Abstract
Significant advances have allowed diffusion MRI (dMRI) to evolve into a powerful tool in the field of movement disorders that can be used to study disease states and connectivity between brain regions. dMRI represents a promising potential biomarker for Parkinson’s disease and other forms of parkinsonism, and may allow for the distinction of different forms of parkinsonism. Techniques such as tractography have contributed to our current thinking regarding the pathophysiology of dystonia and possible mechanisms of penetrance. dMRI measures could potentially assist in monitoring disease progression in Huntington’s disease, and in uncovering the nature of the processes and structures involved the development of essential tremor. The ability to represent structural connectivity in vivo also makes dMRI an ideal adjunctive tool for the surgical treatment of movement disorders. We will review recent studies utilizing dMRI in movement disorders research and present the current state of the science as well as future directions.
Keywords: diffusion magnetic resonance imaging, diffusion tensor imaging, movement disorders, Parkinson’s disease, parkinsonism, dystonia, Huntington’s disease, essential tremor
Introduction
While the clinical use of magnetic resonance imaging (MRI) has revolutionized the diagnosis and management of neurological diseases, its utility in neurodegenerative diseases had initially been limited to the exclusion of other diagnoses [1]. During the past three decades, significant advances have been made in the field of MRI that have increased its value in the study of neurodegenerative diseases, and these advances have occurred on a time course that has largely paralleled the maturation of the field of movement disorders as a neurologic subspecialty [2]. During this time period, diffusion MRI (dMRI) has emerged as a powerful tool in the field of movement disorders both in research and clinical settings for evaluating white matter (WM), gray matter (GM), and connectivity.
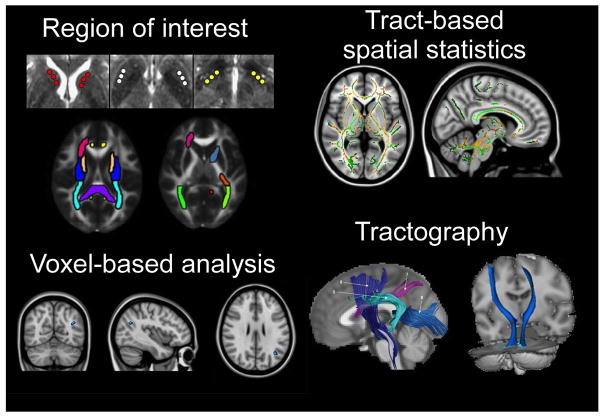
dMRI makes use of the random translational motion of molecules that occurs secondary to thermal energy and is influenced by a variety of microstructural factors, including organelles, neurofibrils, and membranes. Diffusion can be relatively directional (anisotropic) or can occur relatively equally in all directions (isotropic). The degree and direction of diffusion can be used to produce accurate contrast images, and a tensor can be calculated to estimate diffusivity in three-dimensional space [3]. From this tensor one can calculate the mean-squared displacement of molecules (mean diffusivity or MD) and the degree to which diffusion is directional (fractional anisotropy or FA, which ranges between 0 and 1). Additional scalars such as axial (AD) and radial (RD) diffusivity can be used to estimate the magnitude of diffusion parallel and perpendicular to the principal axis of diffusion, respectively. As underlying fiber orientation can be inferred from the orientation of the longest axis of the tensor, fiber orientation in neighboring pixels can be repeatedly reconstructed to produce streamlines to reconstruct WM pathways in a process called tractography, making dMRI especially useful in the analysis of WM tracts in the brain. Figure 1 shows some of the major analytic approaches that have been used and includes region of interest analysis (ROI), voxel-based analysis, tract-based spatial statistics (TBSS), and tractography.
Figure 1.
Visual representations of the major dMRI analysis methods that are currently in use. Clockwise from top left: manual selection of regions of interest (ROI); automated tract-based spatial statistics (TBSS) aligns a combined fractional anisotropy map on an FA skeleton; tractography involves tract reconstruction based on orientation and magnitude of diffusion, after which probabilities of connectivity can be analyzed; automated voxel-based analysis registers diffusion maps into a standard space. (With permission from: Prodoehl J, Li H, Planetta PJ, Goetz CG, Shannon KM, Tangonan R et al. Diffusion Tensor Imaging of Parkinson’s Disease, Atypical Parkinsonism, and Essential Tremor. Movement disorders: official journal of the Movement Disorder Society. 2013 May 14. doi: 10.1002/mds.25491 [19]; Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Human brain mapping. 2013. doi:10.1002/hbm.22256 [21]; Saini J, Bagepally BS, Bhatt MD, Chandran V, Bharath RD, Prasad C et al. Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism & related disorders. 2012;18(5):477–82. doi:10.1016/j.parkreldis.2012.01.006 [73]; Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(31):9740–7. doi:10.1523/JNEUROSCI.2300-09.2009 [27]; Wang HC, Hsu JL, Leemans A. Diffusion tensor imaging of vascular parkinsonism: structural changes in cerebral white matter and the association with clinical severity. Archives of neurology. 2012;69(10):1340–8. doi:10.1001/archneurol.2012.633 [102]; and Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011 Feb;77(2):269–73. doi: 10.1016/j.ejrad.2009.07.032. Epub 2009 Aug 18 [107].)
The interpretation of dMRI measures, including the specific abnormalities that these proxy measures represent and the conclusions that can be drawn from abnormal values in disease, can be controversial [4]. Results can be highly dependent upon the appropriateness of the methods of acquisition and data processing [5]. Keeping these caveats in mind, changes in dMRI scalars can reveal important information about the microstructural properties of underlying tissues. FA is higher in areas of highly coherent fiber structure and is affected by myelination. Reduced AD has been correlated with axonal injury, while increased RD has been suggested to represent incomplete or damaged myelination [5]. Reduced probabilities of tractography are believed to represent compromised WM fiber integrity or a reduction in fiber myelination or number [6].
dMRI can be used to study differences in tissue properties at specific locations in the brain between individuals or groups, to look for relationships between dMRI properties and other variables (such as task performance), and to investigate connectivity between GM and WM or alternatively to attempt to reconstruct specific WM pathways [4]. Recently, dMRI studies have made major contributions in a variety of sub-disciplines within the field of movement disorders, with wide-ranging implications for pathophysiology, differential diagnosis, surgical treatment, and the search for possible biomarkers of disease.
In this review, we discuss the evolving role of dMRI in the study of movement disorders, and we emphasize the most important publications in the field within the past year. As our scope is focused across all movement disorders, this work is not intended to be exhaustive (the reader is directed to disease-specific reviews of dMRI for such purposes). We do however hope to present the state of the science regarding the role of dMRI in movement disorders research, and we will discuss dMRI in the context and framework of prior research within each field.
Diffusion MRI in Parkinsonism
The study of Parkinson’s disease (PD) and parkinsonism has evolved into a multi-faceted field that has many different avenues of approach, and many of these areas of interest are actively making use of dMRI technology to answer research questions. Table 1 reviews the manner in which dMRI has been used in the past year to study parkinsonism. In addition, we recommend a recent systematic review and meta-analysis [7] that includes many studies that predate the studies described.
Table 1.
