Abstract
Objectives
Xanthophyll pigments lutein and zeaxanthin cross the blood-retina barrier to preferentially accumulate in the macular region of the neural retina. There they form macular pigment, protecting the retina from blue light damage and oxidative stress. Lutein and zeaxanthin also accumulate in brain tissue. The objective of the study was to evaluate the relationship between retinal and brain levels of these xanthophylls in non-human primates.
Methods
Study animals included rhesus monkeys reared on diets devoid of xanthophylls that were subsequently fed pure lutein or pure zeaxanthin (both at 3.9 μmol/kg*d, n=6/group) and normal rhesus monkeys fed a stock diet (0.26 μmol/kg*d lutein and 0.24 μmol/kg*d zeaxanthin, n=5). Retina (4 mm macular punch, 4-8 mm annulus and periphery) and brain tissue (cerebellum, frontal cortex, occipital cortex and pons) from the same animals were analyzed by reverse phase HPLC.
Results
Lutein in the macula and annulus were significantly related to lutein levels in the cerebellum, occipital cortex and pons, both in bivariate analysis and after adjusting for age, sex and n–3 fatty acid status. In the frontal cortex the relationship was marginally significant. Macular zeaxanthin was significantly related to zeaxanthin in the cerebellum and frontal cortex, while the relationship was marginally significant in the occipital cortex and pons in a bivariate model.
Discussion
An integrated measure of total macular pigment optical density, which can be measured noninvasively, has the potential to be used as a biomarker to assess brain lutein and zeaxanthin status.
Keywords: brain, cognition, lutein, macula, zeaxanthin
Introduction
Lutein [(3R,3′R,6′R)-β,ε-carotene-3,3′diol or L] and zeaxanthin [(3R,3′R)- β,β-carotene-3,3′diol or Z] are xanthophylls (oxygenated carotenoids) that cross the blood-retina barrier to preferentially accumulate in the macula lutea, the central region in the posterior portion of the primate retina responsible for sharp central vision.1 Meso-zeaxanthin [(3R,3′S)-β,β-carotene-3,3′diol or meso-Z], also a xanthophyll, is generated in the macula from L in a 1:1 ratio.2,3 L, Z and meso-Z are collectively referred to as macular pigment (MP). With an absorption maximum closer to 460 nm, MP attenuates the macula's exposure to potentially damaging blue light.4,5 MP also has an antioxidant role in the photoreceptor outer segments and the retinal pigment epithelium.6,7 Because of these properties, increased MP concentrations (which in this paper will refer to chemically-measured MP in post mortem tissues) has been associated with a reduced risk of age-related macular degeneration (AMD).8 High MP optical density (MPOD, which in this paper will refer to MP measured noninvasively in vivo) also has been associated with risk factors for AMD,9,10 as well as improvements in visual function under disability glare and photostress conditions and better visual acuity in AMD and cataract patients.11,12
Apart from their antioxidant and blue light filtering properties, there is evidence that L and Z as MP improve neural efficiency in the retina. L and Z have been shown to enhance gap junctional communication, which in the retina may be important for light processing and development of neural circuitry in the visual system.13 High MPOD has also been related to increased visual processing speed and reduced scotopic noise.14-17 Furthermore, MPOD has been associated with neural functions in the brain. In an ancillary to the Health, Aging and Body Composition study, MPOD in older adults was significantly related to performance on a variety of cognitive indices designed to assess processing speed, accuracy, and completion ability. These relations remained significant after adjusting for age, sex and ethnicity.18 One possible explanation for the association of MPOD with cognitive function is that MPOD reflects brain status of L and Z or linked factors that enhance brain function.
At present, L and Z concentrations in the brain can only be measured in post mortem tissue, whereas MPOD can be measured noninvasively in humans.19-23 Given the significant association of brain L with cognitive function in older adults 24,25, it is critical to evaluate whether MPOD, measured noninvasively, could be used as a tool to assess brain L and Z status. The objective of the present study was to determine the relationship of L and Z in three retinal regions (4 mm diameter macular region, 4-8 mm annulus and periphery) with brain L and Z in four brain areas (cerebellum, frontal and occipital cortices and pons). The study was done in non-human primates, because these are the only animals possessing a macula.
Methods
Animals and Diets
From birth until completion of the study, 12 rhesus monkeys (Macaca mulatta) were fed one of two semipurified diets that contained adequate levels of all nutrients, including vitamin A and α-tocopherol, but no detectable xanthophylls. The two diets varied only in their fat sources and thus in fatty acid composition, with one containing low levels and one adequate levels of n–3 fatty acids in the form of linolenic acid.26 The animals also received limited amounts of very low xanthophyll foods such as wheat or rice cereals, white rice, sweetened drinks, gelatin, pineapple and banana. Beginning at 7 to 16 years of age, the diet of these monkeys was supplemented with either pure L (n=6) or pure Z (n=6) at 3.9 μmol/kg per day for a duration of 24-101 weeks. These doses were approximately 15 times the levels of the two xanthophylls contained in a standard lab diet (see below). The two supplement groups were balanced to the extent possible with regard to sex, omega-3 status and body weight. L purified by HPLC and synthetic Z were both provided by DSM Nutritional Products, Ltd. (Kaiseraugst, Switzerland). The same monkeys were used in a series of studies that examined the effect of xanthophyll-free diets and L and Z supplementation on macular pigment optical density, retinal xanthophyll levels measured by HPLC and the morphology of the retinal pigment epithelium and photoreceptors, as well as retinal sensitivity to blue light damage.3,5,26-28 The details of the duration and method of supplementation were described in Johnson et al.3
In addition to these monkeys, five normal control monkeys were fed a standard stock diet (Purina 5047 Monkey Chow; Ralston Purina, Richmond, IN) providing a daily carotenoid intake of 0.26 μmol/kg per day L, 0.24 μmol/kg per day Z and 0.035 μmol/kg per day β-carotene. These animals also received an average of ∼3 nmol/kg (<1% of the intake from stock diet) of L plus Z from supplemental fruits and vegetables in their diet. The stock diet-fed monkeys were housed in the same conditions as the L- and Z-fed monkeys.
