Abstract
In the present work attempts have been made to prepare the nanostructured lipid carrier (NLC) gel, by using minoxidil, which is preferably used in case of Alopecia, i.e. baldness pattern as a effective drug. The nine different formulations of Minoxidil-NLC (NLC1–NLC9) were prepared using solid and liquid lipids with Cholesterol and Soya lecithin in different concentrations by the melt dispersion ultrasonication method. Properties of NLC1–NLC9 such as the particle size and its distribution, the scanning electron microscopy (SEM), the drug entrapment efficiency (EE), and the drug release behavior were investigated. The nanoparticulate dispersion was suitably gelled and characterized with respect to drug content, pH, spreadability, rheology, and in vitro release. Safety of the NLC-based gel was assessed using primary skin irritation studies. The formulated NLC3 was spherical in shape, with average particle size of 280 nm, zeta potential of −42.40 mV and entrapment efficiency of 86.09%. Differential Scanning Calorimeter (DSC) measurements revealed that imperfect crystallization occurred in the inner core of the NLC particles. The drug release behavior from the NLC displayed a biphasic drug release pattern with burst release at the initial stage followed by sustained release. These results indicated that the NLC3 is a suitable carrier of minoxidil with improved drug loading capacity and controlled drug release properties. It has been observed that NLC gel produces the gel with good consistency, homogeneity, spreadability and rheological behavior. The developed NLC-based gel showed faster onset and elicited prolonged activity up to 16 h. The present study concluded that the NLC-based gel containing minoxidil dissolved in a mixture of solid lipid and liquid lipid in the nanoparticulate form helped us to attain the objective of faster onset yet prolonged action as evident from in vitro release.
Keywords: Minoxidil, Nanostructured lipid carrier, DSC, SEM
1. Introduction
Pharmaceutical technology has taken the advantage of the advent of Nanotechnology; new pharmaceutical dosage forms are under development to deliver many physicochemically different drug molecules (Hommoss, 2008; Muller and Almeida, 2005). Poor water solubility and insufficient bioavailability of the new drug molecules are main and common problems. Therefore, there is an increasing need to develop a drug carrier system that overcomes this drawback (Hommoss, 2008). As a vehicle for controlled release of active substances and targeting to skin layers, nanodisperse systems such as Liposomes, nano emulsions, and lipid nanoparticles are gaining more and more importance (Utreja and Jain, 2001).
In the beginning of the 1990s, solid lipid nanoparticles (SLN) were developed as an alternative carrier system to the existing traditional carriers, such as emulsions, liposomes, and polymeric nanoparticles (Pardeike et al., 2009; Rajashree et al., 2011). Compared with the traditional carriers, SLN are well-tolerated, have high bioavailability, a nice targeting effect, and can be produced on a large industrial scale. The SLN features have been considered advantageous for topical administration of active substances (Uner and Yener, 2007). Potential problems associated with SLN namely, limited drug loading, risk of gelation and drug leakage during storage caused by lipid polymorphism have been further minimized by a new generation of lipid systems, the nanostructured lipid carriers (NLC) developed at the turn of the millennium (Muller et al., 2002a). NLC consists of a mixture of especially very different lipid molecules, i.e., solid lipid(s) is blended with liquid lipid(s) (oils). The resulting matrix of the lipid particles shows a melting point depression compared to the original solid lipid; however, the matrix remains solid at body temperature (Muller et al., 2000; Saupe et al., 2005). SLN and NLC are colloidal carrier systems providing controlled release profiles for many substances in tropical route. They are composed of physiological and biodegradable lipids as a carrier, exhibiting low systemic toxicity and low cytotoxicity (Muller et al., 1997).
In view of topical administration, these systems possess occlusive properties because of film formation on the skin surface (Wissing and Muller, 2002b). It reduces the transepidermal water loss and therefore enhances the penetration of drugs through the stratum corneum by increased hydration. It has also been reported that the occlusion factor of SLN and NLC is related to their particle size, that is, it increases with the decrease of the mean particle diameter (Wissing and Muller, 2002a, 2003). The small size of the lipid particles ensures close contact with the stratum corneum and can increase the amount of drug penetrating into the mucosa or skin. Due to their solid lipid matrix, a controlled release from these carriers is possible (Muller et al., 2002a). In addition, by controlling the amount of liquid lipids added to the formulation, the NLC remains in its solid state at body temperature and the modulation of drug release profile can be achieved. This becomes an important tool when it is necessary to supply the drug over a prolonged period of time, to reduce systemic absorption, and when drug produces irritation in high concentrations (Muller et al., 2000; Chaudhari, 2012).