Summary of recent studies in parkinsonism using dMRI (2012–13)
| Authors | Hypothesis/Study Intent/Diseases of Interest | dMRI Methods* | N | Regions Studied with dMRI | Select dMRI Findings |
|---|---|---|---|---|---|
| dMRI in Parkinson’s disease (PD) | |||||
| Zhan et al. 2012 [20] | dMRI as a biomarker in PD; correlations with severity & subtype | ROI, TBSS | 12 PD 20 NC |
Whole brain WM; ROI of cortical white matter, IC, EC, SN, PUT, AN. | TBSS- ↓ FA in PD in pre/post central gyri, posterior striatum, frontal WM & projections to SMA, IC, EC, PUT, TH, SN; ROI – confirmed most VBA differences in FA, no differences in MD after correction; SN FA negatively correlated with ↑ UPDRS; spatial correlations b/w sub-regions demonstrated. |
| Prakash et al. 2012 [84] | Investigated asymmetry of SN FA in early PD | ROI | 11 PD 12 NC |
Caudal, middle, & rostral SN | No difference in FA & MD b/w PD & NC; asymmetric FA & MD in the rostral SN in both PD & NC; UPDRS positively correlated with FA in L rostral SN. |
| Planetta et al. 2013 [85] | Analysis of specific thalamic tracts in early PD | ROI | 20 PD 20 NC |
6 sub-regions in the TH | ↓ FA in WM tracks from the AN, VA, DM; UPDRS negatively correlated with FA in the AN. |
| dMRI in genetic PD | |||||
| Agosta et al. 2013 [86] | Compared dMRI measures in PD & GBA mutation carriers with PD | TBSS, ROI (VBM) | 14 PD 15 GBA 16 HC |
Whole brain WM, ROI | No differences in voxel-wise dMRI between GBA-PD & PD; post-hoc ROI showed ↓ FA in CC, olfactory tracts, cingulum, ECs, L anterior IC; FA in the CC, EC, & olfactory tracts correlated with verbal fluency in patients. |
| dMRI in PD - Combining neuroimaging methods | |||||
| Du et al. 2012 [17] | Combined dMRI/R2* study of the SN | ROI | 40 PD 28 NC |
6 rostral>caudal SN sub-regions (3 rostral & 3 caudal) | ↓ FA in PD SN caudal (early) > rostral (late); ↑ RD in caudal SN in PD; ↑ R2* in rostral & caudal SN in PD; no correlations b/w FA & UPDRS or disease duration; caudal R2* correlated with UPDRS, disease duration, & LEDD. |
| Sharman et al. 2013 [87] | Combined dMRI/resting state fMRI | ROI, tractography | 36 PD 45 NC |
3 cortical & 5 sub-cortical; tractography from these connections. | ↓ probability of connectivity in PD in SMC-PUT, SMC-TH, GP-TH, SN-TH; ↓ functional connectivity in GP-PUT, GP-TH, SMC-TH, SN-GP; ↑ functional connectivity in associative-PUT, limbic-TH, PUT-TH. No correlation with UPDRS, disease duration, or H&Y. dMRI & fMRI decreased connectivity overlapped in PD in TH to SMC, GP, & SN. |
| dMRI in PD – non-motor and specific symptoms | |||||
| Carlesimo et al. 2012 [88] | Multi-modal (dMRI/VBM) examining cognitive performance & MRI | ROI & VBA (VBM) | 25 PD 25 HC |
Hippocampus MD | ↑ MD in PD in the hippocampus; ↑ hippocampal MD associated with reduced memory performance in PD patients. |
| Rae et al. 2012 [89] | Compared different two methodologies in the study of cognitive measures | TBSS, VBA | 14 PD 15 HC |
Whole brain WM | TBSS - ↓ FA & ↑ MD throughout cerebral WM; ↓ FA in R splenium of CC correlated with UPDRS score; ↓ FA & ↑ MD in anterior CC & prefrontal WM correlated with cognitive scores. VBA – similar WM pattern differences only at more liberal significance threshold. |
| Deng et al. 2013 [90] | dMRI analysis of WM abnormalities & cognitive impairment in PD, PD-MCI, PD-D | ROI | 64 PD 21 NC |
Cortical WM, cingulate bundles, CC, midbrain CST, IC CST, SLF | ↓ FA in L frontal, R temporal, bilateral anterior cingulate bundles in PD-MCI & PD-D vs. NC; ↓ FA in the L occipital & L anterior cingulate bundle in cognitively normal PD vs. NC; ↓ FA in the L anterior cingulate bundle in PD-D vs. other groups & in the L occipital WM vs. cognitively normal PD; Some WM abnormalities correlated with cognitive dysfunction; no differences in FA in the CST in any group vs. NC. |
| Surdhar et al. 2012 [91] | Examined dMRI measures in PD +/− depression & NC | Tractography (volumetric) | 6 PD 6 PD-dep 6NC |
CC & bilateral uncinate fasciculus | No dMRI differences b/w groups (smaller amygdala volumes in PD-dep vs. NC). |
| Zheng et al. 2013 [21] | Evaluated retrospectively the relationship b/w dMRI measures & cognitive performance | ROI, VBA | 16 PD | 40 ROI encompassing most of cortical & subcortical WM | Cognitive performance in distinct domains correlated with dMRI in different areas; dMRI measures correlated with: executive function & language mostly in the frontal WM tracts; attention diffusely in WM; memory in fornix & anterior cingulate; no correlation was found b/w dMRI measures & visuospatial performance. Post-hoc VBA confirmed most correlations. |
| Gallagher et al. 2013 [92] | Investigated relationship b/w dMRI & cognition (especially executive function) | TBSS | 15 PD 15 NC |
Frontal subcortical WM | ↓ FA in PD vs. NC in portions of the anterior limb of IC & anterior CR, body of CC, inferior ILF & inferior fronto-occipital fasciculus, uncinate fasciculus, deep cerebellar WM. ↑ MD in PD patients present in portions of most tracts. FA correlated with executive function in PD but not NC. Both ↓ FA & ↑ MD correlated with increased susceptibility to interference in Stroop task. |
| Kamagata et al. 2013 [93] | Examined the relationship b/w dMRI measures & cognitive performance | TBSS/tractography | 20 PD 20 PD-D 20 NC |
Whole brain WM/genu of CC | No difference b/w PD & NC in FA & MD; ↓ FA & ↑ MD in major WM tracts of PD-D vs. NC; ↓ FA & ↑ MD in WM adjacent to prefrontal area & genu of CC in PD-D vs. PD; FA correlated with MMSE in combined but not separate PD groups; no correlation b/w dMRI & disease duration, H&Y, or levodopa dose. |
| Ford et al. 2013 [94] | Evaluated dMRI measures in PD +/− RBD by questionnaire | TBSS (VBM) | 124 PD | Whole brain WM | No difference in FA or MD when adjusted for multiple comparisons based on presence of RBD |
| dMRI in the differential diagnosis of parkinsonian syndromes | |||||
| Tsukamoto et al. 2012 [95] | MSA-C&P, PSP, PD, NC (retrospective) | ROI | 5 MSA-P 20 MSA-C 20 PSP 17 PD 18 NC |
CN, TH, PUT, GP, midbrain, pons, SCP, MCP, DEN, cerebellar WM | In MSA: ↑ MD in the pons, MCP, cerebellar WM, & DEN vs. to PSP, PD, NC; ↑ MD in the posterior PUT vs. PSP, NC. MD in MSA-P > MSA-C in PUT, GP, CN; MD in MSA-C > MSA-P in pons, MCP, cerebellar WM. In PSP: ↑ MD in GP & midbrain vs. MSA, PD, NC; ↑ MD in CN & SCP vs. MSA & NC. In PD: no difference in any region vs. NC |
| Agosta et al. 2012 [96] | PSP-RS, PSP-P, NC (retrospective) | TBSS, ROI, (VBM) | 21 PSP-RS 16 PSP-P 42 NC |
Whole brain WM (TBSS), infra- & supra-tentorial WM, TH (ROI) | No corrected difference b/w PSP-P & PSP-RS. |
| Haller et al. 2012 [97] | PD & AP (Retrospective) | TBSS | 17 PD 23 AP |
Whole brain WM | ↑FA & ↓RD & MD in multiple brain areas (especially R frontal WM) on TBSS; correctly classified PD & AP up to 97%. |
| Prodoehl et al. [19] | PD, MSA-P, PSP, ET, NC | ROI | 15 PD 14 MSA-P 12 PSP 14 ET 17 NC |
CN, PUT, GP, SN, RN, SCP, MCP, ICP, DN | AUC & sensitivity/specificity in distinguishing: PD vs. AP - 0.99 (sensitivity = 90%; specificity = 100%); PD vs. ET - 0.96 (sensitivity = 92%; specificity = 87%); PD vs. MSA-P – 0.99 (sensitivity = 94%; specificity = 100%); PD vs. PSP - 0.96 (sensitivity = 87%; specificity = 100%); PSP vs. MSA-P - 0.97 (sensitivity = 90%; specificity = 100%) |
| Nair et al. 2013 [98] | Decision tree analysis using conventional/volumetric & dMRI in PD & MSA | ROI, (volumetric) | 26 PD 13 MSA |
PUT, SN, pons, MCP, CBM | Significant dMRI measures (included in decision tree): rostral SN compacta FA, MCP FA, cerebellar FA, MCP MD. Decision tree diagnostic accuracy: Sensitivity 92 %; specificity 96 %; PPV 0.92; NPV 0.96. |
| dMRI in Progressive supranuclear palsy (PSP) | |||||
| Whitwell et al. 2012 [99] | Examined the association b/w clinical scale & MRI | ROI, (linear & volumetric) | 22 PSP | SCP | SCP FA correlated with clinical scores; No correlations between rates of change in dMRI measures & clinical scores |
| Saini et al. 2012 [100] | Evaluated dMRI measures in PSP-RS & PSP-P | TBSS (VBM) | 13 PSP-RS 11 PSP-P 26 NC |
Whole brain WM | ↓ FA, ↑ MD, ↑ AD, & ↑ RD in various WM areas in PSP vs. NC; ↓ FA in bilateral frontal WM & CC in PSP-RS vs. PSP-P; ↓ AD in R frontal region in PSP-RS vs. PSP-P. No correlation between dMRI & UPDRS. |
| dMRI in Multiple system atrophy (MSA) | |||||
| Lu et al. 2013 [101] | Combined Network analysis & dMRI in MSA-C | Tractography | 19 MSA-C 19 NC |
Whole brain fiber tracking | Small-world architecture/network strength/efficiency (more pronounced in cerebellar vs. cerebral network) was reduced in WM networks of MSA-C vs. controls, with abnormalities correlating with clinical scores. |
| dMRI in other parkinsonian syndromes | |||||
| Wang et al. 2012 [102] | Investigated dMRI measures in VP | Global analysis, VBA, tractography | 12 VP 12 NC |
Whole brain WM | Global ↓ FA & ↑ MD in VP vs. NC; ↓ FA on VBA in L TH, R frontal subcortical WM, L anterior IC vs. NC; ↑ MD on VBA in frontal subcortical WM in VP vs. NC; FA & MD in regions above correlated with PIGD clinical scores in VP patients; FA in fiber tracts in anterior IC negatively correlated with PIGD clinical scores; MD in fiber tracts in anterior CC correlated with PIGD clinical scores. |
( ) indicate non-dMRI methods.