The characteristics of the L-fed, Z-fed and stock diet-fed monkeys are described in Table 1. All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center and conformed to NIH guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Table 1. Characteristics of the lutein-fed, zeaxanthin-fed and stock diet-fed monkeys.
| Group, Animal ID | Sex | Age (y) | Body wt (kg) | n–3 fatty acid status |
|---|---|---|---|---|
| Lutein-fed | ||||
| 602 | F | 10.0 | 6.7 | Low |
| 585 | F | 10.5 | 5.3 | Low |
| 362 | F | 15.0 | 6.8 | Low |
| 397 | F | 14.6 | 8.6 | Adequate |
| 636 | M | 10.2 | 11.5 | Low |
| 463 | M | 13.8 | 12.0 | Adequate |
|
| ||||
| Zeaxanthin-fed | ||||
| 642 | F | 9.0 | 7.1 | Low |
| 567 | F | 10.9 | 7.9 | Low |
| 224 | F | 18.3 | 7.6 | Low |
| 217 | F | 18.4 | 6.9 | Adequate |
| 586 | M | 11.6 | 12.7 | Low |
| 398 | M | 15.4 | 11.1 | Adequate |
|
| ||||
| Stock diet-fed | ||||
| 259 | F | 9.0 | 8.2 | Adequate |
| 038 | F | 12.0 | 9.0 | Adequate |
| 963 | F | 13.0 | 7.6 | Adequate |
| 950 | F | 13.0 | 5.8 | Adequate |
| 025 | F | 12.0 | 5.8 | Adequate |
Retina and Brain collection
The procedure for collecting retinal tissue has been described in detail by Johnson et al.3 Animals were perfused with 4% paraformaldehyde plus 0.5% glutaraldehyde for morphologic studies of the retina.27,28 Our own pilot work showed that fixation had no effects on measurement of xanthophylls.28 Following perfusion, the retinas were dissected into three retinal areas: a 4 mm diameter circular punch centered on the fovea, the surrounding 4-8 mm annulus, and a portion of peripheral retina. Brain tissue was dissected from the dorsolateral frontal cortex/frontal pole, the occipital cortex, the cerebellum and the pons. The two cortical samples consisted of pure gray matter, which represents the outer 1-2 mm of the cortical surface; the cerebellar tissue was also taken from the outer 2 mm and was primarily gray matter. In contrast, the pons was rich in white matter.
Retinal extraction for carotenoids
The retinal samples were weighed and ground with a glass rod while on ice. To this, 3 mL chloroform-methanol (2:1), 1 mL 0.85% saline and 150 μL echinenone in ethanol (internal standard) were added. The mixture was vortexed for 30 seconds and centrifuged at 800g for 15 minutes at 4°C. The chloroform layer was removed and evaporated to dryness under nitrogen. The extraction was repeated using 3 mL hexane, and the mixture was vortexed and centrifuged as described earlier. The hexane layer was combined with the first extract and evaporated to dryness under nitrogen. The dried residue was reconstituted in 75 μL ethanol, vortexed, and sonicated for 30 seconds. A 60 μL aliquot was used for reverse phase HPLC analysis.3
Brain extraction for carotenoids
The brain extraction procedure described below was adapted from Park et al.29 Approximately 200 mg of brain tissue from each region was homogenized with 0.3 mL saline and 0.5 mL ethanol in a 50 mL glass tube. To the homogenate 50 μL of internal standard and 2 mL of ethanol were added and the mixture was vortexed for 2 mins. The sides of the tube were scraped down to ensure all the tissue was incorporated into the ethanol-saline mixture at the bottom of the tube. The mixture was then incubated in a 70°C water bath for 2 minutes. To the mixture 25% sodium ascorbate (0.5 mL) and 5% sodium hydroxide (1 mL) were added. Saponification was carried out by incubating the mixture in a 60°C water bath for 20 minutes. Following incubation, 0.5 mL distilled water was added and the mixture was cooled for 5 minutes. Five mL of hexane was then added and the mixture was vortexed vigorously for 2 minutes. The mixture was then centrifuged at 1000g for 10 minutes at 4°C. The upper hexane layer was removed and evaporated to dryness under nitrogen in a 40°C water bath. The extraction was repeated with 5 mL of hexane and the mixture was vortexed and centrifuged as described earlier. The second hexane layer was combined with the first extract and evaporation was continued until dryness. The sides of the tube were then washed down with 0.5 mL hexane and evaporation was continued. The dried residue was reconstituted with 100 μL of a 1:1 mixture of ethanol and methyl tert butyl ether, vortexed, sonicated (30 seconds) and transferred to HPLC inserts. The inserts were centrifuged in a microfuge (Eppendorf 5415D, Eppendorf, NY) at 2000g for 3 mins to remove any precipitate. The clear supernatant was then transferred to clean inserts and 20 μL was injected into a reverse phase HPLC system with a C30 carotenoid column (3 μm, 150 × 4.6 mm, YMC, Wilmington, NC). The method described by Yeum et al. was used for carotenoid separation by reverse HPLC.30
Statistics
All data are expressed as mean ± SEM. The differences in L and Z concentrations among the 4 regions of the brain (cerebellum, frontal and occipital cortices and pons) and among the 3 regions of the retina (4 mm macula, 4-8 mm annulus and periphery) were analyzed using one-way repeated measures ANOVA followed by pairwise comparisons of the means with alpha value set at 0.05. Student's T-test was used to evaluate if brain and retinal L concentrations in L-fed monkeys were different from brain and retinal Z concentrations, respectively, in Z-fed monkeys. In the monkeys fed stock diet, differences between L and Z for each tissue were evaluated using one-way repeated measures ANOVA.
