Abstract
The objective of the article is to highlight various roles of glutamic acid like endogenic anticancer agent, conjugates to anticancer agents, and derivatives of glutamic acid as possible anticancer agents. Besides these emphases are given especially for two endogenous derivatives of glutamic acid such as glutamine and glutamate. Glutamine is a derivative of glutamic acid and is formed in the body from glutamic acid and ammonia in an energy requiring reaction catalyzed by glutamine synthase. It also possesses anticancer activity. So the transportation and metabolism of glutamine are also discussed for better understanding the role of glutamic acid. Glutamates are the carboxylate anions and salts of glutamic acid. Here the roles of various enzymes required for the metabolism of glutamates are also discussed.
Keywords: Glutamic acid, Glutamine, Glutamate, Anticancer agent
1. Introduction
In Cancer mostly there is depletion of glutamine, as cancer cells consume glutamine at large scale that is why there is lack of glutamine in the skeletal muscle (Kulkarni et al., 2005). Glutamine plays very important roles in tumor cell. Firstly it acts as a nitrogen donor in the nucleotide and amino acid biosynthesis, secondly glutamine helps in the uptake of essential amino acid and it maintains the activation of TOR kinase (Wise and Thompson, 2010). In many cancer cells, glutamine is the primary mitochondrial substrate and it maintains mitochondrial membrane potential and integrity. It provides support for the NADPH production needed for redox control and macromolecular synthesis. Glutamine is the respiratory fuel of tumor cells. Glutamic acid and glutamine both are inter convertible. Glutamic acid comes under the major group of neurotransmitters. It is the major work horse neurotransmitter of the brain. It increases the brain function and mental activity. It detoxifies the brain from ammonia by attaching itself to nitrogen atoms in the brain and also helps in the transportation of potassium across the blood–brain barrier. It is conjectured that glutamate is involved in cognitive functions like learning and memory in the brain, though excessive amounts may cause neuronal damage associated with diseases like amyotrophic lateral sclerosis, lathyrism and Alzheimer’s disease (Dutta et al., 2011).
2. Glutamic acid as anticancer agent
l-Glutamic acid is converted into l-glutamine by l-glutamine synthetase. l-glutamine biosynthesize purines and pyrimidines by contributing 3- and 9-nitrogen groups of purine bases, 2-amino group of guanine, 3-nitrogen group and amino group of cytosine which are the bases of DNA and RNA (Dewald and Moore, 1958). l-Glutamine cannot be synthesized in neoplastic cells due to the lower reactivity of l-glutamine synthetase. Thus an antagonist of this enzyme can interfere with the metabolic process of l-glutamine and act as anticancer agents (Luzzio et al., 2000). Patients of cancer often develop glutamine depletion in the muscles due to uptake by tumors and chronic protein metabolism. On the basis of these, it can be assumed that structural variants of glutamine might possess possible antitumor activity.
