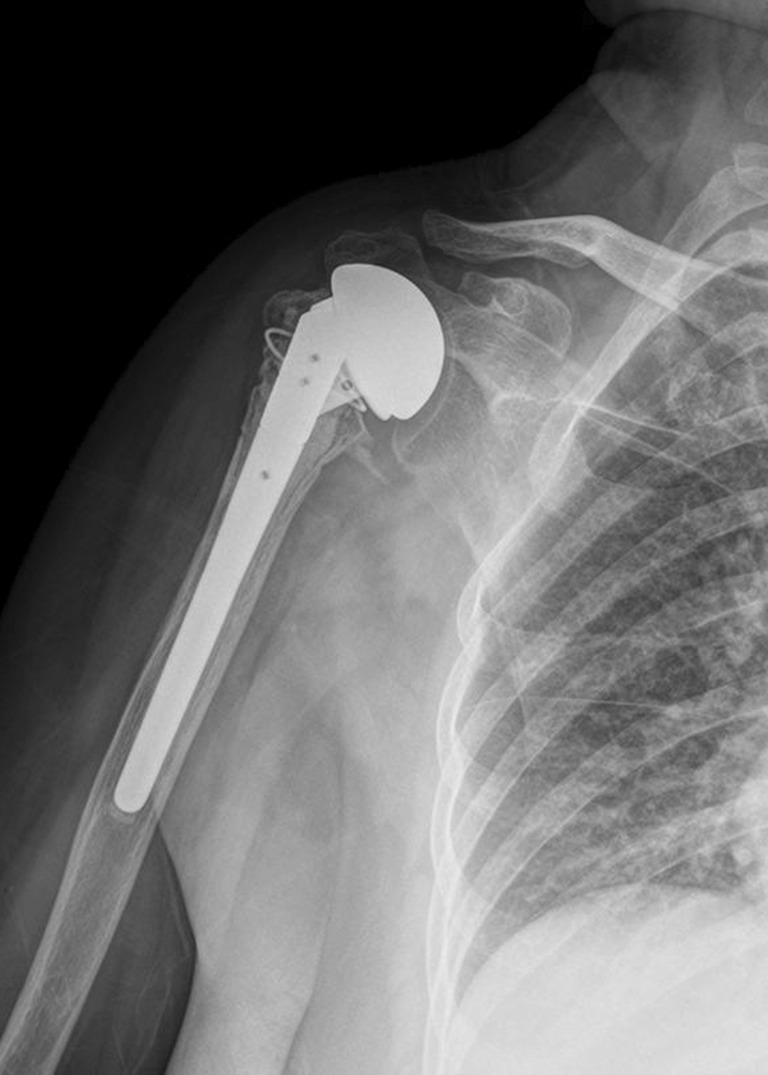
AP view of the right shoulder 3 years after surgery, displaying a well-healed greater tuberosity but a superior migrated prosthetic humeral head, suggesting rotator cuff failure
Discussion
Primary shoulder arthroplasty for proximal humeral fractures is beneficial for pain relief, but its role in restoring post-operative shoulder function remains controversial [3, 4, 7, 8]. In a study conducted by Neer [9], shoulder arthroplasty was used to treat three- or four-part proximal humeral fractures, whereby over 80 % of the patients obtained a good to excellent result. Compito et al. [2], however, only observed an excellent result rate in 48.5 % of their patients. In recent years, good pain relief has been consistently reported in the literature [10–13], but the post-operative shoulder range of motion and functional outcomes have been less satisfying [10–12]. In a multicentre, retrospective study of 167 patients by Kralinger et al. [14], only 41.9 % of the patients demonstrated a post-operative forward elevation of over 90°.
