Abstract
Store-operated calcium entry (SOCE) is activated in response to depletion of the endoplasmic reticulum-Ca2+ stores following stimulation of plasma membrane receptors that couple to PIP2 hydrolysis and IP3 generation. Search for the molecular components of SOCE channels led to the identification of mammalian transient receptor potential canonical (TRPC) family of calcium-permeable channels (TRPC1–TRPC7), which are all activated in response to stimuli that result in PIP2 hydrolysis. While several TRPCs, including TRPC1, TRPC3, and TRPC4, have been implicated in SOCE, the data are most consistent for TRPC1. Extensive studies in cell lines and knockout mouse models have established the contribution of TRPC1 to SOCE. Furthermore, there is a critical functional interaction between TRPC1 and the key components of SOCE, STIM1, and Orai1, which determines the activation of TRPC1. Orai1-mediated Ca2+ entry is required for recruitment of TRPC1 and its insertion into surface membranes while STIM1 gates the channel. Notably, TRPC1 and Orai1 generate distinct patterns of Ca2+ signals in cells that are decoded for the regulation of specific cellular functions. Thus, SOCE appears to be a complex process that depends on temporal and spatial coordination of several distinct steps mediated by proteins in different cellular compartments. Emerging data suggest that, in many cell types, the net Ca2+ entry measured in response to store depletion is the result of the coordinated regulation of different calcium-permeable ion channels. Orai1 and STIM1 are central players in this process, and by mediating recruitment or activation of other Ca2+ channels, Orai1–CRAC function can elicit rapid changes in global and local [Ca2+]i signals in cells. It is most likely that the type of channels and the [Ca2+]i signature that are generated by this process reflect the physiological function of the cell that is regulated by Ca2+.
1. INTRODUCTION
Store-operated calcium entry (SOCE) is a ubiquitous Ca2+ entry pathway that is activated in response to stimulation of plasma membrane receptors that are coupled to PIP2 hydrolysis, IP3 generation, and IP3-mediated Ca2+ release from the endoplasmic reticulum (ER). The primary trigger for activation of SOCE is the depletion of the ER-Ca2+ store, while refilling of this store leads to inactivation. The first store-operated Ca2+ current, ICRAC, was measured in mast cells and T lymphocytes (Parekh & Penner, 1997). Later studies revealed currents with varying characteristics in other cell types (Liu, Groschner, & Ambudkar, 2004; Parekh & Putney, 2005). These observations suggested possible cell-specific differences in the channels mediating SOCE, which likely depend on the components of the channel in different cell types and the physiological function regulated by SOCE in a particular cell. The critical mechanism that senses the status of ER-[Ca2+] and regulates plasma membrane channels, as well as the channels involved in mediating SOCE, remained a challenge to researchers in the field for more than two decades until they were elucidated fairly recently (Hogan, Lewis, & Rao, 2010; Parekh & Putney, 2005; Prakriya, 2009; Venkatachalam & Montell, 2007).
Search for the molecular components of SOCE channels led to the identification of mammalian transient receptor potential canonical (TRPC) family of calcium-permeable channels, which are all activated in response to stimuli that result in PIP2 hydrolysis (Venkatachalam & Montell, 2007). Studies reported by a large number of investigators suggest that TRPC members contribute to SOCE, although data for some TRPCs are not very consistent. For example, activation of TRPC5, TRPC6, and TRPC7 in most cases is achieved via store-independent mechanisms. The available data are most consistent and strongest in support of a role for TRPC1 in SOCE. TRPC3 and TRPC4 have also been shown to be activated by store depletion, although in relatively less number of studies. Very early studies with these channels established that activation of TRPC1 and other TRPC channels generate relatively nonselective cation currents termed ISOC (Liu, Singh, & Ambudkar, 2003) that are distinct from ICRAC, a highly inwardly rectifying Ca2+-selective current mediated by the calcium release activated calcium (CRAC) channel (Cahalan & Lewis, 1990; Hoth & Penner, 1993).
Recent identification of two other critical components of SOCE has transformed and rapidly advanced our knowledge of SOCE. STIM1 is an ER-Ca2+-binding protein that senses ER-[Ca2+] status and transmits the store depletion signal to plasma membrane channels. It was shown that STIM1 aggregates in response to ER-Ca2+ store depletion and translocates to as yet poorly defined microdomains in the cell periphery where it interacts with and activates the channels mediating SOCE (Liou et al., 2005; Roos et al., 2005). Importantly, Orai1, a four-transmembrane channel protein, was identified as the pore-forming component of CRAC channels, and it was confirmed that Orai1 and STIM1 are sufficient for generating ICRAC, thus ending the long search for CRAC channel components (Feske et al., 2006; Vig et al., 2006). Several studies show that STIM1 also gates TRPC1–SOC channels, and furthermore, it has now been established that activation of TRPC1/STIM1 channels requires functional Orai1 (Cheng, Liu, Ong, & Ambudkar, 2008; Huang et al., 2006; Kim, Zeng, et al., 2009). Thus, SOCE appears to be a complex process that depends on temporal and spatial coordination of several distinct steps mediated by proteins in different cellular compartments. Emerging data suggest that, in many cell types, Ca2+ entry measured in response to store depletion is determined by the coordinated regulation of different calcium-permeable ion channels. For example, activation of Orai1 channels can lead to activation of TRPC channels (Fig. 7.1) (Cheng, Liu, Ong, Swaim, & Ambudkar, 2011). Conversely, activation of Orai1–CRAC can be associated with inhibition of another channel, such as the voltage-gated Ca2+ channel (Park, Shcheglovitov, & Dolmetsch, 2010; Wang et al., 2010). Regulation of other Ca2+ signaling components (e.g., IP3Rs, PMCA, or mitochondria) can also modulate the final Ca2+ signal generated in the cell.
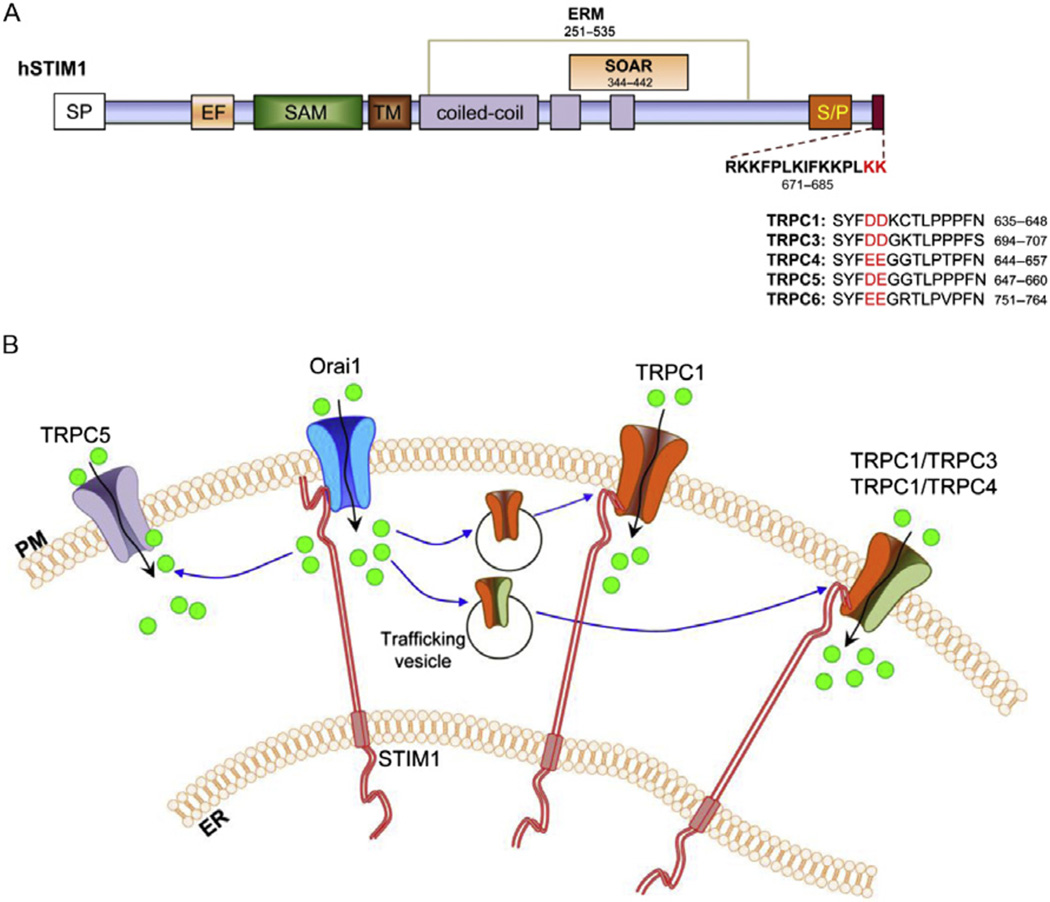
Figure 7.1.