AD = axial diffusivity; AL = ansa lenticularis; AN = anterior nucleus; AP = atypical parkinsonism; BG = basal ganglia; b/w = between; CBT = corticobulbar tract; CC = corpus callosum; CMB = cerebellum; CN = caudate nucleus; CR = corona radiata; CST = corticospinal tract; DaT = brain 123I ioflupane SPECT (DaTSCAN); DM = dorsomedial nucleus; DEN = cerebellar dentate nucleus; Dys = dystonia; EC= external capsule; ET = essential tremor; FA = fractional anisotropy; FCMTE = familial cortical myoclonic tremor with epilepsy; fMRI = functional magnetic resonance imaging; GP = globus pallidus; HARDI = High-angular resolution diffusion-weighted imaging; HD = Huntington’s disease; H&Y = Hohn & Yahr scale score; IC= internal capsule; ICP = inferior cerebellar peduncle; ILF = inferior longitudinal fasciculus; L = left; LEDD = levodopa-equivalent daily dose; MCP = Middle cerebellar peduncle; MD = mean diffusivity (ADC); M-D = myoclonus dystonia; MIBG = 123I-metaiodobenzylguanidine; MMSE = Mini-mental status exam; MRPI = magnetic resonance parkinsonism index; MSA = Multiple system atrophy; MSA-C = Multiple system atrophy - cerebellar subtype; MSA-P = Multiple system atrophy - parkinsonism subtype; NC = normal control; PC-HD – pre-clinical Huntington’s disease; PD-D = Parkinson’s disease with dementia; PD-dep = Parkinson’s disease with depression; PD-MCI = Parkinson’s disease with mild cognitive impairment; PIGD = modified postural instability gait difficulty subscore of UPDRS; PPN = pedunculopontine nucleus; PSP = Progressive supranuclear palsy; PSP-P = Progressive supranuclear palsy - parkinsonism subtype; PSP-RS = Progressive supranuclear palsy - Richardson syndrome subtype; PU = pulvinar; PUT= putamen; Q-BI = Q-ball imaging; Quin = rats infused with quinolinic acid; R = right; R2* = 1/T2* relaxation rate; RBD = REM sleep behavior disorder; RD = radial diffusivity; RN = red nucleus; ROI = region of interest; SCP = Superior cerebellar peduncle; SLF = superior longitudinal fasciculus; SMC = sensorimotor cortex; SN= substantial nigra; TBSS = tract-based spatial statistics; TH = Thalamus; UPDRS = Unified Parkinson’s Disease Rating Scale; VA = ventral anterior nucleus; VBA = voxel-based analysis; VBM = voxel-based morphometry; VL = ventral lateral nucleus; VP = vascular parkinsonism; VPL = ventral posterior lateral nucleus; VPM = ventral posterior medial nucleus; WM= white matter.
One of the most important and exciting aspects of PD research has been the quest for a biomarker of disease that can facilitate diagnosis, allow for objective monitoring of disease progression, and evaluate the efficacy of potential therapeutic and neuroprotective therapies [8]. dMRI is among those modalities being investigated in this regard. Many different GM and WM structures have been investigated using dMRI, and abnormalities in a variety of areas have been reported [7]. However, given what we know about the pathogenesis of PD, it is not surprising that the substantia nigra (SN) has been the most commonly studied area. While not all studies have found a reduction in FA in the SN [9, 10], a recent meta-analysis [7] found a significant pooled effect size for reduction of FA in the SN across studies. One study achieved 100% specificity and sensitivity in distinguishing early medication naïve patients from controls [11], with a caudal ventrolateral > rostral dorsomedial pattern of FA decrease in PD consistent with earlier immunohistochemistry studies. A recent follow-up study [12] demonstrated that, in normal aging, FA decreased and RD increased in the dorsal SN but not in the ventral SN, again consistent with earlier histopathological studies. These studies further the suggestion put forth by animal models that dMRI may act as a proxy for dopaminergic degeneration in the SN [13]. They also highlight the critical importance of where ROI’s are defined in obtaining meaningful results [12] as well as the possible potential of dMRI of the SN as a biomarker for PD [14].
dMRI is also being investigated in combination with other neuroimaging modalities. dMRI measures have been combined with inverse T2* (R2*) [15], another MRI measure that has been shown to be increased in PD patients and is thought to correlate with iron concentration, to see if the combined measures improve separation between PD and controls [16]. Mean FA was reduced and R2* increased in the SN of patients compared to controls, with improved discrimination between groups when the modalities were combined. Further, there was no correlation between the two measures, which the authors suggested could indicate that these measures could reflect independent ongoing pathological processes in the SN. A follow-up study from the same group published this year [17] expanded these earlier findings and showed that the decrease in FA in the SN in PD was significant early in the caudal region of the SN, while in the rostral SN the decrease in FA was only significant in late stages of the disease. R2* in the caudal SN also correlated with clinical scores, disease duration, and levodopa dosage.
Although the most common cause of Parkinsonism is PD, the syndromes of multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration, as well as a variety of other disorders can also result in parkinsonism, and the clinical differentiation between these diseases can sometimes be difficult [18]. In addition to detecting PD, the ability of dMRI to differentiate PD from atypical parkinsonism and the atypicals from each other is also being investigated. A recent study [19] used a multi-target approach based on prior dMRI studies and areas of the brain that are known to be affected by specific diseases in a population of PD, MSA-p (parkinsonism subtype), PSP, essential tremor (ET), and healthy controls. Receiver operating characteristic (ROC) analyses demonstrated an area under the curve (AUC) 0.99 (sensitivity = 90%; specificity = 100%) in distinguishing PD from atypical parkinsonism. AUC’s of 0.99, 0.96, and 0.97 were achieved for distinguishing PD vs. MSAp, PD vs. PSP, and MSA-p vs. PSP respectively, with unique dMRI measures and subcortical ROI’s for each group. The study also found excellent separation between PD and ET. Given the recent finding of increased FA in somatosensory cortex in PD [20], future analyses that include a cortical ROI may further improve classification.
Summary
dMRI in the SN and in other areas of the brain represents a promising potential biomarker for PD and other forms of parkinsonism. It also may provide a powerful method to distinguish PD from atypical parkinsonism, the atypicals from each other, and can possibly answer questions, such as the temporal order of structural abnormalities that occur in parkinsonism, that cannot be answered by neuropathological studies. Diffusion related measures have been correlated with motor dysfunction and cognitive performance in domains such as executive function, language, and attention. [21] dMRI can be useful to evaluate genetic forms of disease, to study specific symptoms, and to compliment other imaging and non-imaging modalities to better understand underlying pathophysiology and network-level dysfunction.
Diffusion MRI in Other Movement Disorders
As in parkinsonism, dMRI has been utilized to investigate the structural and network underpinnings of other movement disorders. Table 2 describes the papers published in the last year involving dMRI in the study of dystonia, Huntington’s disease (HD), and ET. Additionally, the use of dMRI techniques in movement disorders surgery is also described.
Table 2.