Minoxidil, a pyridine-derivative, was initially developed as an oral antihypertensive agent. However, its major clinical attraction is related to its common side effect on the promotion of hair growth (Atrux-Tallau et al., 2009). Alopecia is a common form of hair loss in both men and women. Minoxidil is widely used for the treatment of alopecia (Messenger and Rundegren, 2004). Commercial products containing minoxidil are usually solutions with high percentage of ethyl alcohol and/or propylene glycol. Twice-daily applications are recommended as proper use (Wagner and Kenreigh, 2007; Aronson, 2006). However, repeated applications of high ethyl alcohol and/or propylene glycol content products lead to severe adverse effects such as scalp dryness, irritation, burning, redness and allergic contact dermatitis. Since most of the products containing minoxidil available on the market consist of ethyl alcohol–propylene glycol–water solutions, new dermatological formulations free of organic solvents are needed to minimize adverse effects and optimize androgenic alopecia treatment (Padoisa et al., 2011). Hence to minimize the side effects and to improve the therapeutic efficiency of minoxidil, the development of new systems for topical delivery of such drug is in demand.
Therefore, the aim of the present work was to develop the NLC gel formulation containing minoxidil, using different concentrations of solid and liquid lipids, to evaluate its physicochemical properties and stability.
2. Materials and methods
Minoxidil, was obtained as gift from Dr. Reddy’s Laboratory, Hyderabad, India. Tristearin was provided by Glenmark Generics, Mumbai, India. Oleic acid, Cholesterol and Tween-80 were purchased from Merck Pvt. Ltd., Mumbai, India. Soya lecithin (Phosphatidylcholine), Pluronic F-68, Triton X-100, Carbopol 934 and Triethanolamine were purchased from Hi-Media Laboratories, Mumbai, India. Chemicals and reagents were of the highest purity grade commercially available from Merck Pvt. Ltd., Mumbai, India.
2.1. Preparation of minoxidil loaded NLC
The NLCs were prepared by the melt dispersion ultrasonication method. The lipid (oleic acid and tristearin), cholesterol and phosphatidylcholine (soya lecithin) were blended and melted at 75 °C, along with the minoxidil, to form a uniform and clear oil phase. Meanwhile, the aqueous phase consisting of dispersing surfactant Tween-80 in double distilled water was maintained at 75 °C. The oil phase was added to the aqueous phase, and both phases were mixed by the aid of agitation at 600 rpm for 10 min at this temperature to form a microemulsion. This warm microemulsion was diluted in cold water (2–3 °C) under mechanical stirring to form NLC dispersion such that the concentration of minoxidil in the final dispersion remains 2% w/w (Jain et al., 2009; Wang et al., 2010).
2.2. Preparation of gel formulations
The nanoparticulate dispersion obtained after diluting the warm microemulsion templates was gelled using gelling agents. Based on the compatibility with nanoparticulate dispersion, the esthetic appeal and the ease of spreadability carbopol (2%) was selected as the gelling agent. Carbopol was added to the nanoparticle dispersion under overhead stirring at 800 rpm (Remi, Mumbai, India). Stirring was continued until carbopol was dispersed. The carbopol dispersion was neutralized using 0.05% (w/w) Triethanolamine and pH of gel was adjusted to 7.4 (Joshi and Patravale, 2006).
2.3. Characterization of nanoparticulate dispersion
2.3.1. Determination of particle size, polydispersity index and zeta potential
All measurements were performed in triplicates. The average particle size and polydispersity index (PI) of NLCs were determined by Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at a temperature of 25 ± 2 °C and at 90° to the incident beam applying the principle of photon correlation spectroscopy (PCS). Dispersions were diluted with double distilled water to ensure that the light scattering intensity (between 6e+004 and 1e+006), was within the instrument’s sensitivity range.