For determining the relationship between retinal L and Z and brain L and Z, the L data for the L-fed and stock diet-fed monkeys were combined and the Z data for the Z-fed and stock diet-fed monkeys were combined to obtain a sample size of 11 for each. Retinal meso-Z analyses were not performed for the stock diet fed monkeys. However, three different studies have shown that meso-Z is found in the 4 mm macular region and it forms a significant portion of the macular pigment.2,3,31 The evidence above and our previous finding that meso-Z originates from L and is present in an approximate 1:1 ratio with L in the 4 mm macula was used as the basis for estimating meso-Z concentrations in the macula of monkeys fed stock diet.3 The 4 mm macular Z concentrations for these monkeys were corrected by subtracting the estimated amount of meso-Z (calculated based on the 1:1 ratio with L) from the observed Z values, since meso-Z and Z retention times are the same on a reverse phase HPLC. The sum of L and meso-Z was used to determine the relationship between L in the 4 mm macular region and brain, as this provided a more accurate representation of L that was taken up by the macula.
Normality of the data was assessed using the following criteria before performing correlation tests: (i) histogram showing distribution of data with normal curve superimposed (ii) skewness and kurtosis values between +/− 2 and (iii) statistically non-significant tests of normality. For data that did not satisfy these criteria, correlations were performed after the data were log transformed. Bivariate analysis was used to determine Pearson correlation coefficients, while multivariate analysis was used to determine partial correlation coefficients using age, sex and n–3 fatty acid status as covariates. N–3 fatty acid status was used as a covariate since supplementation of humans with the long-chain n–3 fatty acid docosahexaenoic acid has been shown to increase MPOD in the central retina,32 indicating that n–3 fatty acids may influence macular L and Z uptake. SPSS version 19.0 statistical package was used for all data analyses.
Results
Brain L and Z concentrations
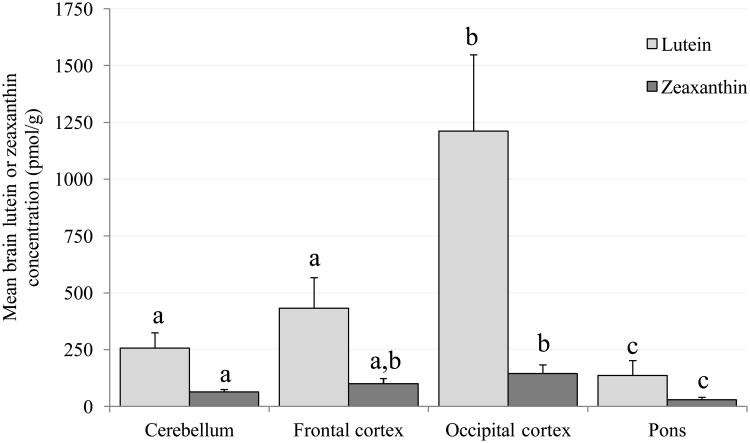
Given the nature of the study design only L was detected in the brain tissues of L-fed monkeys and only Z was detected in Z-fed monkeys. In the stock diet fed monkeys small amounts of β-carotene were detected in tissues of only two brain regions in two monkeys (data not shown). Figure 1A shows the mean concentrations in the cerebellum, frontal and occipital cortices and pons of L and Z in the L-fed and Z-fed monkeys, respectively. L concentration was highest in the occipital cortex and lowest in the pons; concentrations were different (P<0.05) among the four regions, except between the cerebellum and frontal cortex. Similarly, in the Z-fed monkeys, mean Z concentration was lower in the pons than in the other three regions (P<0.05), and lower in the cerebellum than occipital cortex (P=0.036). Despite ingestion of the same dose of L or Z, L concentrations were significantly greater (P<0.05) in the cerebellum and occipital cortex of L-fed monkeys compared to Z concentrations in the Z-fed monkeys. These differences were marginally significant in the frontal cortex (P=0.05). No such difference was observed in the pons between the two groups.