l-Glutamic acid-γ-(4-hydroxyanilide) is isolated from mushroom Agaricus bisporous. It acts as a growth regulatory substance for inhibiting the B16 melanoma cells in culture (Srikanth et al., 2002). Azaserine and 6-diaza-5-oxo-l-norleucine antagonized the metabolic process involving l-glutamine and exhibited antitumor activity in animal models (Vishwanathan et al., 2008). An aryl amide derivative of l-threo-γ-hydroxy glutamic acid which is isolated from Justica ghiesbreghtiana is active against various tumors (Nishiyama et al., 2001). The synthetic amides of l-glutamic acid exhibit activity against Ehrlich ascites carcinoma (Vila et al., 1990). Four N-(benzenesulfonyl)-l-glutamic acid bis(p-substituted phenylhydrazides) have anticancer activity against PC-3 prostate cancer and in COLO-205 colon cancer. l-Glutamic acid-γ-monohydroxamate (GAH) demonstrated complete cytotoxicity against L-1210 cells in the culture and marked antitumoral activity in vivo against L-1210 leukemia and B-16 melanoma (Xu et al., 2005). Glutamate receptor is another important player in hippocampal long-term potentiation and memory. Glutamic acid, a glutamate receptor agonist enhances retention of memory (Cui et al., 2009). Glutamic acid is also useful in lowering blood pressure. According to a study by the Imperial College of London, people who consume more glutamic acid have low blood pressure than those who consume less (Stamler et al., 2009). When the glutamine importer SLC1A5 is impaired then the uptake of essential amino acids is also impaired and without the aid of essential amino acids rapamycin-sensitive mTORC1 is not activated. mTORC1 is responsible for the regulation of cell growth, protein translation and plays an important role in inhibiting macro autophagy (Nicklin et al., 2009). That means if mTORC1 is inactivated then there will be no cell growth and no protein translation. In glioblastoma cells, metabolism of glutamine provides the bulk of oxaloacetic acid (OAA) cellular pool (DeBerardinis et al., 2007). This OAA is one of the substrates in mitochondria that leads to the synthesis of many essential biological macromolecules like cholesterol (Hatzivassiliou et al., 2005). Hence glutamine is the primary substrate in cancer cells that provides precursor molecules to mitochondria for anaplerosis (replenishment of the carbon pool). c-MYC (Myc), a DNA transcription factor regulates three out of the five steps of purine and pyrimidine synthesis at oncogenic level. It also promotes glutaminolysis and this catabolism of glutamine leads to the larger amount of carbon in the cell, which allows the cell to produce more NADPH (Wise and Thompson, 2010). Since cancer cells depend on glutamine lack of glutamine can lead to the death of cancer cells. But as it is also required for some other essential functions in the body such as in the brain therefore that treatment should be adopted which can reduce the ability of the cell to uptake glutamine by targeting Myc and other proteins that are responsible for transporting glutamine into the cell. l-γ-Glutamyl-p-nitroanilide (GPNA) which inhibits SLC1A5 (Esslinger et al., 2005) and BCH (2-aminobicyclo-(2,2,1)heptanes carbozylic acid) which blocks mTORC1 (Nicklin et al., 2009) and that treatment which reprograms the mitochondria so that it no longer depends on glutamine can also be effective, e.g. amino-oxyacetic acid (AOA) is used because it is a transaminase inhibitor (Moreadith and Lehninger, 1984).
3. Glutamic acid as conjugates with anticancer drug
Glutamic acid is used as a conjugate because it increases the efficacy of anticancer drug and decreases its toxicity toward normal cells. Polyglutamic acid is biodegradable, edible and nontoxic toward humans (Shih et al., 2004).
3.1. Conjugate with All-trans retinoic acid (ATRA)
ATRA is an active metabolite of vitamin A. It is used in the treatment of acute promyelocytic leukemia and myelodysplastic syndrome. It has a slow dissolution rate and low bioavailability. Therefore to obtain ATRA with better solubility, transportation and bioavailability, derivatives of ATRA containing glutamic acid or its sodium salt were synthesized. The two derivatives of ATRA, RAE and RAENa, exhibited improved aqueous solubility and were more effective in mice bearing S(180) tumors (Cui et al., 2009).
3.2. Conjugate with paclitaxel
Conjugation of paclitaxel and the water-soluble polyglutamate is known as poly (l-glutamic acid)-paclitaxel (PG-TXL). Observations showed that PG-TXL has more antitumor activity than free paclitaxel. PG-TXL exerts its anticancer activity by the continuous release of free paclitaxel into cells (Oldham et al., 2000).
3.3. Conjugate with cisplatin
Cisplatin [cis-dichlorodiammineplatinum (II), CDDP] is combined with γ-poly(α,l-glutamic acid) (γ-PGA) to form γ-poly(α,l-glutamic acid)-cis-dichlorodiammineplatinum (γ-PGA-CDDP) which is water soluble. γ-PGA–cisplastin conjugate effectively inhibits human breast tumor cells xenografted into nude mice. It also reduces the toxic side effects associated with the use of free CDDP and also produced desirable pharmacokinetics and enhanced antitumor activity (Ye et al., 2006) (Figure 1).