Laser Doppler electrophoresis technique was applied to measure particle electrostatic charge. The zeta potential was analyzed by Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25 ± 2 °C after appropriate dilution with double distilled water (Souto and Muller, 2005).
2.3.2. Scanning electron micrographic studies
Images were recorded on a scanning electron micrograph (magnification: 50×; accelerating voltage: 15.0 kV). Analysis was performed at 25 ± 2 °C. The NLC dispersion was diluted appropriately and sonicated. Few drops of the dispersion were placed on the grid and allowed to dry (Zhao et al., 2010). After the samples were dried thoroughly, the image was captured (Fig. 2).
Figure 2.
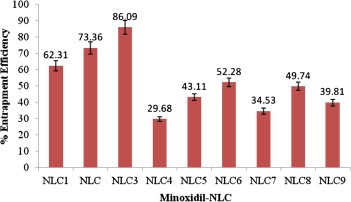
Percentage entrapment efficiency for minoxidil-NLC. Each value represents the mean ± SD (n = 3).
2.3.3. Entrapment efficiency
Determination of the amount of drug incorporated in NLC is of prime importance, since it influences the release characteristics. The amount of drug encapsulated per unit weight of the nanoparticles is determined after separation of the free drug and solid lipids from the aqueous medium. A known dilution of the NLC dispersion was prepared, and 100 μL was placed in the upper chamber of a centrifuge tube matched with an ultrafilter and centrifuged for 20 min at 10,000 rpm (Joshi and Patravale, 2006). The supernatant and the filtrate were diluted appropriately and the amount of drug in both the phases was determined spectrophotometrically at a wavelength of 280 nm. The drug entrapment efficiency in the NLCs was calculated by the following equation.
where, Winitial drug is the weight of total drug in NLC, while Wfree drug the weight of free drug detected in filtrate.
2.3.4. In vitro drug release
In vitro release studies were performed using a modified Franz diffusion cell. Dialysis membrane having pore size 2.4 nm and molecular weight cut-off between 12,000 and 14,000 was used. The membrane was soaked in double distilled water for 12 h before mounting in a Franz diffusion cell. About 0.5 g of semisolid preparation of NLC was applied to the donor compartment, and the receptor compartment was filled with phosphate buffer, pH 7.4 (25 mL). During the experiments, the solution in the receptor side was maintained at 37 ± 0.5 °C and stirred at 800 rpm with Teflon-coated magnetic stirring bars (Chen et al., 2012). At fixed time intervals, 5 mL of the sample was withdrawn from the receiver compartment through a side tube and analyzed spectrophotometrically at 280 nm.
A graph of % cumulative release against time in hours was plotted as depicted in Fig. 4. To describe the kinetics of the drug release from the NLC, mathematical models such as zero-order, first-order, and Higuchi’s were used. The criterion for selecting the most appropriate model was based on a goodness-of-fit test.
Figure 4.
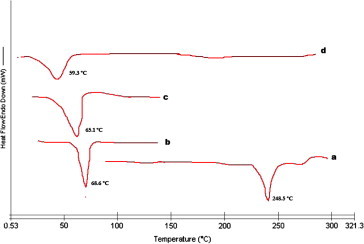
DSC image of Minoxidil-NLC and its ingredients. (a) Minoxidil; (b) tristearin; (c) mixture of tristearin and oleic acid; (d) mixture of minoxidil, tristearin and oleic acid.
2.3.5. Differential scanning calorimetry (DSC) analysis
Thermograms were recorded with DSC (CDR-4P, Shanghai, China). For DSC measurement, 10 mg of sample was put in an open aluminum pan, and then heated at the scanning rate of 10 °C/min between 0 and 400 °C temperature ranges (Zhao et al., 2010).
2.4. Characterization of the gel
2.4.1. Determination of drug content, spreadability, and pH
For determination of drug content, about 500 mg of the gel was weighed in a 100 mL volumetric flask and dissolved in 50 mL of phosphate buffer of pH 7.4. The volumetric flask was kept for 2 h and shaken well in a shaker to mix it properly. It was diluted appropriately and analyzed on a Shimadzu UV-1700 PC UVVIS at a λ-max of 280 nm.