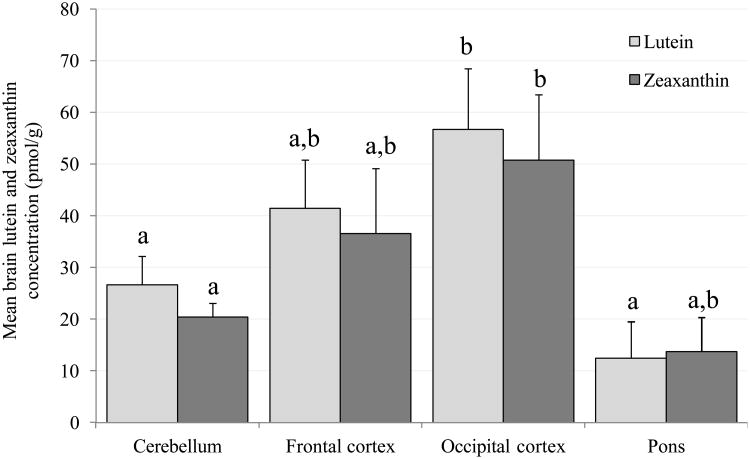
Figure 1.
Mean (± SEM) concentrations of lutein and zeaxanthin in the cerebellum, frontal cortex, occipital cortex and pons. Columns labeled with different letters (a, b or c) represent means that are significantly different at P<0.05, while those labeled with the same letters represent means that are not significantly different from one another (evaluated independently for lutein and zeaxanthin).
A. Xanthophyll-free monkeys fed pure lutein or pure zeaxanthin. Lutein was detected in the brain tissue of the lutein-fed monkeys only, and zeaxanthin was detected in the brain tissue of the zeaxanthin-fed monkeys only.
B. Monkeys fed stock diet. Note that the y-axis scale is different from Figure 1A; lutein and zeaxanthin concentrations are 10-20 times lower for stock diet fed monkeys.
Figure 1B shows the mean L and Z concentrations in the cerebellum, frontal and occipital cortices and pons of the monkeys fed stock diet. Because these animals were fed a normal, non-purified diet, both xanthophylls were present in their brain. Like the L- and Z-fed monkeys, monkeys fed stock diet had the lowest concentrations of L and Z in the pons and the highest in the occipital cortex. Specifically, mean L concentration in the occipital cortex was significantly higher than in the cerebellum (P=0.018) and pons (P=0.047), and mean Z concentration was higher in occipital cortex than in cerebellum (P=0.032). Concentrations of L and Z were not significantly different from each other in any of the four regions.
Compared with the stock diet monkeys, L concentrations in the L-fed monkeys were 10 - 20 times higher (P<0.05) in the cerebellum, frontal and occipital cortices, while Z in the Z-fed monkeys was 2.5 - 3 times higher in the cerebellum (P<0.05), frontal (P=0.05) and occipital (P=0.06) cortices. No significant differences were observed in the pons. Meso-Z analyses were not performed in the brain tissues of stock diet fed monkeys.
Retinal L and Z concentrations
The concentration of L and Z in the 4 mm macular region, 4-8 mm annulus and the periphery of the L-fed and Z-fed monkeys have been previously described in detail by Johnson et al.3 Data are provided again in the context of the relationships with brain xanthophyll concentrations.
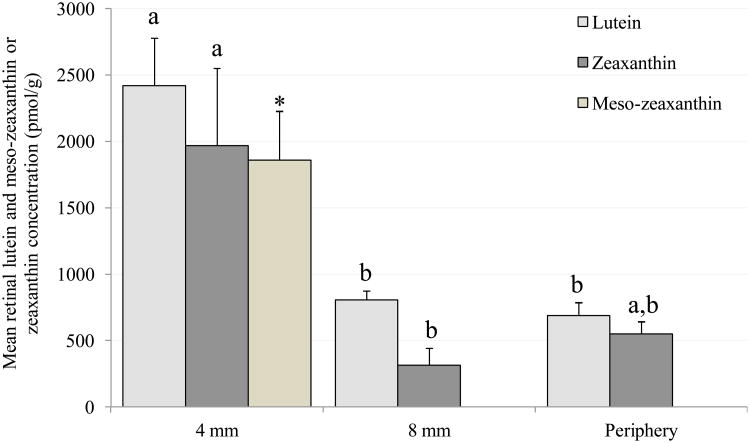
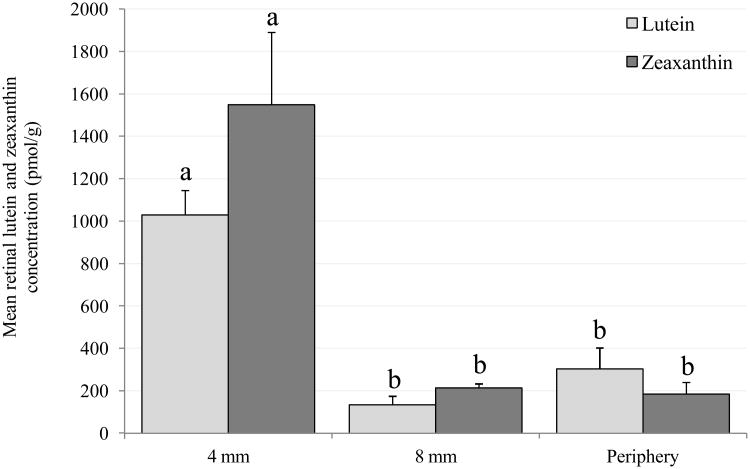
Figure 2A shows mean retinal L and Z concentrations in the L- and Z-fed monkeys, respectively. L concentration was significantly greater in the macular region compared to the annulus (P=0.005) and also the periphery (P=0.004). Meso-Z, which is synthesized from L in the retina, was present only in the 4 mm macular region where its concentration was similar to L. Z concentration in the macula was significantly greater than in the annulus (P=0.041), but in the periphery the difference was only marginally significant (P=0.08). In the macular sample, L concentration in the L-fed monkeys was similar to Z concentration in the Z-fed monkeys; however, the combination of L and meso-Z was approximately twice as high in the L-fed group. In the annular region, L concentration in L-fed monkeys was significantly greater than Z concentration in the Z-fed monkeys.