Figure 1.
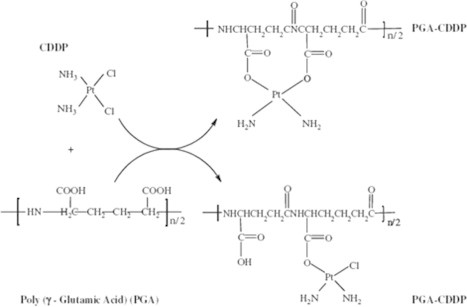
Chemical structures of CDDP, γ-PGA, and PGA–CDDP conjugates.
3.4. Conjugate with curcumin
Curcumin [diferuloylmethane; 1,7-bis-(4-hydroxyl-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the major pigmentary component of turmeric (Curcuma longa). Curcumin with nphthaloyl-glutamoylchloride forms 4,40-(di-o-glutamoyl)-curcumin. According to a study curcumindiglutamoyl derivative was found to be more potent against cancer cell lines, HeLa (cervical cancer) and KB (oral cancer), than other derivatives. It is due to the activation of caspases which is facilitated due to accumulation and better stability of diglutamiccurcumin (Dubey et al., 2008).
3.5. Conjugate with 20(s)-camptothecin (CPT)
Due to the instability of active lactone the therapeutic efficacy of 20(s)-camptothecin (CPT) is limited in humans. By binding one molecule of a drug via the γ-carboxylic acid of each monomeric subunit of poly-(l-glutamic acid) (PGA) it leads to the stability of lactone. Linking of CPT to a high molecular weight anionic polymer that is PGA enhances solubility and improves distribution to the tumor through enhanced permeability and retention (EPR effect) (Singer et al., 2001).
3.6. Conjugate with N-(4-hydroxyphenyl)retinamide (4HPR)
Several studies have indicated that N-(4-hydroxyphenyl)retinamide (4HPR) treatment is associated with the inhibition of angiogenesis and a decreased vascular response in vitro and in vivo. 4HPR was bound to a synthetic polyamino acid, poly(l-glutamic acid) (PG). PG-4HPR was evaluated for its release kinetics and in vitro anti-proliferative and in vivo antitumor activities against ovarian cancer cell lines. It was confirmed that treatments with both 4HPR and PG-4HPR decreased the expression of pre-angiogenic factor VEGF in SKOV3 tumors. In-vivo, PG-4HPR demonstrated significantly enhanced antitumor activities compared to 4HPR in both early treatment and later treatment protocols. Treatments with PG-4HPR suppressed the expression of VEGF and reduced blood flow into the tumor (Zou et al., 2007).
4. Derivatives of glutamic acid as anticancer agent
4.1. Aminopteroylglutamic acid or pteroyl-l-glutamic acid
Aminopterin (4-aminopteroic acid), a 4-amino analog of folic acid, is an antineoplastic drug with immunosuppressive properties used in chemotherapy (Oaks et al., 2010). Folate is involved in DNA synthesis and methylation which may reduce breast cancer risk, particularly among women with greater alcohol consumption. High intake of folate may reduce the risk of colon cancer (Giovannucci et al., 1998), but the dosage and duration relations and the impact of diet compared with supplementary sources are not well understood (Boehm et al., 2009). Folate intake decreased the risk of pancreatic cancer in women but not in men (Percival et al., 2008).
4.2. Methotrexate
Chemically methotrexate is N-[4-[[(2,4-diamino-6-pteridinyl) methyl] methylamino] benzoyl]-l-glutamic acid. Methotrexate tablets are used alone or in combination with other anticancer agents in the treatment of breast cancer, epidermoid cancers of the head and neck, advanced mycosis fungoides (cutaneous T-cell lymphoma), and lung cancer, particularly squamous cell and small cell types. Methotrexate tablets are also used in combination with other chemotherapeutic agents in the treatment of advanced stage non-Hodgkin’s lymphomas. It reduces dihydrofolates to tetrahydrofolates by the help of enzyme dihydrofolic acid reductase which inhibits the synthesis of purines (Skeel, 2008).
