Abstract
The sigma-1 receptor (Sig-1R) is a novel endoplasmic reticulum (ER) molecular chaperone that regulates protein folding and degradation. The Sig-1R activation by agonists is known to improve memory, promote cell survival, and exert an antidepressant-like action in animals. Cutamesine (SA4503), a selective Sig-1R ligand, was shown to increase BDNF in the hippocampus of rats. How exactly the intracellular chaperone Sig-1R or associated ligand causes the increase of BDNF or any other neurotrophins is unknown. We examined here whether the action of Sig-1Rs may relate to the post-translational processing and release of BDNF in neuroblastoma cell lines. We used in vitro assays and confirmed that cutamesine possesses the bona fide Sig-1R agonist property by causing the dissociation of BiP from Sig-1Rs. The C-terminus of Sig-1Rs exerted robust chaperone activity by completely blocking the aggregation of BDNF and GDNF in vitro. Chronic treatment with cutamesine in rat B104 neuroblastoma caused a time- and dose-dependent potentiation of the secretion of BDNF without affecting the mRNA level of BDNF. Cutamesine decreased the intracellular level of pro-BDNF and mature BDNF whereas increased the extracellular level of mature BDNF. The pulse-chase experiment indicated that the knockdown of Sig-1Rs decreased the secreted mature BDNF in B104 cells without affecting the synthesis of BDNF. Our findings indicate that, in contrast to clinically used antidepressants that promote the transcriptional upregulation of BDNF, the Sig-1R agonist cutamesine potentiates the post-translational processing of neurotrophins. This unique pharmacological profile may provide a novel therapeutic opportunity for the treatment of neuropsychiatric disorders.
Keywords: sigma-1 receptor, sigma receptor, BDNF, GDNF, cutamesine, SA4503, antidepressant, depression, cleavage, secretion, neuroprotection
Introduction
Although originally proposed as a subtype of opioid receptors, the sigma-1 receptor (Sig-1R) is now recognized as a non-opioid endoplasmic reticulum (ER) protein (Katz et al 2011; Maurice & Su 2009; Ruscher et al 2011; Hayashi & Su 2007; Su et al 1988). While the Sig-1R is implicated in neuronal differentiation, neuroplasticity and neuroprotection, Sig-1R ligands have been demonstrated by a number of studies to exert therapeutic actions in animal models of depression, drug abuse, stroke, and neurodegenerative disorders (Katz et al 2011; Maurice & Su 2009; Ruscher et al 2011; Su et al 1988). For example, selective Sig-1R agonists exhibit a rapid antidepressant-like action in the forced swimming test as well as a potent neuroprotective/neuroregenerative action against either β-amyloid-induced neurotoxicity or hypoxia (Maurice et al 1998; Ruscher et al 2011). In contrast, Sig-1R antagonists block the drug-induced behavioral sensitization and to attenuate the cancer proliferation (Matsumoto 2009; Spruce et al 2004).
The Sig-1R was recently discovered as a novel molecular chaperone regulating protein folding and degradation at the ER (Hayashi & Su 2007). Sig-1Rs associate with diverse proteins such as IP3 receptor type-3, K+ channel subunit, and binding immunoglobulin protein (BiP) (Aydar et al 2002; Hayashi & Su 2001; 2007) at least in part to prevent the misfolding of proteins. The chaperone activity of Sig-1Rs can be regulated by small synthetic molecules. The Sig-1R in the dormant state forms a complex with another chaperone BiP at the lumen of the ER. The presence of Sig-1R agonists or the depletion of ER Ca2+ can trigger the dissociation of Sig-1Rs from BiP, leading to the activation of Sig-1R chaperones (Hayashi & Su 2007). In light of the growing evidence demonstrating the importance of ER stress and the unfolded protein response (UPR) in the pathophysiology of depression, stroke, Alzheimer’s disease and amyotrophic lateral sclerosis, selective Sig-1R agonists were proposed to serve as novel agents that may ameliorate the accumulation of misfolded proteins in the nervous system (Hayashi et al 2011; Maurice & Su 2009).
Cutamesine (SA4503), a novel Sig-1R agonist with completed Phase II clinical trials in major depression and post-stroke recovery, respectively, improves memory, promotes cell survival, and exerts an antidepressant-like action (Matsuno et al 1996; Ruscher et al 2011). A recent study found that the chronic treatment with cutamesine increased the protein level of BDNF in the hippocampus of the rat brain (Kikuchi-Utsumi & Nakaki 2008). Since BDNF and associated signaling pathways play crucial roles in neuronal survival, plasticity, and mood, the therapeutic effects of cutamesine may be at least in part mediated by promoting the upregulation of neurotrophins.