Figure 2.
Mean (± SEM) concentrations of lutein and zeaxanthin in the retina (4 mm macular region, 4-8 mm annulus and periphery). Columns labeled with different letters (a, b or c) represent means that are significantly different at P<0.05, while those labeled with the same letters represent means that are not significantly different from one another (evaluated independently for lutein and zeaxanthin).
A. Xanthophyll-free monkeys fed pure lutein or pure zeaxanthin. *Meso-zeaxanthin was detected only in the 4 mm macular region of lutein-fed monkeys. Lutein was detected in the retinal tissue of the lutein-fed monkeys only. Zeaxanthin was detected in the retinal tissue of the zeaxanthin-fed monkeys only. These data have been previously reported (3).
B. Monkeys fed stock diet.
Figure 2B shows mean retinal L and Z concentrations in the monkeys fed stock diet. L concentration in the macular region was significantly greater than in the annulus (P=0.002) and periphery (P=0.006). Z (plus meso-Z) concentration was also significantly greater in the macula compared to the annulus (P=0.015) and periphery (P=0.02). Z (plus meso-Z) concentration was greater than L in the macula, while in the periphery L concentration was greater than Z. However, these differences were not statistically significant, probably due to the small sample size.
L concentrations in the macula, annulus and periphery were 2 - 5 times higher (P<0.05) for the L-fed compared to the stock diet-fed monkeys. Z concentrations in the macula were not significantly different between the Z-fed and stock diet fed monkeys. The inability to analyze meso-Z in the stock diet fed monkeys resulted in higher Z (plus meso-Z) concentrations in their macula. A significant (3 fold) difference in Z was observed in the periphery (P=0.049).
Serum L and Z concentrations
Serum was unavailable for the stock diet fed monkeys hence serum L and Z could not be analyzed for these animals. However, serum L and Z was analyzed for the L-fed, Z-fed and a different group of stock diet fed monkeys and these results are described in a previous publication.3 Because these monkeys were fed the same stock diet and were from the same research colony, these published serum values are representative of the stock diet fed monkeys in this study. Serum L and Z were significantly higher in the L-fed and Z-fed monkeys, respectively, compared to the stock diet fed monkeys.3
Relationship between retinal and brain L concentrations
Data for L concentrations in the cerebellum, frontal and occipital cortices, pons and annulus did not meet the normality criteria and were thus log transformed. In the macula, the sum of L and its derivative, meso-Z, was positively related to L in the cerebellum, occipital cortex and pons in a bivariate model. These relationships remained statistically significant after adjusting for age, sex and n–3 fatty acid status. In the frontal cortex the relationship was marginally significant in the age and sex and the age, sex and n–3 status adjusted models (Table 2).
Table 2.
Pearson correlation coefficients between retinal lutein and zeaxanthin and brain lutein and zeaxanthin in the rhesus monkeys (n=11). Data that were not normally distributed were log transformed (indicated in parenthesis) before correlation coefficients were determined.