4.3. l-Theanine
Chemically l-theanine is γ-ethylamino-l-glutamic acid. Limited studies evaluate the effects of l-theanine in the prevention of cancer. The observed anticancer effects are largely attributed to the catechins found in tea, while action on tumors may be due to an enhanced immune response (McPhee et al., 2011).
4.4. Thalidomide
It is a chemotherapeutic agent used against multiple myeloma, myelodysplastic syndrome, leprosy etc. It acts by inhibiting VEGF, TNF-α, GI growth factor, proliferation of NK cells and stimulation of T-cells.
5. Transportation and metabolism of glutamine
Glutamine is the most abundant amino acid present in the body. It is also known as levoglutamide, l-GA 5 amide, l-(+)-2-aminoglutamic acid. Glutamine is formed in the body from GA and ammonia in an energy requiring reaction catalyzed by glutamine synthase (Kulkarni et al., 2005).
Metabolism of glutamine takes place in the mitochondria. Glutamine is transferred from the extra cellular medium of mitochondria to the inner surface of mitochondria through the specific plasma membrane. Malignant cells transport glutamine across their plasma membranes at a faster rate than their non-malignant cells. Malignant cells show an uncontrolled rate of cellular proliferation and therefore require increased supply of precursor amino acids for biosynthesis. Therefore they transport amino acids (glutamine) much faster than normal cells (Medina et al., 1992). It has been seen in human hepatoma cells which transport glutamine at a rate 10–20 times faster than normal hepatocytes (Bannai and Ishii, 1988). Cytoplasm is filled with excess of glutamine, and glutamine is transported to mitochondria. In the mitochondria, glutamine is acted upon by glutaminase, an enzyme requiring high phosphate concentrations to be fully active. The high concentrations of inorganic phosphate found in the mitochondria of tumor cells could explain the high activity of tumor glutaminase in vivo. For cellular metabolism it is essential to transport the amino acids through the plasma membrane. This transportation is mediated by proteins called carriers (Figure 2).
Figure 2.
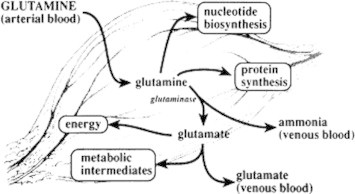
Transportation and metabolism of glutamine.
Glutamine is converted into glutamate and ammonia by phosphate dependent glutaminase. This is the first step in the series of reaction that generates the metabolic intermediate which is essential for cell growth. The high rate of intracellular glutaminolysis results in high release of ammonia. Glutamine is transported into cells mainly by sodium ion dependent systems A and ASC. After an excess of deposition of glutamine in the cell cytoplasm it is transferred to mitochondria otherwise it will be hydrolyzed by phosphate dependent glutaminase.
6. Glutamate and its metabolism enzymes
The carboxylate anions and salts of glutamic acid are known as glutamates. It is also important for metabolism.
6.1. Glutamine synthetase
It acts as a catalyst in this reaction
The enzyme from mammalian brain has two important functions: assimilation of ammonia and biosynthesis of glutamine (Boksha et al., 1995).
6.2. Glutaminase
Glutaminase catalyzes the reaction of glutamine catabolism to produce glutamate and ammonia. Its activity can be observed both in neurones and astroglial cells, but glutaminase is more active in neurons (Nimmo and Tipton, 1979), predominantly in mitochondria. The enzyme becomes free from the inhibitory influence of glutamate only when the concentration of glutamic acid falls below normal values (Plaitakis et al., 1984).
6.3. Glutamate dehydrogenase
Glutamate dehydrogenase (GDH) catalyzes a reversible reaction:
The GDH reaction is reversible but equilibrium is shifted closer to direct reaction (i.e. to synthesis of glutamic acid) (Zaganas et al., 2009). Consequently, GDH in brain takes part in the synthesis of glutamate from 2-oxoglutaric acid, more than in oxidation of the amino acid. This is the way of continuous maintenance for changing of free ammonia into amino nitrogen of amino acids.