The spreadability of the gel was determined using the following technique: 0.5 g of gel was placed within a circle of 1 cm diameter premarked on a glass plate over which a second glass plate was placed. A weight of 500 g was allowed to rest on the upper glass plate (Joshi and Patravale, 2006). The increase in the diameter due to spreading of the gels was noted.
The pH of the gel was determined by using a digital pH meter Model EQ 610, standardized using pH 4.0 and 7.0 standard buffers before use. One gram of gel was dissolved in 100 mL of distilled water and stored for 2 h (Joshi and Patravale, 2006). The results of characterization of gel are summarized in Table 1.
Table 1.
Quantity of raw material as per formulation (in mg).
| Ingredient | Formulations |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NLC1 | NLC2 | NLC3 | NLC4 | NLC5 | NLC6 | NLC7 | NLC8 | NLC9 | |
| Minoxidil | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Tristearin | 200 | 200 | 200 | 150 | 150 | 150 | 250 | 250 | 250 |
| Oleic acid | 50 | 75 | 100 | 50 | 75 | 100 | 50 | 75 | 100 |
| Cholestrol | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 |
| Soya Lecithin | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 |
| Tween-80 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Triton-X 100 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Pluronic F-68 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
2.4.2. Rheological studies on the gel
Brookefield Synchro-Lectric Viscometer (Model RVT) with helipath stand was used for rheological studies. The sample (30 g) was placed in a beaker and was allowed to equilibrate for 5 min before measuring the dial reading using T-C spindle at 0.5, 1, 2.5, and 5 rpm. At each speed, the corresponding dial reading on the viscometer was noted. The spindle speed was successively lowered and the corresponding dial reading was noted. The measurements were carried out in duplicate at ambient temperature. Direct multiplication of the dial readings with factors given in the Brookefield viscometer catalog gave the viscosity in centipoises (Joshi and Patravale, 2006). The consistency index and the flow index were calculated from the Power law equation:
where: τ is shear stress; r is shear rate; K is consistency index; and n is flow index.
Taking log of both sides,
2.4.3. Skin irritation test
The developed formulations were tested for primary skin irritation on albino mice of either sex weighing 20–22 gm. The hair was removed from the mice 3 days before the experiment. The animals were divided into two batches and each batch was again divided into two groups. The gel containing drugs was used on the test animal. A piece of cotton wool soaked in saturated drug solution was placed on the back of albino mice taken as control. The animals were treated daily up to 7 days and finally the treated skin was examined visually for erythema and edema (Joshi and Patravale, 2006).
2.5. Statistical analysis
All values obtained were expressed as mean ± standard error mean (SEM). Statistical comparisons were performed by analysis of variance and Student’s t test using SAS Version 8.0 software.
3. Results and discussion
3.1. Preparation of NLCs
The Minoxidil-loaded NLCs were prepared using varying concentrations of solid lipid and liquid lipid by the melt dispersion ultrasonication technique. The quantity of ingredient used and the properties of resultant Minoxidil-loaded NLC are shown in Tables 1 and 2.
Table 2.
Particle size, PDI, zeta potential for F1–F9 formulation.
| Minoxidil-NLC | Particle size | Polydispersity index | Zeta potential |
|---|---|---|---|
| NLC1 | 524.8 | 0.718 | −39.20 |
| NLC2 | 608.6 | 0.450 | −15.30 |
| NLC3 | 280.4 | 0.367 | −42.40 |
| NLC4 | 508.9 | 0. 486 | −44.50 |
| NLC5 | 469.9 | 0.604 | −19.47 |
| NLC6 | 446.2 | 0.530 | −32.60 |
| NLC7 | 997.0 | 0.604 | −21.90 |
| NLC8 | 2163.2 | 1.000 | −23.60 |
| NLC9 | 946.3 | 0.507 | −28.80 |
From Table 2, the results demonstrated that on increasing the concentration of solid lipid, it enhances the size of particles. However the mixture of tristearin and oleic acid (2:1) decreases the particle size up to 280.4 nm in NLC3. Additionally from the result it has been observed that the mixture of tristearin and oleic acid (10:3) increases the particle size up to 2163.2 nm in NLC8. This result was probably related with the different viscosities of NLC produced by mixing different concentrations of tristearin and oleic acid. It indicates that the higher oleic acid content reduced the viscosity inside NLC, and consequently, reduced the surface tension to form smaller and smoother surface particles. On the other hand, polydispersity is a measure of particle homogeneity and it varies from 0 to 1. The nearer the value of polydispersity to zero, the higher is the homology between the particles. The polydispersity index of NLC3 was 0.367 indicating wide particle size distributions (Jia et al., 2010). These results suggest that the ratio of tristearin and oleic acid of NLC3 was favored to form nanoparticles with more homogenous size distributions due to the size reduction.