| Bivariate analysis | Adjusted for age and sex | Adjusted for age, sex and n–3 status | |
|---|---|---|---|
| LUTEIN (4 mm macula) | |||
| Cerebellum (log) | 0.78 (0.005)* | 0.82 (0.007)* | 0.79 (0.019)* |
| Frontal cortex (log) | 0.49 (0.13) | 0.64 (0.066)** | 0.70 (0.055)** |
| Occipital cortex (log) | 0.83 (0.002)* | 0.85 (0.004)* | 0.82 (0.012)* |
| Pons (log) | 0.79 (0.011)* | 0.81 (0.027)* | 0.86 (0.029)* |
|
| |||
| LUTEIN (8 mm annulus log) | |||
| Cerebellum (log) | 0.94 (0.000)* | 0.93 (0.000)* | 0.84 (0.009)* |
| Frontal cortex (log) | 0.49 (0.13) | 0.41 (0.27) | 0.61 (0.11) |
| Occipital cortex (log) | 0.91 (0.000)* | 0.89 (0.001)* | 0.80 (0.017)* |
| Pons (log) | 0.90 (0.001)* | 0.89 (0.007)* | 0.71 (0.113) |
|
| |||
| LUTEIN (periphery) | |||
| Cerebellum (log) | 0.78 (0.005)* | 0.72 (0.028)* | 0.36 (0.39) |
| Frontal cortex (log) | 0.42 (0.21) | 0.20 (0.61) | 0.28 (0.50) |
| Occipital cortex (log) | 0.71 (0.014)* | 0.65 (0.06)** | 0.29 (0.48) |
| Pons (log) | 0.54 (0.13) | 0.46 (0.30) | -0.52 (0.30) |
|
| |||
| ZEAXANTHIN (4 mm macula) | |||
| Cerebellum | 0.70(0.037)* | 0.27 (0.55) | 0.20 (0.71) |
| Frontal cortex | 0.72 (0.018)* | 0.51 (0.24) | 0.41 (0.42) |
| Occipital cortex (log) | 0.59 (0.07)** | 0.63 (0.13) | 0.41 (0.43) |
| Pons | 0.63 (0.05)** | 0.35 (0.45) | 0.26 (0.62) |
|
| |||
| ZEAXANTHIN (8 mm annulus log) | |||
| Cerebellum | −0.46 (0.18) | −0.38 (0.35) | −0.56 (0.19) |
| Frontal cortex | −0.45 (0.16) | −0.22 (0.57) | −0.47 (0.24) |
| Occipital cortex (log) | −0.26 (0.44) | −0.13 (0.75) | −0.18 (0.66) |
| Pons | −0.58 (0.062)** | −0.34 (0.37) | −0.48 (0.23) |
|
| |||
| ZEAXANTHIN (periphery) | |||
| Cerebellum | 0.52 (0.13) | 0.31 (0.46) | 0.33 (0.48) |
| Frontal cortex | 0.23 (0.49) | 0.18 (0.67) | 0.37 (0.41) |
| Occipital cortex (log) | 0.56 (0.07)** | 0.55 (0.16) | 0.61 (0.15) |
| Pons | 0.003 (0.99) | 0.002 (0.10) | 0.05 (0.92) |
Values are correlation coefficients ‘r’ with ‘p’ values in parenthesis.
P<0.05,
P<0.1(marginally significant)
Similarly, L in the annulus was positively associated with L levels in the cerebellum, occipital cortex and pons in a bivariate analysis and also after adjusting for all covariates, except in the pons where the association was not significant after adjusting for age, sex and n–3 status. L in the periphery was significantly associated with L in the cerebellum and occipital cortex in a bivariate model. In the age and sex adjusted model this relationship remained statiscally significant only in the cerebellum, while in the occipital cortex the significance was marginal (Table 2). L in the frontal cortex and pons were not related to lutein in the periphery.
Correlations of macular L alone, without including meso-Z, gave similar results (data not shown).
Relationship between retinal and brain Z concentrations
Data for Z concentrations in the occipital cortex and annulus did not meet the normality criteria and were thus log transformed. The association between Z in the macula (corrected for meso-Z) and brain was statistically significant for the cerebellum, frontal cortex and pons and marginally significant for the occipital cortex in a bivariate analysis (Table 2). However, these relationships were not significant when adjusted for covariates.
No significant relationships were observed between Z in the annulus and brain except in the pons, where there was a marginally significant negative association in the bivariate model. There was a marginally significant association between Z in the periphery and occipital cortex in the bivariate model. No other significant correlations were observed for the periphery (Table 2).
Discussion
The present study is the first to report that L and Z in the macular region of the retina are related to brain L and Z levels. This association is plausible given that the retina is part of the central nervous system. In addition to their close genetic homology to humans (92.5% to 95% for rhesus monkeys), higher primates are the only animals that have a retinal structure similar to humans including the presence of a macula. This makes them the most appropriate model for studying macular diseases and the characteristics of macular xanthophylls.33,34
In both the xanthophyll-supplemented monkeys and those fed stock diet, the highest concentration of L and/or Z was found in the occipital cortex, the primary visual processing area of the brain. Second highest in each case was the frontal cortex, which is responsible for several aspects of higher cognitive function, followed by the cerebellum, which is crucial for motor control and some types of learning.35,36 The pons, a region not associated with visual processing or cognitive function,37 had the lowest levels of both L and Z. The presence and observed distribution of L and Z in these four regions could possibly be an indication of a functional role of L and Z in the brain. Transneuronal transport from the retina may also have contributed to the higher L and Z concentration in the occipital cortex.38,39 In addition, the cortical samples consisted entirely of gray matter, and the cerebellum sample was predominantly gray matter, whereas the pons was rich in white matter. Therefore the differences among tissues could also reflect differences in the content of xanthophylls in these two types of tissue. However, a previous study of human brain tissue did not find substantial differences in xanthophyll content between white and gray matter.24
Even though the xanthophyll-free monkeys ingested the same dose of either L or Z, the L-fed monkeys had almost 10-20 times more L in the cerebellum, frontal cortex and occipital cortex compared to Z in the Z-fed monkeys. Thus, there appeared to be differential uptake of L and Z in the brain tissue at the supraphysiologic doses used in this study. This was not the case at the physiological doses of L and Z present in the stock diet. Brain L concentrations tended to be higher than Z in the monkeys fed stock diet but the differences were not statistically significant. Possible explanations for the observed limitation in Z uptake in the brain of Z-fed monkeys may be (i) decreased expression of Z binding proteins compared to L in the brain, (ii) differences in uptake mechanisms, (iii) greater functional usage of Z and/or (iv) a consequence of life-long deficiency of xanthophylls in these monkeys.