(A) Schematic model showing the different domains of STIM1. The polybasic tail in the C-terminal end is expanded, with the lysines mediating electrostatic interactions with TRPCs in red. The negatively charged residues in the C-terminus of TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 that binds electrostatically to the lysines are also in red. The ezrin/radixin/moesin (ERM) and STIM1-Orai1-activating region (SOAR) domains are denoted in the model. SP, signaling peptide; EF, EF hand; SAM, sterile α motif; TM, transmembrane; and S/P, serine/proline-rich. (B) Model showing cellular events activated Orai1-mediated Ca2+ entry. Following depletion of the ER-Ca2+ stores, STIM1 aggregates and translocates to the ER–PM junctional regions where it binds to and activates the Orai1 channel. The Orai1-mediated Ca2+ entry may induce the trafficking of homomeric TRPC channels or heteromeric TRPC channels (e.g., TRPC1/TRPC3 and TRPC1/TRPC4) to the cell surface, following by insertion into the plasma membrane and subsequent gating by STIM1. Additionally, the extracellular Ca2+ entering via Orai1 may also directly activate other channels, such as TRPC5. ER, endoplasmic reticulum; PM, plasma membrane.
Typical reagents used for activation of SOCE include physiologically relevant agonists as well as agents (ER-Ca2+ pump inhibitors) that lead to passive depletion of the ER Ca2+ store such as thapsigargin (Tg) and di-tert-butylhydroquinone. The current criteria used to identify SOCE are (i) activation of Ca2+ entry and calcium currents by agonists, Tg, or IP3 (the latter is included in the pipette solution for current measurements); (ii) inhibition of both activities by 1–5 µM Gd3+ and 10–20 µM 2APB; and (iii) requirement of STIM1. The best-characterized channel among the store-operated calcium channels is the CRAC channel, which generates ICRAC, an inwardly rectifying current with a reversal potential (Erev) of >+50 mV (Parekh & Putney, 2005). There is now relatively detailed understanding of the pore characteristics of the Orai1–CRAC channel (Hogan et al., 2010; Prakriya, 2009). More recently, the crystal structure of a hexameric Orai1 channel has been resolved (Hou, Pedi, Diver, & Long, 2012). Details of recent studies of STIM1–Orai1 interactions as well as regulation of CRAC channel function will be discussed in further detail in other chapters of this issue. In the following sections of this chapter, we will describe and discuss the contribution of TRPC channels to SOCE, with a focus on TRPC1 and the mechanism(s) of activation as well as the functional interactions between TRPC and Orai1 channels.
2. PHYSIOLOGICAL FUNCTIONS OF TRPC CHANNEL-MEDIATED SOCE
There are six TRPC proteins in humans (TRPC1, TRPC3–TRPC7), which have been divided into two subfamilies, TRPC1/TRPC4/TRPC5 and TRPC3/TRPC6/TRPC7, based on biochemical and functional similarities. The remaining member, trpc2, is a pseudogene in humans but is expressed in other species, with a rather selective expression in some tissues (Vannier et al., 1999). Furthermore, a wide range of physiological functions have been attributed to SOCE mediated by various TRPC channels (Ambudkar et al., 2006; Feske, 2009; Hogan et al., 2010; Venkatachalam & Montell, 2007). TRPC proteins have six transmembrane domains, with a proposed pore domain spanning the region between the fifth and sixth transmembrane domains. The involvement of this region in ion channel permeability has been confirmed in a number of studies with different TRPC proteins (Liu et al., 2003; Owsianik, Talavera, Voets, & Nilius, 2006). The first evidence that endogenous TRPC1 is involved in SOCE, as well as confirmation of its contribution to Ca2+ permeability, came from studies in human submandibular gland (HSG) ductal cells showing that knockdown of TRPC1 decreases SOCE and mutations in the putative pore domain either changes the cation permeability or completely blocks pore function (Liu et al., 2003, 2000). Although exogenously expressed TRPC1 does not consistently result in an increase in SOCE, knockdown of TRPC1 has been consistently associated with reduction of store depletion-mediated Ca2+ entry in a variety of cell types, including keratinocytes, platelets, smooth, skeletal, and cardiac muscles, DT40, HEK293, salivary gland, neuronal, intestinal, and endothelial cells (Beech, 2005; Cai et al., 2006; Dietrich, Chubanov, Kalwa, Rost, & Gudermann, 2006; Fiorio Pla et al., 2005; Liu, Cheng, et al., 2007; Liu et al., 2000; Mehta et al., 2003; Mori et al., 2002; Rao et al., 2006; Tiruppathi, Ahmmed, Vogel, & Malik, 2006; Vandebrouck, Martin, Colson-Van Schoor, Debaix, & Gailly, 2002; Zagranichnaya, Wu, & Villereal, 2005). Conclusive evidence has been provided by studies using a knockout mouse model which showed that salivary gland and pancreatic acinar cells from TRPC1−/− mice display strong reductions in SOCE and Ca2+-dependent regulation of cell function (fluid secretion and Ca2+-dependent K+ channel activation) (Hong et al., 2011; Liu, Cheng, et al., 2007). Kochukov, Balasubramanian, Noel, and Marrelli (2013) reported that TRPC1 contributes to Ca2+ influx and vasorelaxation of aorta in primary aortic endothelial cells and TRPC1-mediated Ca2+ entry is required in skeletal muscles for maintenance of force (Zanou et al., 2010). Conversely, upregulation of TRPC1 channel function was associated with proliferation of airway smooth muscle cells (Sweeney et al., 2002) and embryonic rat neural stem cells (Fiorio Pla et al., 2005), as well as modulation of endothelial barrier permeability (Ahmmed et al., 2004; Paria et al., 2004). Interestingly, some tissues in the knockout mice do not demonstrate any changes in SOCE (Dietrich et al., 2007; Varga-Szabo et al., 2008), whereas a recent study showed enhanced calcium signaling in bone marrow-derived mast cells (Medic et al., 2013). Such discrepancies in the apparent redundancy of TRPC1 in different tissues remain to be fully understood.
TRPC3, TRPC4, and TRPC7 have also been implicated in SOCE. While overexpression of TRPC3 increases SOCE in HEK293 cells, the mode of regulation appears to depend on its level of expression. Nonetheless, knockdown of TRPC3 transcript in cell lines or deletion of the protein in mouse models decreases SOCE with consequent effects on cell function (Kim et al., 2006; Zagranichnaya et al., 2005). Loss of TRPC3 in pancreatic acinar and submandibular gland cells harvested from knockout mice exhibited significantly reduced (>50%) SOCE. Furthermore, severity of acute pancreatitis was less in the TRPC3−/− mice, as indicated by the low levels of pancreatic edema and serum amylase (Kim, Hong, et al., 2009). While TRPC3−/− mice have also been reported to exhibit impaired motor coordination and abnormal walking behavior (Hartmann et al., 2008), whether TRPC3 mediates SOCE following activation of metabotropic glutamate receptors in neurons remains to be elucidated. A role for TRPC4 in SOCE has been proposed based on the suppression of function by knockdown of TRPC4 expression and inhibition by TRPC4 antibodies in mouse mesangial cells and smooth muscle cells, respectively. In addition, TRPC4−/− mice display loss of store-operated current in adrenal, endothelial, and smooth muscle cells, resulting in impaired endothelial cell function, vascular smooth muscle contractility, and lung microvascular permeability (Freichel et al., 2001, 2004; Philipp et al., 2000; Tiruppathi et al., 2002). SOCE was attenuated following knockdown of TRPC5 in human monocytes and thus the channel may have an important role in the pathogenesis of hypertension (Liu, Thilo, et al., 2007). Even though TRPC6 and TRPC7 have been implicated in SOCE in a few studies, these channels are largely believed to be receptor-activated (Ambudkar, Ong, Liu, Bandyopadhyay, & Cheng, 2007; Venkatachalam & Montell, 2007). Another interesting, but complicated, aspect of TRPC channels appears to be their ability to form heteromeric channels. While studies using heterologously expressed channels show that TRPC1 can interact with TRPC4/TRPC5, and TRPC3 can interact with TRPC6/TRPC7 (Goel, Sinkins, & Schilling, 2002; Hofmann, Schaefer, Schultz, & Gudermann, 2002; Strubing, Krapivinsky, Krapivinsky, & Clapham, 2003), it is important to note that very little is reported thus far regarding the status of endogenous heteromeric TRPC channels and the physiological functions that may be ascribed to these channels. Endogenous TRPC1/TRPC3 involvement in SOCE has been reported in human parotid gland ductal cells (Liu, Bandyopadhyay, Singh, Groschner, & Ambudkar, 2005) and rat H19-7 hippocampal cell lines (Wu, Zagranichnaya, Gurda, Eves, & Villereal, 2004); TRPC1/TRPC5 in vascular smooth muscle (Beech, 2005); TRPC1/TRPC4 in endothelial cells (Sundivakkam et al., 2012); and TRPC1/TRPC3/TRPC7 in HEK293 cells (Zagranichnaya et al., 2005).
