Abstract
Aged dogs spontaneously develop progressive decline in both cognitive and behavioral function, in addition to neuropathological changes, that collectively parallel several aspects of human aging and Alzheimer’s disease progression and likely contribute to the development of canine cognitive dysfunction syndrome. In the current study, ethologically relevant spatial learning, retention, and reversal learning tasks were conducted, with the goal of expanding canine neuropsychological testing to pet dogs. Initially, dogs (N = 44, aged 7.8 ± 2.8 years, mean ± SD) had to learn which of two alternative routes successfully led out of a T-maze. Two weeks later, long-term memory retention was assessed, immediately followed by a reversal learning task in which the previously correct route out of the maze was reversed compared with the initial learning and memory retention tasks. No effects of age were evident on the learning or retention tasks. However, older (≥8 years) dogs were significantly impaired on the reversal learning task compared with younger ones (<8 years). Moreover, trial response latency was significantly increased in aged dogs across both the initial and reversal learning tasks but not on the retention task, which suggests that processing speed was impaired by increasing age during the acquisition of novel spatial information but not during performance of previously learned responses. Overall, the current study provides a framework for assessing cognitive function in pet dogs, which should improve understanding of the effects of aging on cognition in the dog population.
Keywords: Aging, Cognitive impairment, Pet dog, Navigation task, Spatial cognition
Introduction
Canine aging is associated with neuropathological changes as well as cognitive decline and behavioral alterations that parallel several aspects of human cognitive aging and Alzheimer’s disease (AD) progression. For example, β-amyloid (Aβ) deposits progressively accumulate in the brain of aged dogs in a region-specific manner; deposition occurs earliest and most consistently in the prefrontal cortex and ultimately progresses to posterior brain regions (Borras et al. 1999; Head et al. 2000; Uchida et al. 1992). Additional age-related neuropathological parallels of human aging in dogs include localized neuronal loss (Morys et al. 1994; Siwak-Tapp et al. 2008; Su et al. 2005; Tapp et al. 2004), cortical atrophy (Gonzalez-Soriano et al. 2001; Su et al. 2005; Tapp et al. 2004), increased ventricular volume (Gonzalez-Soriano et al. 2001), increased oxidative stress (Head et al. 2002; Papaioannou et al. 2001; Rofina et al. 2004), and cholinergic deficits (Araujo et al. 2005). Collectively, neuropathological changes observed in aged dogs range from that seen in normal human aging to those seen in early stages of AD (Cotman and Head 2008).
The similarity between human and canine senescence has stimulated substantial interest in assessing the effects of age on canine cognitive function. Indeed, age-related cognitive decline and neuropathological changes in the dog is relevant to the study of human aging and AD (Cotman and Head 2008; Studzinski et al. 2005), as well as the study of aging and cognitive dysfunction syndrome (CDS) in pet dogs (Landsberg 2005, Ruehl et al. 1995). Standardized laboratory-based neuropsychological tests are generally used to evaluate cognitive domain-specific effects of canine aging (Milgram et al. 1994; Studzinski et al. 2006; Tapp et al. 2003a). Basic cognitive processes, such as those underlying procedural and simple associative learning are not consistently affected by age (Adams et al. 2000; Milgram et al. 1994). By contrast, reversal learning and short-term visuospatial working memory tasks, which rely on executive processes that are supposed to have neuroanatomical substrates within the prefrontal cortex, are highly age sensitive (Studzinski et al. 2006; Tapp et al. 2003a, b). Impairment in these tasks occurs relatively earlier (Studzinski et al. 2006; Tapp et al. 2003a) than impairment on other complex tasks presumably independent of prefrontal function, such as allocentric and egocentric visuospatial learning (Christie et al. 2005). Moreover, short-term working memory impairments occur as early as 6 years of age in dogs (Studzinski et al. 2006), thereby preceding prefrontal atrophy and amyloid deposition, which, generally, are not observed before the age of 8 years (Head et al. 1998; Tapp et al. 2005).
The high degree of variability and lack of systematic CDS evaluation criteria hinders the exact determination of CDS prevalence in pet dogs. Symptoms may be present in as few as 5 % of pet dogs aged 10–12 years and a formal veterinary diagnosis of CDS is likely to be lower (Salvin et al. 2011), although higher prevalence rates are also reported (see Landsberg et al. 2012 for a review). Current views propose that age-related neurodegenerative processes and associated decline in cognitive function likely precedes the clinical diagnosis of CDS (Landsberg 2005; Landsberg et al. 2012; Ruehl et al. 1995). For example, cognitively impaired aged laboratory dogs demonstrate behavioral alterations consistent with CDS (i.e., altered interspecific interactions and exploratory behavior; Siwak et al. 2001). Therefore, the postulation that CDS occurs subsequent to age-related cognitive changes and represents late stages of a neurodegenerative process is warranted (Landsberg et al. 2012). However, the extensive training required to assess cognitive ability in dogs has impeded the application of neuropsychological tests in pet dog populations. Therefore, very little is known about the effects of normative ageing on cognitive decline in pet dogs.