Expression of BDNF is tightly regulated at the transcriptional level (Brunoni et al 2008; Calabrese et al 2010; Racagni & Popoli 2008; Sen et al 2008; Shimizu et al 2003; Yasui-Furukori et al 2011). However, the level of BDNF is also post-translationally regulated by elaborated intracellular systems. BDNF is synthesized as a pre-proBDNF in the ER. Newly synthesized BDNF is post-translationally processed via multiple steps involving glycosylation, sorting, proteolytic cleavage and secretion (Lu et al 2005). ProBDNF is cleaved for maturation both intracellularly and extracellularly by several proteases such as plasmin, matrix metalloproteinases (MMP)-3, MMP-7 and furin (Lee et al 2001; Nikoletopoulou et al 2010). Despite recent advances in understanding the mechanisms of the post-translational processing of BDNF, few studies have been, however, directed in exploring the post-translational processing of BDNF as a potential avenue for therapeutic purpose. In light of the unique ‘ligand-activated’ property of Sig-1R chaperones, we hypothesized in this study that selective Sig-1R agonists may serve as novel agents that may pharmacologically augment the post-translational processing and secretion of neuroprophins. In this study, we examined specifically the action of the Sig-1R agonist cutamesine on the secretion of BDNF with a particular goal to see whether Sig-1Rs may regulate the post-translational processing of BDNF. We reported here that cutamesine possesses bona fide Sig-1R agonist property and that cutamesine potentiates the post-translational processing of BDNF.
MATERIALS AND METHODS
Materials
Minimal essential medium (MEM)-α Glutamax, F-12 and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Eagle’s minimunm essential medium was from ATCC (Manassas, VA). Specific antibodies were purchased as follows: anti-BiP from BD Biosciences (San Jose, CA); anti-green fluorescent protein (GFP) from Clontech (Mountain View, CA); anti-metalloproteinase-3 (MMP-3) from AbCam (Cambridge, MA); anti-rat metalloproteinase-7 (MMP-3) from Cell Signaling Technology (Danvers, MA); chicken anti-BDNF from Promega (Madison, WI); rabbit anti-BDNF (sc-546) and anti-ERK from Santa Cruz (Santa Cruz, CA). Anti-Sig-1R antibodies were developed as described previously (Hayashi and Su, 2007). Chemicals were from Sigma-Aldrich. (+)Pentazocine was synthesized at the Division of Basic Research at the National Institute on Drug Abuse. Cutamesine (SA4503) was synthesized by M’s Science Corporation (Hyogo, Japan). NE100 was donated by Taisho Pharmaceutical Co. Ltd. (Saitama, Japan). B104 rat neuroblastoma was provided by Dr. F. Cambi (Thomas Jefferson University)6. The expression vectors for enhanced yellow fluorescent protein (EYFP)-tagged Sig-1Rs were constructed by ligating mouse Sig-1R cDNA in the pEYFP-N1 vector (Hayashi & Su 2007). The proBDNF pcDNA3.1 vector was donated by Dr. Y.P. Loh (National Institute of Child Health and Human Development)6. The small interfering RNA (siRNA) targeting the human Sig-1R was purchased from Dharmacon (Lafayette, CO).
Cell culture
Chinese hamster ovary (CHO) cells (ATCC, Manassas, VA) were maintained in MEM-α Glutamax containing 10% (v/v) heat-inactivated FBS at 37°C with 5% CO2. The CHO cell line stably expressing EYFPs or Sig-1R-EYFP was established by transiently transfecting pEYFP-N1 or pEYFP- Sig-1R vectors followed by colony selections with G418 (Geneticin; Invitrogen). SH-SY5Y human neuroblastoma cells (ATCC) and B104 rat neuroblastoma cells were maintained in 1:1 mixture of Eagle’s MEM and F12 containing 10% FBS at 37°C with 5% CO2.
Sig-1R-BiP association assay to characterize the agonist-antagonist properties of Sig-1R ligands
CHO cells stably expressing EYFP or Sig-1R-EYFP were grown in 6-well plates and treated with Sig-1R ligands in culture medium at 37°C. CHO cells were harvested and suspended in 50 mM Hepes (pH7.4) followed by cross-linking with 100 μg/ml of dithiobis succinimidyl propionate (Thermo Fisher Scientific, Waltham, MA) for 30 min at 4°C. Reaction was stopped by adding Tris-HCl (pH 8.8, final 50 mM). Fifteen minutes after incubation on ice, cells were lysed with RIPA buffer [50 mM Tris (pH7.4), 150mM NaCl, 1% Triton X-100, 0.3% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail (Sigma-Aldrich)]. After centrifugation at 12,000g, the supernatant was incubated overnight at 4°C with polyclonal anti-GFP antibodies. The cell lysate was incubated with Sepharose protein-A (GE Healthcare, Buckinghamshire, UK) for 90 min. After washing with RIPA buffer, immunoprecipitants were boiled in 30 μl of 2 x sample buffer (130 mM Tris-HCl, pH 6.8, 4.2% SDS and 20% glycerol) and applied to Western blotting. Immunoprecipitated Sig-1R-EYFP and co-immunoprecipitated BiP were detected by immunoblotting using monoclonal antibodies against GFP and BiP, respectively. Intensities of BiP were normalized to those from Sig-1R-EYFP for quantification of the activity of Sig-1R ligands.