Summary of recent studies in movement disorders other than parkinsonism using dMRI (2012–13)
| Authors | Hypothesis/Study Intent | dMRI Methods* | N | Regions Studied with dMRI | Select dMRI Findings |
|---|---|---|---|---|---|
| dMRI in Dystonia | |||||
| Horovitz et al., 2012 [103] | Combined VBM/dMRI study of blepharospasm | TBSS (VBM) | 14 Dys 14 NC |
Whole brain, CBT | No significant difference in dMRI b/w groups. |
| van der Meer et al., 2012 [104] | dMRI study of myoclonus-dystonia (M-D) | ROI (WM VBM) | 16 M-D 18 NC |
Cortical, BG, brainstem, CBM | ↑ FA in the R subthalamic brainstem & thalamocortical WM of M-D vs. NC; ↓ MD in the WM underlying the SMC & near the subthalamic areas & R thalamus in M-D vs. NC. |
| Blood et al., 2012 [6] | Investigated dMRI measures in DYT1-negative cervical dystonia patients | Tractography | 12 Dys 12 NC |
bilateral pallidum & AL for tractography | ↓ FA in the WM near the L SCP & ↑ FA near & in the L SN; reduced probability of connectivity in Dys vs. NC in the L AL projections to the ipsilateral brainstem; increased probability of connectivity in Dys vs. NC in connections b/w the pallidum & brainstem |
| Cheng et al., 2012 [105] | Evaluated dMRI measures in DYT6 dystonia & subcellular distribution of THAP1 protein | ROI | 6 Dys 6 NC |
SMC, SLF, cingulate, CC, CTS, IC, SCP, MCP, cerebellar WM | ↓ FA in the SMC area in Dys vs. NC; ↑ MD in the R SLF & R supracapsular CST. |
| dMRI in Huntington’s disease | |||||
| Delmaire et al., 2012 [62] | Evaluated relationship b/w dMRI measures & clinical measures in early HD patients from TRACK-HD study | VBA | 27 HD 24 NC |
SVC – BG, IC, EC, centrum ovale, cingulate bundle, SLF, & CC | Detailed list of motor & cognitive tasks were correlated with abnormal dMRI (increased MD & decreased FA) measures in multiple distinct areas of gray & WM in the brain. |
| Di Paola et al., 2012 [106] | dMRI of corpus callosum in preclinical & early HD | TBSS | 17 PC-HD 17 HD 17 NC |
WM of CC | ↓ FA & ↑ AD in the isthmus of CC in PC-HD vs. NC; ↓ FA, ↑ AD, ↑ RD in HD vs. NC; ↓ FA, ↑ AD, ↑ RD in CC of HD vs. PC-HD. |
| Van Camp et al., 2012 [58] | Combined histopathology & dMRI study of quinolinic-acid rat model of HD | VBA, ROI | 9 Quin 6 Sham 5 NC |
IC, EC, 3 sub- region of CN/PUT | dMRI discriminated Quin rats +/− cortical lesions; dMRI was more sensitive than histology in detecting microstructural changes in the caudate, putamen, & IC/EC in cortically-lesioned rats. |
| Dumas et al., 2012 [53] | Investigated dMRI in HD & PC-HD as part of TRACK-HD study | ROI, tractography | 16 HD 27 PC-HD 28 NC |
CN, TH, CC, WM pathways. | ↑ MD in the CC & WM fibers of the SMC in PC-HD vs. NC; no differences in FA b/w PC-MD & NC; ↑ MD in CC, CN, & WM tracts of CC, TH, SMC, & prefrontal regions in HD vs. NC; ↓ FA in the CC & WM tracts of the CC & motor & prefrontal cortices; MD in multiple regions correlated with motor/cognitive tasks, as well as probability of onset & burden of pathology. |
| Georgiou-Karistianis et al., 2013 [40] | Quadratic discriminant analysis study using combined dMRI & volumetric data in PC-HD & HD | ROI | 33 HD 35 PC-HD 36 NC |
CN, PUT, pallidum, nucleus accumbens, TH | Group differences in: FA in CN, PUT, & R pallidum & accumbens; MD in CN, PUT, pallidum, & accumbens; the highest level of discriminative accuracy (78%) was attained when clinical motor scores were added to dMRI & volumetric measures, with MD the most accurate measure. |
| Matsui et al., 2013 [63] | Examined dMRI measures in sub-regions of the prefrontal cortex in relation to measures of disease burden in PC-HD | ROI | 53 PC-HD 34 NC |
Prefrontal cortex | Differences were found for RD & MD, but not FA or AD, in inferior & lateral sub-regions of the prefrontal cortex b/w groups. Within pre-symptomatic patients, abnormalities largely followed disease burden as defined by the age-CAG length calculation. |
| Marrakchi-Kacem et al., 2013 [64] | Investigated cortico-striatal connectivity using dMRI | TBSS, HARDI, Q-BI, | 15 HD 15 NC |
Cortico-striatal connections | Reduced inferred connectivity from CN to parietal & frontal lobes, & from the putamen to the associative temporal, dorsal & ventral frontal, parietal, & SMC cortices; % difference b/w groups in connectivity b/w areas of the striatum & cortex reported; relative preservation of limbic connections; reduced inferred connectivity in primary sensory vs. motor connections. |
| dMRI in essential tremor | |||||
| Saini et al., 2012 [73] | Studied dMRI measures of WM in ET patients | TBSS, ROI | 22 ET 17 NC |
ROI – CC, SCP, MCP, ICP, CST, anterior limb of IC | TBSS - ↑ MD in the R hemispheric WM & ↑ AD throughout the bilateral cortical WM, IC, EC, TH, brainstem, CBM; no differences in FA b/w ET patients & NC. ROI analysis - ↓ FA in the L SCP & R CST & ↑ MD in the R anterior limb of the IC & L CST; ↑ AD in the bilateral SCP & R ICP, CST, & anterior limb of IC. No correlations were found b/w tremor severity or disease duration & dMRI measures. |
| Buijink et al., 2013 [72] | Studied dMRI measures in FCMTE compared to ET & NC | Tractography, ROI | 7 FCMTE 8 ET 5 NC |
CBM | Mean FA was decreased in FCMTE vs. ET & NC, but FA was not different b/w ET patients & NC. |
( ) indicate non-dMRI methods. See table 1 for explanations of abbreviations.
Dystonia
Though traditionally considered a disease of the BG, the pathophysiology of dystonia is now thought to involve multiple levels of the neuraxis, with a loss of motor inhibition as well as disordered sensory processing, neuroplasticity, and somatotopic organization [18]. Neuroimaging studies have been crucial in the development of a broader network model of dystonia pathophysiology that includes the multiple brain regions that are likely involved in its development. Structural abnormalities giving rise to secondary dystonia have been demonstrated throughout the brain, and the results of nuclear imaging studies, fMRI findings of brain activation patterns, and voxel-based morphometry studies of regional GM volumes have varied based on the type of dystonia studied (for extensive review including dMRI studies see Neychev et al. 2011 [22] and Zoons et al. [23]).
dMRI has been useful in evaluating WM connectivity and integrity in both hereditary and idiopathic forms of dystonia. In young onset hereditary dystonia, the evolving notion of the disease as a neurodevelopmental disorder involving pathways of the BG, cortex, and cerebellum has been heavily influenced by dMRI studies [24]. The earliest dMRI study in DYT1 carriers (manifesting and non-manifesting) found decreases in FA in the WM underlying the sensorimotor cortex compared to age matched controls [25]. Subsequent studies [26] in DYT1 and DYT6 patients confirmed this finding and expanded reduced FA findings to the dorsal pontine brainstem. One of the most promising studies described to date [27] used probabilistic tractography to show reduced probability of connectivity in the proximal cerebello-thalamic pathway near the dentate nucleus in mutation carriers, with penetrance regulated by an additional connectivity abnormality in the sub-rolandic WM of the thalamocortical projections. The authors hypothesized that reduced penetrance in asymptomatic gene carriers may be due to a protective effect of the thalamocortical pathway disruption in altering the effect of the more caudal abnormality. This report has been further strengthened by abnormalities found in thalamocortical and cerebello-cortical pathways in torsinA DYT1 knock-in mice [28]. In addition to genetic dystonia, abnormalities in dMRI measures in focal dystonias such as torticollis [29–32], writer’s cramp [33], and spasmodic dysphonia [34] have also been described in the WM connections of the pathways of the BG, cortex, and cerebellum, suggesting an important role of these areas in gene-negative primary dystonias.