One strategy for overcoming the limitations of object-based neuropsychological testing is to utilize spatial cognition tasks, which exploit ethologically relevant behavior and, therefore, are readily acquired with limited training (Tolman and Honzik 1930). Spatial cognition encompasses neuropsychological processes involved in recognizing, coding, storing and retrieving spatial arrangements and route navigation (Carrillo-Mora et al. 2009), which are essential for environmental survival. In rodents, age-related learning and memory deficits are found on a variety of navigation-based tasks (Begega et al. 2001; Carrillo-Mora et al. 2009; Gallagher and Pelleymounter 1988; Ingram 1988; Lukoyanov et al. 1999; McLay et al. 1999; Sharma et al. 2010). In humans, normative aging negatively impacts navigation skills, such as learning new routes, inferring both distances and directions between locations, and acquiring/retaining information required to navigate controlled environments (Cushman et al. 2008; Iachini et al. 2009; Moffat 2009; Newman and Kaszniak 2000). Moreover, impaired spatial processing is a key characteristic in the diagnosis of mild cognitive impairment and early stage dementia (Braak and Braak 1991; Hort et al. 2007; Klein et al. 1999). Similarly, healthy aged pet dogs (8 years and above) demonstrate deficits in spatial reference memory, but not in spatial acquisition, compared with young dogs (1–4 years), in an appetitive sand version of the Morris Water Maze (Salvin et al. 2011), which is the only study we are aware of investigating spatial skills in pet dogs. While limitations of the sand maze paradigm include the apparatus itself and confounds of age on motivation, the results indicate spatial navigation tasks are rapidly and reliably acquired by pet dogs and may be used to dissociate cognitive domain-specific age effects.
The current study, therefore, sought to develop a practical and rapid methodology for cognitive assessment of pet dogs using a spatial navigation paradigm. Based on previous findings described above, we hypothesized that: (1) a spatial navigation learning task would be easily acquired by pet dogs and (2) aged dogs would perform more poorly than young, particularly on a subsequent reversal learning task.
Materials and methods
Animal subjects
Forty-four pet dogs were included in this study. Recruitment was done by word of mouth and advertisements targeting clients of local veterinary clinics and students of the University of Padova. Inclusion criteria consisted of a minimum age of 2.5 years and the absence of CDS or other medical condition that could negatively impact the current study, which was determined by a specialized veterinary practitioner based on the combination of results from a physical examination, evaluation of historical records, and behavioral assessment, including owner interview, in all dogs conducted prior to the study. The recruited sample (Table 1) included 16 intact males, 2 orchiectomized males, 8 intact females, and 18 ovariectomized females of different breeds; age ranged from 3 to 12.5 years (mean ± SD = 7.8 ± 2.8 years).
Table 1.
Age and sex of dogs enrolled in the study
| Name | Sex | Age (years) | Name | Sex | Age (years) |
|---|---|---|---|---|---|
| Luna | Female | 3.0 | Milka | Femalea | 8.0 |
| Max | Male | 3.2 | Zoe | Femalea | 8.0 |
| Camilla | Female | 3.8 | Pitti | Male | 8.1 |
| Jorgo | Male | 3.9 | Nocciola | Male | 8.3 |
| Spigola | Femalea | 4.0 | Gilda | Femalea | 8.5 |
| Ska | Female | 4.3 | Amur | Male | 9.0 |
| Aries | Male | 4.4 | Baby | Female | 9.0 |
| Aky | Femalea | 4.5 | Susanna | Femalea | 9.0 |
| Lana | Femalea | 5.2 | Samba | Female | 9.1 |
| Fei | Femalea | 5.8 | Notte | Female | 9.2 |
| Fiocco | Malea | 6.0 | Kimi | Femalea | 9.4 |
| Freud | Male | 6.1 | Wendy | Femalea | 10.3 |
| Geppo | Male | 6.1 | Tobia | Male | 10.8 |
| Kira | Femalea | 6.2 | Lucky | Male | 11.0 |
| Peggy | Femalea | 6.2 | Arturo | Male | 11.5 |
| Zizza | Female | 6.3 | Pucci | Female | 12.0 |
| Turbo | Male | 6.5 | Ringo | Malea | 12.0 |
| Chucky | Femalea | 6.7 | Birba | Femalea | 12.1 |
| Jack | Male | 6.8 | Trudy | Femalea | 12.3 |
| Kirk | Male | 7.0 | Pippo | Male | 12.4 |
| Lilly | Femalea | 7.0 | Cora | Femalea | 12.5 |
| Hook | Male | 7.3 | Farida | Femalea | 12.5 |
aGonadectomized dog
Experimental procedure
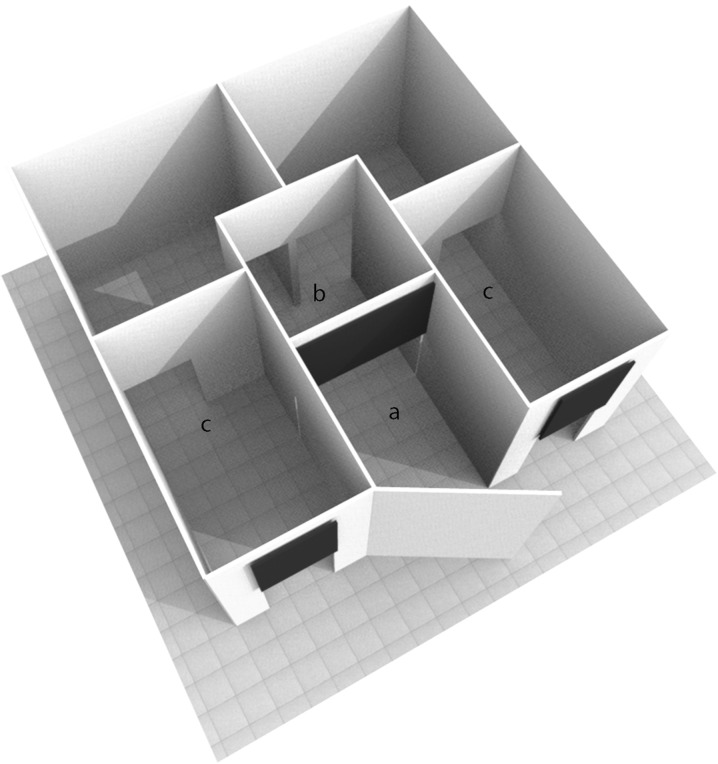