Immunoprecipitation of BDNF
Plasmids (e.g., BDNF pcDNA3.1) or siRNA were transfected into B104 cells using PolyJet or PepMute (SignaGen Laboratories, Ijamsville, MD). Two days later, cells and culture medium were harvested. One tenth of cells were lysed in 2x sample buffer for detections of Sig-1R and ERK in the total cell lysate (see below). For immunoprecipitation, nine tenths of cells were suspended by lysis buffer (10mM Tris pH7.4, 150 mM NaCl, 1% Triton X-100) containing protease inhibitors. After centrifugation at 12,000×g, the supernatant was incubated overnight at 4°C with primary chicken anti-BDNF IgY antibody (1 : 75). Immunocomplexes were precipitated by incubating the lysates with agarose-coupled goat anti-chicken IgY antibodies (PrecipHen®, Aves Labs Inc., Tigard, OR) for 3 h at 4°C. After washing with lysis buffer 3 times, BDNF immunoprecipitants were boiled in 30 μl of 2 x sample buffer for 5 min and applied to Western blotting. One tenth of culture medium was used for immunoprecipitation of secreted BDNF with the same combination of primary antibodies and beads.
Western Blotting
Cells were briefly washed and harvested in ice-cold PBS. Cell pellets after a centrifuge at 1300g for 10 min were suspended into 2 x sample buffer. After a brief sonication, protein concentrations in lysates were measured by a BCA kit (Thermo Fisher Scientific, Rockford, IL) and 15 μg of total proteins were resolved by SDS-PAGE. Samples of immunoprecipitation were prepared as described above. After SDS-PAGE, proteins were transblotted onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA) by Mini Trans-Blot Cell (Bio-Rad Laboratories). The membrane was blocked with 10% non-fat dry milk in Tris-based saline buffer with Tween-20 (20 mM Tris base, 500 mM NaCl, 0.005% Tween 20, pH 7.5) for 1 hour followed by incubation overnight with primary antibodies (Sig-1R, 1:500; ERK, 1:1500; BiP, 1:2000 with 5% milk; GFP, 1:3000; MMP-3, 1:1000; MMP-7, 1:500). After washing with Tris-based saline buffer with Tween-20, the membrane was incubated for 1 hour with secondary antibodies conjugated with horseradish peroxidase (Thermo Fisher Scientific). Protein bands were visualized by a Kodak IS4000 MM Pro imaging system (Carestream Health, Rochester, NY) with a chemiluminescent reagent SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific).
Pulse-chase experiment
Vectors were transfected into B104 cells 2 days before experiments. Cells were preincubated with methionine-cysteine (Met/Cys)-free Dulbecco’s Modified Eagle Medium for 30 minutes followed by metabolically labeling for 60 minutes with 150 μCi/ml of 35S-Met/Cys (MP Biomedicals, Solon, OH). After changing the medium to the regular culture medium containing 10% FCS and 2.25 mg/ml of L-Met and L-Cys, cells were incubated for chase at 37°C. Medium and cells were collected for BDNF immunoprecipitation and SDS-PAGE. The bands were visualized by direct autoradiography.
ELISA
Cells at 9 × 105 cells/well were seeded onto 6-well plates coated with poly-D-lysine and cultured for 24 hours in 3 ml of growth medium. After replacing the medium with 2 ml of serum-free growth medium, cells were maintained in a 5% CO2 incubator for 1–9 days with or without drug. Conditioned medium was collected after a centrifugation at 1300g for 10 minutes. Levels of BDNF in conditioned media (both pro- and mature forms), were determined using ELISA (Promega). In brief, 96-well, flat-bottomed ELISA plates [Costar #3696 plate (Cambridge, MA)] were coated with anti-BDNF monoclonal antibodies diluted in carbonate coating buffer, then incubated overnight at 4°C. After emptying the coating buffer from the wells, plates were blocked for 1 hour at room temperature with 1 X Block and Sample Buffer (Promega). Samples and standards were incubated overnight at 4°C. The captured BDNF was incubated with BDNF polyclonal antibodies at room temperature. Horseradish peroxidase-conjugated anti-IgY rabbit antibodies and TMB One solution (Promega) was added successively at room temperature. The reaction was stopped by adding hydrochloric acid and absorbance at 450 nm was recorded by a microplate reader.
Light scattering assay
Purified polypeptides [citrate synthase (CS), BDNF or GDNF at 75 nM] in 40 mM HEPES (pH 7.4) were heated to 43°C to induce protein aggregation. Formation of protein aggregation was monitored by a spectrophotometer at absorbance of 320 nm every 10 seconds. In some experiments the same concentration of purified Sig-1R polypeptides (C-terminus a.a. 116-223) was mixed with substrate peptides at 4 °C prior to incubation at 43 °C. Sig-1R polypeptides were purified with a glutathione affinity-column chromatography from E. Coli lysates expressing GST-fusion Sig-1Rs as described previously (Hayashi and Su, 2007).