In addition to interest in the role of cerebello-thalamo-cortical connections in hereditary dystonia described above [27], recent interest has also been focused on collateralized pallidal connections to the thalamus and brainstem and hemispheric differences in dMRI findings in gene-negative primary focal dystonias [35, 6, 36]. In a 2006 study, Blood and colleagues compared patients with primary focal dystonia and healthy controls and demonstrated increased hemispheric asymmetry in FA in the WM fibers between the pallidum/putamen and the thalamus. Further, this asymmetry was no longer different from controls after botulinum toxin injections [36]. The authors hypothesized [35] that these differences might represent abnormal functioning of a distributed (and possibly lateralized) postural control system that could result in dystonia. Recently, the authors utilized dMRI measures (FA and MD) and probabilistic tractography to further investigate pallidal connections to the brainstem in 12 DYT1 negative patients with cervical dystonia and 12 healthy matched controls [6]. Focusing on the bilateral pallidum and ansa lenticularis (AL) as their seed ROI, they found reduced FA in the WM near the left SCP and increased FA near the left SN. A reduced probability of connectivity was shown in the left AL projections to the ipsilateral brainstem in dystonia patients compared to controls, with the greatest difference demonstrated in the area between the AL and the region of the red nucleus and SN. In the right hemisphere, increased probability of connectivity was found in the connections between the pallidum and brainstem.
Summary
dMRI (and especially tractography) has made major contributions to our current thinking regarding the pathophysiology of dystonia, and helped to expand our focus beyond the BG to include related connections to and between the brainstem, cerebellum, and cortex. These studies have proposed models to explain reduced penetrance, directed attention to possible hemispheric differences in the disease, and fueled hypotheses incorporating dysfunction of postural control in models of dystonia. Examination of the similarities and differences between dMRI abnormalities in the various forms of dystonia may shed light on a shared pathophysiology as well as how dystonia can be focal in presentation [23]. dMRI studies in patients treated with botulinum toxin might elucidate the manner in which the toxin exerts a central effect though motor afferent feedback, and may suggest new treatment modalities. Expanding dMRI analyses to include dystonia-plus syndromes and secondary dystonias may further elucidate characteristics that are shared and that differentiate the various dystonia subtypes.
Huntington’s Disease
Huntington’s disease (HD) is a neurodegenerative disorder that produces progressive degeneration and volume loss of the striatum and other GM and WM structures in a process that begins well before the onset of clinical symptoms. The symptoms of HD are progressive and include motor dysfunction, cognitive decline, and neuropsychiatric disturbances [18].
As would be expected based on neuropathological data, conventional and volumetric brain imaging have shown reductions in striatal and putaminal volume in symptomatic and pre-symptomatic HD, as well as cortical GM loss and whole brain atrophy in some studies [37]. These findings of volume loss in the striatum have been confirmed in large multicenter studies such as PREDICT-HD [38] and TRACK-HD [39], and caudate volume abnormalities have been correlated with cognitive function, repeat length, and age of manifestation of clinical symptoms [40]. However, not all T1-weighted structural imaging studies have yielded consistent results [41].
A number of studies have investigated diffusion characteristics in symptomatic and pre-clinical HD [42–51], demonstrating microstructural changes in multiple areas of the brain (for a comprehensive review see Esmaeilzadeh et al. [52]). Some caution has however been raised that the changes in FA reported in some of these studies might be due to misregistration of images due to neurodegenerative changes [41], and dMRI findings have also not been consistent across all studies [53]. Among the studies that have looked at dMRI measures longitudinally, some studies [54] have found worsening abnormalities in dMRI measures over time, while some have not [55]. Many of the dMRI scalar abnormalities have been shown to correlate with clinical features [55, 54, 56]. Unified Huntington’s Disease Rating Scale (UHDRS) score has been shown to correlate with MD in the corpus callosum in an area demonstrated by tractography to connect to pre- and supplementary motor areas, and radial diffusivity in areas of the corpus callosum projecting to the prefrontal cortices has also been demonstrated to correlate with cognition [51]. In tractography studies of pre-symptomatic HD patients, a reduction of streamlines directed to the caudate was demonstrated, with the degree of impairment of voluntary saccades correlated with fewer fiber tracking streamlines between the caudate and frontal cortex [57].
The studies published within the past year using dMRI techniques to study HD have spanned across human and animal models of the disease, and have included established as well as new dMRI techniques in a variety of different areas of the brain. The first dMRI study [58] of quinolinic-acid induced excitotoxicity, a commonly used lesioning model of HD in rats [59], found that dMRI discriminated between rats that developed cortical lesions from those that did not secondary to lesioning. In addition, dMRI measures were more sensitive than histology in detecting microstructural changes in the caudate, putamen, and internal and external capsules, complementing data from the same group [60] that presented the first dMRI data in a transgenic mouse model of HD.
One study [40] combined motor and cognitive measures with dMRI and volumetric analysis in patients with and without symptoms and healthy controls and found the highest level of discriminative accuracy (78%) was attained when motor and cognitive scores were added to neuroimaging measures. This level of accuracy was slightly higher than that (76%) found using machine learning approaches and voxel-based volumetric analysis [61]. Another study [62] found that motor and cognitive tasks were correlated with abnormal dMRI measures in multiple areas of GM and WM, suggesting that dysfunction in extra-striatal areas of the brain are related to clinical manifestations in a region-specific manner. Dumas and colleagues [53] found more widespread dMRI abnormalities in early manifesting compared to pre-manifesting HD, with correlations between dMRI and clinical measures. A study [63] that examined dMRI measures in the sub-regions of the prefrontal cortex in pre-symptomatic HD patients and controls found that dMRI abnormalities largely followed “disease burden” as defined by the age-CAG length calculation, and a recent tractography study [64] examined symptomatic HD patients and healthy controls and developed detailed maps and percentages of reduced inferred connectivity from the striatum to the cortex.
Summary
Diffusion MRI has the potential to contribute to the study of HD on a number of different fronts. Though genetic testing generally negates the need for a biomarker for diagnosis, a quantitative method of monitoring disease progression would be useful should more effective treatment options become available [65, 37]. It could potentially allow objective investigation into the relationship between specific clinical findings and microstructural changes and function as a means of predicting time to symptom onset and monitoring disease progression [40]. Combining dMRI measures with other neuroimaging measures, as well as clinical data, might be better than dMRI alone. As the sequence of pathogenic events that occur in HD remain poorly understood [66], specific measures of dMRI may be useful as in vivo representations of these processes and are being investigated as such [54]. In addition, dMRI studies in animal models of disease may also be useful in developing novel connectivity-based markers of HD-related pathological processes [66].
Essential tremor
Compared to other movement disorders, relatively few studies investigating essential tremor (ET) with dMRI have been published. The first report that used diffusion weighted imaging (DWI) to study patients with ET used an ROI approach and found no differences in MD values between ET patients and normal controls in the cerebellum, BG, and frontal WM [67]. Subsequent studies utilizing dMRI found reduced FA in areas of the cerebellum, pons, and the WM of the midbrain and cerebral cortex in a voxel-wise analysis [68] and reduced FA in the dentate nucleus and the superior cerebellar peduncle (SCP, along with increased MD), with no overlap in FA values between patients and controls in the dentate [69]. A 2011 study found an increase in MD in the red nucleus but no differences in FA amongst the BG, thalamus, red nucleus, and SN of ET patients compared to controls [70]. Another group the same year [71] showed increased MD in the bilateral inferior cerebellar peduncle (ICP), adjacent to the left parieto-occipital sulcus, and in bilateral frontal and parietal WM, with decreased FA in the right ICP and a correlation between tremor scores and MD values in some WM regions in patients with ET.
Two papers have used dMRI techniques in the study of ET within the past year [72, 73]. The first of these [73] used TBSS and ROI analysis of specific WM tracts in 22 patients with definite or probable ET and 17 normal matched controls. TBSS showed increased asymmetric MD changes and increased AD throughout the bilateral cortical WM, potentially suggesting axonal damage. No correlations were found between dMRI measures and tremor severity or disease duration. The most recent work [72] to analyze dMRI measures in ET did so as part of a study to evaluate cerebellar WM in familial cortical myoclonic tremor with epilepsy (FCMTE), showing that mean FA was significantly decreased in FCMTE patients compared to the other groups, but FA was not different between ET patients and controls.
Summary
The body of dMRI literature in ET is not as developed as in other movement disorders, which may be due to both a smaller study number and the nature of the disease. The syndrome of ET itself is likely more heterogeneous [74] and the abnormalities present in ET might be more subtle and more difficult to identify. Despite these challenges, a number of useful observations have been made to guide future research. Studies that focus on the cerebellum might be best approached with ROI analysis, as involvement of these regions are guided by strong hypotheses and data from the greater literature and thus may produce more robust results than voxel-wise comparisons [71]. Laterality of tremor and handedness may also be important factors and should be carefully considered in future studies [73], and certainly larger sample sizes are needed. While some reports have interpreted their findings as supporting the notion of ET as a neurodegenerative disease [68], it is important to consider that microstructural WM abnormalities suggested by dMRI do not differentiate between changes due to a neurodegenerative process and those that might occur secondary to ongoing abnormal oscillatory activity in a tremorogenic network. Further correlation with neuropathologic data might shed light on the underlying microstructural changes that the dMRI abnormalities represent in the ET brain.