Cognitive testing was conducted in a room of approximately 5 × 5 m, which housed the test apparatus (Fig. 1). The apparatus was an adapted version of the apparatus already employed for testing spatial learning and memory in a different species (Regolin et al. 1995; Regolin and Rose 1999) and was constructed of white plastic panels. Externally, the maze measured 3 m in both width and length and 1.5 m in height. The entrance to the apparatus consisted of a 1-m wide door, leading into the start compartment, (1 × 1 m; see compartment “a” in Fig. 1). A chair for the owner was placed outside of the entrance door. The apparatus contained three sliding panels remotely controlled by an experimenter located in an adjacent room; one panel permitted access to the central compartment (1 × 1 m; see compartment “b” in Fig. 1) from the start compartment and the remaining two served as exits at the end of each lateral arm (3 × 1 m at their narrowest point; see compartments “c” in Fig. 1). The dogs’ responses within the apparatus were monitored in real time using video cameras and were also recorded (WV-GP250, Panasonic, Osaka, Japan) for subsequent analysis. The position of the apparatus within the room was randomly rotated (180°) among subjects in a balanced design to prevent fixed external cues (i.e., cues within test room) from biasing subject responses.
Fig. 1.
Representation of the T-maze employed in the study. A door in the center of the front wall leads into the start compartment (1 × 1 m; a). A sliding panel divides the start and the central compartment (1 × 1 m; b), from where two identical and symmetrical openings lead into the lateral arms of the maze (3 × 1 m at their narrowest point; c). Each of these arms terminates with an aperture (0.8 m in height), equipped with a remotely operated sliding panel. Sliding panels are represented in black in the picture
The procedure consisted of four stages. The first stage was a single direction-determination trial, which was used to determine the correct exit path for each subject in subsequent learning and memory retention stages. The second stage of the procedure was the learning task, in which dogs were required to learn which of the two arm choices resulted in the correct exit path from the apparatus. Retention of this information was assessed after 2 weeks in the third long-term retention test stage. In the last stage, the reversal learning task, dogs were required to learn to exit from the path opposite to that reinforced in the learning task.
Direction-determination trial
The trial began when the dog was in the start compartment and with all doors and panels closed. The owner then called the dog and immediately thereafter the experimenter raised the sliding panel leading into the central compartment, which remained lifted until the end of the trial. The dog could freely enter either of the lateral arms. The arm entered first by the dog became the incorrect arm entry for that dog in the subsequent learning task and memory retention test. Accordingly, the sliding panel at the end of the lateral arm initially entered by the dog remained closed throughout the trial, as well as all of the trials of the subsequent learning task. Therefore, the dog had to navigate back to the central compartment and then to the opposite arm to exit from the apparatus. When the dog reached the half-way point of the correct arm, the sliding panel at the end of this was raised and the trial ended when the dog stepped out of the apparatus. This procedure was used to rule out any possible side preference of the individual dogs and to oblige the dog to explore the entire maze at least once. This also reduced the possibility of the subjects using olfactory cues in subsequent trials of the learning task.
Learning task
Immediately following the direction-determination trial, the dogs underwent one session of continuous consecutive trials during which the exit panel of the correct lateral arm remained lifted. To reduce the possibility of developing a negative association between stepping out of the apparatus and subsequent re-introduction, the owner praised, pet or played with the dog for about 45 s during the inter-trial intervals. On each trial, a correct response was recorded when the dog entered the correct side compartment first and an incorrect response was recorded when the dog entered the incorrect side first. Each dog was tested until it achieved the learning criterion of three consecutive correct trials within the maximum 15 trials. If the learning criterion was not achieved within 15 trials, the dog failed the task and was not included in the subsequent stages.