RT-PCR
Total RNA were extracted by a NucleoSpin II kit (Clontech) from B104 cells. The level of BDNF mRNA was measured by RT-PCR (33 cycles at 56 °C with a Titanium One-Step RT-PCR kit, Clontech) with primers 5′-gatgccgcaaatatgtctatga-3′ and 5′-taatactgtcacacacgctcagctc-3.′ The level of BDNF mRNA was normalized to that of β-actin.
Statistics
Data were analyzed by a Prism 3.0cx software (GraphPad Software Inc., San Diego, CA). For comparison of two groups, Two-tailed Student t test was used. For data with multiple groups, one-way ANOVA followed by Dunnett’s multiple comparison test or two-way ANOVA followed by Bonferroni post hoc test was employed. Data were presented as means ± SEM. The significance level was set at p < 0.05.
RESULTS
Cutamesine is a bona fide Sig-1R agonist that dissociates BiP from Sig-1R
By employing a novel in vitro assay recently developed by us (Hayashi & Su 2007), we examined whether cutamesine possesses bona fide agonist property for the Sig-1R. This assay quantifies the ability and potency of Sig-1R agonists in promoting the dissociation of Sig-1Rs from BiP (Fig. 1A). The association between Sig-1Rs and BiP was measured by immunoprecipitation using CHO cells stably expressing EYFP-tagged Sig-1Rs. Similar to the prototypic Sig-1R agonist (+)pentazocine, cutamesine applied to the culture medium promoted a dose-dependent dissociation of Sig-1Rs from BiP (Fig. 1A). The potency of cutamesine in causing the dissociation of Sig-1Rs from BiP (EC50 ≈ 1 μM) was slightly less than that of (+)pentazocine. The dissociation of Sig-1Rs from BiP caused by cutamesine became apparent as early as 10 minutes after its application to the culture medium (Fig. 1B). The action of (+)pentazocine in dissociating Sig-1Rs from BiP lasted for at least 2 days (Fig. 1C). The effect of cutamesine promoting the dissociation of Sig-1Rs from BiP was blocked by the selective Sig-1R antagonist NE100 (1 μM) (Fig. 1D), indicating that the action of cutamesine is mediated via Sig-1Rs. Thus, these results clearly demonstrated that cutamesine possesses the bona fide Sig-1R agonist property that dissociates Sig-1R chaperones from BiP in living CHO cells.
Fig. 1. Cutamesine is a bona fide agonist of the Sig-1R.
A. Cutamesine dose-dependently dissociates Sig-1Rs from BiP. CHO cells stably expressing EYFP or EYFP-tagged Sig-1R (Sig-1R-EYFP) were grown in 6-cm dishes. Cutamesine (1 μM, n=9) or (+)pentazocine (PTZ; 0.3 μM, n=7) was applied to the culture medium followed by incubation at 37 °C for 30 min. EYFP or Sig-1R-EYFP were immunoprecipitated with polyclonal GFP-antibodies in total cell lysates of CHO cells. Co-immunoprecipitated BiP was detected by monoclonal anti-BiP antibodies. B. Cutamesine time-dependently dissociates Sig-1Rs from BiP. Cutamesine (1 μM) or (+)pentazocine (0.3 μM) was applied to the culture medium for up to 60 min. N=4. C. The long-lasting effect of (+)pentazocine or cutamesine promoting the dissociation of Sig-1Rs form BiP. BiP associated with Sig-1Rs was measured as described in A. D. Inhibition of the effect of cutamesine by the Sig-1R antagonist NE100. Cutamesine (1 μM for 30 min) and NE100 (10 μM for 35 min) were applied to the culture medium. BiP associated with Sig-1Rs was measured by immunoprecipitation.
Purified Sig-1R polypeptides block the aggregation of neurotrophins
The C-terminus of the Sig-1R exerts the chaperone activity (Hayashi & Su 2007). Here we examined whether purified C-termini of the Sig-1R inhibit the aggregation of neurotrophins such as BDNF and GDNF. Protein aggregates formed at 43°C were monitored by the light scattering assay (see Method). Purified C-termini comprising the amino acid residues 116–223 (75 nM) of the Sig-1Rs significantly reduced heat-induced aggregation of citrate synthase (75 nM), an assay substrate that is prone to heat-induced aggregation (Fig. 2A). This result confirmed the anti-aggregation activity of Sig-1R polypeptides. At 43°C, both BDNF and GDNF formed protein aggregates (Fig. 2B, C). Sig-1R polypeptides (75 nM) completely blocked the aggregation of either neurotrophin (75 nM) (Fig. 2B, C).
Fig. 2. Prevention of BDNF and GDNF aggregation by the purified GST-fused C-terminus of the Sig-1R polypeptide.

Protein aggregates formed at 43°C were monitored every 10 sec by a light scattering assay. Citrate synthase (CS) (A), BDNF (B), or GDNF (C) at 75 nM was incubated at 43 °C in the presence or absence of purified GST-fused C-terminus (a.a. 116-223) of the Sig-1R polypeptide (75 nM). GST-fused Sig-1R 166-223 was purified as described previously (Hayashi and Su, 2007).