3. CHARACTERISTICS OF TRPC CHANNELS
TRPC channels have been reported to be nonselective cation channels with low Ca2+ selectivity in contrast to CRAC channels that are highly Ca2+ selective. The channels display permeability to K+, Na+, Cs+, Ca2+, Ba2+, and other divalents to varying degrees (Parekh & Penner, 1997). When activated by agonist and store-depleting conditions, TRPC channels typically display currents with relatively linear current–voltage relationship that have been called ISOC, to differentiate them from ICRAC (Ong & Ambudkar, 2011). Reversal potentials for ISOC range from zero to slightly positive (Kim, Zeng, et al., 2009; Liu et al., 2000; Seth et al., 2009), which differs from the Erev of >+50 mV for ICRAC. The electrophysiological characteristics of currents measured in various cells depend on the type of TRPC channels involved. For example, in HSG cells, the macroscopic current primarily determined by TRPC1 displays a slight inward rectification with Erev≈ +15 mV (Liu et al., 2003). In addition to TRPC1, TRPC5 and TRPC4 channels are also relatively Ca2+ selective. TRPC5 displays a distinct current–voltage relationship with double rectification due to a Mg2+ block that is relieved at +mV (Blair, Kaczmarek, & Clapham, 2009; Obukhov & Nowycky, 2008). TRPC4 is the only TRPC channel that was reported to display an inwardly rectifying cation current in bovine adrenal cells (Philipp et al., 2000). However, this property of TRPC4 has not been observed in other cell types. In most other cases, TRPC4-associated currents are relatively nonselective. Overexpressed TRPC3 and TRPC6 channels when directly activated by DAG or via agonist stimulation display linear currents (Dietrich, Kalwa, Rost, & Gudermann, 2005; Parekh & Putney, 2005; Venkatachalam & Montell, 2007). Relatively fewer studies have been done with TRPC7.
TRPCs are capable of forming multiple heteromeric and/or homomeric channels, which could potentially lead to a plethora of channels displaying a wide variety of properties. For example, a heteromeric store-operated TRPC1/TRPC4 channel has been identified in endothelial cells, which conducts an inwardly rectifying ISOC current with a Erev near +40 mV. Orai1 interacts with both TRPC4 and TRPC1 upon ER-Ca2+ store depletion (Cioffi et al., 2012). Loss of Orai1 coincides with a left shift in the reversal potential suggesting that Orai1 either contributes to the measured macroscopic current or influences the calcium selectivity of the TRPC1/TRPC4 channel. On the other hand, TRPC1 and TRPC3 heteromeric channel mediates a nonselective cation channel that exhibits a linear current– voltage relationship with a Erev of about 0 mV in parotid salivary gland cell line (Liu et al., 2005). The heteromeric TRPV4/TRPC1 channel displays distinct electrophysiological properties that differ from those generated by homomeric TRPV4 channels (Ma, Nilius, Wong, Huang, & Yao, 2011). Heteromeric TRPV4/TRPC1 channels show a strong field strength cation binding site, in contrast to a weak field strength binding site reported for homomeric TRPV4 channels. Moreover, heteromeric TRPV4/TRPC1 channels are slightly more permeable to Ca2+ with reduced sensitivity to inhibition by extracellular Ca2+ than homomeric TRPV4 channels (Ma et al., 2011).
SOC and CRAC channels markedly vary in their single-channel conductance. Direct and conclusive measurements of the single-channel conductance for ICRAC have not yet been reported, although an extremely small single-channel conductance (<<1 pS) has been suggested based on noise analysis (Parekh & Putney, 2005). TRPC channel activity has been recorded at the single-channel level for TRPC1, TRPC3, and TRPC5. TRPC1 was reported to have a single-channel conductance of 22.8 pS in HEK293 cells (Zhang et al., 2009); 16–17 pS in rat sympathetic neuron cells and HEK293 cells (Skopin et al., 2013); and 20 pS in HSG cells (Liu et al., 2003). On the other hand, heteromeric expression of TRPC1/TRPC5 channel proteins produces single-channel currents with a unitary conductance of about 5 pS (Strubing, Krapivinsky, Krapivinsky, & Clapham, 2001), which is similar to endogenous TRPC1 + TRPC5 channels found in vascular smooth muscle (2–5 pS) (Golovina et al., 2001; Trepakova et al., 2001). TRPV4 and TRPC1 in HEK293 cells display conductance of ~95 pS for outward currents and ~83 pS for inward currents (Ma et al., 2011).
As discussed further in this review, the ISOC current might not be solely mediated by TRPC channels as has been believed thus far. In fact, the whole cell current measured is likely to be a mixed current with contributions from different channels. For example, the current in HSG cells is not solely mediated by TRPC1 but also has contributions from Orai1. Since TRPC1 function is lost when Orai1 activity is suppressed, the exact properties of TRPC1 channels per se following store depletion have not yet been resolved. Similar conclusions can be made regarding cells that have other TRPC channel activities. Based on recent studies elucidating the regulation of TRPC channels following store depletion, it is important to view previously reported characteristics of ISOC currents with some caution. Further studies are necessary to conclusively describe currents exclusively mediated by TRPC channels.
4. TRPC1 CHANNEL IN SOCE
TRPC1, the first mammalian TRPC protein to be identified, is widely expressed in both neuronal as well as nonneuronal tissues (Ambudkar et al., 2007; Venkatachalam & Montell, 2007). Support for a role for TRPC1 in SOCE was provided by several earlier observations showing that knockdown of endogenous TRPC1 resulted in a decrease in SOCE in several tissues (Ahmmed et al., 2004; Fiorio Pla et al., 2005; Paria et al., 2004; Sweeney et al., 2002). This was further confirmed using the TRPC1 knockout mouse model. Severe loss of salivary gland fluid secretion and SOCE was observed in TRPC1−/− mice (Liu, Cheng, et al., 2007). Further, pancreatic acinar cells from these mice displayed defects in SOCE and Ca2+-activated Cl− channel function (Hong et al., 2011). Decreased SOCE was also detected in dopaminergic neurons (Fiorio Pla et al., 2005) and smooth muscle cells (Sweeney et al., 2002). However, some tissues in this mouse model did not have SOCE defects, indicating possible cell or and tissue-specific functions for TRPC1.
The mechanism by which TRPC1 gets activated following store depletion was established by studies showing that TRPC1 binds and clusters with STIM1 and is gated via electrostatic interaction of STIM1(684KK685) with TRPC1(639DD640) residues (Zeng et al., 2008). The acidic residues involved in the gating by STIM1 are conserved in all TRPC channels and all TRPC channels have the inherent ability to bind and be gated by STIM1. This was demonstrated in using heterologously expressed TRPC channels with STIM1 (Lee, Yuan, So, Worley, & Muallem, 2010) where the individual TRPC channels showed activation by STIM1. However, it has been reported that not all TRPC channels are gated by STIM1 even when they appear to contribute to SOCE, for example, TRPC5 (Yuan, Zeng, Huang, Worley, & Muallem, 2007). Nevertheless, there is strong evidence that activation of TRPC channels following store depletion is dependent on Orai1. The exact mode by which Orai1 determines TRPC1 function has also been established (further discussed below). While TRPC1 can form homomeric channels, it has also been shown to interact with other TRPCs members including TRPC3, TRPC4, and TRPC5. This association is mediated via interactions between the N-terminal domains of the monomers (Liu et al., 2005). An interesting hypothesis suggests that association of other, nonstore-dependent, TRPC members with TRPC1 can potentially endow the former with store-dependent regulation (Ambudkar et al., 2007; Yuan et al., 2007). The concept proposed was that TRPC1 in the channel complex will be the component that binds to STIM1 and mediate gating by STIM1. Alternatively, since other TRPCs have the capacity to bind and be activated by STIM1, they need to have the correct localization in the cell to be activated by STIM1. As discussed above, the channels need to be localized in the microdomain where STIM1 puncta are tethered in the cell periphery. Further, they will also need to be located in close vicinity to Orai1. Since TRPC1 has a propensity to be localized in this region and also be translocated into these domains upon stimulation (Cheng et al., 2011; Ong et al., 2007; Pani & Singh, 2009), heteromerically interacting TRPCs will be trafficked together with TRPC1. In the absence of binding to TRPC1, these other TRPCs can possibly be localized in different regions of the cell where they cannot be regulated by STIM1. Thus, TRPC1 can serve as an important determinant of the regulation of other TRPC channels. More significantly, ion permeability of heteromeric TRPC channels will be determined by the relative composition of the channels, and this could have a significant impact on the downstream functions that are being regulated. Whether all TRPC channels are gated by STIM1 and are dependent on Orai1 for function under physiological conditions is yet to be established. Moreover, current exclusively mediated by TRPC1/STIM1 channels has not yet been measured.