Retention test
This test took place 2 weeks (mean ± SD = 15 ± 1.9 days) after the learning phase with the aim of determining if dogs retained the information acquired in the learning phase over a long-term delay. The retention test consisted of four trials in which the exit panels for both lateral arms were open, thereby allowing the dog to exit the apparatus from either lateral arm and preventing new learning from confounding the results of the retention test. A dog successfully passed the retention test if it initially entered the correct lateral arm, as acquired during the learning task, at least three out of the four trials.
Reversal learning task
Only dogs that passed the retention test were included in this phase, which took place immediately after the retention test. The reversal learning task was intended to evaluate the dogs’ ability to contrast and modify previously acquired behavioral responses, i.e., navigating the previously reinforced direction. Thus, the protocol and learning criterion were identical to that of the learning task, but the arm choice resulting in a path out of the apparatus was reversed. Since the dogs were expected to respond incorrectly on the first trial, this was not included in the 15 trial maximum, nor was it considered in the statistical analysis.
Data collection and statistical analysis
Data were collected from video-recordings by a single observer. It was unnecessary to verify interobserver reliability as the determination of the dog's choice within the maze was unequivocal. More specifically, a side choice was recorded when both fore limbs first entered a lateral arm of the maze. For each trial, latency (time in seconds from the owner’s call to the side choice), duration (time in seconds from the dog’s side choice to exit from the apparatus) and side choice were recorded. Maximal errors or errors to reach criterion served as the dependent variable for statistical analyses of learning, retention and reversal-learning performance accuracy. Latency and duration were also analyzed as respective measures of performance speed, which in turn could reflect processing speed, locomotion speed or motivation. Data are reported as mean ± SD and all statistical analyses were performed using Statistica 11.0 (StatSoft Inc., Tulsa, OK).
Initially, linear regression analyses were conducted to examine the relationship between age and performance parameters (mean trial latency, duration and errors) in the learning, retention and reversal learning stages. To determine the relationship between performance accuracy across stages, Pearson-product moment correlations between task errors were determined. Moreover, an identical analysis was conducted between task errors and mean trial latency and duration measures within each task.
To further investigate the effect of age on performance parameters across the learning and reversal learning tasks, subjects were divided in a cohort of younger (<8 years, N = 22, mean age ± SD = 5.5 ± 1.3) and older dogs (≥8 years, N = 22, mean age ± SD = 10.3 ± 1.7). Repeated-measures analyses of variance (ANOVA) were conducted with the dog serving as subject variable, age group (younger and older) as a between-subject factor and task (learning and reversal) as a within-subject factor. Post hoc Tukey’s test was used to examine significant main effects and interactions as appropriate. To examine the effects of age on memory retention performance, independent t-tests between age groups were conducted.
Results
Overall performance in the spatial navigation tasks
Dogs performed the direction-determination trial with a latency of 10.6 ± 14.9 s and completed the remainder of the trial in 48.5 ± 50.4 s. The first choice was similarly distributed between the left (53.5 % of the sample) and the right arm of the maze (46.5 %).
The mean latency and duration of learning task trials were 5.1 ± 5.9 s and 18.1 ± 21.9 s, respectively; the learning criterion was reached by 75.0 % (33/44) of subjects in 7.5 ± 3.5 trials. The subjects that achieved the learning criterion were 7.3 ± 2.5 years of age on average compared with an average age of 9.2 ± 3.5 years for those that failed the task.
In the retention test, mean trial latency was 3.3 ± 2.6 s and mean trial duration was 3.7 ± 5.5 s. Twenty-seven of 33 (81.8 %; age = 7.1 ± 2.5 years) dogs showed successful retention of the task after 2 weeks, with 40.1 % of successful subjects committing no errors. The mean age of the unsuccessful dogs was 8.7 ± 2.1 years.
Reversal learning trials had a mean latency of 7.6 ± 11.5 s and a mean duration of 20.6 ± 17.0 s. The reversal learning criterion was successfully achieved by 20 out of the 27 dogs (74.1 %) that passed the retention task, in an average of 7.8 ± 3.1 trials. The mean age of the successful and unsuccessful dogs was 6.2 ± 2.0 and 9.4 ± 2.2 s, respectively. Overall, the results demonstrate the tasks were successfully acquired rapidly in the majority (i.e., ≥74 % for each tasks) of tested subjects.
Effects of age on performance in the spatial navigation task
Success and failure rate for younger and older dogs in the different stages are presented in Table 2.
Table 2.
Number of dogs in each age group that succeeded and failed each stage
| Age group | Successful | Unsuccessful | Total |
|---|---|---|---|
| Learning task | |||
| <8 years | 17 | 5 | 22 |
| ≥8 years | 16 | 6 | 22 |
| Total | 33 | 11 | 44 |
| Retention test | |||
| <8 years | 15 | 2 | 17 |
| ≥8 years | 12 | 4 | 16 |
| Total | 27 | 6 | 33 |
| Reversal task | |||
| <8 years | 14 | 1 | 15 |
| ≥8 years | 6 | 6 | 12 |
| Total | 20 | 7 | 27 |
Significant effects of age were found by linear regression on reversal errors (F1, 25 = 6.07; p = 0.021) but not on learning or retention errors. No effect of age was detected on mean trial latency or duration measures.