Cutamesine potentiates the secretion of BDNF
Next, the effect of cutamesine treatment on the secretion of BDNF was examined. The human neuroblastoma SH-SY5Y is the most commonly used cell line for screening drug effects on the BDNF secretion (Baj & Tongiorgi 2009; Donnici et al 2008; Serres & Carney 2006). However, we found that B104 rat neuroblastoma cells secrete much more BDNF than SH-SY5Y human neuroblastoma cells under serum-free conditions (Fig. 3A). Further, in contrast to SH-SY5Y cells that are often differentiated for the reliable detection of BDNF (Baj & Tongiorgi 2009; Garzon & Fahnestock 2007), treatment to induce differentiation was not necessary to detect BDNF secreted from B104 cells. By simply culturing in the serum-free medium for a few days, B104 cells significantly increased BDNF mRNA as well as the secretion of BDNF (data not shown). Thus, B104 cells represent an ideal tool for characterizing compounds on the BDNF secretion. The following experiments were therefore performed mainly by using B104 cells.
Fig. 3. Cutamesine potentiates the BDNF secretion from neuroblastoma cells.
A. The comparison of activity to secrete BDNF between two neuroblastoma cell lines. SH-SY5Y cells (n=2) and B104 cells (n=3) were cultured for indicated days without changing the medium. Concentrations of BDNF in the culture medium were measured by ELISA. B. Cutamesine treatment time-dependently enhances the secretion of BDNF. B104 cells were treated with 1 μM of cutamesine for up to 9 days. BDNF concentrations in the culture medium were measured by ELISA. Control and cutamesine-treated samples collected on the same day were compared by two-tailed Student t test. n=5–6. *p<0.05. C. Cutamesine treatment dose-dependently enhances the secretion of BDNF. The x-axis indicates the concentration of cutamesine (μM). B104 cells were cultured with cutamesine for 7 days. BDNF concentrations in the culture medium were measured by ELISA. Control and cutamesine-treated samples were compared by one-way ANOVA followed by Dunnett’s multiple comparison test. n=6. *p < 0.05, **p < 0.01. D. Blockade of the effect of cutamesine by the Sig-1R antagonist NE100. B104 cells were cultured with 1 μM of cutamesine and/or 1 μM of NE100 for 7 days. BDNF concentrations in the culture medium were measured by ELISA. Control and cutamesine-treated samples were compared by one-way ANOVA followed by Dunnett’s multiple comparison test. n=9. **p < 0.01. E. Effect of cutamesine on the BDNF mRNA level. B104 were treated with cutamesine (1 mM, 7 days) or vehicle (control), and BDNF mRNA in extracts were measured by RT-PCR. The level of BDNF mRNA was normalized to β-actin mRNA (N=5).
We found that cutamesine (1 μM) time-dependently increased the release of BDNF (pro plus mature BDNF) from B104 cells as shown by the ELISA assay (Fig. 3B). Cutamesine at 1 μM or higher concentrations significantly increased the secretion of BDNF (Fig. 3C). Notably, the effect of cutamesine potentiating the BDNF secretion was blocked by the Sig-1R antagonist NE100 (Fig. 3D), indicating that the effect involves the activation of Sig-1Rs. Cutamesine did not affect the cell viability and proliferation during chronic treatment for 10 days (data not shown). Cutamesine (7 days) also did not affect the mRNA level of BDNF (Fig. 3E). Since cutamesine causes the dissociation of Sig-1Rs from BiP for a considerably long period of time as demonstrated in Fig. 1C, the chronic treatment with cutamesine may potentiate the release of BDNF by promoting the long-lasting activation of Sig-1R chaperones at the ER that might affect the post-translational processing of BDNF.
Effect of cutamesine on the post-translational processing of BDNF
We examined the effect of cutamesine on the processing of BDNF. Since the level of endogenous intracellular pro- and mature BDNF are still below the detection limit even under serum-free conditions, we employed B104 cells that had been transiently transfected with human wild-type BDNF cDNA to enhance the level of BDNF. Levels of pro- and mature forms of BDNF were measured by immunoprecipitation followed by immunoblotting in both cell lysates (inside cells) and culture medium (outside cells). It is noteworthy that, under our transfection condition, the cleaved form (i.e., mature form) represents the majority of BDNF inside of the cell and that a much greater amount of mature BDNF was detected in the culture medium than in cell lysates (Fig. 4). Thus, overexpressed human BDNF was actively processed and then released in B104 cells. In contrast, expression of BDNF with an epitope tag (e.g., Flag) hampered the processing of BDNF in B104 as shown by an obvious accumulation of pro-BDNF inside the cell and fewer released mature BDNF (data not shown). Apparently, B104 cells transfected with human wild-type BDNF appear to physiologically process the BDNF maturation and secretion.
