Abstract
Hippocampal neurogenesis, important for memory and mood function, wanes greatly in old age. Studies in rat models have implied that this decrease is not due to loss of neural stem cells (NSCs) in the subgranular zone of the dentate gyrus (DG) but rather due to an increased quiescence of NSCs. Additional studies have suggested that changes in the microenvironment, particularly declines in the concentrations of neurotrophic factors, underlie this change. In this study, we compared the expression of 84 genes that are important for NSC proliferation and neurogenesis between the DG of young (4 months old) and aged (24 months old) Fischer 344 rats, using a quantitative real-time polymerase chain reaction array. Interestingly, the expression of a vast majority of genes that have been reported previously to positively or negatively regulate NSC proliferation was unaltered with aging. Furthermore, most genes important for cell cycle arrest, regulation of cell differentiation, growth factors and cytokine levels, synaptic functions, apoptosis, cell adhesion and cell signaling, and regulation of transcription displayed stable expression in the DG with aging. The exceptions included increased expression of genes important for NSC proliferation and neurogenesis (Stat3 and Shh), DNA damage response and NF-kappaB signaling (Cdk5rap3), neuromodulation (Adora1), and decreased expression of a gene important for neuronal differentiation (HeyL). Thus, age-related decrease in hippocampal neurogenesis is not associated with a decline in the expression of selected genes important for NSC proliferation and neurogenesis in the DG.
Keywords: Aging, Hippocampus, Dentate gyrus, Dentate neurogenesis, Genes, Gene expression, Hippocampal neurogenesis, Neural stem cells, Stem cells and aging, qRT-PCR
Introduction
Hippocampal neurogenesis, characterized by the proliferation of neural stem/progenitor cells (NSCs) residing in the subgranular zone (SGZ) of the dentate gyrus (DG) and differentiation of newly born cells into granule cells in the granule cell layer (GCL) of the DG, occurs all through life (Gage 2002; Gould 2007; Leuner and Gould 2010). This phenomenon has received widespread attention because of its perceived role in functions such as learning, memory, and mood (Aimone et al. 2006; Clelland et al. 2009; Drapeau et al. 2003; Dupret et al. 2007; Eisch et al. 2008; Imayoshi et al. 2008; Jessberger et al. 2009; Kee et al. 2007; Kuhn et al. 1996; Leuner et al. 2006; Sahay and Hen 2007; Santarelli et al. 2003). However, the extent of neurogenesis progressively declines with age resulting in ~90 % decline in old age (Kuhn et al. 1996; Rao et al. 2005, 2006). As old age is also associated with decreased ability for hippocampal-dependent learning and memory function and increased incidence of depression, there is a widespread interest to understand the mechanisms underlying the age-related decrease in neurogenesis and to develop strategies that increase neurogenesis in the aged hippocampus (Bachstetter et al. 2008; Bernal and Peterson 2004; Lie et al. 2005; Drapeau and Abrous 2008; Hattiangady et al. 2007; Jessberger and Gage 2008; Miranda et al. 2012).
Multiple studies have shown that age-related decrease in neurogenesis is a result of proliferation of far fewer NSCs in the SGZ (Hattiangady and Shetty 2008; Kuhn et al. 1996; Nacher et al. 2003; Olariu et al. 2007; Rao et al. 2005, 2006). Examination of the putative NSCs using markers such as Sox-2, GFAP, and vimentin over the course of aging has revealed no changes in NSC numbers in the rodent SGZ with aging (Aizawa et al. 2011; however, see Encinas et al. 2011; Hattiangady and Shetty 2008). Interestingly, a combined analysis using a birth-dating marker 5′-bromodeoxyuridine and an endogenous proliferation marker Ki-67 has suggested an increased quiescence of NSCs in the aged rat’s SGZ (Hattiangady and Shetty 2008). Additional studies suggest that changes in the milieu, particularly declines in the concentrations of multiple neurotrophic factors that are mitogenic to NSCs in the hippocampus and changes in neurotrophic factor receptors and stem cell niche, likely underlie this change (Chadashvili and Peterson 2006; Hattiangady et al. 2005; Shetty et al. 2005). These include decreases in the concentration of brain-derived neurotrophic factor (Bdnf), fibroblast growth factor (Fgf-2), insulin-like growth factor (Igf-1), vascular endothelial growth factor (Vegf), and an increased expanse between NSCs and vasculature. However, it is unknown whether the expression of groups of genes that are important for NSC function and neurogenesis is altered in the aged DG. With an interest to investigate the molecular processes that are potentially involved in the age-related decline of hippocampal neurogenesis, we investigated changes in the expression of genes that are important for NSC proliferation, differentiation, and neurogenesis in the DG with aging. We specifically compared the expression of stem cell- and neurogenesis-related genes between the young (4 months old) DG and the aged (24 months old) DG, using a quantitative real-time polymerase chain reaction (qRT-PCR) array. For this, we employed “The Rat Neurogenesis and Neural Stem Cells RT2 ProfilerTM PCR Array” from SABiosciences, which profiles the expression of 84 genes that are important for cell proliferation, cell cycle, cell migration, cell differentiation, synaptic functions, apoptosis, growth factors, and cytokines.