To determine the long-term physical stability of the colloidal systems, the zeta potential was evaluated. The nanoparticle emulsion had the higher zeta potential value, indicating the better stability of this colloidal system of the lipid particles. This resulted from the electrostatic repulsion, which could prevent the biocolloids from aggregation of nanoparticles of emulsion. Zeta potential values higher than −30 mV show good physical stability, being optimized when they reach approximately −60 mV, exhibiting a very good physical stability during the shelf-life. In this study, zeta potential of NLC1 to NLC9 was in the range of −15.30 to −44.50 mV. While the concentration of oleic acid present in NLC3 is appropriate it brings the zeta potential up to −42.40 mV, demonstrating that NLC3 should possess a good physical stability since particle aggregation is not likely to occur owing to electrostatic repulsion. Additionally the coating of hydrophilic Tween 80 could further improve the stability of the lipid present in NLC by hydration in the surface layer (Teeranachaideekul et al., 2007; Lim and Kim, 2002).
3.2. Scanning electron microscopy (SEM)
To obtain more information about the particle size and shape, SEM analysis was also performed. The micrograph of the NLC3 illustrated spherical droplets in the nanometer range (Fig. 1) which was in agreement with the size data determined by PCS. The results indicated that the particles were spherical and no drug crystal of particles visible in the figure. The picture shows agglomeration of particles due to the lipid nature of the carriers and sample preparation prior to SEM analysis. Some particle shapes deviating from sphericity might be due to the lipid modification during the drying process of sample treatment. In addition, the particle shape depends on the purity of the lipid. Highly pure tristearin particles are more cuboid (Liu et al., 2007).
Figure 1.

Scanning electron microscopy of NLC.
3.3. Entrapment efficiency
The percentage of incorporated drug in the lipid matrix (entrapment efficiency) was evaluated. Fig. 2 depicted the high amount of drug entrapment efficiency in the NLC3 was found to be 86.09%, probably because of its lipophilic character. The NLC3 is responsible for higher entrapment efficiency in comparison with other NLC formulation. This result is due to the binary mixture of liquid and solid lipids, resulting in only a very weak crystallization. The NLC4 indicated the low drug entrapment efficiency, i.e. 29.68% is mainly due to the partitioning of the drug between the oil phase and the aqueous phase.
The NLC3 and NLC6 have the same amount of lipid matrix but both formulations produce different drug entrapment efficiency. The drug entrapment efficiency in NLC3 and NLC6 were found to be 86.09% and 52.28%, respectively. The change in drug entrapment efficiency of both formulations could be due to the different ratio of solid lipid and liquid lipid present in the mixture. NLC8 and NLC9 have a higher amount of lipid as compared to all other formulations but the entrapment efficiency was low. Hence it confirms that maximum entrapment efficiency of drug in the lipid matrix depends on the optimum ratio of solid lipid and liquid lipid mixture. Here NLC3 showed maximum drug entrapment efficiency so that it was selected for in vitro drug release study.
3.4. Assessment of drug release
To elucidate the mechanism of drug release from NLC for topical administration, in vitro release studies using vertical Franz diffusion cells have been performed for NLC3 and temperature maintained at 32 °C. In vitro release curves of NLC3 type drug-loaded nanoparticles are shown in Fig. 3. For NLC3 formulation, a biphasic drug release pattern was observed, that was drug burst release at the initial stage and followed by sustained release at a constant rate. This phenomenon might be explained in two aspects, i.e. higher oleic acid content and smaller size of nanoparticles. Firstly, the obtained nanoparticles were prepared by the method of melt dispersion ultrasonication at a high temperature and solidification at a low temperature. During the solidification at a low temperature, due to the solid lipid (tristearin) owning a higher melting point, it would rapidly solidify to form a solid lipid core in which liquid lipid was randomly distributed. When the liquid lipid (oleic acid) content is higher, liquid lipids would be located at the outer shell of the nanoparticles besides being distributed in the solid lipid core, which led to drug-enriched shell related with drug burst release at the initial stage (Jia et al., 2010; Hu et al., 2005). In addition, as liquid lipid was distributed in solid lipid, the crystalline structure of NLC became more imperfect and allowed drugs loaded to release more easily, thus increasing the rate of drug release. Secondly, it was of smaller size. When the particle size decreased, the specific surface area of nanoparticles increased, therefore, the release rate became fast. Therefore, the fastest release rate in the initial stage, emerged in the nanoparticles of NLC3, resulted from both the smaller size and higher oleic acid content.