4.1. TRPC–STIM1–Orai1 complex
In addition to interacting with other TRPC channels, TRPC1 is also assembled in a complex with several key Ca2+ signaling proteins. Evidence has been provided for association of TRPC1 with calmodulin (CaM), IP3R, PMCA, Caveolin1 (Cav1), Gq/11, and Homer (Ambudkar et al., 2007). The domains in TRPC1 that are involved in some of these interactions have been defined, as are the effects on TRPC1 function. However, the actual gating mechanism of TRPC1 was only identified recently. It has now been demonstrated that TRPC1 dynamically interacts with STIM1 and Orai1 in response to store depletion and that all three proteins are involved in SOCE (Ong & Ambudkar, 2012). The three proteins are colocalized in the plasma membrane region of cells and can be coimmunoprecipitated following store depletion by agonists or Tg (Ong et al., 2007). Recruitment of TRPCs and Orai1 in this complex is dependent on STIM1 (Cheng et al., 2011). These results have been confirmed in different cell types by various research groups. SOCE involving TRPC1/STIM1/Orai1 complex has been observed in human salivary gland cell (Ong et al., 2007), mouse pulmonary arterial smooth muscle cells (Ng et al., 2009), human liver cells (Zhang, Pan, & Zhang, 2010), human parathyroid cells (Lu et al., 2010), and rat kidney fibroblasts (Almirza, Peters, van Zoelen, & Theuvenet, 2012). In addition to TRPC1, other members of the TRPC family also display functional interactions with STIM and Orai proteins in SOCE. Physical interaction of Orai1 with the N- and C-termini of TRPC3 and TRPC6 has been reported (Liao et al., 2007), TRPC6/STIM1/Orai1 association was shown in human platelets (Jardin, Gomez, Salido, & Rosado, 2009), TRPC1/TRPC4/STIM1 complex in glomerular mesangial cells (Sours-Brothers, Ding, Graham, & Ma, 2009) and endothelial barrier function (Sundivakkam et al., 2012), and TRPC5/STIM1/Orai1 association in mast cell degranulation (Ma et al., 2008).
4.2. TRPC1–STIM1 interactions
Interaction of STIM1 with TRPC1 has been demonstrated by (i) clustering and coimmunoprecipitation of TRPC1 and STIM1 following store depletion; (ii) attenuation of TRPC1-mediated Ca2+ entry in response to store depletion by knockdown of STIM1 expression in several cell types; (iii) increase in SOCE following coexpression of TRPC1 and STIM1; (iv) suppression of TRPC1-mediated SOCE by dominant-negative STIM1 constructs; and (v) spontaneous activation of TRPC1 by the constitutively active STIM1-D76A mutant. Together, these data suggest a crucial, non-redundant role for STIM1 in store-dependent activation of TRPC channels (Yuan et al., 2007; Zeng et al., 2008). STIM1 domains involved in binding and gating of TRPC1 have now been identified. The ERM (ezrin/radixin/moesin) domain in the cytosolic carboxyl terminus of STIM1 is required for the binding to TRPC1 and a few other TRPCs (TRPC2, TRPC4), but not TRPC3, TRPC6, or TRPC7 (Huang et al., 2006). The requirement of STIM1 for activity of TRPC3 and TRPC6, channels it normally does not bind to, is determined by the ability of these channels to interact with TRPC1 or TRPC4. These data provide a feasible explanation for the observed function of TRPC3 and TRPC6 in SOCE.
Binding of the STIM1–ERM domain to TRPC1 is not sufficient to activate the channel. A lysine-rich region (referred to as polybasic tail or K domain, aa 671–685; Fig. 7.1) is present at the C-terminal end of STIM1, which has been proposed to be important in anchoring STIM1 to the plasma membrane, possibly via interaction with PIP2 and PIP3 domains. Data in support of the distinct binding and gating domains in STIM1 come from studies showing that overexpression of the cytosolic C-terminal region of STIM1 results in constitutive activation of endogenous TRPC1, while expression of a truncated STIM1, lacking the K domain, binds but does not activate TRPC1. The molecular mechanism by which STIM1 gates TRPC channel has now been established by Zeng et al. (Worley et al., 2007; Zeng et al., 2008). This elegant study demonstrated that STIM1 gates Orai1 and TRPC channels through distinct domains in its C-terminus. While STIM1 interacts with and gates Orai1 via a cytosolic SOAR domain (aa 344–442) (Yuan et al., 2009), gating of TRPC1 by STIM1 is achieved by electrostatic interaction between highly conserved, negatively charged aspartate residues in TRPC1 (639DD640) with the positively charged lysines in the STIM1 C-terminus (684KK685). Mutation of DD or KK in either of the two proteins attenuates TRPC1 channel activation, whereas aspartate–lysine charge swapping between TRPC1 and STIM1 completely rescues channel function. Since these negatively charged residues of TRPC1 are highly conserved in TRPC3, TRPC4, TRPC5, and TRPC6, these channels also have the ability to be gated by intermolecular electrostatic interaction with STIM1 (Zeng et al., 2008).
4.3. TRPC–Orai1 interactions
A somewhat puzzling and intriguing aspect of TRPC regulation is the functional requirement of Orai1 in the activation of the channel by store depletion. The original observations demonstrate that knockdown of endogenous Orai1 or transfection of cells with functionally defective Orai1 mutants (R91W, E106Q) attenuates the increase in SOCE induced by TRPC1 + STIM1 overexpression (Cheng et al., 2008; Kim, Zeng, et al., 2009). In addition, endogenous SOCE in HSG cells, which is primarily contributed by TRPC1, is suppressed by similar experimental maneuvers (Ong et al., 2007). Together, these data indicated that abrogation of Orai1 function eliminates SOCE and activation of TRPC channels. Several studies have addressed this critical functional interaction between Orai1 and TRPC1, and it has been proposed that Orai1 physically modulates TRPC channels and confers STIM1-mediated activation in response to store depletion (Liao et al., 2007). This study hypothesized that native SOCE is mediated by TRPC channels, with Orai acting as a regulatory subunit. The suggestion was based on the findings that Orai1 physically interacts with the N- and C-termini of TRPC3 and TRPC6 in coimmunoprecipitation experiments (Liao et al., 2007). Another prediction was that Orai1 and TRPC1 might form heteromeric channels, with properties distinct from that mediated by Orai1 or TRPC alone (Liao et al., 2008). However, neither of these proposals has been further supported by studies or conclusive data.
Identification of distinct domains of STIM1 that are involved gating TRPC1 and Orai1 provided an important tool for assessing whether TRPC1 and Orai1 contribute to single heteromeric channel or whether these proteins form different channels. A key study was carried out using the HSG cell line which contains both Orai1 and TRPC1 channels and in which TRPC1 is major contributor of SOCE. The current typically activated by store depletion in these cells is a relatively Ca2+-selective current, ISOC, with Erev = +15 mV. Importantly, activation of TRPC1 by STIM1 following store depletion is dependent on Orai1. However, expression of the SOAR domain in these cells results in generation of a spontaneous ICRAC, which is not affected by knockdown of TRPC1. Furthermore, expression of the dominant-negative STIM1-EE mutant that cannot gate TRPC1, but retains activation of Orai1, also results in activation of ICRAC (instead of ISOC) in response to store depletion. These important findings suggest that Orai1 and TRPC1 form distinct channels and that ISOC activated by store depletion in these cells is composed of TRPC1 + STIM1-mediated nonselective cation current and Orai1 + STIM1-mediated ICRAC (Cheng et al., 2011). The smaller ICRAC current is most likely masked by the larger nonselective TRPC1 + STIM1-mediated current. The underlying mechanism has been revealed by data showing that Ca2+ entry via Orai1 triggers recruitment of TRPC1 to the plasma membrane. Increase of TRPC1 expression in the surface membrane is prevented by blocking SOCE with 1 µM Gd3+, removal of extracellular Ca2+, knockdown of Orai1, or expression of dominant-negative mutant Orai1 lacking a functional pore (E106Q). This study also revealed that while Orai1-mediated Ca2+ entry can trigger recruitment of TRPC1, gating of the channel is achieved by electrostatic interaction with STIM1, as previously reported. Together, these data provide an important insight into the critical interactions between Orai1 and TRPC1 that are required for TRPC1 function. These data also suggest that coordinated regulation of the surface expression of TRPC1 by Orai1 and gating by STIM1 provides a mechanism for rapidly modulating and maintaining SOCE-generated Ca2+ signals. By recruiting ion channels and other signaling pathways, Orai1 and STIM1 concertedly impact a variety of critical cell functions that are initiated by SOCE.