Materials and methods
Animals and dissection of DG tissue
Young adult (4 months old) and aged (24 months old) male Fischer 344 (F344) rats, obtained from the National Institute of Aging colony at Harlan Sprague Dawley Inc. (Indianapolis, IN), were used in this study. Prior to the experiment, all animals were housed for 2 weeks in an environmentally controlled room (~23 °C) with a 12:12-h light–dark cycle and were given food (commercial rat chow) and water ad libitum. We chose F344 rats for this aging study because the genetic background, the normal life span, and the development of these rats are reasonably well defined (Coleman et al. 1977). Institutional animal care and use committees of Duke University Medical Center, Durham Veterans Affairs Medical Center, and Texas A&M Health Science Center approved the experiments performed in this study. Both young adult and aged animals (n = 5 per group) were killed by an overdose of anesthesia, the brains were rapidly removed from the skull, and the entire hippocampus was swiftly dissected from each cerebral hemisphere (Hattiangady et al. 2005). Using a dissection microscope, the DG was then separated from the hippocampal CA1 and CA3 subfields using microscissors. However, the dissected DG also contained the hippocampal CA3c subregion, as the part of CA3 pyramidal cell layer that is inserted between the two blades of the dentate granule cell layer could not be removed. Coronal slicing and examination of the dissected DG (from additional samples) revealed the existence of appropriate cell layers in the dissected pieces of tissues.
RNA extraction from DG tissues and first-strand cDNA synthesis
The DG samples obtained from the hippocampus of intact young adult rats and intact aged rats (n = 5/group) were used for qRT-PCR array. For this, we first extracted total RNA from individual DG tissues belonging to different animals of the two groups. The total RNA was extracted using Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA) using the manufacturer’s instructions. The RNA concentration (A260) and quality (A260/A280 ratio) were determined using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE). The total RNA was used to synthesize the template cDNA. For this, we used “The RT2 First Strand Kit” from SABiosciences (Qiagen, Valencia, CA) following the manufacturer’s protocol.
Rat neurogenesis and neural stem cells RT2 ProfilerTM PCR Array
The template cDNA (102 μl) from each sample was mixed with 1,350 μl of 2X RT2 qPCR master mix (SABiosciences, Qiagen, Valencia, CA) and 1,248 μl of dH2O for a total volume of 2,700 μl. Twenty-five microliters of this experimental cocktail was then transferred to each well on the 96-well PCR array plate comprising pre-dispensed gene-specific primer sets (i.e., “The Rat Neurogenesis and NSCs RT2 ProfilerTM PCR Array” from SABiosciences, Catalog # PARN404, Qiagen, Valencia, CA). Each array consisted of primer sets of 84 genes related to neurogenesis and NSCs. This comprised of genes that are important for cell proliferation, cell cycle, cell migration, cell differentiation, synaptic functions, apoptosis, growth factors, and cytokines. Each array also comprised five housekeeping genes and three RNA and three PCR quality controls. Housekeeping genes from this point forward will be referred to as “HKGs.” Reactions were carried out in PCR array kits using a CFX96 real-time system (Bio-Rad, Hercules, CA). The PCR amplification followed a two-step cycling program: 10 min denaturation at 95 °C, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. At the end of the amplification assay, a melt curve analysis was conducted to evaluate the specificity of the reaction. The melting temperature of a DNA double helix depends on its base composition (and its length if it is very short). At the melting point, the two strands of DNA will separate and the fluorescence rapidly decreases. The conditions for the melt curve were 95 °C for 10 s, followed by incremental increase of 0.5 °C every 5 s from 65 to 95 °C.
Fold change calculations and data analyses
Analysis of the expression of individual genes was determined by the defined template developed by SABiosciences (Qiagen) using instructions at their PCR Array Data Analyses website. The fold change calculation involved the following: First, due to the inverse proportional relationship between threshold cycle (Ct) and the original gene expression level, and doubling of the amount of product in every cycle, the original expression level (L) for each gene of interest was expressed as L = 2−Ct. Second, to normalize the expression level of a gene of interest (GOI) to HKGs, the expression level of the GOI is divided by the expression level of HKG: 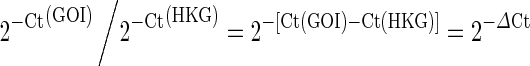 . 2−ΔCt values (n = 5/group) were next compared between young and aged DG using a two-tailed, unpaired Student’s t test.