No significant correlations were found between tasks on errors committed, suggesting that performance accuracy on each task was independent of the other. Moreover, errors were not correlated with mean trial latency or duration on any task, indicating that accuracy was independent of performance speed.
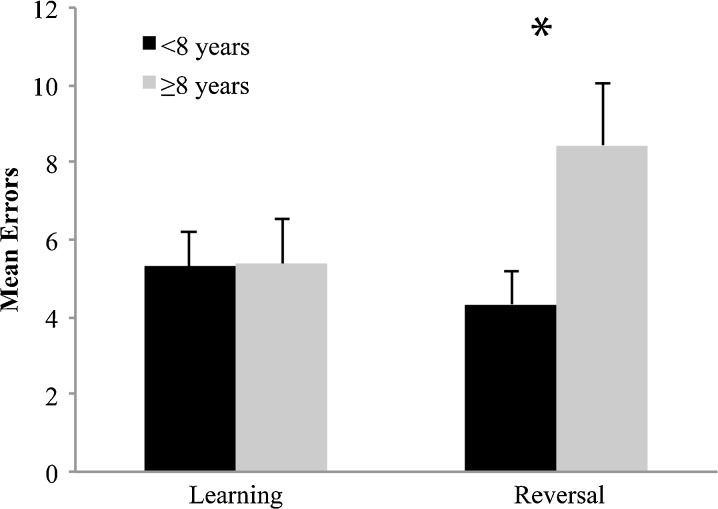
ANOVAs examining errors across the learning and reversal tasks revealed significant effects of age group (F1, 25 = 6.13; p = 0.02), task (F1, 25 = 11.14; p = 0.003), and an interaction between the two (F1, 25 = 7.11; p = 0.01). Older dogs committed significantly more errors on the reversal learning task compared with all other conditions (p < 0.001 in all cases) consistent with an age-related deficit in reversal learning (Fig. 2). The individual ANOVAs examining age group effect on mean trial latency and duration revealed significant effects of age on mean trial latency (F1, 25 = 4.66; p = 0.04), but not duration. Moreover, no effects of task or an interaction between task and age group were evident on duration or latency measures. This indicates that older dogs made a choice more slowly than younger dogs independent of task, but completed the remainder of the trial in the same time as younger dogs. Lastly, there was no effect of age group on errors, mean trial latency or mean trial duration during the retention test. Collectively, these results suggest processing speed may be reduced in aged subjects, while time to complete the task is independent of age, which is discussed further below.
Fig. 2.
Mean errors ± SD committed by younger (<8 years, N = 15) and older (≥8 years, N = 12) dogs in the learning and reversal learning tasks. Tukey’s post hoc test; *P < 0.001
Discussion
The current study sought to assess the learning, retention and reversal learning performance of aged pet dogs in a simple spatial navigation task. Forty-four dogs were initially trained on a spatial navigation learning task and data were obtained from all dogs within a single session. Additionally, 61.3 % (27/44) of the subjects successfully moved through the procedure to the reversal learning task. Older dogs showed reversal learning accuracy deficits, which were not evident on the learning and memory retention tasks. Overall, the spatial navigation paradigm employed here successfully permitted the practical assessment of spatial cognitive abilities of pet dogs.
Studies investigating age-related cognitive decline in dogs are generally restricted to laboratory populations, primarily due to the absence of practical methods for testing cognitive function in pet dogs. The rationale of the methodology employed in the current study was to develop a novel spatial navigation paradigm, based on ethologically relevant owner seeking behavior, that ultimately could be used to assess spatial cognitive ability in pet dogs. In contrast to the object based testing paradigms employed in the laboratory, ethologically relevant tasks are expected to confer more predictable responses with little or no training (Miklosi 2009), which was evident in the present study as dogs navigated the entire maze in a short time even on the first trial. Moreover, each task was in most cases completed in less than half an hour. Collectively, the relative ease of acquisition makes this spatial navigation paradigm practical for both pet dogs and their owners.
The second aim of this study was to determine if age negatively impacted performance across the three tasks of this spatial navigation paradigm. Reversal learning ability declined with increasing age and was impaired in the older sub-group compared with the younger subgroup; however, there was no evidence of similar deficits on the learning task and retention test. The age-dependent reversal learning deficits reported here are consistent with those previously reported in canine cognitive studies (Adams et al. 2000; Christie et al. 2005). Specifically, more errors were committed by the older dog sub-group on the reversal task compared with younger dogs and this age effect was not evident on the spatial learning task. In contrast to discrimination learning, age-sensitive reversal learning deficits in dogs are independent of contextual cues, being reported consistently across a variety of tasks including shape (Milgram et al. 1994), size (Tapp et al. 2003a) and egocentric visuospatial discrimination (Christie et al. 2005). The high degree of genetic and environmental heterogeneity of the canine population in the present study suggests reversal learning deficits reported in laboratory Beagles are applicable to the general dog population. In this respect, this phenomenon is consistent in mammalian species as impaired reversal learning is reported in aged primates (Herndon et al. 1997; Lai et al. 1995; Tsuchida et al. 2002; Voytko 1999) and rodents (Rahner-Welsch et al. 1995; Stephens et al. 1985).