In addition to TRPC1, other TRPC subtypes also appear to be regulated by Orai1, such as TRPC4 and TRPC5. TRPC5 forms a nonselective cation channel that can be directly activated by increases in [Ca2+]i induced by store depletion or by other mechanisms. TRPC5 activation by Orai1–CRAC channel-mediated Ca2+ entry has been reported. Extracellular Ca2+ entry mediated by Orai1 + STIM1 is required for a sustained activation of TRPC5, while Tg-induced release of Ca2+ from internal stores produces a transient channel activation (Gross et al., 2009). These findings demonstrate an additional mode by which Orai1 function can be coupled to regulation of TRPC channels, and this can explain the requirement for Orai1 and TRPC5 previously reported in SOCE in mast cells (Ma et al., 2008). TRPC4 channels can also be similarly modulated by Ca2+, although exactly how Orai1 determines TRPC4 channel activity is not yet known. As noted above, channels that heteromerically interact with TRPC1 can be activated by STIM1 and the possibility that these heteromeric channels are also recruited to the plasma membrane along with TRPC1 cannot be ruled out. It is interesting to note a recent study showing Orai1-dependent trafficking of TRPC1/TRPV4 channel in smooth muscle cells, where both channels contribute to SOCE (Ma et al., 2011).
5. PLASMA MEMBRANE DOMAINS INVOLVED IN SOCE
Lipid raft domains (LRDs) which are enriched in such lipids can serve as platforms for recruiting and anchoring STIM1/channel complexes in the cell periphery. LRDs are biochemically distinct plasma membrane lipid domains that are enriched in cholesterol, sphingolipids, PIP2, PIP3, and key calcium-signaling protein components (e.g., Cav1, EGFRs, G-proteins, PMCA pumps, Homer, and PKC). SOCE has been proposed to occur within LRDs, as disruption of these domains attenuates SOCE. The dependence of TRPC1 channel function on intact LRDs has been shown in many cell types, such as HSG cells, C2C12 skeletal myoblasts, polymorphonuclear neutrophils, endothelial cells, and human platelets (Ong & Ambudkar, 2012). Further evidence for the involvement of LRD in assembly of functional TRPC1 channels was provided by data demonstrating an increase in the partitioning of TRPC1 into lipid rafts following stimulation of cells and Ca2+ store depletion (Lockwich et al., 2000; Pani et al., 2008). Consistent with the suggestion that STIM1 may be anchored to the plasma membrane via interaction with LRDs, partitioning of STIM1 into LRD is increased during activation of SOCE. More importantly, coimmunoprecipitation of TRPC1 + STIM1 is achieved in the LRD, but not in non-LRD, fractions. When these domains are disrupted, the partitioning and coimmunoprecipitation of TRPC1 and STIM1, as well as SOCE, are attenuated. The polybasic tail of STIM1 contains a consensus sequence that can potentially mediate its binding to PIP2 in the plasma membrane (Liou, Fivaz, Inoue, & Meyer, 2007). This has been confirmed in experiments showing that deletion of the polybasic tail results in loss of SOCE as well as STIM1 puncta formation in the ER–plasma membrane (PM) junctional regions. The exact interactions between STIM1 and plasma membrane proteins or lipids have not yet been resolved. It is also unclear whether other scaffolding proteins are involved in targeting the STIM1 clusters to specific plasma membrane regions. It is likely that these regions have specific biochemical, structural, and spatial characteristics since ion channels and possibly other effector proteins regulated by SOCE, such as CaM and calcineurin, are recruited and regulated within this domain. Thus, the rate and specificity of these processes need to be strictly controlled.
Cav1, a cholesterol-binding protein that is localized within and organizes LRD, has been proposed to be involved in the regulation of SOCE via both Orai1 and TRPC1, and to serve as a scaffold for recruitment of various proteins into LRDs. In Xenopus oocytes, Orai1 actively recycles between the endosomal compartment and the plasma membrane. Following cell stimulation, Orai1 is actively trafficked to the plasma membrane from the endosomal compartments. A putative Cav1-binding site is present in the N-terminus of Orai1 and Cav1 has been shown to play a role in the endocytosis of Orai1 during meiosis (Yu, Sun, & Machaca, 2010). While further studies are required to define the role of LRDs in modulating Orai1 channel function, a number of studies published demonstrated a role for Cav1 in the regulation of TRPC1 (Ong & Ambudkar, 2012; Pani & Singh, 2009). TRPC channels have conserved Cav1-binding domains located within the N- and C-termini. The N-terminal Cav1-binding motif (aa 322 and 349 of TRPC) binds to the scaffolding domain in Cav1 (aa 82 and 101). TRPC1 coimmunoprecipitates with Cav1 in HSG (Lockwich et al., 2000) and pulmonary artery endothelial cells (Kwiatek et al., 2006). Further, the N-TRPC1/Cav1 interaction serves to scaffold TRPC1 in the plasma membrane region and determines its subsequent activation by store depletion. A concerted role for Cav1 and STIM1 in the regulation of TRPC1 has been demonstrated. The suggested mode of regulation for TRPC1 is that in resting cells, it is present in recycling endosomes. Cav1 interacts with and scaffolds TRPC1 vesicles near the plasma membrane. This scaffolding likely represents a short retention of the channel at this location. From here, it either recycles back into the trafficking pathway or if store depletion is initiated, the channel is recruited to the plasma membrane, interacts with STIM1 and is activated. Following ER-Ca2+ store depletion, STIM1 translocates to the periphery of the cells, interacts with and activates Orai1, and Orai1-mediated Ca2+ influx drives insertion of TRPC1 into the plasma membrane. Following insertion, STIM1 gates and activates TRPC1. The binding of STIM1 to TRPC1 also induces dissociation of TRPC1/Cav1 complex (Pani et al., 2009). The present data suggest that STIM1/TRPC1 forms a stable complex that helps to retain active TRPC1 channels in the plasma membrane. Refilling of the ER-Ca2+ stores leads to inactivation of the channel, due to the dissociation of STIM1 from TRPC1. At this point, TRPC1 can reassociate with Cav1 (Pani et al., 2009) or, alternatively, it can be endocytosed via a different route. More studies are required to further delineate protein–protein interactions involved in the progress of TRPC1 through the different steps involved in its activation (trafficking, insertion into the plasma membrane, scaffolding, and activation by STIM1). Interestingly, Homer-1 has been reported to bind TRPC1 channel and facilitate rapid reassembly of the TRPC1/Homer/IP3R complex, following refilling of the ER-Ca2+ stores (Worley et al., 2007). How exactly Homer-1, Cav1, STIM1, and IP3R regulate the trafficking and function of TRPC1 in a single cell has yet to be elucidated. Another interesting hypothesis that needs to be further examined is the proposal that recruitment of Orai/TRPC/STIM1 complexes into the LRDs is required for store-dependent regulation but that the same complexes can function as receptor-operated channels when localized outside lipid rafts (Liao et al., 2009). Thus, several proteins have the capacity to critically affect TRPC functions. Whether these are ubiquitous in all cell types or whether TRPC1–SOCE is regulated in a cell-specific way needs to be established.
6. DISTINCT PHYSIOLOGICAL FUNCTIONS OF TRPC1 AND ORAI1
SOCE mediates a wide variety of cellular functions in various tissues. As discussed above, several distinct channels can contribute to the net [Ca2+]i increase in cells following store depletion. Even more significant is the observation that there are functional differences between two channels that are simultaneously activated. For example, both TRPC1 and Orai1 are gated by STIM1 and activated following store depletion. However, once activated, the two channels have distinct functional contributions (Cheng et al., 2011). Orai1-mediated Ca2+ influx is sufficient for regulation of Ca2+-dependent gene expression via activation of the transcription factor, nuclear factor activated T cells (NFAT). Nuclear translocation of NFAT occurs in an “all-or-none” manner and requires the activation of calcineurin, a Ca2+-CaM-dependent phosphatase that dephosphorylates NFAT prior to translocation (Parekh, 2011). By contrast, activation of the transcription factor, nuclear factor kappa-light-chain enhancer of activated B cells (NFκB) is primarily dependent on TRPC1 function (Cheng et al., 2011; Ong, Jang, & Ambudkar, 2012). KCa channel activity in salivary gland cells is also dependent on TRPC1 activation. However, NFκB and KCa activation are lost by elimination of Orai1 function since TRPC1 activation requires Orai1. In B cells, additional channels activated downstream from Orai1 or via other mechanisms associated with cell activation can lead to higher levels of [Ca2+]i exceeding that achieved by CRAC channel activity alone. This results in a different gene expression pattern (Scharenberg, Humphries, & Rawlings, 2007). Thus, it can be hypothesized that channels like TRPC1 that are activated in response to store depletion and Orai1 activation can modulate the initial Ca2+ signal generated by Orai1–CRAC channels. The downstream cellular function that can be regulated under these conditions would then depend on the nature of individual [Ca2+]i signals generated by the two types of channels as well as the resultant pattern of [Ca2+]i increase in the cell when both are simultaneously activated.