. 2−ΔCt values (n = 5/group) were next compared between young and aged DG using a two-tailed, unpaired Student’s t test.
Results
Neural stem cell- and neurogenesis-related gene expression in the young and aged DG
The expression of genes in the young and aged DG in this study was determined using five stable HKGs (Rplp1, similar to 60S acidic ribosomal protein P1; Hprt, hypoxanthine guanine phosphoribosyl transferase; Rpl13a, ribosomal protein L13A; Ldha, lactate dehydrogenase A; Actb, actin, beta). The expression of all HKGs was stable between the young and aged DG (data not shown). Furthermore, the expression of a vast majority of genes pertaining to NSC function and neurogenesis was found to be stable with aging in the DG. The expression levels of various genes belonging to different categories are described below.
Cell proliferation genes
To determine age-related changes in the expression of genes involved in positive and negative regulation of cell proliferation, the expression of anaplastic lymphoma kinase (Alk), brain-specific angiogenesis inhibitor 1 (Bai1), chemokine C-X-C motif ligand 1 (Cxcl1), epidermal growth factor (Egf), V-er-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma-derived oncogene homolog (Erbb2), fibroblast growth factor-2 (Fgf-2), interleukin-3 (IL-3), leukocyte-specific transcript 1 (Lst1), necdin homolog (Ndn), neurophilin 1 (Nrp1), notch homolog 2 (Notch2), pleiotrophin (Ptn), and vascular endothelial growth factor A (Vegfa) was measured and compared between the young and aged DG (Table 1). Among these genes, Egf, Fgf-2, and Vegf are well known as NSC mitogenic factors (Cheng et al. 2002; Jin et al. 2003), whereas Notch2 plays a role in a variety of developmental processes by controlling cell fate decisions (Larsson et al. 1994). Ptn, initially recognized as a neurite outgrowth-promoting factor present in the developing rat brain (Rauvala and Pihlaskari 1987), is expressed in an activity-dependent manner in the adult hippocampus where it can suppress long-term potentiation induction (Lauri et al. 1996; Pavlov et al. 2002). Statistical analyses revealed that none of the above genes exhibited change in their expression with aging (Table 1).
Table 1.
Statistical analyses of the expression pattern of different genes
| Gene | Young DGa | Aged DGa | ||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Acetylcholinesterase (Ache) | 0.072917 | 0.0127814 | 0.062817 | 0.0088255 |
| Adenosine A 2 receptor (Adora2a) | 0.015123 | 0.0031573 | 0.013242 | 0.0022333 |
| Anaplastic lymphoma kinase (Alk) | 0.000935 | 0.0002156 | 0.001409 | 0.0003053 |
| Amyloid beta precursor protein-binding, family B, member 1 (Apbb1) | 0.222637 | 0.0379586 | 0.257551 | 0.0397114 |
| Apolipoprotein E (Apoe) | 4.411265 | 0.5905114 | 5.501489 | 0.6288423 |
| Aryl hydrocarbon receptor nuclear translocator 2 (Arnt2) | 0.177054 | 0.0263049 | 0.200545 | 0.0306795 |
| Artemin (Artn) | 0.006275 | 0.0008704 | 0.005381 | 0.0011527 |
| Achaete–scute complex homolog (Ascl1) | 0.021616 | 0.0041798 | 0.015039 | 0.0027787 |
| Brain-specific angiogenesis inhibitor 1 (Bai1) | 0.204503 | 0.0306093 | 0.29986 | 0.0468465 |
| Brain-derived neurotrophic factor (Bdnf) | 0.072874 | 0.0063572 | 0.08628 | 0.0054741 |
| Bone morphogenetic protein 15 (Bmp15) | 0.001472 | 0.0002891 | 0.001474 | 0.0001678 |
| Bone morphogenetic protein 2 (Bmp2) | 0.000562 | 4.29E−05 | 0.000571 | 5.519E−05 |
| Bone morphogenetic protein 4 (Bmp4) | 0.004914 | 0.000359 | 0.006282 | 0.0006074 |