Figure 3.
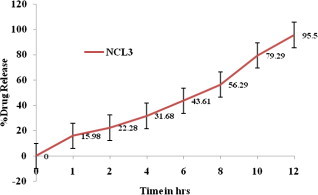
In vitro release profile of Minoxidil from NLC. Each value represents the mean ± SD (n = 3).
3.5. DSC
DSC gives the information regarding the crystalline or amorphous nature of the drug using the fact that different lipid modifications possess different melting points. The DSC thermograms of minoxidil, tristearin, mixture of tristearin and oleic acid (2:1) and ternary bulk mixture of minoxidil, tristearin and oleic acid (2:1) are shown in Fig. 4d. The minoxidil and tristearin illustrated an endothermic peak at 248.5 and 68.6 °C (Fig. 4a and b). The bulk mixture of tristearin and oleic acid exhibited an endothermic peak at 65.1 °C (Fig. 4c) with a widening of melting peak which indicates that oleic acid is dissolved in tristearin, inducing a less pronounced crystalline structure. From the figure, the ternary mixture of minoxidil, tristearin and oleic acid in the ratio used in minoxidil loaded NLC was investigated in order to obtain information about the ability of the lipid matrix to dissolve minoxidil. This mixture does not produce any endothermic peak for minoxidil incorporated in NLC. It has been reported that when the drug does not show its endothermic peak in the nanoparticulate formulations, it is said to be in the amorphous state (Liu et al., 2007; Sarmento et al., 2006). Hence, it could be concluded that the drug was present in the amorphous phase and may have been homogeneously dispersed in the NLCs.
3.6. Characterization of NLC gel
The average particle size of the NLC3 dispersion was estimated to be 280.4 nm with a polydispersity index of 0.367 indicating wide particle size distributions. Polydispersity is a measure of particle homogeneity and it varies from 0 to 1. The drug encapsulation efficiency within the NLC3 was found to be 35% and comparatively it was maximum than other formulations. The evaluation study of Minoxidil-loaded NLC3 exhibited good result as compared to all other formulations. Hence, NLC3 was selected for preparation of gel by using the gelling agent carbopol 934. The NLC gel showed an optimum viscosity at the concentration of 2% of carbopol 934. The drug content of the NLC based gel was found to be 89.80 ± 0.82% and pH was found to be 7.29 ± 0.18, which are within the acceptable limits. Spreadability is an important property of topical formulation from a patient’s compliance point of view. Application of the formulation to inflamed skin is more comfortable if the base spreads easily, exhibiting maximum slip and drag. The prepared gel produces excellent spreadability. In general, the gels that possess a high consistency index are less spreadable. The release of the drug from the formulation is governed by its components as well as by the consistency of the formulation. Viscosity of the NLC gel was found to be 32,540 ± 7.63 cps at 5 rpm. It is essential for any formulation to study its rheological behavior to be used for topical drug delivery applications. It is important for its efficacy in delivering molecules onto or across the skin. Consistency index is a measure of consistency and is equivalent to apparent viscosity at a shear rate of 1 s−1. The consistency index of the formulation was found to be 10,103. The flow index n is a measure of the deviation of a system from Newtonian behavior (n = 1). A value of n < 1 indicates pseudoplastic flow or shear thinning; n > 1 indicates dilatant or shear thickening flow. The gel showed a flow index of 0.361, indicating the pseudoplastic flow behavior. Flow index reflects the mobility of the gel from the container.