A large number of reports have suggested that the healing status of the greater tuberosity after shoulder arthroplasty is crucial for functional recovery of the shoulder [14–21]. In a study by Tanner and Cofield [22], post-operative displacement of the greater tuberosity was thought to be the most common complication after shoulder arthroplasty for the treatment of proximal humeral fractures. Additionally, the incidence of nonunion or malunion of the tuberosity has been reported to be between 12.5 and 50 % [4, 12, 13]. Boileau et al. [4] demonstrated that migration and malunion of the greater tuberosity could lead to subacromial impingement, superior migration of the prosthesis, joint stiffness and consistent pain in the shoulder. A poor greater tuberosity position has also been shown to change the lever arm in the glenohumeral joint abduction, alter the local biomechanical characteristics, accelerate the process of prosthesis and glenoid wear and finally lead to prosthetic failure [23, 24]. In 2007, we conducted a study that included 59 proximal humeral fracture patients treated with standard humeral head replacements with a mean follow-up time of 22.2 months [25]. The tuberosity disappearance rate was 13.6 % (8/59). Confirmed by the computed tomography (CT) scan, six of the eight patients with disappearing tuberosities were found to have a posteriorly migrated malunion, while the other two were found to have resorbed tuberosities.
Several attempts to avoid tuberosity complications after shoulder arthroplasty have been reported, including cerclage fixation, decreased humeral retroversion, placement of a post-operative neutral position brace and delayed post-operative rehabilitation [14, 17, 19]. The application of bone ingrowth materials is also an approach. Tantalum TM has a highly porous three-dimensional structure with up to 80 % porosity. This type of material increases biomechanical strength by enhancing the potential for bone ingrowth. Tantalum TM has already been applied in hip and knee arthroplasties, and in spinal surgery, with promising clinical outcomes [26–28]. A review of the literature, however, has revealed no reports on its efficacy in tuberosity healing after shoulder arthroplasty for the treatment of proximal humeral fractures. This study, for the first time, retrospectively analysed the efficacy of the TM shoulder prosthesis for the treatment of proximal humeral fractures in a series of 42 patients with a minimum follow-up time of two years. We also precluded the patients with a greater tuberosity that was initially malpositioned to determine the healing potential of TM prostheses on well-reconstructed greater tuberosities. Finally, the post-operative radiographs in this study displayed an anatomically healed greater tuberosity in 93 % (39/42) of the patients (Figs. 3, 4, 5 and 6).
Fig. 3.
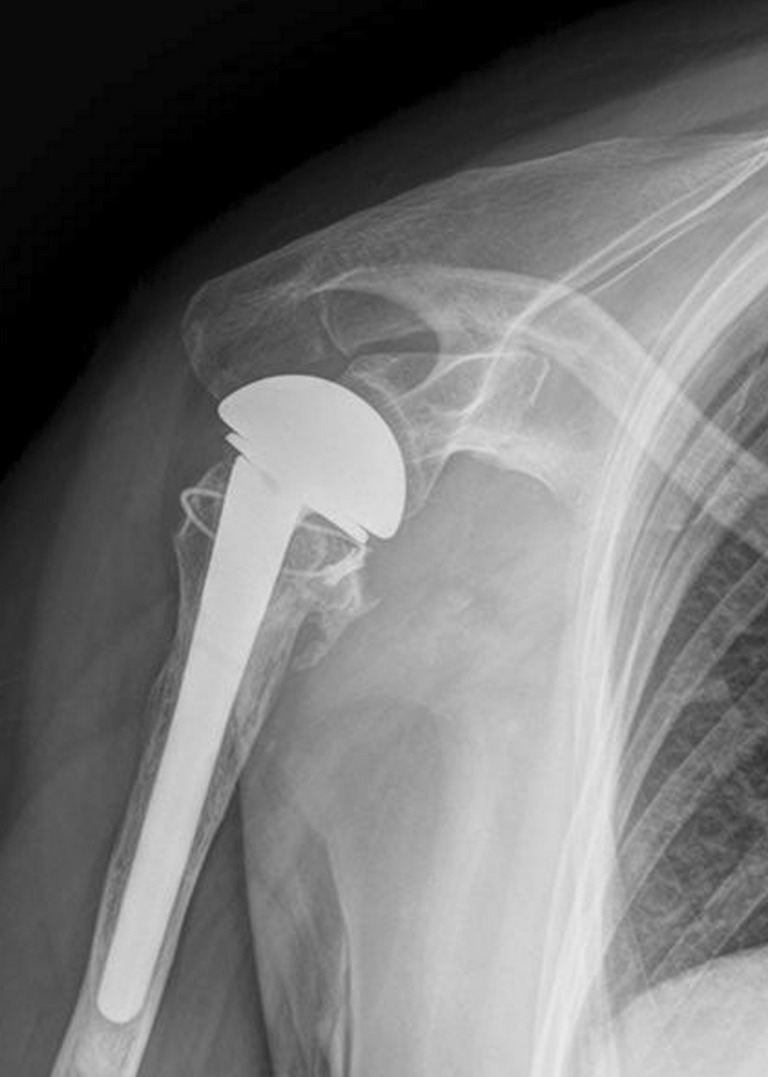
Axillary view of the right shoulder displaying a well-attached greater tuberosity 4 years after surgery
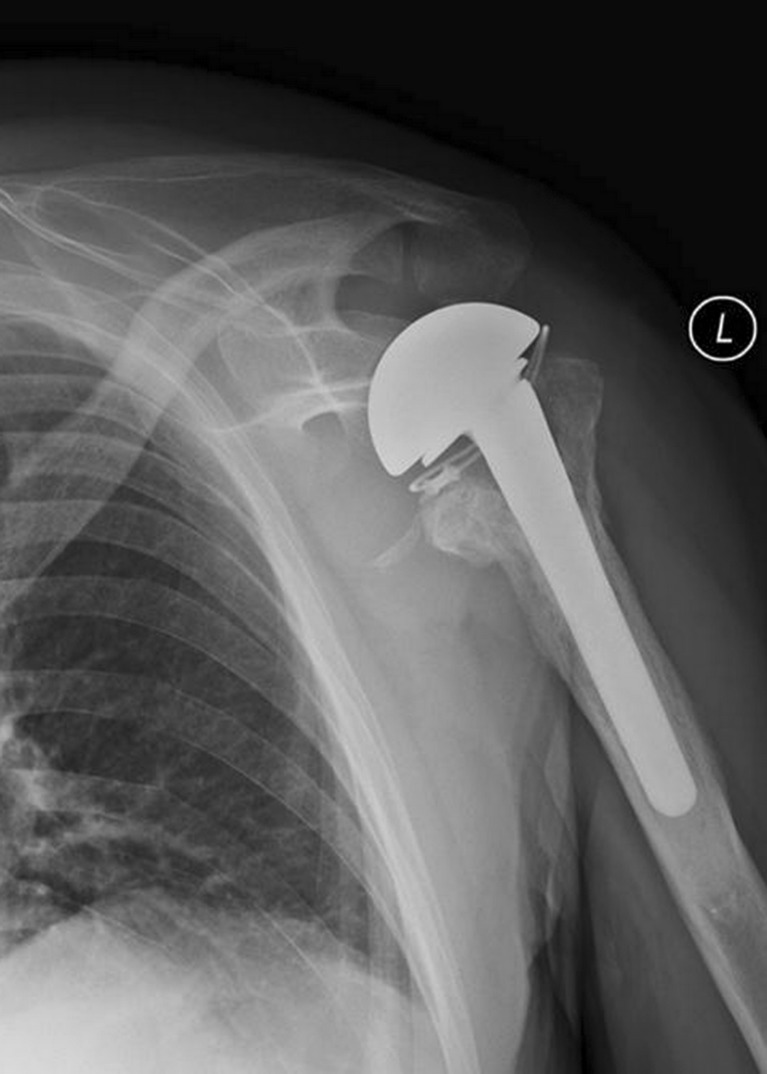
Fig. 4.