The accumulation of L and Z was highest in the macular region for both the supplemented and the stock diet monkeys. This is consistent with reported literature that the concentration of xanthophylls decreases with increasing eccentricity from the central foveal region.7 It is noteworthy that even though the supplemented monkeys received approximately 15 times more L and Z compared to the monkeys fed stock diet, the retinal L and Z levels in the supplemented monkeys were only about 2-5 times greater than monkeys fed stock diet. This contrasts with levels in brain tissue, which were 10-20 times higher in the supplemented animals. Thus, in the monkeys fed stock diet retinal concentrations of L and Z exceeded brain concentrations by an order of magnitude. Saturation of carotenoid binding proteins in the retina could be a possible explanation for the limitation in retinal uptake of L and Z at supraphysiological doses.
The results indicate that L levels in the macula and annulus of the retina reflect L status in the cerebellum, frontal and occipital cortices and the pons. These relationships were less strong for Z. The level of significance was marginal, probably due to the small sample size, although the correlation coefficients indicate that the strength of the association was large. Unlike L, the relationships between macular Z and brain Z were attenuated after adjusting for covariates. This was probably due to the observed significant association of age with Z concentrations in the cerebellum, frontal and occipital cortices and macula. The Z-fed monkeys were slightly older (mean: 13.9, SD: 4.0, range: 9.0-18.4) compared to the L-fed monkeys (mean: 12.4, SD: 2.4, range 10.0-15.0). However, the small sample size limits further exploration of the differences in uptake of L and Z into the neural tissue with age. Another possibility for the weaker correlation with Z could be the lower concentrations of Z in the brain compared to L. One limiting factor of this study was that meso-Z analyses were not performed in the retina of the stock diet fed monkeys due to the unavailability of the HPLC instrumentation required for measuring meso-Z at the time. However, published results on the ratio of L to meso-Z in the macula provided a rational approach for estimating meso-Z levels.2,3,31 Another limitation of this study was the evaluation of two different study populations (monkeys fed stock diet and xanthophyll-free monkeys supplemented with L or Z). However, the wide range of L and Z intakes and the subsequently resulting wide range in tissue concentrations may have strengthened our ability to measure correlations between retina and brain xanthophyll concentrations.
MPOD can be measured using psychophysical techniques such as heterochromatic flicker photometry, which require active perceptual judgments by the subject, or other techniques such as fundus reflectometry, fundus autofluorescence and resonance Raman spectroscopy. An integrated measure of total MPOD has been shown to highly correlate with macular L and Z measured by HPLC.40 Given our findings of a positive association between macular and brain L and the trend for such an association with Z, an integrated measure of total MPOD has the potential to be used as a tool to assess brain L and Z status. Assessing brain L and Z status noninvasively has become relevant with the emerging data on the relationship of these xanthophylls to cognitive function. Brain L concentrations were positively associated with pre-mortem measures of global cognitive function and executive function in centenarians.25 Brain L concentration was also significantly lower in centenarians with mild cognitive impairment compared to those with normal cognitive function.25 In an exploratory, randomized, double-blinded placebo controlled trial, older women taking L supplementation (12 mg/d) for 4 months significantly improved their scores on a verbal fluency test, showing the ability of L to improve cognitive function measures possibly through increasing brain L concentrations.41 There is also epidemiological evidence for the beneficial effects of L on cognition.42,43
In conclusion, our results demonstrate that biochemically measured concentrations of L and possibly Z in the macula are a biomarker of brain L and Z status in primates. Therefore, an integrated measure of total MPOD may be a useful tool in evaluating the role of xanthophylls in cognitive function.
Acknowledgments
The authors would like to thank Noelle Landauer, Lauren Renner and Alison Weiss for tissue collection, Emily Eggert for help with some of the tissue extractions and Gerard Dallal and Tammy Scott for advice on statistical analysis.