| Bone morphogenetic protein 8a (Bmp8a) | 0.000497 | 5.336E−05 | 0.00047 | 9.48E−05 |
| Cyclin-dependent kinase 5 regulatory subunit 1 (Cdk5r1) | 0.697317 | 0.1339584 | 0.717158 | 0.1427335 |
| Cyclin-dependent kinase (Cdk) regulatory subunit protein 1 (Cdk5rap1) | 0.058138 | 0.0103947 | 0.057914 | 0.0094744 |
| Cdk regulatory subunit protein 2 (Cdk5rap2) | 0.018268 | 0.0045408 | 0.023589 | 0.0047671 |
| Cholinergic receptor muscarinic 2 (Chrm2) | 0.001031 | 0.0002005 | 0.000966 | 0.0001495 |
| Chemokine C-X-C motif ligand 1 (Cxcl1) | 0.001449 | 0.0003703 | 0.002391 | 0.0005866 |
| Discs large homolog 4 (Dlg4) | 0.334712 | 0.0659295 | 0.356174 | 0.0697842 |
| Delta-like 1 (Dll1) | 0.001153 | 0.0001214 | 0.001208 | 0.0002196 |
| Dopamine receptor d2 (Drd2) | 0.003742 | 0.0011114 | 0.002628 | 0.0002956 |
| Dopamine receptor genes d5 (Drd5) | 0.000739 | 0.0001828 | 0.000505 | 7.352E−05 |
| Disheveled dsh homolog 3 (Dvl3) | 0.018894 | 0.0031255 | 0.014643 | 0.0019315 |
| Ephrin-B1 (Efnb1) | 0.008467 | 0.0012573 | 0.00677 | 0.0006678 |
| Epidermal growth factor (Egf) | 0.000467 | 7.623E−05 | 0.000451 | 0.0001045 |
| E1A binding protein p300 (Ep300) | 0.096154 | 0.0093849 | 0.093288 | 0.0093212 |
| V-er-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma-derived oncogene homolog (Erbb2) | 0.003705 | 0.0003308 | 0.004397 | 0.0004698 |
| Fasciculation and elongation protein zeta-1 (Fez1) | 0.345012 | 0.051167 | 0.342668 | 0.0480434 |
| Fibroblast growth factor-13 (Fgf-13) | 1.067819 | 0.2105958 | 1.145277 | 0.1785256 |
| Fibroblast growth factor-2 (Fgf-2) | 0.003106 | 0.0004588 | 0.004303 | 0.0008229 |
| Filamin alpha (Flna) | 0.047025 | 0.0065577 | 0.063761 | 0.0087398 |
| Glial cell line-derived neurotrophic factor (Gdnf) | 0.000496 | 5.401E−05 | 0.000409 | 6.747E−05 |
| Guanine nucleotide-binding protein alpha O polypeptide (Gnoa1) | 0.239916 | 0.0260209 | 0.210701 | 0.0241759 |
| Glucose phosphate isomerase (Gpi) | 1.241085 | 0.2544971 | 0.930615 | 0.1914184 |
| Glutamate receptor ionotropic N-methyl-d-aspartate (Grin1) | 0.363912 | 0.0409108 | 0.33386 | 0.0430336 |
| Histone deacetylase 4 (Hdac4) | 0.14423 | 0.0241545 | 0.146017 | 0.0204341 |
| Histone deacetylase 7 (Hdac7) | 0.018382 | 0.0028146 | 0.017647 | 0.0028586 |
| Hairy enhancer of split 1 (Hes1) | 0.030004 | 0.0043203 | 0.023733 | 0.0037094 |
| Hairy enhancer of split related with YRPW motif 1 (Hey1) | 0.301132 | 0.0565931 | 0.25531 | 0.0551592 |
| Hairy enhancer of split related with YRPW motif 2 (Hey2) | 0.020951 | 0.0023701 | 0.013903 | 0.0014546 |
| Interleukin-3 (IL-3) | 0.000467 | 7.623E−05 | 0.000459 | 9.998E−05 |
| Inhibin beta A (Inhba) | 0.023158 | 0.0055115 | 0.015706 | 0.0021757 |
| Leukocyte-specific transcript 1 (Lst1) | 0.001339 | 0.0002217 | 0.001437 | 0.0002394 |
| Midkine (Mdk) | 0.096365 | 0.0180305 | 0.087922 | 0.0140347 |
| Myocyte enhancer factor 2C (Mef2c) | 0.194882 | 0.0131694 | 0.187893 | 0.0139817 |
| Myeloid/leukemia or mixed-lineage leukemia 1 (Mll1) | 0.155738 | 0.023403 | 0.179161 | 0.0209434 |
| Nuclear receptor coactivator 6 (Ncoa6) | 0.140425 | 0.023143 | 0.146143 | 0.0234583 |
| Necdin homolog (Ndn) | 0.470386 | 0.0865611 | 0.373892 | 0.0740088 |
| Norrie disease—pseudoglioma (Ndp) | 0.044403 | 0.0056247 | 0.036789 | 0.0029199 |
| Neurogenic differentiation 1 (Neurod1) | 0.056289 | 0.0068586 | 0.051557 | 0.0060995 |
| Noggin (Nog) | 0.020408 | 0.0028609 | 0.016399 | 0.0022719 |
| Notch homolog 2 (Notch2) | 0.046888 | 0.0104926 | 0.04167 | 0.0091267 |