To some extent, this has been tested physiologically using mouse models that lack TRPC1. Previous studies using TRPC1−/− mice showed a substantial decrease in SOCE in acinar cells from salivary glands, which accounted for the decrease in KCa activation and fluid secretion from the glands (Liu, Cheng, et al., 2007). Similarly, pancreatic acinar cells from TRPC1−/− mice demonstrated decreased SOCE and Ca2+ -activated Cl− channel activity (Hong et al., 2011). Hence, it can be concluded that TRPC1 serves a nonredundant function in these cells and that residual Orai1 alone cannot support these cellular functions. What is important to note is that, unlike in cell lines, these channels are not distributed uniformly in exocrine acinar cells. While TRPC1 is localized primarily in the lateral membrane with some basal localization, Orai1 is mainly concentrated in the lateral membrane toward the apical pole of the cell (Hong et al., 2011). STIM1 relocates to regions of the cells containing both channels after stimulation. Thus, the three proteins overlap in the lateral membrane toward the apical end while TRPC1–STIM1 overlap can be seen in the lateral membrane toward the basal pole of the cell. One report suggested that Orai1 might be located in intracellular compartments in the apical region of acinar cells; however, this has not been substantiated by further studies. In terms of [Ca2+]i signals generated by agonist stimulation in acinar cells, it has been well established that the initial increase in [Ca2+]i occurs in the apical region. In pancreatic acinar cells, these increases are oscillatory at very low levels of stimulation and are retained within the apical pole of the cells. However, at higher levels of stimulation, they are initiated in the apical region but spread to the basal region. In salivary gland acinar cells, at both low and high levels of stimuli, Ca2+ increases are initiated at the apical end and then spread in a wave-like pattern to the basal end of the cell (Fig. 7.2). During regulation of fluid or protein secretion in salivary or pancreatic acini, respectively, various ion channels, transporters, and granule fusion (in pancreatic acini) are activated in different cellular regions. Thus, to maintain the secretion in either cell type, it is imperative that the Ca2+ signal required for regulation of the various processes is achieved or is initiated at the proper cellular location. The exact temporal and spatial contributions of TRPC1 and Orai1 to agonist-stimulated Ca2+ signals and how these are utilized for regulation of specific cellular functions need to be studied in greater detail.
Figure 7.2.
Ca2+ signaling mechanisms regulating salivary gland fluid secretion. The figure shows Ca2+-mobilizing events in acinar cells that are initiated by a stimulus and lead to fluid secretion. The coordinated regulation (spatial and temporal) of Ca2+ signaling as well as channel function (KCa, TMEM16A, AQP5 insertion) achieves fluid secretion via the apical membrane of acinar cells. Release of calcium via apically localized IP3Rs triggers the initial apical rise in cytosolic [Ca2+] (denoted in the figure as Ca2+ signal, red stellate area). The Ca2+ signal is then propagated toward the basal region of the cell. It is suggested that apically and basolaterally localized Orai1 and TRPC1 channels mediate Ca2+ entry that is critical for the maintenance of sustained elevation of [Ca2+]i which is required to drive fluid secretion.
7. ASSEMBLY AND FUNCTION OF TRPC/STIM1/ORAI1 MICRODOMAINS
A wide range of cellular functions are regulated by [Ca2+]i changes mediated by one or more Ca2+ channels; further, these need to have the exact spatiotemporal characteristics. An important question that needs to be considered is how the cell decodes a specific [Ca2+]i signal for regulation of cellular functions. There is increasing evidence to show that such precise coordination is achieved by the assembly and segregation of channels and their accessory proteins (e.g., Ca2+ sensors, scaffolding proteins, Ca2+-dependent effectors) into complexes that are localized within discrete cellular or membrane domains. Formation of such Ca2+-signaling microdomains allows for generation of compartmentalized Ca2+ signals with distinct amplitude, pattern, and frequency, which are then either locally decoded for the regulation of downstream effectors or propagated into the cytoplasmic milieu in the form of Ca2+ waves or oscillations (Berridge, Bootman, & Roderick, 2003; Parekh, 2011). Localization of accessory proteins within the microdomains facilitates the sensing of Ca2+ signals by Ca2+ sensors and rapid transduction of these signals by downstream effector proteins to induce both acute and long-term cellular events. Ca2+ signals that are situated very close to the channel pore are called “local Ca2+ signals,” whereas the bulk cytoplasmic Ca2+ signals are considered as “global Ca2+ signals.” It has been suggested that [Ca2+] within these microdomains can rise to micromolar concentrations, often without significantly changing the bulk cytoplasmic [Ca2+]. There are two main advantages of this: (i) high [Ca2+] can be achieved without inducing cell toxicity and (ii) initiation of downstream cellular events can occur with minimal channel opening times. An appropriate sensor (e.g., CaM) can rapidly sense these changes and regulate the function of other proteins, some of which can induce long-term effects on cell functions such as gene expression (e.g., via calcineurin/NFAT pathway) (Parekh, 2011). Changes in gene expression are seen long after the initial Ca2+ signal has decayed and are most often determined by the slow off-rate of effector components that are involved in sensing and transducing the signal. In contrast, acute modulation of regulatory processes, such as the activation of KCa channels, Ca2+-activated Cl− channels, or protein secretion, results from more direct effects of Ca2+ and thus their activity correlates with changes in [Ca2+]i. The spatial and structural architecture of microdomains ensure the specificity, rate, and efficacy of protein–protein interactions required for the generation of distinct Ca2+ signals and transduction of these signals for regulation of cell functions.
Several studies have provided conclusive evidence that Orai1- and TRPC1-mediated SOCE occur within calcium-signaling microdomains (Derler, Madl, Schutz, & Romanin, 2012; Ong & Ambudkar, 2012). In addition to these channels, accessory proteins involved in generating the intracellular Ca2+ signals and modulating the cellular level of [Ca2+] have also been reported to be localized within these microdomains. For example, PMCA, which plays a vital role in regulating [Ca2+]i by pumping Ca2+ out of the cell, has been shown to associate with TRPC1 (Singh, Liu, Tang, Zhu, & Ambudkar, 2002) and also to regulate CRAC channel function (Bautista & Lewis, 2004). Homer-1 and IP3R were also reported to be associated with TRPC1 and involved in channel activation following store depletion (Yuan, Lee, Hong, & Muallem, 2012). Additionally, TRPC1 was shown to interact with cytoskeletal modulators such as RhoA, suggesting the possibility that local cytoskeletal rearrangements might be involved in SOCE on TRPC1 regulation (Galan, Dionisio, Smani, Salido, & Rosado, 2011; Mehta et al., 2003). The critical functional interaction between Orai1 and TRPC1 is also determined by the assembly and localization of these channels and STIM1 within specific ER/PM junctional domains. Recruitment of Orai1 into regions where STIM1 puncta accumulates is an essential requirement for activation of CRAC channels (Hogan et al., 2010). A number of proteins have been identified that interact with either Orai1 or STIM1 and modulate the activity of Orai1 or its activation by STIM1 (Soboloff, Rothberg, Madesh, & Gill, 2012; Srikanth et al., 2012). It has been suggested that TRPC1-containing vesicles are scaffolded near the Orai1 channels so that Ca2+ entry via the CRAC channel is sensed via an as yet unidentified mechanism that triggers insertion of TRPC1 into the plasma membrane. As noted above, this insertion is blocked when Orai1 function is abrogated (Cheng et al., 2011). Localization of TRPC1 near the plasma membrane also facilitates its subsequent interaction with STIM1. As discussed above, LRD and Cav1 have important roles in the assembly of TRPC1/STIM1 complex. The possible role of LRD in Orai1–CRAC activity needs to be further elaborated.