Diffusion MRI in Movement Disorders Surgery
The ability to represent structural connectivity in vivo makes dMRI an ideal adjunctive tool for deep brain stimulation (DBS) surgery for movement disorders, and dMRI will likely be increasingly used in the planning of DBS surgeries in the future [5]. As DBS technology advances, the ability to target specific sub-regions of GM in a given individual for stimulation will be important in optimizing clinical benefit and minimizing side effects [75, 76]. dMRI is already being used to investigate inter-individual variability in DBS targeting [77] in patient-specific partitioning of thalamic areas based on probability of connectivity with motor cortices [78], a concept that has been validated [79] and for which refinement of methodologies is continuously being sought [80]. Further, as WM tracts may sometimes be more ideal targets for neurostimulation than GM structures, dMRI may allow direct targeting of WM pathways [81]. Tractography studies guided by effective DBS placement can be used as a starting point to elucidate network connectivity such as in tremor [82].
Conclusions
DTI has evolved into an invaluable tool in the study of movement disorders, and is used in studies of disease states, connectivity between brain regions, and brain development [3]. In a variety of disorders, dMRI is showing promise as a biomarker of disease, which is encouraging since MRI is non-invasive, widely available, and generates reproducible data that can be analyzed offline if required [83]. However, in the absence of standardized techniques, variability in methods of acquisition, image processing, and analysis can affect the reproducibility of findings. Sensitivity and specificity of potential dMRI biomarkers may vary throughout the course of the disease [43], and the future role of DTI as a biomarker could be as part of a battery of tests. Future studies will help to determine the degree to which dMRI measures can serve as a proxy for disease presence and progression in movement disorders.
Acknowledgments
Christopher W. Hess has received fellowship funding from the Parkinson’s Disease Foundation. Michael S. Okun has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, TSA, DMRF, and the UF Foundation. David E. Vaillancourt has received grant support from NIH, Michael J. Fox, Bachmann-Strauss Foundation, and Tyler’s Hope.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Christopher W. Hess declares that he has no conflict of interest.
Edward Ofori declares that he has no conflict of interest.
Umer Akbar declares that he has no conflict of interest.
Michael S. Okun serves as a consultant for the National Parkinson Foundation. He has received royalties for publications with Demos, Manson, Amazon, and Cambridge (movement disorders books). He has participated in CME activities on movement disorders sponsored by the USF CME office, PeerView, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. He has participated as a site PI and/or co-I for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria.
David E. Vaillancourt consults for projects at UT Southwestern Medical Center, University of Illinois, and Great Lakes NeuroTechnologies.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, et al. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain: a journal of neurology. 2006;129(Pt 2):538–42. doi: 10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- 2.Stoessl AJ, Brooks DJ, Eidelberg D. Milestones in neuroimaging. Movement disorders: official journal of the Movement Disorder Society. 2011;26(6):868–978. doi: 10.1002/mds.23679. [DOI] [PubMed] [Google Scholar]
- 3.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–39. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 5.Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Frontiers in neuroscience. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blood AJ, Kuster JK, Woodman SC, Kirlic N, Makhlouf ML, Multhaupt-Buell TJ, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PloS one. 2012;7(2):e31654. doi: 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80(9):857–64. doi: 10.1212/WNL.0b013e318284070c. This systematic review and meta-analysis describes many of the important dMRI studies that predated those described in Table 1 and describes a significant effect size for decreased FA in the SN in PD patients compared to controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherer TB. Biomarkers for Parkinson’s disease. Science translational medicine. 2011;3(79):79ps14. doi: 10.1126/scitranslmed.3002488. [DOI] [PubMed] [Google Scholar]
- 9.Menke RA, Scholz J, Miller KL, Deoni S, Jbabdi S, Matthews PM, et al. MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. NeuroImage. 2009;47(2):435–41. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M. Connectivity-based segmentation of the substantia Nigra in human and its implications in Parkinson’s disease. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.05.086. [DOI] [PubMed] [Google Scholar]
- 11.Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–84. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiology of aging. 2012;33(1):35–42. doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, et al. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiology of disease. 2007;26(3):590–6. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skorpil M, Soderlund V, Sundin A, Svenningsson P. MRI diffusion in Parkinson’s disease: Using the technique’s inherent directional information to study the olfactory bulb and substantia nigra. Journal of Parkinson’s disease. 2012;2(2):171–80. doi: 10.3233/JPD-2012-12091. [DOI] [PubMed] [Google Scholar]
- 15.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology. 2008;70(16 Pt 2):1411–7. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 16••.Du G, Lewis MM, Styner M, Shaffer ML, Sen S, Yang QX, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2011;26(9):1627–32. doi: 10.1002/mds.23643. This study combined dMRI with R2* imaging and showed abnormalities in both measures in the SN, with combined measures showing the greatest sensitivity and specificity in distinguishing patients from controls. Further, the two measures were not correlated, suggesting they might represent different pathological processes in PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du G, Lewis MM, Sen S, Wang J, Shaffer ML, Styner M, et al. Imaging nigral pathology and clinical progression in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2012;27(13):1636–43. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahn S, Jankovic J, Hallett M. Principles and practice of movement disorders. 2. Edinburgh; New York: Elsevier/Saunders; 2011. [Google Scholar]
- 19••.Prodoehl J, Li H, Planetta PJ, Goetz CG, Shannon KM, Tangonan R, et al. Diffusion Tensor Imaging of Parkinson’s Disease, Atypical Parkinsonism, and Essential Tremor. Movement disorders: official journal of the Movement Disorder Society. doi: 10.1002/mds.25491. In press This study used a multi-targeted approach using basal ganglia and cerebellar ROI to study the discriminative capability of dMRI in PD, MSA-p, PSP, ET, and healthy controls. Excellent differentiation was achieved between the differentiation of disease from each other and from controls, and the pattern of dMRI targets and measures was unique for each disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan W, Kang GA, Glass GA, Zhang Y, Shirley C, Millin R, et al. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Movement disorders: official journal of the Movement Disorder Society. 2012;27(1):90–7. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Human brain mapping. 2013 doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiology of disease. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. This review describes current thinking regarding the neuroanatomic substrates of dystonia, and provides as a critical appraisal of the evidence from pathology, imaging (including dMRI), and physiologic studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia--a review. NeuroImage. 2011;56(3):1011–20. doi: 10.1016/j.neuroimage.2011.02.045. This extensive review focuses on the role of neuroimaging specifically in focal dystonia, and includes descriptions of many of the important studies published prior to those listed in Table 2 in this review. [DOI] [PubMed] [Google Scholar]
- 24.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiology of disease. 2011;42(2):202–9. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Annals of neurology. 2004;56(2):283–6. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- 26.Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Movement disorders: official journal of the Movement Disorder Society. 2008;23(2):234–9. doi: 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(31):9740–7. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Ulug AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6638–43. doi: 10.1073/pnas.1016445108. This animal model of dystonia study is important because it demonstrated cerebellothalamic dMRI changes in mutant compared to controls that correlated with measures of cortical metabolic activity, somewhat similar to the findings of Argyelan et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. Journal of neurology, neurosurgery, and psychiatry. 2005;76(11):1591–3. doi: 10.1136/jnnp.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2008;15(2):185–9. doi: 10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- 31.Bonilha L, de Vries PM, Vincent DJ, Rorden C, Morgan PS, Hurd MW, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Movement disorders: official journal of the Movement Disorder Society. 2007;22(8):1110–6. doi: 10.1002/mds.21295. [DOI] [PubMed] [Google Scholar]