6.4. Aspartate transaminase or glutamate oxaloacetate transaminase
Aspartate transaminase catalyzes the reversible transfer of amino group to 2-oxoglutarate, as a result oxaloacetate and glutamate are formed. This is the most active transaminase in the brain (Kelly and Stanley, 2001). Oxaloacetate is quickly transformed into malic acid which enters from the cytoplasm into mitochondria. In this way the aspartate-malate bypath for transfer redox equivalents from the cytosol to mitochondria is formed. This bypath is the main way for transferring redox equivalents to mitochondria in neurons. Influx of aspartate through the mitochondrial membrane is bound with an efflux of a glutamate in such a way influx of a malate is bound with the efflux of 2-oxoglutarate, too.
6.5. Glutamic acid decarboxylase
Glutamic acid decarboxylase (GDA) catalyzes the separation of a carboxylic group from glutamate with the formation of gamma-aminobutyric acid (GABA). When GAD acts on glutamate which has an excitatory influence on neurons, then GABA is formed which is a main inhibitory neurotransmitter in the brain (Kelly and Stanley, 2001).
7. Applications
7.1. Pharmacological uses of glutamic acid
7.1.1. Fuel
Glutamic acid takes part in more metabolic reactions than any other amino acid. It is a source of glucose which is the chief source of fuel. It maintains the normal blood glucose level and also the acidity. It serves as a source of fuel for the intestinal epithelium. Glutamine increases the body’s secretion of human growth hormone (HGH). It also plays a major role in removing excess ammonia from the bloodstream.
7.1.2. Muscle and other cell components
Glutamine is present in more than three-fifths of the human muscle tissue. It synthesizes both DNA and RNA and is essential for the synthesis of all proteins. By stimulating the release of the anabolic hormone HGH, it plays an indirect role in building the skeletal muscle. Glutamine also protects the integrity of the gastrointestinal tract when ulcers, chemotherapy agents or non-steroidal anti-inflammatory drugs are present.
7.1.3. Immune function
Glutamine is very essential for proper immune function. It is required for the proliferation of lymphocytes and cytokines. It increases the efficiency of macrophages, large immune cells which engulf and degrade foreign substances ranging from microbes to inorganic molecules. In many situations in which the immune function is compromised, for example, severe burns, sepsis or when athletes overexert themselves, glutamine depletion is at least partly responsible for the immune suppression. The administration of glutamine to patients receiving bone marrow transplants results in a lower incidence of post-operative infections and a shorter stay in the hospital.
7.1.4. Neurotransmitter
It is an important excitatory neurotransmitter. It helps in the transportation of potassium across the blood–brain barrier. It also shows promise in the future treatment of neurological conditions, ulcers, hypoglycemic coma, muscular dystrophy, epilepsy, Parkinson’s and mental retardation.
7.2. Other uses of glutamic acid
7.2.1. It is used as surfactants
It is hydrophilic by nature and can combine with hydrophobic fatty acids easily to form molecules with both water-soluble and water repelling portions that can be used as surfactants. There are many applications of GA derivatives as surfactants, especially due to its lack of harmful effects to skin and their general smooth appearance. It is very much favored by the cosmetic, moisture containing hair shampoo product manufacturers.
7.2.2. It is used as buffer
GA is an amphopteric substance that contains both acidic and basic functional groups and thus a natural buffer by itself.
7.2.3. It is used as Chelating agents
GA has two carboxylic groups, which can form chelates with many metal cations. Such chelating reaction is useful in the removal of heavy-metal contaminants in the wastewater treatment processes.
7.2.4. It is used as flavor enhancer
Monosodium glutamate (MSG), the single largest amino acid product, has been used as a flavor enhancer throughout the world for the past forty years. MSG is used worldwide in huge quantities as a flavor enhancer in foods. MSG is known to produce a unique taste sensation termed ‘UMAMI’ the fifth taste, i.e. savory or brothy taste present in tomatoes and cheese. Free glutamate content is said to increase during the process of natural ripening and brings about a fuller taste in many foods, the basis behind is not known.