Axillary view of the left shoulder demonstrating a posteriorly migrated greater tuberosity 2 years after surgery
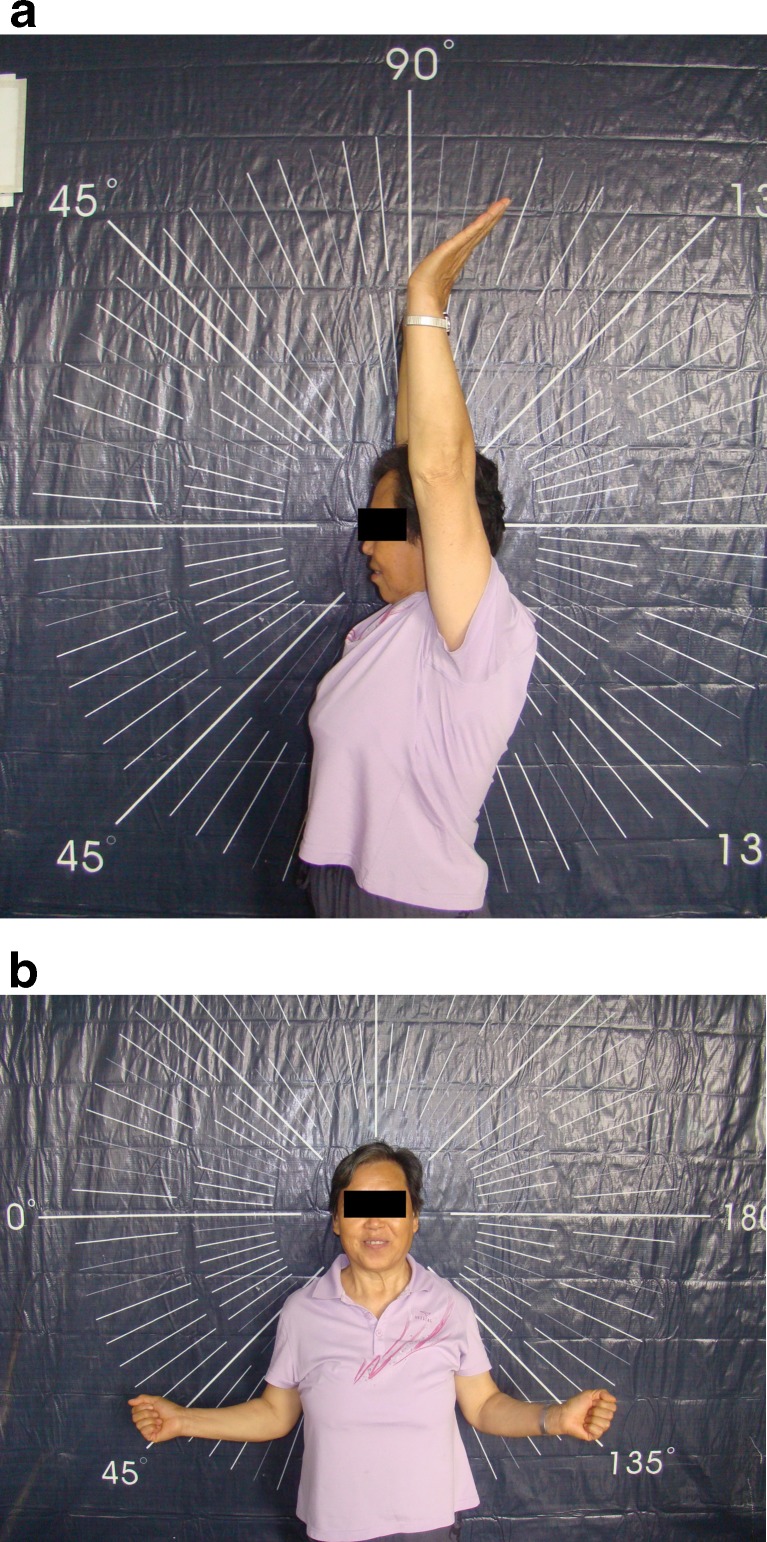
Fig. 5.