| Neuronal pentraxin 1 (Nptx1) | 3.354199 | 0.5150424 | 3.915687 | 0.7066843 |
| Neuronal cell adhesion molecule (Nrcam) | 0.174036 | 0.0175506 | 0.170775 | 0.0173962 |
| Neuregulin 1 (Nrg1) | 0.00581 | 0.0008401 | 0.004288 | 0.0006205 |
| Neurophilin 1 (Nrp1) | 0.104465 | 0.0121486 | 0.128387 | 0.0116148 |
| Neuropilin 2 (Nrp2) | 0.078064 | 0.0060422 | 0.080348 | 0.0064152 |
| Netrin1 (Ntn1) | 0.013655 | 0.0021402 | 0.015404 | 0.0024558 |
| Platelet-activating factor acetylhydrolase isoform 1b alpha subunit (Pafah1b1) | 1.667748 | 0.3399504 | 1.629038 | 0.3032395 |
| Partitioning defective 3 homolog (Pard3) | 0.023974 | 0.0030629 | 0.024091 | 0.0028423 |
| partitioning defective 6 homolog beta (Pard6b) | 0.010405 | 0.0023436 | 0.01649 | 0.0036767 |
| Paired box 2 (Pax2) | 0.000527 | 3.468E−05 | 0.000594 | 3.539E−05 |
| Paired box 3 (Pax3) | 0.000467 | 7.623E−05 | 0.000451 | 0.0001045 |
| Paired box 6 (Pax6) | 0.018361 | 0.0028532 | 0.016875 | 0.0028726 |
| POU class 3 homeobox 3 (Pou3f3) | 0.1413 | 0.0226035 | 0.145334 | 0.0214729 |
| POU class 4 homeobox 1 (Pou4f1) | 0.00047 | 7.322E−05 | 0.000471 | 9.298E−05 |
| Pleiotrophin (Ptn) | 0.667952 | 0.1447678 | 0.465668 | 0.1070898 |
| Ras-related C3 botulinum toxin substrate 1 (Rac1) | 1.049361 | 0.1729229 | 0.957117 | 0.1212237 |
| Roundabout homolog 1 (Robo1) | 0.103739 | 0.0224133 | 0.082614 | 0.0158471 |
| Reticulon-4 (Rtn4) | 2.845402 | 0.6348741 | 2.261293 | 0.6094182 |
| S100 calcium-binding protein A6 (S100a6) | 0.028494 | 0.004679 | 0.034261 | 0.0049849 |
| S100 calcium-binding protein b (S100b) | 3.383875 | 0.9789574 | 3.630579 | 0.8744802 |
| Semaphorin-4D (Sem4d) | 0.122598 | 0.017455 | 0.134256 | 0.0187546 |
| Slit homolog 2 (Slit2) | 0.097756 | 0.0043197 | 0.090677 | 0.0042266 |
| SRY (sex-determining region Y)-box 8 (Sox8) | 0.017244 | 0.0003738 | 0.017603 | 0.0005031 |
| Tenascin-R (Tnr) | 0.333668 | 0.0653956 | 0.341642 | 0.0716827 |
| Vascular endothelial growth factor A (Vegfa) | 0.139126 | 0.0281957 | 0.154283 | 0.0260234 |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (Ywhah) | 5.473344 | 1.5134014 | 6.374386 | 1.4288888 |
aNumbers denote the extent of expression of various genes (mean ± SEM, n = 5/group) normalized to the expression of housekeeping genes (HKGs) and labeled as 2−ΔCt
Cell cycle genes and genes regulating cell motility and migration
The expression pattern of different cell cycle genes in the young and aged DG is shown in Table 1. These include cell cycle arrest genes such as the amyloid beta precursor protein-binding, family B, member 1 (Apbb1), inhibin beta A (Inhba), myeloid/leukemia or mixed-lineage leukemia 1 (Mll1), Notch2, and other regulators of cell cycle, namely, E1A-binding protein p300 (Ep300), histone deacetylase 4 (Hdac4), histone deacetylase 7 (Hdac7), midkine (Mdk), S100 calcium-binding protein A6 (S100a6), and partitioning defective 6 homolog beta (Pard6b). None of these genes exhibited statistically significant changes in their expression with aging (Table 1). We also measured the expression of several genes important for the regulation of cell motility and migration. Most genes belonging to this category such as filamin alpha (Flna), netrin1 (Ntn1), platelet-activating factor acetylhydrolase isoform 1b alpha subunit (Pafah1b1), and slit homolog 2 (Slit2) displayed no changes in their expression between the young and aged DG (Table 1). However, signal transducer and activator of transcription 3 (Stat3) gene showed a significant increase in its expression with aging (Fig. 1).