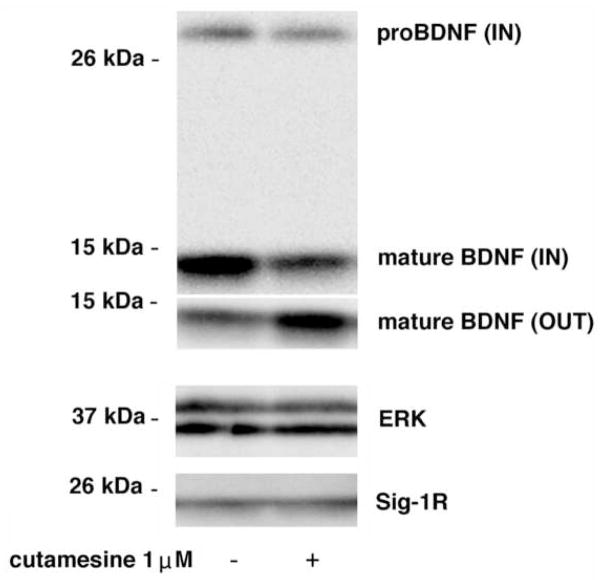
Fig. 4. Effect of cutamesine on protein levels of BDNF inside and outside cells.
One day after transfection with human proBDNF cDNA, B104 cells were treated with 1 μM of cutamesine overnight. Pro- and mature BDNF were immunoprecipitated in both cell lysate (IN) and culture medium (OUT), and detected by immunoblotting. The level of Sig-1Rs and ERK (the loading control) were measured in total cell lysates (bottom). Images represent data from 3 repeated experiments. Mean intensities of mature BDNF bands (% of control) are: control=100.0±7.1, cutamesine=70.6±8.8 (IN); control=100.0±6.2, cutamesine=174.7±21.4 (OUT).
Accordingly, B104 cells expressing human BDNF were treated with cutamesine and the pro and mature forms of BDNF were detected. We found that the cutamesine treatment (1 μM, 14 hours) decreased both pro- and mature BDNF inside the cell, whereas it greatly increased the mature BDNF released to the culture medium (Fig. 4). These findings suggest that cutamesine facilitates the secretion of BDNF, and as a result fewer BDNF is retained inside the cell.
Although it is well-known that cleavage of pro-BDNF is processed by MMP-3 and -7, the treatment with cutamesine (1 μM, 14 hours) did not alter the protein level of MMP in Western blotting (control, 100±10.1; cutamesine, 105.8 ±7.4, N=6, p=0.65). The expression of MMP-7 in B104 cells was under the detection limit in Western blotting.
Knockdown of Sig-1Rs suppresses the secretion of BDNF from B104 cells
To further confirm the involvement of Sig-1Rs in the secretion of BDNF, the effect of Sig-1R knockdown on the BDNF secretion was examined. Two days after the transfection of B104 cells with Sig-1R siRNA, the levels of pre and mature forms of BDNF was measured both intracellularly and extracellularly as demonstrated in Fig. 5. We found that the Sig-1R knockdown decreases the level of mature BDNF in the medium (Fig. 5A). However, despite causing a decreased release of mature BDNF into the medium, the knockdown of Sig-1Rs did not affect the level of intracellular BDNF (Fig. 5A).
Fig. 5. Effect of knockdown of Sig-1Rs on the secretion of BDNF from B104 cells.

A. Effect of Sig-1R knockdown on the BDNF protein levels inside and outside cells. B104 cells were transfected with proBDNF cDNA with or without Sig-1R siRNA and incubated for 2 days. CTR: control scramble siRNA. Pro- and mature BDNF inside (IN) and outside (OUT) cells were measured by immunoprecipitation as described in Fig. 5. The level of Sig-1Rs and ERK (the loading control) were measured in total cell lysates (bottom). Images represent data from 4 repeated experiments. Mean intensities of mature BDNF bands (% of CTR) are: CTR siRNA=100.0±4.2, Sig-1R siRNA=88.78±6.7 (IN); CTR siRNA=100.0±7.54, Sig-1R siRNA=65.8±12.7 (OUT). B. Pulse-chase experiment for monitoring synthesis and secretion of BDNF. B104 cells transfected with human BDNF cDNA for 1 day were pulse-labeled with 35S-Met/Cys for 60 min followed by a chasing for 180 min. CTR: control scramble siRNA. BDNF were immunoprecipitated in both cell lysates (IN) and culture medium (OUT), and visualized by direct autoradiography. ERK and Sig-1Rs were measured in total cell lysates with immunoblotting. Images represent data from 3 repeated experiments. Mean intensities of mature BDNF bands (% of CTR) 180 min after chasing are: CTR siRNA=100.0±0.1, Sig-1R siRNA=113.6±27.9 (IN); CTR siRNA=100.0±8.9, Sig-1R siRNA=53.4±8.0 (OUT).