Pictures of a female patient 3 years after hemiarthroplasty of her left shoulder, displaying good ranges of motion in forward elevation (a) and external rotation (b)
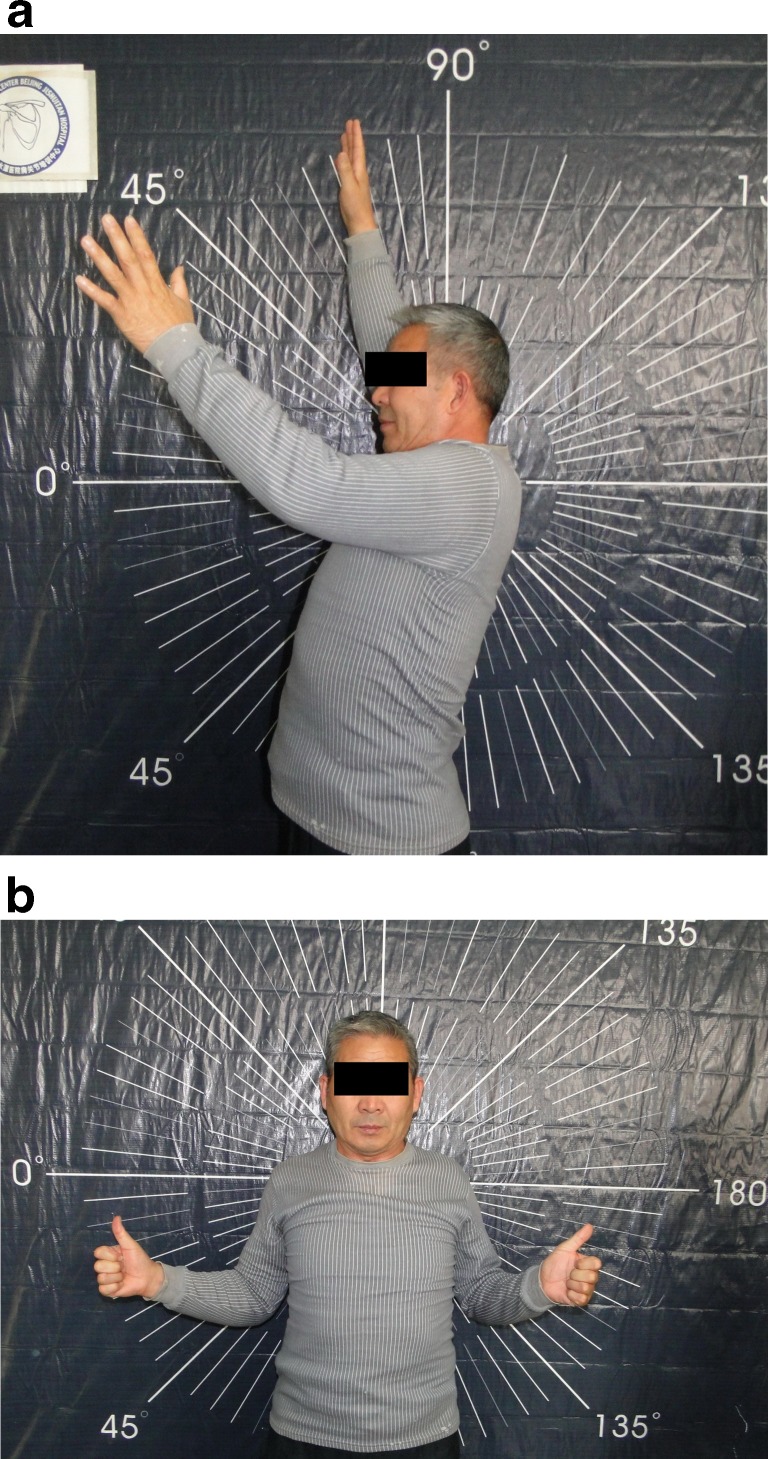
Fig. 6.