Fig. 1.
Comparison of the expression of Stat3 between the young and aged DG. The y-axis numbers denote the expression level of Stat3 (mean ± SEM) normalized to the expression of housekeeping genes (HKGs) and labeled as 2−ΔCt. *p < 0.05
Genes regulating cell differentiation
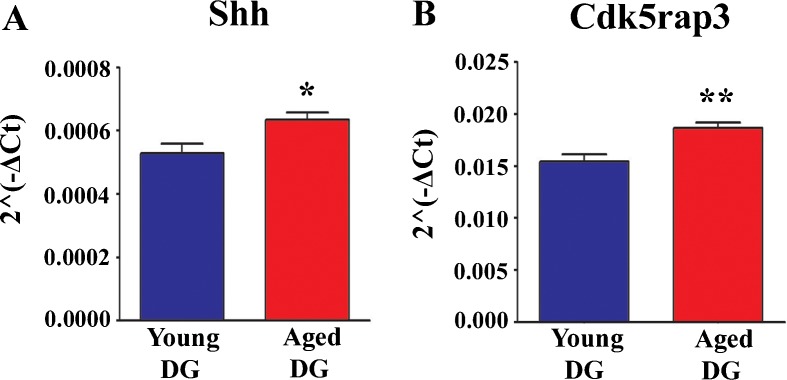
We measured genes that regulate neuronal differentiation, namely, cyclin-dependent kinase (Cdk) regulatory subunit protein 1 (Cdk5rap1), Cdk regulatory subunit protein 2 (Cdk5rap2), cyclin-dependent kinase 5 regulatory subunit 1 (Cdk5r1), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (Ywhah). Interestingly, none of these genes exhibited a significant change in expression with aging, implying that genes mediating neuronal differentiation remain stable with aging in the DG. A similar trend was mostly observed for the expression of genes that are involved in cell fate determination and regulation of cell differentiation. These include the expression of achaete–scute complex homolog (Ascl1), delta-like 1 (Dll1), Hdac4, Hdac7, Inhba, Mdk, neurogenic differentiation 1 (Neurod1), neuronal cell adhesion molecule (Nrcam), noggin (Nog), neuregulin 1 (Nrg1), Notch 2, paired box 2 (Pax2), and paired box 6 (Pax6) (Table 1). However, genes encoding sonic hedgehog (Shh) and Cdk regulatory subunit protein 3 (Cdk5rap3, a putative tumor suppressor) exhibited significantly increased expression with aging (Fig. 2).
Fig. 2.
Comparison of the expression of Shh (a) and Cdk5rap3 (b) between the young and aged DG. The y-axis numbers denote the expression level of genes (mean ± SEM) normalized to the expression of housekeeping genes (HKGs) and labeled as 2−ΔCt. *p < 0.05; **p < 0.01
Growth factor and cytokine genes and genes involved in synaptic functions
We measured the expression of genes of multiple growth factors and cytokines as a function of age. These include artemin (Artn), Bdnf, bone morphogenetic protein (Bmp) 15, Bmp2, Bmp4, Bmp8a, Cxcl1, Egf, Fgf-2, Fgf-13, glial cell line-derived neurotrophic factor (Gdnf), glucose phosphate isomerase (Gpi), IL-3, Inhba, Mdk, Norrie disease—pseudoglioma (Ndp), Nrg1, Ptn, and Vegfa. None of these genes exhibited a significant change in expression with aging in the DG. Furthermore, genes involved in synaptic functions such as acetylcholinesterase (Ache), apolipoprotein E (Apoe), cholinergic receptor muscarinic 2 (Chrm2), discs, large homolog 4 (Dlg4), dopamine receptor genes Drd2 and Drd5, glutamate receptor ionotropic N-methyl-d-aspartate (Grin1), neuronal pentraxin 1 (Nptx1), Nrcam, POU class 4 homeobox 1 (Pou4f1), S100 calcium-binding protein b (S100b), and Ywhah exhibited no changes in their expression with aging.
Genes involved in apoptosis and cell adhesion
Most of the genes involved in apoptosis and cell death exhibited no changes in expression with aging. These include the expression of adenosine A2 receptor (Adora2a), Apoe, Ep300, Gdnf, Inhba, Notch2, Ntn1, Pax3, reticulon-4 (Rtn4), S100b, semaphorin-4D (Sem4d), Vegfa, and Ywhah (Table 1). However, one exception in this group is the adenosine A1 receptor (Adora1), the expression of which was significantly increased with aging (Fig. 3a). Furthermore, examination of the expression of genes involved in cell adhesion revealed no changes in their expression with aging in the DG. The genes studied in this group included Ache, Bai1, Dll1, ephrin-B1 (Efnb1), fasciculation and elongation protein zeta-1 (Fez1), neuropilin 2 (Nrp2), Nrcam, Nrp1, partitioning defective 3 homolog (Pard3), Ras-related C3 botulinum toxin substrate 1 (Rac1), roundabout homolog 1 (Robo1), Sem4d, Slit2, and tenascin-R (Tnr) (Table 1).