7.2.5. It is used in culture medium
GA happens to be one of the main components in the cell wall of Gram-positive bacteria. It is also one of the most essential amino acids for other microorganisms to grow on. In most cases, it is often necessary to add GA into culture media to effect normal growth.
7.2.6. It is used in agriculture
GA is one of the major amino acids in plant proteins and plays a role of the major nitrogen storage for plants. That is why GA is often one of the more prominent ingredients in many plant growth supplements. Besides, GA is vital in the nitrogen metabolism in plants.
8. Overview and conclusion
The main objective of this article is to highlight the anticancer activity of glutamic acid. From the above discussion it has been concluded that glutamic acid is endogenic in nature hence will produce a fewer side effects. Some times it is seen that it increases the anticancer activity of that drug with which it is combined, as in the case of γ--poly (α,l-glutamic acid)-cis-dichlorodiammineplatinum. This conjugate produces less side effect and increased anticancer activity when compared with cisplastin. Glutamine is the primary substrate in cancer cells that provide precursor molecules which allows the cell to produce more NADPH. Since cancer cells depend on glutamine, if glutamine synthesis and metabolism is blocked the anticancer activity can be produced. But as glutamine is required for several other functions of body like in the brain therefore blockage of glutamine is not a smart way to achieve anticancer activity. Rather the treatment should be done in such a way which can reduce the ability of the cell to uptake glutamine, such as by targeting Myc which is responsible for the synthesis of purine and pyrimidine at oncogenic level and other proteins that are responsible for transporting glutamine into the cell, or drugs which block mTORC1. It is also a source of glucose, essential for proper immune function. It also shows promise in the future treatment of neurological conditions, ulcers, hypoglycemic coma, muscular dystrophy, epilepsy, Parkinson’s, and mental retardation. Hence it can be concluded that important therapeutic applications of glutamic acid are just waiting to be discovered and can be implicated in cancer treatment.
Footnotes
Peer review under responsibility of King Saud University
References
- Bannai S., Ishii T. A novel function of glutamine in cell culture: utilization of glutamine for the uptake of cystine in human fibroblast. J. Cell. Physiol. 1988;137(2):360–366. doi: 10.1002/jcp.1041370221. [DOI] [PubMed] [Google Scholar]
- Boehm K., Borrelli F., Ernst E., Habacher G., Hung S.K., Milazzo S., Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2009;(3) doi: 10.1002/14651858.CD005004.pub2. CD005004. doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksha I.S., Tereshkina E.B., Burbayeva G.S. Isolation and some properties of glutamine synthetase from human brain. Biokhimiia (Mosc) 1995;60(10):1697–1705. (Rus) [PubMed] [Google Scholar]
- Cui C., Zhang Y., Wang L., Liu H., Cui G. Enhanced anticancer activity of glutamate prodrugs of all-trans retinoic acid. J. Pharm. Pharmacol. 2009;61(10):1353–1358. doi: 10.1211/jpp/61.10.0012. [DOI] [PubMed] [Google Scholar]
- DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald H.A., Moore A.M. 6-Diazo-5-oxo-l-norleucine, a new tumor-inhibitory substance. Preparation of l-, d- and DL-forms. J. Am. Chem. Soc. 1958;80(15):3941–3945. [Google Scholar]