To confirm the results shown in Fig. 5A, we performed pulse-chase experiments to monitor the protein synthesis, cleavage and secretion of BDNF. B104 cells transfected with human BDNF were pulse-labeled with 35S-methionin/cysteine for 60 minutes, then chased for 180 minutes. Data clearly demonstrated that the production of pro-BDNF during the 60 minute pulse labeling is unaffected by knockdown of Sig-1Rs (30 kDa bands in the first and second lanes of the upper panel in Fig. 5B), suggesting that knockdown of Sig-1Rs does not affect the protein synthesis of BDNF in our system. Newly synthesized pro-BDNF seems to be rapidly cleaved in B104 cells as seen from the appearance of cleaved 15-kDa BDNF bands during the 60 minute pulse (in the first and second lanes of the upper panel in Fig. 5B). During the chase for 180 minutes, the 15-kDa bands inside cells decreased, but the 15-kDa bands in the culture medium increased concomitantly (Fig. 5B), suggesting that mature BDNF is actively released during the 180 minute chase. Notably, the knockdown Sig-1Rs significantly decreased the level of mature BDNF secreted to the outside of cells during the chase (Fig. 5B). Again, despite a decreased level of the secreted BDNF caused by the Sig-1R knockdown, no accumulation of intracellular BDNF was observed.
DISCUSSION
A recent study demonstrated that cutamesine induces the upregulation of BDNF proteins in the rat hippocampus (Kikuchi-Utsumi & Nakaki 2008). However, our findings indicate that cutamesine does not merely increase the total amount of BDNF, but potentiates the post-translational processing of BDNF proteins related to the protein secretion. We demonstrated that cutamesine decreased both pro- and mature BDNF inside the cell whereas cutamesine concomitantly increased the level of mature BDNF in the extracellular space (Fig. 4). Further, the knockdown of Sig-1Rs decreases the release of mature BDNF without significantly affecting the protein synthesis of BDNF (Fig. 5). Those results clearly indicate that the activation of Sig-1Rs leads to increased release of BDNF from B104 cells. On the other hand, whether cutamesine by activating Sig-1Rs may also potentiate the cleavage of BDNF remains unknown at present since neither the Sig-1R knockdown nor the pulse-chase experiment showed a clear change in the ratio between pro- and cleaved form of intracellular BDNF. Despite causing a decrease in the extracellular level of mature BDNF, the knockdown of Sig-1Rs did not cause any apparent accumulation of intracellular BDNF (Fig. 5A). We cannot provide an explanation for this result. However it is speculated that the knockdown of Sig-1R chaperones might lead to the instability and subsequently the degradation of intracellularly stored BDNF. Further studies including the examination of the effect of Sig-1R knockdown on the degradation of pro- or mature BDNF are certainly needed.
The exact molecular mechanism underlying the Sig-1R-related activation of the BDNF secretion is unknown at this moment. Specifically, whether Sig-1Rs regulate the ‘constitutive’ (i.e. spontaneous) or the ‘regulated’(i.e. depolarization-induced) pathway of the BDNF secretion (Lou et al 2005; Lu 2003) needs to be examined in the future. Further, considering diversity of signaling pathways observed in different cell types, the post-translational action of cutamesine should be examined in primary neurons. Nonetheless, inasmuch as Sig-1Rs are chaperone proteins localized predominantly at the ER, it is reasonable to speculate that Sig-1Rs may be involved in the early stage of intracellular BDNF processing such as folding, ER transport, and degradation. The potent inhibition of neurotrophin aggregation by Sig-1Rs (Fig. 2) supports this possibility although future studies are required to confirm whether the same action can be seen in vivo.
In this study, we employed an in vitro assay to confirm the agonist property of cutamesine. This assay employs the immunoprecipitation prior to the BiP immunoblotting, allowing thus a rapid assessment of the agonist-antagonist property as well as the potency of Sig-1R agonists in activating the chaperone activity of the Sig-1R. Our assay clearly showed that cutamesine is a bona fide agonist causing the dissociation of Sig-1Rs from BiP and that the cutamesine action is blocked by the selective Sig-1R antagonist NE100. In agreement with previous binding assays showing that (+)pentazocine has a higher Sig-1R affinity than cutamesine [Ki (nM): 4.63±0.21 for cutamesine, 1.62±0.15 for (+)pentazocine] (Lever et al 2006), (+)pentazocine dissociated Sig-1Rs from BiP at lower concentrations when compared to cutamesine. The effect of those Sig-1R ligands causing the dissociation of Sig-1Rs from BiP was observed as early as 10 minutes after their administration to the culture medium. The action of Sig-1R ligands lasted for up to at least 2 days if the medium was not changed. Thus, the Sig-1R agonists are rapidly and efficiently taken up by the cells, reach the ER in short time, and promote a rapid and continuous activation of intracellularly localized Sig-1R chaperones. The long-lasting action of Sig-1R agonists in this regard may be particularly important for the potential therapeutic efficacy of Sig-1R agonists.