3.7. In vitro drug release studies of NLC gel
The study of drug release from gel is an important step in the development stages of new formulations, as well as a routine quality-control test for assuring uniformity of the finished product. In the in vitro release studies the NLC gel (Fig. 5) showed burst release in the first one hour followed by a steady release. This could be due to the diffusion of unencapsulated drug in the first hour followed by diffusion from the NLC surface and thereafter from the core. As can be seen in Fig. 4, 92.18% of drug encapsulated within the core of NLC was released. Burst release as well as sustained release both are of interest for dermal application. Burst release can be useful to improve the penetration of drug. Sustained release applied the drug over a prolonged period of time.
Figure 5.
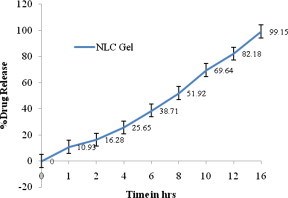
In vitro release profile of Minoxidil from NLC-Gel. Each value represents the mean ± SD (n = 3).
The release data from NLC gel were fitted to the different models. The value of r2 was found to be highest for the Higuchi model (r2 = 0.99). This indicates that the test product follows matrix diffusion based release kinetics.
4. Conclusion
The Minoxidil-loaded NLCs were prepared using different concentrations of solid lipid and liquid lipid by the melt dispersion ultrasonication technique. NLC3 exhibited a good physical stability indicated by zeta potential value and a high entrapment efficiency value. The drug release behavior from the NLC displayed a biphasic drug release pattern with burst release at the initial stage followed by sustained release. These results indicated that the NLC3 is a suitable carrier of minoxidil with improved drug loading capacity and controlled drug release properties.
Therefore, NLC3 was selected and the NLC based gel containing minoxidil was formulated by using the gelling agent carbopol 934. It has been observed that NLC gel produces the gel with good consistency, homogeneity, spreadabiliity and rheological behavior. It was found that NLC gel showed a biphasic release pattern, and provided a fast release initially for skin saturation followed by a slow and prolonged release profile to maintain the skin concentration.
The present study concluded that the NLC-based gel containing minoxidil dissolved in a mixture of solid lipid and liquid lipid in the nanoparticulate form helped us to attain the objective of faster onset yet prolonged action as evident from in vitro release.
Acknowledgments
This study was supported by the Oriental College of Pharmacy, Bhopal, India. The authors also sincerely thank Dr. D.P. Chatterjee, Director, Oriental College of Pharmacy, Bhopal, India for providing Lab facility and support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aronson J.K. Minoxidil. In: Aronson J.K., editor. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. Elsevier; Amsterdam: 2006. pp. 2354–2356. [Google Scholar]
- Atrux-Tallau N., Falson F., Pirot F. Nanotherapeutics for skin diseases. In: Lamprecht A., editor. Nanotherapeutics: Drug Delivery Concepts in Nanoscience. Pan Stanford; Singapore: 2009. pp. 125–161. [Google Scholar]
- Chaudhari Y.S. Nanoparticles – a paradigm for topical drug delivery. Chronicles of Young Scientists. 2012;3:82–85. [Google Scholar]
- Chen Y., Zhou L., Yuan L., Zhang Z., Liu X., Wu Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. International Journal of Nanomedicine. 2012;7:3023–3033. doi: 10.2147/IJN.S32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommoss, A., 2008. Nanostructured lipid carriers (NLC). In: Dermal and Personal Care Formulations, Inaugural-Dissertation, Department of Biology, Chemistry and Pharmacy, Freie Universitat Berlin, p. 14.