- Dubey S.K., Sharma A.K., Narain U., Misra K., Pati U. Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur. J. Med. Chem. 2008;43(9):1837–1846. doi: 10.1016/j.ejmech.2007.11.027. < http://dx.doi.org/10.1016/j.ejmech.2007.11.027>. [DOI] [PubMed] [Google Scholar]
- Dutta S., Ray S., Nagarajan K. Glutamic acid analogues used as potent anticancer: a review. Der Pharm. Chem. 2011;3(2):263–272. [Google Scholar]
- Esslinger C.S., Cybulski K.A., Rhoderick J.F. Nγ-Aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 2005;13(4):1111–1118. doi: 10.1016/j.bmc.2004.11.028. < http://dx.doi.org/10.1016/j.bmc.2004.11.028>. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Stampfer M.J., Colditz G.A., Hunter D.J., Fuchs C., Rosner B.A., Speizer F.E., Willett W.C. Multivitamin use, folate and colon cancer in women in the Nurses’ health study. Ann. Intern. Med. 1998;129(7):517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G., Zhao F., Bauer D.E., Andreadis C., Shaw A.N., Dhanak D., Hingorani S.R., Tuveson D.A., Thompson C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–321. doi: 10.1016/j.ccr.2005.09.008. < http://dx.doi.org/10.1016/j.ccr.2005.09.008>. [DOI] [PubMed] [Google Scholar]
- Kelly A., Stanley C.A. Disorders of glutamate metabolism. Ment. Retard. Dev. Disabil. Res. Rev. 2001;7(4):287–295. doi: 10.1002/mrdd.1040. [DOI] [PubMed] [Google Scholar]
- Kulkarni C., Kulkarni K.S., Hamsa B.R. l-Glutamic acid and glutamine: exciting molecules of clinical interest. Indian J. Pharmacol. 2005;37(3):148–154. [Google Scholar]
- Luzzio F.A., Mayorov A.V., Figg W.D. Thalidomide metabolites. Part 1: derivatives of (+)-2-(N-phthalimido)-γ-hydroxyglutamic acid. Tetrahedron Lett. 2000;41(14):2275–2278. < http://dx.doi.org/10.1016/S0040-4039(00)00160-X>. [Google Scholar]
- McPhee S.J., Papadakis M.A., Rabow M.W. 50 ed. Lange Medical Books/McGraw-Hill; New York: 2011. Current Medical Diagnosis and Treatment 2011. [Google Scholar]
- Medina M.A., Sanchez-Jimenez F., Marquez J., Quesada A.R., Castro I.N. Relevance of glutamine metabolism to tumour cell growth. Mol. Cell. Biochem. 1992;113(1):1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- Moreadith R.W., Lehninger A.L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984;259(10):6121–6215. [PubMed] [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., Myer V.E., MacKeigan J.P., Porter J.A., Wang Y.K., Cantley L.C., Finan P.M., Murphy L.O. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. < http://dx.doi.org/10.1016/j.cell.2008.11.044>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G.A., Tipton K.F. The distribution of soluble and membrane-bound forms of glutaminase in pig brain. J. Neurochem. 1979;33(5):1083–1084. doi: 10.1111/j.1471-4159.1979.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Kato Y., Sugiyama Y., Kataoka K. Cisplatin-loaded polymer–metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm. Res. 2001;18(7):1035–1041. doi: 10.1023/a:1010908916184. [DOI] [PubMed] [Google Scholar]
- Oaks B.M., Dodd K.W., Meinhold C.L., Jiao L., Church T.R., Stolzenberg-Solomon R.Z. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the prostate, lung, colorectal and ovarian cancer screening trial. Am. J. Clin. Nutr. 2010;91(2):449–455. doi: 10.3945/ajcn.2009.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham E.A., Lic K.S., Wallace S., Huang P. Comparison of action of paclitaxel and poly(l-glutamic acid)–paclitaxel conjugate in human breast cancer cells. Indian J. Oncol. 2000;16:125–132. [PubMed] [Google Scholar]
- Percival S.S., Bukowski J.F., Milner J. Bioactive food components that enhance γδ T cell function may play a role in cancer prevention. J. Nutr. 2008;138(1):1–4. doi: 10.1093/jn/138.1.1. [DOI] [PubMed] [Google Scholar]