Funding sources: USDA 581950-7-07 and DSM Nutritional Products Ltd., NIH grants DK29930 and RR000163, and The Foundation Fighting Blindness
References
- 1.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29(6):843–849. [PubMed] [Google Scholar]
- 2.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- 3.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46(2):692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 4.Junghans A, Sies H, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys. 2001;391(2):160–164. doi: 10.1006/abbi.2001.2411. [DOI] [PubMed] [Google Scholar]
- 5.Barker FM, Snodderly DM, Johnson EJ, et al. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n–3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci. 2011 Jun 1;52(7):3934–3942. doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krinsky NI. Antioxidant function of carotenoids. Free Radic Biol Med. 1989;7(6):617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed SS, Lott MN, Marcus DM. The macular xanthophylls. Surv Ophthalmol. 2005;50(2):183–193. doi: 10.1016/j.survophthal.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study.[Erratum appears in Invest Ophthalmol Vis Sci 2001 Mar;42(3):548] Invest Ophthalmol Vis Sci. 2001 Jan;42(1):235–240. [PubMed] [Google Scholar]
- 9.Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001 Feb;42(2):439–446. [PubMed] [Google Scholar]
- 10.Nolan JM, Stack J, O' Donovan O, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84(1):61–74. doi: 10.1016/j.exer.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci. 2008;85(2):82–88. doi: 10.1097/OPX.0b013e318162266e. [DOI] [PubMed] [Google Scholar]
- 12.Richer S, Stiles W, Statkute L, et al. Double masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veteran's LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75(4):216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 13.Stahl W, Seis H. Effects of carotenoids and retinoids on gap junctional communication. Biofactors. 2001;15(2-4):95–98. doi: 10.1002/biof.5520150209. [DOI] [PubMed] [Google Scholar]
- 14.Hammond BR, Wooten BR. CFF thresholds: relation to macular pigment optical density. Ophthalmic Physiol Opt. 2005;25(4):315–319. doi: 10.1111/j.1475-1313.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Renzi LM, Hammond BR. The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol Opt. 2010;30(4):351–357. doi: 10.1111/j.1475-1313.2010.00720.x. [DOI] [PubMed] [Google Scholar]
- 16.Zimmer JP, Hammond BR. Possible influences of lutein and zeaxanthin on the developing retina. Clin Ophthalmol. 2007;1(1):11. [PMC free article] [PubMed] [Google Scholar]
- 17.Gutherie AH, Hammond BR. Macular pigment and scotopic noise. ARVO Abstracts [abstract] Invest Ophthalmol Vis Sci. 2005:E1784. [Google Scholar]
- 18.Renzi LM, Iannaccone A, Johnson EJ, Kritchevsky SB. The relation between serum xanthophylls, fatty acids, macular pigment and cognitive function in the Health ABC Study (abstract) FASEB J. 2008;22 [Google Scholar]
- 19.Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40(11):2481–2489. [PubMed] [Google Scholar]
- 20.Bernstein PS, Zhao DY, Sharifzadeh M, Ermakov IV, Gellermann W. Resonance Raman measurement of macular carotenoids in the living human eye. Arch Biochem Biophys. 2004;430(2):163–169. doi: 10.1016/j.abb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Bone RA, Brener B, Gibert JC. Macular pigment, photopigments, and melanin: Distributions in young subjects determined by four-wavelength reflectometry. Vision Res. 2007;47(26):3259–3268. doi: 10.1016/j.visres.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A. 2001;18(6):1212–1230. doi: 10.1364/josaa.18.001212. [DOI] [PubMed] [Google Scholar]
- 23.Berendschot TTJM, van Norren D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Arch Biochem Biophys. 2004;430(2):149–155. doi: 10.1016/j.abb.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8(3):156–162. [PubMed] [Google Scholar]
- 25.Johnson EJ, Vishwanathan R, Schalch W, et al. Brain levels of lutein (L) and zeaxanthin (Z) are related to cognitive function in centenarians. FASEB J. 2011;25(975.21) [Google Scholar]
- 26.Neuringer M, Snodderly DM, Sandstrom M, Johnson E, Schalch W. Nutritional manipulation of primate retinas: I. Effects of lutein or zeaxanthin supplements of serum and macular pigment of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2004;45(9):3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 27.Leung IYF, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: Effects of age, n–3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004 Sep 1;45(9):3244–3256. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- 28.Leung IYF, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas. IV. Effects of n-3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp Eye Res. 2005;81(5):513–529. doi: 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Hwang HJ, Kim MK, Lee-Kim YC. Effects of dietary fatty acids and vitamin E supplementation on antioxidant vitamin status of the second generation rat brain sections. Korean J Nutr. 2001;34(7):754–761. [Google Scholar]
- 30.Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64(4):594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 31.Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997 Feb;64(2):211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 32.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008 May;87(5):1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- 33.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004 Sep 3;305(5689):1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 34.Wald G. Human vision and the spectrum. Science. 1945;101(2635):6. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- 35.Thach WT. What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci. 1998;2(9):331–337. doi: 10.1016/s1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- 36.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2(9):338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 37.American Association of Neurological Surgeons. [Accessed August 22, 2011];Anatomy of the Brain. 2006 Jun; Available from: http://www.aans.org/PatientInformation/ConditionsandTreatments/AnatomyoftheBrain.aspx. Available at.
- 38.Wiesel TN, Hubel DH, Lam DMK. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Research. 1974;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- 39.Hubel DH, Wiesel TN, LeVay S. Plasticity of Ocular Dominance Columns in Monkey Striate Cortex. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 40.Handelman GJ, Snodderly DM, Krinsky NI, Russett MD. Biological control of primate macular pigment. Biochemical and densitometric studies. Invest Ophthalmol Vis Sci. 1991;32:257–267. [PubMed] [Google Scholar]
- 41.Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008 Apr;11(2):75–83. doi: 10.1179/147683008X301450. [DOI] [PubMed] [Google Scholar]
- 42.Morris MC, Evans MD, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005;57(5):713–720. doi: 10.1002/ana.20476. [DOI] [PubMed] [Google Scholar]