Fig. 3.
Comparison of the expression of Adora1 (a) and HeyL (b) between the young and aged DG. The y-axis numbers denote the expression level of genes (mean ± SEM) normalized to the expression of housekeeping genes (HKGs) and labeled as 2−ΔCt. *p < 0.05
Cell signaling genes important for neurogenesis and genes involved in regulation of transcription
We examined the possible changes in the expression of cell signaling genes involved in neurogenesis with aging in the DG. These include Adora2a, Bai1, Chrm2, Cxcl1, Dll1, disheveled dsh homolog 3 (Dvl3), guanine nucleotide-binding protein alpha O polypeptide (Gnoa1), Notch2, and nuclear receptor coactivator 6 (Ncoa6). None of these genes exhibited significant changes in their expression between the young and aged DG. We also quantified changes in the expression of genes involved in the regulation of transcription with aging. This comprised measurement of the expression of Apbb1, aryl hydrocarbon receptor nuclear translocator 2 (Arnt2), Ascl1, Ep300, Hdac7, hairy enhancer of split 1 (Hes1), and hairy enhancer of split related with YRPW motif 1 (Hey1), Hey2, hairy enhancer of split related with YRPW motif-like (HeyL), Mll1, myocyte enhancer factor 2C (Mef2c), Ncoa6, Ndn, Neurod1, Notch2, Pax2, Pax3, Pax6, Pou4f1, POU class 3 homeobox 3 (Pou3f3), SRY (sex-determining region Y)-box 8 (Sox8), and Stat 3. With the exception of Stat3 and HeyL, all other genes in this category exhibited no differences in their expression between the young and aged DG (Table 1). The expression of Stat3 was increased (as shown in Fig. 1 earlier), and the expression of HeyL was significantly decreased (Fig. 3b).
Discussion
We investigated the molecular processes that are potentially involved in NSC proliferation, differentiation, and neurogenesis in order to determine whether the age-related decline of neurogenesis has any link to changes in the expression of genes in the DG. Our study provides novel evidence that greatly diminished neurogenesis in the aged DG is not associated with declined expression of selected genes that mediate NSC proliferation and neurogenesis. However, this study suggests that a few genes important for NSC proliferation and neurogenesis (Stat3 and Shh), DNA damage response and NF-kappaB signaling (Cdk5rap3), and neuromodulation (Adora1) exhibit an increased expression, and a gene involved in neuronal differentiation (HeyL) displays decreased expression in the aged DG.
Aged DG displays stable expression of genes that are known to regulate NSC proliferation
Measurement of the expression of 12 genes (Alk, Bai1, Cxcl1, Egf, Fgf-2, IL-3, Lst1, Ndn, Notch2, Nrp1, Ptn, and Vegfa) important for NSC proliferation in the DG revealed no significant changes between young and old age. This finding is surprising in the light of earlier observations in rat models that a dramatic decline in the proliferation of NSCs occurs in the DG between young and old age (Olariu et al. 2007; Rao et al. 2006), but NSC numbers are preserved in the SGZ during the course of aging (Hattiangady and Shetty 2008). Furthermore, most studies on rodent models point out that proliferation of far fewer NSCs in the aged DG underlies the age-related reduction in net hippocampal neurogenesis (Cameron and McKay 1999; Hattiangady and Shetty 2008; Heine et al. 2004; Kuhn et al. 1996; McDonald and Wojtowicz 2005; Rao et al. 2005; Seki and Arai 1995; Walter et al. 2011 but see Encinas et al. 2011). Consistent with this, other studies have shown that aging in the hippocampus is associated with alterations in NSC proliferation factors such as decreased concentrations of neurotrophic factors that are mitogenic to NSCs (Hattiangady et al. 2005; Shetty et al. 2005; Zeng et al. 2011).