- Plaitakis A., Berl S., Yahr M.D. Neurological disorders associated with deficiency of glutamate dehydrogenase. Ann. Neurol. 1984;15(2):144–153. doi: 10.1002/ana.410150206. [DOI] [PubMed] [Google Scholar]
- Shih I.L., Van Y.T., Shen M.H. Biomedical applications of chemically and microbiologically synthesized poly(glutamic acid) and poly(lysine) Mini Rev. Med. Chem. 2004;4(2):179–188. doi: 10.2174/1389557043487420. [DOI] [PubMed] [Google Scholar]
- Singer J.W., Bhatt R., Tulinsky J., Buhler K.R., Heasley E., Klein P., de Vries.P. Water-soluble poly-(l-glutamic acid)-gly-camptothecin conjugates enhance camptothecin stability and efficacy in vivo. J. Controlled Release. 2001;74(1–3):243–247. doi: 10.1016/s0168-3659(01)00323-6. < http://dx.doi.org/10.1016/S0168-3659(01)00323-6>. [DOI] [PubMed] [Google Scholar]
- Skeel R.T. seventh ed. Lippincott, Williams & Wilkins; New York: 2008. Hand Book of Cancer Chemotherapy. [Google Scholar]
- Srikanth K., Kumar C.A., Ghosh B., Jha T. Synthesis, screening and quantitative structure-activity relationship (QSAR) studies of some glutamine analogues for possible anticancer activity. Bioorg. Med. Chem. 2002;10(7):2119–2131. doi: 10.1016/s0968-0896(02)00079-2. < http://dx.doi.org/10.1016/S0968-0896(02)00079-2>. [DOI] [PubMed] [Google Scholar]
- Stamler J., Brown I.J., Daviglus M.L., Chan Q., Kesteloot H., Ueshima H., Zhao L., Elliott P. Glutamic acid—the main dietary amino acid and blood pressure: the INTERMAP study (international collaborative study of macronutrients, micronutrients and blood pressure) Circulation. 2009;120(3):221–228. doi: 10.1161/CIRCULATIONAHA.108.839241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila J., Thomasset N., Navarro C., Doré J.F. In vitro and in vivo anti-tumor activity of l-glutamic acid γ-monohydroxamate against L1210 leukemia and B16 melanoma. Int. J. Cancer. 1990;45(4):737–743. doi: 10.1002/ijc.2910450428. [DOI] [PubMed] [Google Scholar]
- Vishwanathan C.L., Deb S., Jain A., Lokhande T., Juvekar A. Synthesis and evaluation of l-glutamic acid analogs as potential anticancer agents. Indian J. Pharm. Sci. 2008;70(2):245–249. doi: 10.4103/0250-474X.41467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. < http://dx.doi.org/10.1016/j.tibs.2010.05.003>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jiang M., Li H., Lu D., Ouyang P. Efficient production of poly(γ-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem. 2005;40(2):519–523. < http://dx.doi.org/10.1016/j.procbio.2003.09.025>. [Google Scholar]
- Ye H., Jin L., Hu R., Yi Z., Li J., Wu Y., Xi X., Wu Z. Poly (γ,l-glutamic acid)–cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials. 2006;27(35):5958–5965. doi: 10.1016/j.biomaterials.2006.08.016. < http://dx.doi.org/10.1016/j.biomaterials.2006.08.016>. [DOI] [PubMed] [Google Scholar]
- Zaganas I., Kanavouras K., Mastorodemos V., Latsoudis H., Spanaki C., Plaitakis A. The human GLUD2 glutamate dehydrogenase: localization and functional aspects. Neurochem. Int. 2009;55(1-3):52–63. doi: 10.1016/j.neuint.2009.03.001. < http://dx.doi.org/10.1016/j.neuint.2009.03.001>. [DOI] [PubMed] [Google Scholar]
- Zou C., Brewer M., Cao X., Zang R., Lin J., Deng Y., Li C. Antitumor activity of 4-(N-hydroxyphenyl) retinamide conjugated with poly (l-glutamic acid) against ovarian cancer xenografts. Gynecol. Oncol. 2007;107(3):441–449. doi: 10.1016/j.ygyno.2007.07.077. < http://dx.doi.org/10.1016/j.ygyno.2007.07.077>. [DOI] [PubMed] [Google Scholar]