How Ca2+ signals generated by channels that are in the close proximity to each other within a cell are delineated and decoded to regulate distinct cellular functions is an important question that has been receiving quite a bit of attention lately. It has been suggested that signaling specificity is achieved by specific accessory proteins that can differentially detect the Ca2+ entry via different channels. For example, although TRPC1 and Orai1 are localized in close proximity to each other, only Orai1-mediated Ca2+ entry is utilized for NFAT activation. While this is believed to occur via the scaffolding of CaM-calcineurin close to the Orai1 channel, conclusive data are required to establish this. In contrast, KCa activation is primarily dependent on TRPC1 and NFκB activation requires Ca2+ influx mediated by both channels (Cheng et al., 2011). Furthermore, it is also possible that the channels contribute distinct Ca2+ signals that can be decoded by the cellular machinery. Concurrent with this, a recent study (Ong et al., 2012) demonstrated that endogenous Orai1 and TRPC1 channels contribute distinct local and global [Ca2+]i signals following agonist stimulation of HSG cells, in which both channels contribute to the net SOCE (Fig. 7.3). At relatively high [CCh] (>1 µM), Orai1-mediated Ca2+ entry generates baseline [Ca2+]i oscillations (seen in cells where TRPC1 channel activation is supported). When TRPC1 is also functional, this pattern is altered to oscillations over a sustained baseline [Ca2+]i. Interestingly, at very low [CCh] (300 nM), the baseline [Ca2+]i oscillations are Orai1-dependent but require Ca2+ entry via TRPC1 to maintain the oscillation frequency. Thus, Orai1 channel function in these cells results in the generation of [Ca2+]i oscillations, whereas TRPC1 channel function appears to increase signal amplitude and frequency. More importantly, cell functions that are Orai1-dependent (e.g., NFAT activation) are unaffected by the TRPC1-mediated global Ca2+ signals. Hence, despite the close proximity of Orai1 and TRPC1 channels to each other, the Ca2+ entering via these channels contribute to different [Ca2+]i signals (local vs. global). Activation of NFAT nuclear translocation and NFAT-dependent gene expression is exclusively driven by local [Ca2+]i mediated by Orai1. In contrast, Ca2+ entry via TRPC1 is the primary determinant in the activation of NFκB-dependent gene expression and KCa channel activation (Ong et al., 2012). It is unclear whether these are determined by local or global [Ca2+]i signals generated by TRPC1. The stimulus intensity most likely determines the number of channels that are activated within the cell which then control the magnitude of local [Ca2+]i increase. The exact patterns of Ca2+ signals generated due to Ca2+ entry via Orai1, TRPC1, or other TRPCs in different cell types have not yet been fully elucidated. Nevertheless, it is reasonable to speculate that all cells are likely to have mechanisms to decode the Ca2+ signals generated by different channels for regulation of various cellular processes.
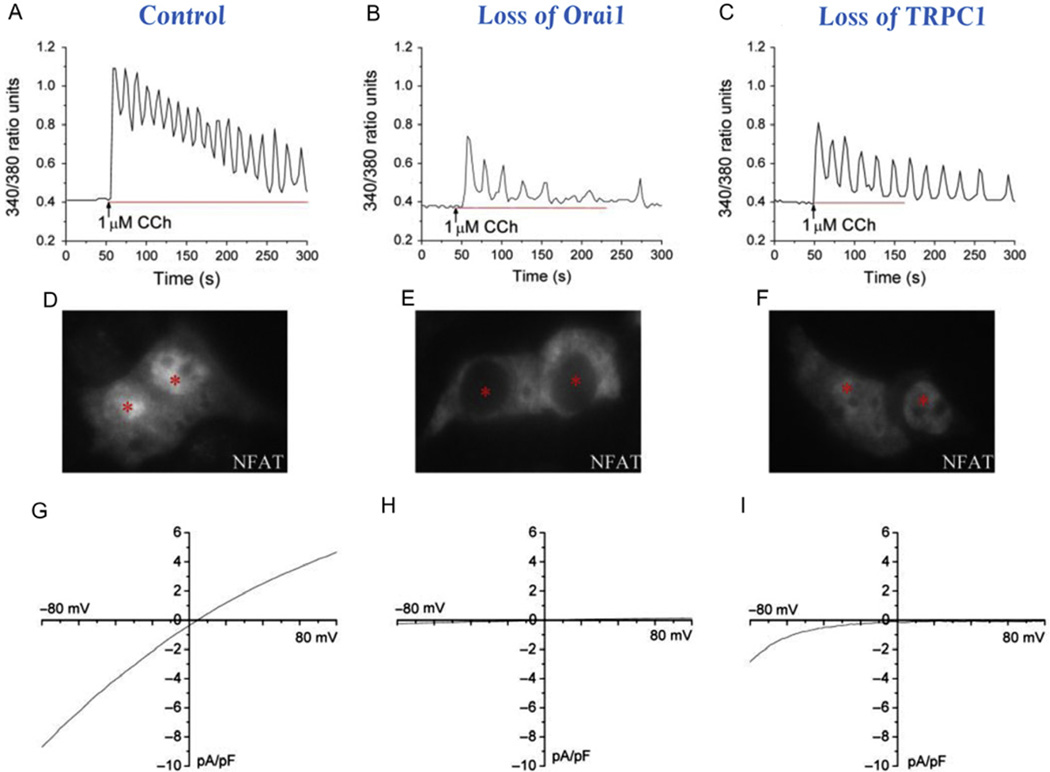
Figure 7.3.
Distinct Ca2+ signals and currents, as well as downstream cellular events associated with Orai1- and TRPC1-mediated Ca2+ entry. (A–C) Distinct Ca2+ signals induced by 1 µM CCh. The pattern shown in control cells reflects the activation of both Orai1 and TRPC1 channels. No response was seen following knockdown ofOrai1. In the case of cells lacking TRPC1, baseline oscillations were seen and these are attributed to the still functional Orai1 channel. (D–F) Activation of NFAT translocation from the cytoplasm into the nuclear (denoted by *) following store depletion. Nuclear translocation of a GFP-tagged NFAT could be clearly seen in control cells but not in cells lacking Orai1, pointing to Ca2+ entry via Orai1 as the primary determinant of NFAT activation. This was further supported by the observation of nuclear translocation of NFAT in cells lacking TRPC1, where the Orai1 channel could still be activated following store depletion. (G–I) Currents activated following store depletion. The ISOC measured in control cells is a combination of currents carried by Ca2+ entry via both TRPC1 and Orai1 channels. Loss of Orai1 expression resulted in no currents, as neither Orai1 nor TRPC1 was functional. However, an ICRAC could be seen in cells lacking TRPC1.
8. CONCLUSION
SOCE is a complex process that is determined by temporal and spatial coordination of several distinct steps that involve interactions and regulation of proteins in different cellular compartments. Several channels impact SOCE, for example, TRPCs, Orai1, and voltage-gated calcium channels. Of these, Orai1 and TRPCs contribute to the net [Ca2+]i increase. TRPC-mediated Ca2+ entry (via TRPC1, TRPC3, and TRPC4) has a nonredundant role in regulation of function in a variety of tissues, including exocrine glands. The very critical role of Orai1/STIM1–CRAC channels is now emerging. Orai1-mediated Ca2+ entry can regulate several different channels. For example, TRPC1 is recruited to the plasma membrane by an as yet unidentified mechanism dependent on Orai1-mediated Ca2+ entry where it is gated by STIM1. Alternatively, Orai1-mediated Ca2+ entry also induces direct Ca2+-dependent activation of channels such as TRPC5. In addition, STIM1 can also modulate the function of Ca2+-signaling proteins, such as PMCA. Together, this leads to rapid and specific effects on [Ca2+]i that are decoded for regulation of the physiological functions of the cell that are regulated by Ca2+. Several interesting questions remain to be answered with regard to how these channels are clustered and what determines the formation of microdomains. Even more critical is to elucidate the mechanisms that sense the Ca2+ entry via different channels and decode them for the regulation of specific cellular functions. It can be speculated that several scaffolding, trafficking, and signaling proteins might be involved in this process. Future studies should be directed toward identifying these and resolving their role in SOCE and cell function. It is now accepted that both lipids and protein components are involved in determining the assembly of Ca2+-signaling complexes and spatiotemporal patterning of Ca2+ signal. It has also been suggested that both the dose and type of agonists can determine the pattern of [Ca2+]i signals that are generated by SOCE. Finally, it is important that the endogenous status of the channels in native cell types is studied in further detail to fully elucidate the physiological implications of their function.