- 32.Bonilha L, de Vries PM, Hurd MW, Rorden C, Morgan PS, Besenski N, et al. Disrupted thalamic prefrontal pathways in patients with idiopathic dystonia. Parkinsonism & related disorders. 2009;15(1):64–7. doi: 10.1016/j.parkreldis.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Archives of neurology. 2009;66(4):502–8. doi: 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- 34.Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain: a journal of neurology. 2008;131(Pt 2):447–59. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blood AJ. New hypotheses about postural control support the notion that all dystonias are manifestations of excessive brain postural function. Bioscience hypotheses. 2008;1(1):14–25. doi: 10.1016/j.bihy.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blood AJ, Tuch DS, Makris N, Makhlouf ML, Sudarsky LR, Sharma N. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport. 2006;17(12):1251–5. doi: 10.1097/01.wnr.0000230500.03330.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgiou-Karistianis N, Scahill R, Tabrizi SJ, Squitieri F, Aylward E. Structural MRI in Huntington’s disease and recommendations for its potential use in clinical trials. Neuroscience and biobehavioral reviews. 2013;37(3):480–90. doi: 10.1016/j.neubiorev.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of neurology, neurosurgery, and psychiatry. 2008;79(8):874–80. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet neurology. 2012;11(1):42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- 40.Georgiou-Karistianis N, Gray MA, Dominguez DJ, Dymowski AR, Bohanna I, Johnston LA, et al. Automated differentiation of pre-diagnosis Huntington’s disease from healthy control individuals based on quadratic discriminant analysis of the basal ganglia: the IMAGE-HD study. Neurobiology of disease. 2013;51:82–92. doi: 10.1016/j.nbd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Kloppel S, Henley SM, Hobbs NZ, Wolf RC, Kassubek J, Tabrizi SJ, et al. Magnetic resonance imaging of Huntington’s disease: preparing for clinical trials. Neuroscience. 2009;164(1):205–19. doi: 10.1016/j.neuroscience.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60(10):1615–20. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 43.Mascalchi M, Lolli F, Della Nave R, Tessa C, Petralli R, Gavazzi C, et al. Huntington disease: volumetric, diffusion-weighted, and magnetization transfer MR imaging of brain. Radiology. 2004;232(3):867–73. doi: 10.1148/radiol.2322030820. [DOI] [PubMed] [Google Scholar]
- 44.Reading SA, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, et al. Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry research. 2005;140(1):55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Movement disorders: official journal of the Movement Disorder Society. 2006;21(9):1317–25. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 46.Seppi K, Schocke MF, Mair KJ, Esterhammer R, Weirich-Schwaiger H, Utermann B, et al. Diffusion-weighted imaging in Huntington’s disease. Movement disorders: official journal of the Movement Disorder Society. 2006;21(7):1043–7. doi: 10.1002/mds.20868. [DOI] [PubMed] [Google Scholar]
- 47.Magnotta VA, Kim J, Koscik T, Beglinger LJ, Espinso D, Langbehn D, et al. Diffusion Tensor Imaging in Preclinical Huntington’s Disease. Brain imaging and behavior. 2009;3(1):77–84. doi: 10.1007/s11682-008-9051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoffers D, Sheldon S, Kuperman JM, Goldstein J, Corey-Bloom J, Aron AR. Contrasting gray and white matter changes in preclinical Huntington disease: an MRI study. Neurology. 2010;74(15):1208–16. doi: 10.1212/WNL.0b013e3181d8c20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, et al. Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. NeuroImage. 2010;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller HP, Glauche V, Novak MJ, Nguyen-Thanh T, Unrath A, Lahiri N, et al. Stability of white matter changes related to Huntington’s disease in the presence of imaging noise: a DTI study. PLoS currents. 2011;3:RRN1232. doi: 10.1371/currents.RRN1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohanna I, Georgiou-Karistianis N, Sritharan A, Asadi H, Johnston L, Churchyard A, et al. Diffusion tensor imaging in Huntington’s disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain imaging and behavior. 2011;5(3):171–80. doi: 10.1007/s11682-011-9121-8. [DOI] [PubMed] [Google Scholar]
- 52•.Esmaeilzadeh M, Ciarmiello A, Squitieri F. Seeking brain biomarkers for preventive therapy in Huntington disease. CNS neuroscience & therapeutics. 2011;17(5):368–86. doi: 10.1111/j.1755-5949.2010.00157.x. This review highlights the neuroimaging literature (including dMRI) and details the findings of previous studies aimed at developing imaging-based potential boimarkers for HD, and includes an interesting figure highlighting potential biomarkers according to each period of disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumas EM, van den Bogaard SJ, Ruber ME, Reilman RR, Stout JC, Craufurd D, et al. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Human brain mapping. 2012;33(1):203–12. doi: 10.1002/hbm.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weaver KE, Richards TL, Liang O, Laurino MY, Samii A, Aylward EH. Longitudinal diffusion tensor imaging in Huntington’s Disease. Experimental neurology. 2009;216(2):525–9. doi: 10.1016/j.expneurol.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sritharan A, Egan GF, Johnston L, Horne M, Bradshaw JL, Bohanna I, et al. A longitudinal diffusion tensor imaging study in symptomatic Huntington’s disease. Journal of neurology, neurosurgery, and psychiatry. 2010;81(3):257–62. doi: 10.1136/jnnp.2007.142786. [DOI] [PubMed] [Google Scholar]
- 56.Della Nave R, Ginestroni A, Tessa C, Giannelli M, Piacentini S, Filippi M, et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR American journal of neuroradiology. 2010;31(9):1675–81. doi: 10.3174/ajnr.A2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain: a journal of neurology. 2008;131(Pt 1):196–204. doi: 10.1093/brain/awm275. [DOI] [PubMed] [Google Scholar]
- 58.Van Camp N, Blockx I, Camon L, de Vera N, Verhoye M, Veraart J, et al. A complementary diffusion tensor imaging (DTI)-histological study in a model of Huntington’s disease. Neurobiology of aging. 2012;33(5):945–59. doi: 10.1016/j.neurobiolaging.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. Journal of the neurological sciences. 2012;323(1–2):1–8. doi: 10.1016/j.jns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Blockx I, Van Camp N, Verhoye M, Boisgard R, Dubois A, Jego B, et al. Genotype specific age related changes in a transgenic rat model of Huntington’s disease. NeuroImage. 2011;58(4):1006–16. doi: 10.1016/j.neuroimage.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Rizk-Jackson A, Stoffers D, Sheldon S, Kuperman J, Dale A, Goldstein J, et al. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington’s disease using machine learning techniques. NeuroImage. 2011;56(2):788–96. doi: 10.1016/j.neuroimage.2010.04.273. [DOI] [PubMed] [Google Scholar]
- 62.Delmaire C, Dumas EM, Sharman MA, van den Bogaard SJ, Valabregue R, Jauffret C, et al. The structural correlates of functional deficits in early huntington’s disease. Human brain mapping. 2012 doi: 10.1002/hbm.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui JT, Vaidya JG, Johnson HJ, Magnotta VA, Long JD, Mills JA, et al. Diffusion weighted imaging of prefrontal cortex in prodromal huntington’s disease. Human brain mapping. 2013 doi: 10.1002/hbm.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marrakchi-Kacem L, Delmaire C, Guevara P, Poupon F, Lecomte S, Tucholka A, et al. Mapping cortico-striatal connectivity onto the cortical surface: a new tractography-based approach to study Huntington disease. PloS one. 2013;8(2):e53135. doi: 10.1371/journal.pone.0053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohanna I, Georgiou-Karistianis N, Hannan AJ, Egan GF. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington’s disease. Brain research reviews. 2008;58(1):209–25. doi: 10.1016/j.brainresrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet neurology. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 67.Martinelli P, Rizzo G, Manners D, Tonon C, Pizza F, Testa C, et al. Diffusion-weighted imaging study of patients with essential tremor. Movement disorders: official journal of the Movement Disorder Society. 2007;22(8):1182–5. doi: 10.1002/mds.21287. [DOI] [PubMed] [Google Scholar]
- 68.Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR American journal of neuroradiology. 2008;29(1):151–3. doi: 10.3174/ajnr.A0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74(12):988–94. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 70.Jia L, Jia-Lin S, Qin D, Qing L, Yan Z. A diffusion tensor imaging study in essential tremor. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2011;21(4):370–4. doi: 10.1111/j.1552-6569.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 71.Klein JC, Lorenz B, Kang JS, Baudrexel S, Seifried C, van de Loo S, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Human brain mapping. 2011;32(6):896–904. doi: 10.1002/hbm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buijink AW, Caan MW, Tijssen MA, Hoogduin JM, Maurits NM, van Rootselaar AF. Decreased cerebellar fiber density in cortical myoclonic tremor but not in essential tremor. Cerebellum. 2013;12(2):199–204. doi: 10.1007/s12311-012-0414-2. [DOI] [PubMed] [Google Scholar]