- Hu F.Q., Jiang S.P., Du Y.Z., Yuan H., Ye Y.Q., Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids and Surfaces, B: Biointerfaces. 2005;45:167–173. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Jain P., Mishra A., Yadav S.K., Patil U.K., Baghel U.S. Formulation development and characterization of solid lipid nanoparticles containing nimesulide. International Journal of Drug Delivery Technology. 2009;1:24–27. [Google Scholar]
- Jia L., Zhang D., Li Z., Feng F., Wang Y., Dai W., Duan C., Zhang Q. Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Delivery. 2010;17(1):11–18. doi: 10.3109/10717540903431586. [DOI] [PubMed] [Google Scholar]
- Joshi M., Patravale V. Formulation and evaluation of nanostructured lipid carrier (NLC)-based Gel of Valdecoxib. Drug Development and Industrial Pharmacy. 2006;32:911–918. doi: 10.1080/03639040600814676. [DOI] [PubMed] [Google Scholar]
- Lim S.J., Kim C.K. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. International Journal of Pharmaceutics. 2002;243:135–146. doi: 10.1016/s0378-5173(02)00269-7. [DOI] [PubMed] [Google Scholar]
- Liu J., Gong T., Wang C., Zhong Z., Zhang Z. Solid lipid nanoparticles loaded with insulin by sodium cholate–phosphatidylcholine-based mixed micelles: preparation and characterization. International Journal of Pharmaceutics. 2007;340:153–162. doi: 10.1016/j.ijpharm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Messenger A.G., Rundegren J. Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology. 2004;150:186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- Muller, R.H., Almeida, A.J., 2005, SLN and NLC for Topical Antifungals for Drug Delivery, Inaugural-Dissertation, Department of Biology, Chemistry and Pharmacy, Freie Universitat Berlin, pp. 32–40.
- Muller R.H., Ruh D., Runge S., Schulze-Forster K., Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharmaceutical Research. 1997;14:458–462. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- Muller, R.H., Mader, K., Lippacher, A., Jenning, V., 2000. Solid–liquid (semi-solid) liquid particles and method of producing highly concentrated lipid particle dispersions, in PCT/EP00/04565.
- Muller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews. 2002;54:131–155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Padoisa K., Cantienia C., Berthollea V., Bardel C., Pirot F., Falson F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. International Journal of Pharmaceutics. 2011;416:300–304. doi: 10.1016/j.ijpharm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. International Journal of Pharmaceutics. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Rajashree H., Harshal G., Vilasrao K. Solid lipid nanoparticles and nanostructured lipid carriers: a review. Current Drug Therapy. 2011;6(4):240–250. [Google Scholar]
- Sarmento B., Ferreira D., Veiga V., Ribeiro A. Characterization of insulin loaded alginate nanoparticles produced by ionotropic pregelation through DSC and FTIR studies. Carbohydrate Polymers. 2006;66:1–7. [Google Scholar]
- Saupe A., Wissing S.A., Lenk A., Schmidt C., Müller R.H. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) – structural investigations on two different carrier systems. Bio-medical Materials and Engineering. 2005;15:393–402. [PubMed] [Google Scholar]
- Souto E.B., Muller R.H. SLN and NLC for topical delivery of ketoconazole. Journal of Microencapsulation. 2005;22(5):501–510. doi: 10.1080/02652040500162436. [DOI] [PubMed] [Google Scholar]
- Teeranachaideekul V., Muller R.H., Junyaprasert V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—effects of formulation parameters on physicochemical stability. International Journal of Pharmaceutics. 2007;340:198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Uner M., Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. International Journal of Nanomedicine. 2007;2(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- Utreja S., Jain N.K. Solid lipid nanoparticles. CBS Publishers; New Delhi, India: 2001. (Advances in Controlled and Novel Drug Delivery). pp. 408–425. [Google Scholar]
- Wagner L., Kenreigh C. Minoxidil. In: Enna S.J., David B.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York: 2007. pp. 1–5. [Google Scholar]
- Wang M., Jin Y., Yang Y., Zhao C., Yang H., Xu X., Qin X., Wang Z., Zang Z., Jian Y., Huang Y. In vivo biodistribution, anti-inflammatory, and hepatoprotective effects of liver targeting dexamethasone acetate loaded nanostructured lipid carrier system. International Journal of Nanomedicine. 2010;5:487–497. doi: 10.2147/ijn.s10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissing S.A., Muller R.H. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. Journal of Controlled Release. 2002;81:225–233. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- Wissing S.A., Muller R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. International Journal of Pharmaceutics. 2002;242:377–379. doi: 10.1016/s0378-5173(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Wissing S.A., Muller R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—in vivo study. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(1):67–72. doi: 10.1016/s0939-6411(03)00040-7. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang C., Yang K., Li K., Hu H., Chen D. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Development and Industrial Pharmacy. 2010;36(7):773–780. doi: 10.3109/03639040903485716. [DOI] [PubMed] [Google Scholar]