Pictures of a male patient 2 years after hemiarthroplasty of his left shoulder, displaying limited ranges of motion in forward elevation (a) and external rotation (b)
Although the anatomical healing of the greater tuberosity may be crucial to the post-operative shoulder function, it is not the only determining factor. In our study, superior migration of the humeral head still occurred in two patients with properly healed greater tuberosities (Fig. 2), resulting in a relatively poor range of motion and functional outcome. After reviewing the operation records, these two patients were determined to have pre-existing degenerative rotator cuff tears that were present during the surgery. Although rotator cuff repairs were meticulously carried out after implantation of the prostheses and reconstruction of the tuberosities, post-operative superior migration of the prosthetic humeral heads still indicates failure of the rotator cuff repair. Therefore, reverse shoulder arthroplasty might be a better choice for elderly patients with complex proximal humeral fractures with pre-existing degenerative rotator cuff tears.
Our study has several limitations. First, this report describes a relatively small case series, whereby only 42 eligible patients were enrolled with short follow-up times. Future large case series that evaluate the long-term effectiveness of TM prostheses for the treatment of proximal humeral fractures should be warranted based on the findings from this study. Another limitation is that this report describes a retrospective case series. A study with higher level of evidence is required for further evaluation.
In conclusion, satisfactory results can be expected with the TM shoulder prosthesis for the treatment of complex proximal humeral fractures. The post-operative radiographs reported in this study displayed an anatomically healed greater tuberosity in 93 % of the patients with a minimum follow-up time of two years.
References
- 1.Iannotti JP, Ramsey ML, Williams GR, Jr, Warner JJ. Nonprosthetic management of proximal humeral fractures. Instr Course Lect. 2004;53:403–416. [PubMed] [Google Scholar]
- 2.Compito CA, Self EB, Bigliani LU. Arthroplasty and acute shoulder trauma. Reasons for success and failure. Clin Orthop Relat Res. 1994;307:27–36. [PubMed] [Google Scholar]
- 3.Kontakis G, Koutras C, Tosounidis T, Giannoudis P. Early management of proximal humeral fractures with hemiarthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90:1407–1413. doi: 10.1302/0301-620X.90B11.21070. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–412. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 5.Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74:491–500. [PubMed] [Google Scholar]
- 6.Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audigé L. Fracture and dislocation classification compendium-2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1–S133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bastian JD, Hertel R. Osteosynthesis and hemiarthroplasty of fractures of the proximal humerus: outcomes in a consecutive case series. J Shoulder Elbow Surg. 2009;18:216–219. doi: 10.1016/j.jse.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan SG, Bennion PW, Reineck JR, Burkhead WZ. Hemiarthroplasty for proximal humeral fracture: restoration of the Gothic arch. Orthop Clin North Am. 2008;39:441–450. doi: 10.1016/j.ocl.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Neer CS., 2nd Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52:1090–1103. [PubMed] [Google Scholar]
- 10.Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12:569–577. doi: 10.1016/S1058-2746(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 11.Anjum SN, Butt MS. Treatment of comminuted proximal humerus fractures with shoulder hemiarthroplasty in elderly patients. Acta Orthop Belg. 2005;71:388–395. [PubMed] [Google Scholar]