By contrast to reversal learning, data from previous studies examining the effect of age on simple associative learning are inconsistent, with reports of both age sensitivity (Christie et al. 2005; Milgram et al. 2002a; Tapp et al. 2003a) and age insensitivity (Christie et al. 2005; Milgram et al. 1994; Salvin et al. 2011). The primary difference among these studies is the use of different test paradigms that presumably vary in difficulty. Therefore, the absence of age-related spatial learning deficits in the current study may be due to the use of a two-choice spatial navigation discrimination task that is too simple to reliably actualize potential age effects on learning. By way of comparison, the current results are similar to those of the task used by Salvin et al. (2011), which also was insensitive to the effects of age on spatial learning ability.
The aged group also demonstrated longer latencies in making a choice compared with young dogs across the learning and reversal tasks, but not on the memory retention task. However, the absence of an interaction between age group and task on mean trial latency, in conjunction with the absence of significant correlations between task latency and accuracy suggests that the age-dependent slowing in making a choice is independent from the accuracy deficits observed in aged dogs on the reversal learning task. Moreover, both mean trial latency and duration were comparable between the original learning task and the reversal learning task, which suggests procedural memory, speed of locomotion and/or motivation, are preserved across the two tasks. The absence of age effects on mean trial latency during the retention test and presence of age effects across both the learning and reversal tasks more likely reflects inherent age-related differences in processing speed, that emerge during the acquisition of novel spatial information, but are not evident when retrieving previously acquired information. Therefore, the current results suggest processing speed is impaired by aging during acquisition of novel information and that this effect is independent of age-related accuracy deficits on similar tasks.
Retention was assessed 2 weeks after the initial learning task by repeatedly letting the dogs choose freely between the arms of the maze. The high proportion (81.8 %) of dogs that chose the direction previously acquired on the spatial learning task has two relevant implications. First, it supports the validity of the learning criterion used in the current spatial learning task opposed to the more stringent criterion often used with laboratory dogs, e.g., at least 80 % correct responses in 20 trials (Milgram et al. 1994). Second, this result suggests age-insensitivity of long-term spatial memory retention in the dog. On the other hand, twice the number of aged dogs failed the retention test (i.e., four aged dogs failed compared with two young dogs), which suggests the current study may have been under-powered to accurately evaluate spatial memory. Regarding the characterization of memory functions in dogs, current data are mostly limited to short(er)-term memory (e.g., Fiset 2007; Fiset et al. 2003; Salvin et al. 2011). The lack of scientific data on canine long-term memory is quite surprising, especially as some symptoms of CDS may be ascribed to long-term memory deficits (e.g., getting lost in familiar places, failure to recognize known people, loss of previously known commands; Landsberg 2005; Osella et al. 2007). There is only one report of age-related impairment in long-term memory in Beagle dogs (Milgram et al. 2002b), which, however, is not directly comparable to the current study due to substantial methodological differences.
A limitation of the current study is the attrition of subjects unable to achieve the task-specific learning criterion, which was likely linked to the limited number of trials afforded for completing each task. The rationale for the a priori selection of a maximum of 15 acquisition trials for the learning and reversal stages was twofold. First, previous work indicated adult pet dogs learn to detour around a barrier to reach a goal in as few as 6 trials (Pongracz et al. 2001) and laboratory dogs can learn a spatial search task with a stringent learning criterion in an average of 14 trials (Ashton and De Lillo 2011). Therefore, we postulated that 15 trials would be sufficient for both the learning and reversal tasks, due to the expected simplicity associated with this ethologically relevant navigation paradigm. Second, we wanted to ensure that the duration of the test was sufficiently short that the attention and motivation of both the dog and owner was maintained over the course of the session and over the sessions of the whole study. While future studies may help further refine the learning criteria employed in the current study, the impact of these limitations in the current study were partially relegated by including errors over the maximum number of sessions for subjects that failed a specific task in the statistical analyses. Regardless, the procedure and learning criteria in the present study likely resulted in the exclusion of the most cognitively impaired subjects before the last stage, suggesting that age-related impairments limited to the reversal learning task in the current study may be an overly conservative conclusion.