REFERENCES
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, et al. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. The Journal of Biological Chemistry. 2004;279(20):20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Almirza WH, Peters PH, van Zoelen EJ, Theuvenet AP. Role of Trpc channels, Stim1 and Orai1 in PGF(2alpha)-induced calcium signaling in NRK fibroblasts. Cell Calcium. 2012;51(1):12–21. doi: 10.1016/j.ceca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40(5–6):495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: The link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42(2):213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Lewis RS. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. The Journal of Physiology. 2004;556(Pt. 3):805–817. doi: 10.1113/jphysiol.2003.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clinical and Experimental Pharmacology and Physiology. 2005;32(8):597–603. doi: 10.1111/j.1440-1681.2005.04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nature Reviews. Molecular Cell Biology. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. The Journal of General Physiology. 2009;133(5):525–546. doi: 10.1085/jgp.200810153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Lewis RS. Functional roles of ion channels in lymphocytes. Seminars in Immunology. 1990;2(2):107–117. [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflügers Archiv. 2006;452(1):43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. The Journal of Biological Chemistry. 2008;283(19):12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca signals required for specific cell functions. PLoS Biology. 2011;9(3):e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, et al. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circulation Research. 2012;110(11):1435–1444. doi: 10.1161/CIRCRESAHA.112.269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derler I, Madl J, Schutz G, Romanin C. Structure, regulation and biophysics of I(CRAC), STIM/Orai1. Advances in Experimental Medicine and Biology. 2012;740:383–410. doi: 10.1007/978-94-007-2888-2_16. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: Their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacology and Therapeutics. 2006;112(3):744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: Functional characterization and physiological relevance. Pflügers Archiv. 2005;451(1):72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Salanova O, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Archiv. 2007;455(3):465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Feske S. ORAI1 and STIM1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunology Reviews. 2009;231(1):189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, et al. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. The Journal of Neuroscience. 2005;25(10):2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nature Cell Biology. 2001;3(2):121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Hoffmann M, Muller C, Stolz S, et al. Functional role of TRPC proteins in vivo: Lessons from TRPC-deficient mouse models. Biochemical and Biophysical Research Communications. 2004;322(4):1352–1358. doi: 10.1016/j.bbrc.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Galan C, Dionisio N, Smani T, Salido GM, Rosado JA. The cytoskeleton plays a modulatory role in the association between STIM1 and the Ca2+ channel subunits Orai1 and TRPC1. Biochemical Pharmacology. 2011;82(4):400–410. doi: 10.1016/j.bcp.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. The Journal of Biological Chemistry. 2002;277(50):48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, et al. Upregulated TRP and enhanced capacitative Ca(2+) entry in human pulmonary artery myocytes during proliferation. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280(2):H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Gross SA, Guzman GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, et al. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. The Journal of Biological Chemistry. 2009;284(49):34423–34432. doi: 10.1074/jbc.M109.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59(3):392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual Review of Immunology. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, et al. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12(2):232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. The Journal of Physiology. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nature Cell Biology. 2006;8(9):1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Jardin I, Gomez LJ, Salido GM, Rosado JA. Dynamic interaction of hTRPC6 with the Orai1-STIM1 complex or hTRPC3 mediates its role in capacitative or non-capacitative Ca(2+) entry pathways. The Biochemical Journal. 2009;420(2):267–276. doi: 10.1042/BJ20082179. [DOI] [PubMed] [Google Scholar]
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, et al. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137(4):1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, et al. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. The Journal of Biological Chemistry. 2006;281(43):32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. The Journal of Biological Chemistry. 2009;284(15):9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. Journal of Vascular Research. 2013;50(1):11–20. doi: 10.1159/000342461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Molecular Pharmacology. 2006;70(4):1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. The Journal of Biological Chemistry. 2010;285(49):38666–38673. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(22):9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current Biology. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. The Journal of Biological Chemistry. 2005;280(22):21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(44):17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Groschner K, Ambudkar IS. Distinct Ca(2+)-permeable cation currents are activated by internal Ca(2+)-store depletion in RBL-2H3 cells and human salivary gland cells, HSG and HSY. The Journal of Membrane Biology. 2004;200(2):93–104. doi: 10.1007/s00232-004-0698-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5–S6 region. The Journal of Biological Chemistry. 2003;278(13):11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- Liu DY, Thilo F, Scholze A, Wittstock A, Zhao ZG, Harteneck C, et al. Increased store-operated and 1-oleoyl-2-acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. Journal of Hypertension. 2007;25(4):799–808. doi: 10.1097/HJH.0b013e32803cae2b. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, et al. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. The Journal of Biological Chemistry. 2000;275(5):3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. The Journal of Biological Chemistry. 2000;275(16):11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- Lu M, Branstrom R, Berglund E, Hoog A, Bjorklund P, Westin G, et al. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. Journal of Molecular Endocrinology. 2010;44(5):285–294. doi: 10.1677/JME-09-0138. [DOI] [PubMed] [Google Scholar]
- Ma X, Nilius B, Wong JW, Huang Y, Yao X. Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochimica et Biophysica Acta. 2011;1808(12):2789–2797. doi: 10.1016/j.bbamem.2011.07.049. [DOI] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. The Journal of Immunology. 2008;180(4):2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic N, Desai A, Olivera A, Abramowitz J, Birnbaumer L, Beaven MA, et al. Knockout of the Trpc1 gene reveals that TRPC1 can promote recovery from anaphylaxis by negatively regulating mast cell TNF-alpha production. Cell Calcium. 2013;53(5–6):315–326. doi: 10.1016/j.ceca.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, et al. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. The Journal of Biological Chemistry. 2003;278(35):33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, et al. Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes. The Journal of Experimental Medicine. 2002;195(6):673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, et al. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. The Journal of Physiology. 2009;587(Pt. 11):2429–2442. doi: 10.1113/jphysiol.2009.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukhov AG, Nowycky MC. TRPC5 channels undergo changes in gating properties during the activation-deactivation cycle. Journal of Cellular Physiology. 2008;216(1):162–171. doi: 10.1002/jcp.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Ambudkar IS. The dynamic complexity of the TRPC1 channelosome. Channels (Austin, Texas) 2011;5(5):424–431. doi: 10.4161/chan.5.5.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Ambudkar IS. Role of lipid rafts in the regulation of store-operated Ca2+ channels. In: Levitan I, Barrantes F, editors. Cholesterol regulation of ion channels and receptors. Hoboken, NJ: John Wiley & Sons; 2012. pp. 69–90. [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. The Journal of Biological Chemistry. 2007;282(12):9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Jang SI, Ambudkar IS. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca(2+)](i) signals determine specificity of Ca(2+)-dependent gene expression. PLoS One. 2012;7(10):e47146. doi: 10.1371/journal.pone.0047146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annual Review of Physiology. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, et al. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) The Journal of Biological Chemistry. 2008;283(25):17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45(6):625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends in Biochemical Sciences. 2011;36(2):78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77(4):901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiological Reviews. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, et al. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;287(6):L1303–L1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330(6000):101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- Philipp S, Trost C, Warnat J, Rautmann J, Himmerkus N, Schroth G, et al. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. The Journal of Biological Chemistry. 2000;275(31):23965–23972. doi: 10.1074/jbc.M003408200. [DOI] [PubMed] [Google Scholar]
- Prakriya M. The molecular physiology of CRAC channels. Immunology Reviews. 2009;231(1):88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, et al. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006;290(4):G782–G792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of Cell Biology. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nature Reviews. Immunology. 2007;7(10):778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circulation Research. 2009;105(10):1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Molecular Cell. 2002;9(4):739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- Skopin A, Shalygin A, Vigont V, Zimina O, Glushankova L, Mozhayeva GN, et al. TRPC1 protein forms only one type of native store-operated channels in HEK293 cells. Biochimie. 2013;95(2):347–353. doi: 10.1016/j.biochi.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: Dynamic calcium signal transducers. Nature Reviews. Molecular Cell Biology. 2012;13(9):549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Experimental Biology and Medicine (Maywood, N.J.) 2009;234(6):673–682. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1) Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8682–8687. doi: 10.1073/pnas.1200667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29(3):645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. The Journal of Biological Chemistry. 2003;278(40):39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, et al. The Ca(2+) sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca(2+) entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Molecular Pharmacology. 2012;81(4):510–526. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, et al. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. Journal of Applied Physiology. 2002;92(4):1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13(8):693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, et al. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circulation Research. 2002;91(1):70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. The Journal of Biological Chemistry. 2001;276(11):7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. The Journal of Cell Biology. 2002;158(6):1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, et al. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Szabo D, Authi KS, Braun A, Bender M, Ambily A, Hassock SR, et al. Store-operated Ca(2+) entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflügers Archiv. 2008;457(2):377–387. doi: 10.1007/s00424-008-0531-4. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annual Review of Biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312(5777):1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330(6000):105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, et al. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42(2):205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. The Journal of Biological Chemistry. 2004;279(42):43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. The Journal of Cell Biology. 2010;191(3):523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Lee KP, Hong JH, Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiologica (Oxford, England) 2012;204(2):238–247. doi: 10.1111/j.1748-1716.2011.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nature Cell Biology. 2009;11(3):337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nature Cell Biology. 2007;9(6):636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. The Journal of Biological Chemistry. 2005;280(33):29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, et al. Role of TRPC1 channel in skeletal muscle function. American Journal of Physiology. Cell Physiology. 2010;298(1):C149–C162. doi: 10.1152/ajpcell.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Molecular Cell. 2008;32(3):439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Luo Y, Chasan B, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, et al. The multimeric structure of polycystin-2 (TRPP2): Structural-functional correlates of homo- and hetero-multimers with TRPC1. Human Molecular Genetics. 2009;18(7):1238–1251. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Pan LJ, Zhang ZM. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of SOCs in HL-7702 cells. Amino Acids. 2010;39(1):195–204. doi: 10.1007/s00726-009-0398-5. [DOI] [PubMed] [Google Scholar]