- 12.Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:428–430. doi: 10.1067/mse.2002.126615. [DOI] [PubMed] [Google Scholar]
- 13.Demirhan M, Kilicoglu O, Altinel L, Eralp L, Akalin Y. Prognostic factors in prosthetic replacement for acute proximal humerus fractures. J Orthop Trauma. 2003;17:181–188. doi: 10.1097/00005131-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kralinger F, Schwaiger R, Wambacher M, Farrell E, Menth-Chiari W, Lajtai G, Hübner C, Resch H. Outcome after primary hemiarthroplasty for fracture of the head of the humerus. A retrospective multicentre study of 167 patients. J Bone Joint Surg Br. 2004;86:217–219. doi: 10.1302/0301-620X.86B2.14553. [DOI] [PubMed] [Google Scholar]
- 15.Fialka C, Stampfl P, Arbes S, Reuter P, Oberleitner G, Vécsei V. Primary hemiarthroplasty in four-part fractures of the proximal humerus: randomized trial of two different implant systems. J Shoulder Elbow Surg. 2008;17:210–215. doi: 10.1016/j.jse.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Gerber C, Hersche O, Berberat C. The clinical relevance of posttraumatic avascular necrosis of the humeral head. J Shoulder Elbow Surg. 1998;7:586–590. doi: 10.1016/S1058-2746(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 17.Nijs S, Broos P. Outcome of shoulder hemiarthroplasty in acute proximal humeral fractures: a frustrating meta-analysis experience. Acta Orthop Belg. 2009;75:445–451. [PubMed] [Google Scholar]
- 18.Huffman GR, Itamura JM, McGarry MH, Duong L, Gililland J, Tibone JE, Lee TQ. Neer Award 2006: Biomechanical assessment of inferior tuberosity placement during hemiarthroplasty for four-part proximal humeral fractures. J Shoulder Elbow Surg. 2008;17:189–196. doi: 10.1016/j.jse.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Misra A, Kapur R, Maffulli N. Complex proximal humeral fractures in adults—a systematic review of management. Injury. 2001;32:363–372. doi: 10.1016/S0020-1383(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 20.Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86:575–580. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85:1215–1223. doi: 10.1302/0301-620X.85B7.13959. [DOI] [PubMed] [Google Scholar]
- 22.Tanner MW, Cofield RH. Prosthetic arthroplasty for fractures and fracture-dislocations of the proximal humerus. Clin Orthop Relat Res. 1983;179:116–128. doi: 10.1097/00003086-198310000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Boileau P, Trojani C, Walch G, Krishnan SG, Romeo A, Sinnerton R. Shoulder arthroplasty for the treatment of the sequelae of fractures of the proximal humerus. J Shoulder Elbow Surg. 2001;10:299–308. doi: 10.1067/mse.2001.115985. [DOI] [PubMed] [Google Scholar]
- 24.Rietveld AB, Daanen HA, Rozing PM, Obermann WR. The lever arm in glenohumeral abduction after hemiarthroplasty. J Bone Joint Surg Br. 1988;70:561–565. doi: 10.1302/0301-620X.70B4.3403598. [DOI] [PubMed] [Google Scholar]
- 25.Jiang CY, Zhu YM, Lu Y. Postoperative shoulder function and imaging changes of the greater tuberosity after humeral head replacement. Chin J Orthop. 2007;27:505–508. [Google Scholar]
- 26.Stiehl JB. Trabecular metal in hip reconstructive surgery. Orthopedics. 2005;28:662–670. doi: 10.3928/0147-7447-20050701-13. [DOI] [PubMed] [Google Scholar]
- 27.Dunbar MJ, Wilson DA, Hennigar AW, Amirault JD, Gross M, Reardon GP. Fixation of a trabecular metal knee arthroplasty component. A prospective randomized study. J Bone Joint Surg Am. 2009;91:1578–1586. doi: 10.2106/JBJS.H.00282. [DOI] [PubMed] [Google Scholar]
- 28.Löfgren H, Engquist M, Hoffmann P, Sigstedt B, Vavruch L. Clinical and radiological evaluation of Trabecular Metal and the Smith-Robinson technique in anterior cervical fusion for degenerative disease: a prospective, randomized, controlled study with 2-year follow-up. Eur Spine J. 2010;19:464–473. doi: 10.1007/s00586-009-1161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]