Implications and future directions
The present study demonstrates the feasibility of employing a spatial navigation paradigm for the practical assessment of cognitive function, including learning, long-term memory retention and reversal learning, in pet dogs. Age-related spatial impairment was only evident in reversal learning, which is consistent with previous canine cognitive studies thereby providing validation of this cognitive assessment strategy. Therefore, the current study extends neuropsychological cognitive test protocols, which have generally been limited to laboratory-housed Beagles, to pet dogs. This should offer several complimentary benefits for aging studies (Waters 2011). First, pet dogs are exposed to a wider range of environmental factors likely to impact cognitive aging, which are hardly reproducible in a laboratory-confined model. Second, extending canine cognitive aging studies to dogs with more variable life-expectancy, genetic background, dietary experience, health management, social stimuli, and learning experiences should improve understanding of canine aging and improve generalization of study results to the dog population. Third, the fact that pet dogs share both the social and physical environment of humans should ultimately increase the value of the canine model for studying human aging and AD progression, regardless of the potential confounds associated with reduced control of the dogs’ living conditions. Overall, the addition of aging studies in pet dogs should prompt the investigation of normative and pathological aging as it applies to veterinary and human medicine.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
β-amyloid
References
- Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:675–692. doi: 10.1016/S0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Ashton RL, De Lillo C. Association, inhibition, and object permanence in dogs' (Canis familiaris) spatial search. J Comp Psychol. 2011;125:194–206. doi: 10.1037/a0022584. [DOI] [PubMed] [Google Scholar]
- Begega A, Cienfuegos S, Rubio S, Santin JL, Miranda R, Arias JL. Effects of ageing on allocentric and egocentric spatial strategies in the Wistar rat. Behav Processes. 2001;53:75–85. doi: 10.1016/S0376-6357(00)00150-9. [DOI] [PubMed] [Google Scholar]
- Borras D, Ferrer I, Pumarola M. Age-related changes in the brain of the dog. Vet Pathol. 1999;36:202–211. doi: 10.1354/vp.36-3-202. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Carrillo-Mora P, Giordano M, Santamaria A. Spatial memory: theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009;203:151–164. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Christie LA, Studzinski CM, Araujo JA, Leung CSK, Ikeda-Douglas CJ, Head E, Cotman CW, Milgram NW. A comparison of egocentric and allocentric age-dependent spatial learning in the beagle dog. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:361–369. doi: 10.1016/j.pnpbp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimer Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset S. Landmark-based search memory in the domestic dog (Canis familiaris) J Comp Psychol. 2007;121:345–353. doi: 10.1037/0735-7036.121.4.345. [DOI] [PubMed] [Google Scholar]
- Fiset S, Beaulieu C, Landry F. Duration of dogs' (Canis familiaris) working memory in search for disappearing objects. Anim Cogn. 2003;6:1–10. doi: 10.1007/s10071-002-0157-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Pelleymounter MA. An age-related spatial-learning deficit—choline uptake distinguishes impaired and unimpaired rats. Neurobiol Aging. 1988;9:363–369. doi: 10.1016/S0197-4580(88)80082-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Soriano J, Garcia PM, Contreras-Rodriguez J, Martinez-Sainz P, Rodriguez-Veiga E. Age-related changes in the ventricular system of the dog brain. Anat Anz. 2001;183:283–291. doi: 10.1016/S0940-9602(01)80236-3. [DOI] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/S0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/S0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/S0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hort J, Laczo J, Vyhnalek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Complex maze-learning in rodents as a model of age-related memory impairment. Neurobiol Aging. 1988;9:475–485. doi: 10.1016/S0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Klein DA, Steinberg M, Galik E, Steele C, Sheppard JM, Warren A, Rosenblatt A, Lyketsos CG. Wandering behaviour in community-residing persons with dementia. Int J Geriatr Psychiatry. 1999;14:272–279. doi: 10.1002/(SICI)1099-1166(199904)14:4<272::AID-GPS896>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey—spatial and object reversal-learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Landsberg G. Therapeutic agents for the treatment of cognitive dysfunction syndrome in senior dogs. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:471–479. doi: 10.1016/j.pnpbp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Landsberg GM, Nichol J, Araujo JA (2012) Cognitive dysfunction syndrome: a disease of canine and feline brain ageing. Vet Clin N Am Sm Anim Pract 42:749–768. doi: 10.1016/j.cvsm.2012.04.003 [DOI] [PubMed]
- Lukoyanov NV, Andrade JP, Madeira MD, Paula-Barbosa MM. Effects of age and sex on the water maze performance and hippocampal cholinergic fibers in rats. Neurosci Lett. 1999;269:141–144. doi: 10.1016/S0304-3940(99)00442-5. [DOI] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Harlan RE, Kastin AJ, Zadina JE. Tests used to assess the cognitive abilities of aged rats: their relation to each other and to hippocampal morphology and neurotrophin expression. Gerontology. 1999;45:143–155. doi: 10.1159/000022077. [DOI] [PubMed] [Google Scholar]
- Miklosi A. Dog behaviour, evolution and cognition. New York: Oxford University Press; 2009. pp. 137–150. [Google Scholar]
- Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog—acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037/0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neurosci Biobehav Rev. 2002;26:679–695. doi: 10.1016/S0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/S0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Moffat SD. Aging and spatial navigation: what do we know and where do we go. Neuropsychol Rev. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Morys J, Narkiewicz O, Maciejewska B, Wegiel J, Wisniewski HM. Amyloid deposits and loss of neurons in the claustrum of the aged dog. Neuroreport. 1994;5:1825–1828. doi: 10.1097/00001756-199409080-00035. [DOI] [PubMed] [Google Scholar]
- Newman MC, Kaszniak AW. Spatial memory and aging: performance on a human analog of the Morris water maze. Aging Neuropsychol Cogn. 2000;7:86–93. doi: 10.1076/1382-5585(200006)7:2;1-U;FT086. [DOI] [Google Scholar]
- Osella MC, Re G, Odore R, Girardi C, Badino P, Barbero R, Bergamasco L. Canine cognitive dysfunction syndrome: prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl Anim Behav Sci. 2007;105:297–310. doi: 10.1016/j.applanim.2006.11.007. [DOI] [Google Scholar]
- Papaioannou N, Tooten PCJ, van Ederen AM, Bohl JRE, Rofina J, Tsangaris T, Gruys E. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 2001;8:11–21. doi: 10.3109/13506120108993810. [DOI] [PubMed] [Google Scholar]
- Pongracz P, Miklosi A, Kubinyi E, Gurobi K, Topal J, Csanyi V. Social learning in dogs: the effect of a human demonstrator on the performance of dogs in a detour task. Anim Behav. 2001;62:1109–1117. doi: 10.1006/anbe.2001.1866. [DOI] [Google Scholar]
- Rahner-Welsch S, Frolich L, Stoll S, Hoyer S. Decline and preservation of reversal-learning abilities and acquisition in the course of senescence. Neurosci Lett. 1995;194:121–123. doi: 10.1016/0304-3940(95)11712-6. [DOI] [PubMed] [Google Scholar]
- Regolin L, Rose SPR. Long-term memory for a spatial task in young chicks. Anim Behav. 1999;57:1185–1191. doi: 10.1006/anbe.1999.1097. [DOI] [PubMed] [Google Scholar]
- Regolin L, Vallortigara G, Zanforlin M. Object and spatial representations in detour problems by chicks. Anim Behav. 1995;49:195–199. doi: 10.1016/0003-3472(95)80167-7. [DOI] [Google Scholar]
- Rofina JE, Singh K, Skoumalova-Vesela A, van Ederen AM, van Asten A, Wilhelm J, Gruys E. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid. 2004;11:90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- Ruehl WW, Bruyette DS, DePaoli A, Cotman CW, Head E, Milgram NW, Cummings BJ. Canine cognitive dysfunction as a model for human age-related cognitive decline, dementia and Alzheimer's disease: clinical presentation, cognitive testing, pathology and response to 1-deprenyl therapy. Prog Brain Res. 1995;106:217–225. doi: 10.1016/S0079-6123(08)61218-2. [DOI] [PubMed] [Google Scholar]
- Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. The canine sand maze: an appetitive spatial memory paradigm sensitive to age-related change in dogs. J Exp Anal Behav. 2011;95:109–118. doi: 10.1901/jeab.2011.95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak CT, Tapp PD, Milgram NW. Effect of age and level of cognitive function on spontaneous and exploratory Behaviors in the beagle dog. Learn Mem. 2001;8:317–325. doi: 10.1101/lm.41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29:39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Weidmann R, Quartermain D, Sarter M. Reversal-learning in senescent rats. Behav Brain Res. 1985;17:193–202. doi: 10.1016/0166-4328(85)90043-9. [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Araujo JA, Milgram NW. The canine model of human cognitive aging and dementia: pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:489–498. doi: 10.1016/j.pnpbp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. Visuospatial function in the beagle dog: an early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem. 2006;86:197–204. doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Su MY, Tapp PD, Vu L, Chen YF, Chu Y, Muggenburg B, Chiou JY, Chen CQ, Wang J, Bracco C, Head E. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:389–397. doi: 10.1016/j.pnpbp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Memory. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Holowachuk D, Milgram NW. Effects of age on measures of complex working memory span in the beagle dog (Canis familiaris) using two versions of a spatial list learning paradigm. Learn Memory. 2003;10:148–160. doi: 10.1101/lm.56503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp D, Chu Y, Vu L, Chiou JY, Milgram NW, Nalcioglu O, Su MY. Age-dependent changes in regional brain volume and cerebral blood volume in white matter of the canine brain measured using dynamic susceptibility contrast MRI. Neurobiol Aging. 2004;25:S380–S381. doi: 10.1016/S0197-4580(04)81247-8. [DOI] [Google Scholar]
- Tapp PD, Chu Y, Araujo JA, Chiou JY, Head E, Milgram NW, Su MY. Effects of scopolamine challenge on regional cerebral blood volume. A pharmacological model to validate the use of contrast enhanced magnetic resonance imaging to assess cerebral blood volume in a canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:399–406. doi: 10.1016/j.pnpbp.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Tolman EC, Honzik CH. Introduction and removal of reward, and maze performance in rats. Univ Calif Pub Psychol. 1930;4:257–275. [Google Scholar]
- Tsuchida J, Kubo N, Kojima S. Position reversal learning in aged Japanese macaques. Behav Brain Res. 2002;129:107–112. doi: 10.1016/S0166-4328(01)00336-9. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakayama H, Tateyama S, Goto N. Immunohistochemical analysis of constituents of senile plaques and cerebrovascular amyloid in aged dogs. J Vet Med Sci. 1992;54:1023–1029. doi: 10.1292/jvms.54.1023. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20:617–627. doi: 10.1016/S0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Waters D. Aging research 2011: exploring the pet dog paradigm. ILAR J. 2011;52:97–105. doi: 10.1093/ilar.52.1.97. [DOI] [PubMed] [Google Scholar]