A number of studies demonstrated that neurotrophins such as BDNF are intimately involved in various neuropsychiatric diseases such as depression (Otsuki et al 2008), drug addiction (Ghitza et al 2010), and Parkinson’s disease (Rangasamy et al 2010), thus leading recently to the conceptualization of the ‘neurotrophin hypothesis’ in those diseases. The neurotrophin hypothesis of antidepressant drug action postulates that a reduced activity of neurotrophins plays a role in the pathophysiology of major depression and that the restoration of neurotrophins may be the underlying action exerted by antidepressants (Martinowich et al 2007). In fact, clinically used antidepressants activate transcription of BDNF mRNA partly via their action on the monoaminergic systems (MacQueen et al 2001; Martinowich et al 2007; Saarelainen et al 2003). However, since the proBDNF and mature BDNF can elicit opposite neurobiological effects (i.e, proBDNF promotes apoptosis via the pan-neurotrophin receptor p75NTR, whereas mature BDNF promotes cell survival and LTP via the Trk receptor tyrosine kinases) (Figurov et al 1996), the post-translational processes promoting the cleavage of pro-BDNF or the secretion of mature BDNF may represent another important element in the antidepressant action of drugs. In this viewpoint, the action of cutamesine promoting the activation of the post-translational processes of BDNF may provide a novel concept to the drug discovery of new antidepressants.
Despite of the lack of affinities for monoamine transporters and receptors, Sig-1R ligands have been consistently demonstrated to regulate neurotrophin levels and their signaling pathways (Penas et al 2011; Takebayashi et al 2002; Yagasaki et al 2006). Sig-1Rs potentiate BDNF-induced activation of the PLC-gamma/IP3/Ca2+ pathway that results in the glutamate release from cortical neurons (Yagasaki et al 2006). Sig-1R agonists potentiated NGF-induced neurite sprouting of PC-12 cells (Takebayashi et al 2002). The selective Sig-1R agonist PRE084 [2-(4-morpholinethyl)1 phenylcyclohexanecarboxylate] also increases the expression of GDNF proteins as well as the ER chaperone BiP in rat brains (Penas et al 2011). Moreover, our present study found that, in contrast to clinically used antidepressants that increase the transcription of BDNF mRNA, the Sig-1R agonist cutamesine facilitates the post-translational processing of BDNF that relates to the peptide secretion. Thus, the unique post-translational action of Sig-1R ligands on BDNF may provide a great opportunity for developing the next generation of psychotherapeutic drugs.
Footnotes
We thank Drs. F. Cambi and Y.P Loh for providing us B104 cells and the proBDNF/pcDNA3.1 vector, respectively.
References
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Baj G, Tongiorgi E. BDNF splice variants from the second promoter cluster support cell survival of differentiated neuroblastoma upon cytotoxic stress. J Cell Sci. 2009;122:36–43. doi: 10.1242/jcs.033316. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–80. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol. 2010;77:846–53. doi: 10.1124/mol.109.063081. [DOI] [PubMed] [Google Scholar]
- Donnici L, Tiraboschi E, Tardito D, Musazzi L, Racagni G, Popoli M. Time-dependent biphasic modulation of human BDNF by antidepressants in neuroblastoma cells. BMC Neurosci. 2008;9:61. doi: 10.1186/1471-2202-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J Neurosci. 2007;27:2628–35. doi: 10.1523/JNEUROSCI.5053-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–71. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–6. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsai SY, Mori T, Fujimoto M, Su TP. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2011;15:557–77. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, et al. A Role for Sigma Receptors in Stimulant Self Administration and Addiction. Pharmaceuticals (Basel) 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Utsumi K, Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neurosci Lett. 2008;440:19–22. doi: 10.1016/j.neulet.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–8. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–55. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–53. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–93. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol. 2009;2:351–8. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Kobayashi T, Tanaka MK, Mita S. Sigma 1 receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. Eur J Pharmacol. 1996;312:267–71. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–28. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467:59–63. doi: 10.1038/nature09336. [DOI] [PubMed] [Google Scholar]
- Otsuki K, Uchida S, Watanuki T, Wakabayashi Y, Fujimoto M, et al. Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res. 2008;42:1145–53. doi: 10.1016/j.jpsychires.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Penas C, Pascual-Font A, Mancuso R, Fores J, Casas C, Navarro X. Sigma receptor agonist 2-(4-morpholinethyl)1 phenylcyclohexanecarboxylate (Pre084) increases GDNF and BiP expression and promotes neuroprotection after root avulsion injury. J Neurotrauma. 2011;28:831–40. doi: 10.1089/neu.2010.1674. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci. 2008;10:385–400. doi: 10.31887/DCNS.2008.10.4/gracagni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson’s disease. Prog Brain Res. 2010;184:237–64. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–46. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–57. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–32. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006;1101:36–42. doi: 10.1016/j.brainres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–5. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, et al. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:4875–86. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune sytems. Science. 1988;240:219–21. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227–37. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281:12941–9. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Yasui-Furukori N, Tsuchimine S, Nakagami T, Fujii A, Sato Y, et al. Association between plasma paroxetine concentration and changes in plasma brain-derived neurotrophic factor levels in patients with major depressive disorder. Hum Psychopharmacol. 2011 doi: 10.1002/hup.1192. [DOI] [PubMed] [Google Scholar]