- 73.Saini J, Bagepally BS, Bhatt MD, Chandran V, Bharath RD, Prasad C, et al. Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism & related disorders. 2012;18(5):477–82. doi: 10.1016/j.parkreldis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Elble RJ. What is Essential Tremor? Current neurology and neuroscience reports. 2013;13(6):353. doi: 10.1007/s11910-013-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunenberg EJ, Moeskops P, Backes WH, Pollo C, Cammoun L, Vilanova A, et al. Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PloS one. 2012;7(6):e39061. doi: 10.1371/journal.pone.0039061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sedrak M, Gorgulho A, Frew A, Behnke E, DeSalles A, Pouratian N. Diffusion tensor imaging and colored fractional anisotropy mapping of the ventralis intermedius nucleus of the thalamus. Neurosurgery. 2011;69(5):1124–9. doi: 10.1227/NEU.0b013e3182296a42. discussion 9–30. [DOI] [PubMed] [Google Scholar]
- 77.Kincses ZT, Szabo N, Valalik I, Kopniczky Z, Dezsi L, Klivenyi P, et al. Target identification for stereotactic thalamotomy using diffusion tractography. PloS one. 2012;7(1):e29969. doi: 10.1371/journal.pone.0029969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. Journal of neurosurgery. 2011;115(5):995–1004. doi: 10.3171/2011.7.JNS11250. [DOI] [PubMed] [Google Scholar]
- 79.Elias WJ, Zheng ZA, Domer P, Quigg M, Pouratian N. Validation of connectivity-based thalamic segmentation with direct electrophysiologic recordings from human sensory thalamus. NeuroImage. 2012;59(3):2025–34. doi: 10.1016/j.neuroimage.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 80.Sudhyadhom A, McGregor K, Okun MS, Foote KD, Trinastic J, Crosson B, et al. Delineation of motor and somatosensory thalamic subregions utilizing probabilistic diffusion tractography and electrophysiology. Journal of magnetic resonance imaging: JMRI. 2013;37(3):600–9. doi: 10.1002/jmri.23861. [DOI] [PubMed] [Google Scholar]
- 81.Henderson JM. “Connectomic surgery”: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Frontiers in integrative neuroscience. 2012;6:15. doi: 10.3389/fnint.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Klein JC, Barbe MT, Seifried C, Baudrexel S, Runge M, Maarouf M, et al. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology. 2012;78(11):787–95. doi: 10.1212/WNL.0b013e318249f702. This study used probabilistic tractography to demonstrate structural connectivity between effective thalamic stimulation sites and areas of the brain known to be part of a tremor-generating network. This network was consistent across patients and consistent with prior functional imaging studies and animal models. [DOI] [PubMed] [Google Scholar]
- 83.Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington’s disease. Lancet neurology. 2011;10(6):573–90. doi: 10.1016/S1474-4422(11)70070-9. [DOI] [PubMed] [Google Scholar]
- 84.Prakash BD, Sitoh YY, Tan LC, Au WL. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson’s disease. Parkinsonism & related disorders. 2012;18(9):1029–33. doi: 10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 85.Planetta PJ, Schulze ET, Geary EK, Corcos DM, Goldman JG, Little DM, et al. Thalamic projection fiber integrity in de novo Parkinson disease. AJNR American journal of neuroradiology. 2013;34(1):74–9. doi: 10.3174/ajnr.A3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agosta F, Kostic VS, Davidovic K, Kresojevic N, Sarro L, Svetel M, et al. White matter abnormalities in Parkinson’s disease patients with glucocerebrosidase gene mutations. Movement disorders: official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25397. [DOI] [PubMed] [Google Scholar]
- 87.Sharman M, Valabregue R, Perlbarg V, Marrakchi-Kacem L, Vidailhet M, Benali H, et al. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Movement disorders: official journal of the Movement Disorder Society. 2013;28(4):447–54. doi: 10.1002/mds.25255. [DOI] [PubMed] [Google Scholar]
- 88.Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78(24):1939–45. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 89.Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB. White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. NeuroImage. 2012;62(3):1675–84. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, et al. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. American journal of Alzheimer’s disease and other dementias. 2013;28(2):154–64. doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson’s disease with mild depressive symptoms. Parkinsonism & related disorders. 2012;18(7):809–13. doi: 10.1016/j.parkreldis.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Gallagher C, Bell B, Bendlin B, Palotti M, Okonkwo O, Sodhi A, et al. White matter microstructural integrity and executive function in Parkinson’s disease. Journal of the International Neuropsychological Society: JINS. 2013;19(3):349–54. doi: 10.1017/S1355617712001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamagata K, Motoi Y, Tomiyama H, Abe O, Ito K, Shimoji K, et al. Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: tract-based spatial statistics and tract-specific analysis. European radiology. 2013 doi: 10.1007/s00330-013-2775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ford AH, Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Burn DJ, et al. Rapid eye movement sleep behavior disorder in Parkinson’s disease: Magnetic resonance imaging study. Movement disorders: official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25367. [DOI] [PubMed] [Google Scholar]
- 95.Tsukamoto K, Matsusue E, Kanasaki Y, Kakite S, Fujii S, Kaminou T, et al. Significance of apparent diffusion coefficient measurement for the differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and Parkinson’s disease: evaluation by 3.0-T MR imaging. Neuroradiology. 2012;54(9):947–55. doi: 10.1007/s00234-012-1009-9. [DOI] [PubMed] [Google Scholar]
- 96.Agosta F, Pievani M, Svetel M, Jecmenica Lukic M, Copetti M, Tomic A, et al. Diffusion tensor MRI contributes to differentiate Richardson’s syndrome from PSP-parkinsonism. Neurobiology of aging. 2012;33(12):2817–26. doi: 10.1016/j.neurobiolaging.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Haller S, Badoud S, Nguyen D, Garibotto V, Lovblad KO, Burkhard PR. Individual detection of patients with Parkinson disease using support vector machine analysis of diffusion tensor imaging data: initial results. AJNR American journal of neuroradiology. 2012;33(11):2123–8. doi: 10.3174/ajnr.A3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nair SR, Tan LK, Mohd Ramli N, Lim SY, Rahmat K, Mohd Nor H. A decision tree for differentiating multiple system atrophy from Parkinson’s disease using 3-T MR imaging. European radiology. 2013 doi: 10.1007/s00330-012-2759-9. [DOI] [PubMed] [Google Scholar]
- 99.Whitwell JL, Xu J, Mandrekar J, Gunter JL, Jack CR, Jr, Josephs KA. Imaging measures predict progression in progressive supranuclear palsy. Movement disorders: official journal of the Movement Disorder Society. 2012;27(14):1801–4. doi: 10.1002/mds.24970. [DOI] [PubMed] [Google Scholar]
- 100.Saini J, Bagepally BS, Sandhya M, Pasha SA, Yadav R, Pal PK. In vivo evaluation of white matter pathology in patients of progressive supranuclear palsy using TBSS. Neuroradiology. 2012;54(7):771–80. doi: 10.1007/s00234-011-0983-7. [DOI] [PubMed] [Google Scholar]
- 101.Lu CF, Soong BW, Wu HM, Teng S, Wang PS, Wu YT. Disrupted cerebellar connectivity reduces whole-brain network efficiency in multiple system atrophy. Movement disorders: official journal of the Movement Disorder Society. 2013;28(3):362–9. doi: 10.1002/mds.25314. [DOI] [PubMed] [Google Scholar]
- 102.Wang HC, Hsu JL, Leemans A. Diffusion tensor imaging of vascular parkinsonism: structural changes in cerebral white matter and the association with clinical severity. Archives of neurology. 2012;69(10):1340–8. doi: 10.1001/archneurol.2012.633. [DOI] [PubMed] [Google Scholar]
- 103.Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, Hallett M. Anatomical correlates of blepharospasm. Translational neurodegeneration. 2012;1(1):12. doi: 10.1186/2047-9158-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Meer JN, Beukers RJ, van der Salm SM, Caan MW, Tijssen MA, Nederveen AJ. White matter abnormalities in gene-positive myoclonus-dystonia. Movement disorders: official journal of the Movement Disorder Society. 2012;27(13):1666–72. doi: 10.1002/mds.25128. [DOI] [PubMed] [Google Scholar]
- 105.Cheng FB, Wan XH, Feng JC, Ma LY, Hou B, Feng F, et al. Subcellular distribution of THAP1 and alterations in the microstructure of brain white matter in DYT6 dystonia. Parkinsonism & related disorders. 2012;18(8):978–82. doi: 10.1016/j.parkreldis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 106.Di Paola M, Luders E, Cherubini A, Sanchez-Castaneda C, Thompson PM, Toga AW, et al. Multimodal MRI analysis of the corpus callosum reveals white matter differences in presymptomatic and early Huntington’s disease. Cereb Cortex. 2012;22(12):2858–66. doi: 10.1093/cercor/bhr360. [DOI] [PubMed] [Google Scholar]
- 107.Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011 Feb;77(2):269–73. doi: 10.1016/j.ejrad.2009.07.032. Epub 2009 Aug 18. [DOI] [PubMed] [Google Scholar]