However, age-related changes in the concentration of NSC proliferation factors in the hippocampus do not positively correlate with the extent of their gene expression found in this study. For instance, earlier studies have shown significant age-related decreases in the concentration of Fgf-2 and Bdnf proteins in the hippocampus (Hattiangady et al. 2005; Shetty et al. 2005). In contrast, the current study shows that the expression of Fgf-2 and Bdnf genes is unchanged in the aged DG. A recent study has also found discrepancy between gene expression and protein concentration for Vegf and Fgf-2 in the DG of aged rats (Bernal and Peterson 2011). As proteins are the actual cellular players for most genes, one would expect to have a positive correlation between expression levels of genes and concentrations of proteins they make. However, it is not uncommon to see only a partial correlation between gene expression and the amount of functional protein in a given region (Brockmann et al. 2007). This is because of the following: First, numerous posttranscriptional regulation mechanisms such as mRNA processing, export, localization, and translation could affect the overall translational efficiency (Gingold and Pilpel 2011; Mata et al. 2005). Second, factors such as the transcript decay rate, translation rates, ribosome occupancy, and ribosome density can also influence the levels of proteins (Dever 2002; Gebauer and Hentze 2004; Raghavan et al. 2002). Third, the concentrations of proteins at posttranslational time points depend on the degree of protein modifications and degradation. Fourth, multiple micro-RNAs (miRNAs, single stranded small noncoding RNAs) can modulate mRNA functions and result in changes in the overall protein production. For example, target mRNA recognition by the miRNA leads to a decrease in the abundance of proteins encoded by the target mRNA. This has been explained by several mechanisms including posttranscriptional degradation of the target, translational repression, and deadenylation-dependent target decay through partially complementary miRNA target sites in mRNA untranslated regions (Maroney et al. 2006; Petersen et al. 2006; Schouten et al. 2012). Thus, it is likely that one or more of the above posttranscriptional modifications underlie the discrepancy observed in the measured gene expression and protein concentration (Lindner and Demarez 2009).
Expression of most genes important for cell cycle, cell migration, and differentiation is unaltered in the aged DG
The expression of genes that regulate cell cycle function (Apbb1, Ep300, Hdac4, Hdac7, Inhba, Mdk, Mll1, Notch2, Pard6b, and S100a6) was unaltered with aging in the DG. Furthermore, a vast majority of genes related to cell migration and cell differentiation examined in this study showed no changes in expression with aging. However, the aging DG exhibited decreased expression of HeyL and increased expression of Cdk5rap3. HeyL is expressed in multiple neural structures during development and is believed to be important for neuronal differentiation (Gray et al. 2004; Jalali et al. 2011). A recent study further demonstrates that overexpression of HeyL in NSCs promotes neuronal differentiation by activating proneural genes and inhibiting other Hes and Hey proteins (Jalali et al. 2011). In the adult DG, expression of HeyL is low but appears to be localized to some cells in the SGZ-GCL region (based on Allen Brain Atlas). Cdk5rap3 encodes a protein called Cdk5 regulatory subunit-associated protein 3. This protein binds to p25NCK5A, an activating subunit of neuronal CDC2-like kinase involved in neuronal differentiation (Ching et al. 2000), DNA damage response, and NF-kappaB signaling (Wu et al. 2010). In the DG, Cdk5rap3 is localized robustly to cells in the SGZ-GCL; however, some expression is also seen in the dentate hilus and the CA3c pyramidal cell layer (based on Allen Brain Atlas). Increased expression of Cdk5rap3 and unaltered expression of many other genes important for cell fate determination and neuronal differentiation (Cdk5r1, Cdk5rap1, Cdk5rap2, Ywhah, Dll1, Shh, Hdac4, Hdac7, Inhba, Mdk, Neurod1, Nog, Nrg1, and Pax6) likely maintain the neuronal differentiation of newly born cells in the aged DG closer to levels seen in the young DG. This gene expression pattern is consistent with the earlier observation that neuronal fate-choice decision of newly born cells in the DG is unaltered with aging (Rao et al. 2005, 2006). Furthermore, Cdk5rap3 is involved in modulating the G2/M DNA damage checkpoint and can associate with and regulate the Cdk1–cyclin B1 complex, which is the driving force for mitotic entry (Jiang et al. 2009). Studies suggest that Cdk5rap3 promotes Cdk1 activation and mitotic entry in both unperturbed cell cycle progression and DNA damage response (Jiang et al. 2009). In this context, increased expression of Cdk5rap3 in the aged DG may be to counteract the senescent changes (such as increased quiescence) that are believed to occur in NSCs of the aged DG (Hattiangady and Shetty 2008; Encinas et al. 2011).
Expression of Stat3 and Shh genes is enhanced in the aged DG
Stat3 is a transcription factor that is known to promote cytokine- and growth factor-induced signals in cells that result in responses such as proliferation, differentiation, and apoptosis (Ihle 2001). In the DG, Stat3 expression is very low and localized to a small fraction of cells that are randomly distributed in the SGZ-GCL and molecular layer, based on illustrations in Allen Brain Atlas. However, a previous study reported that conditional ablation of Stat3 in SGZ-NSCs reduces neurogenesis, implying that Stat3 signaling is essential for achieving normal levels of neurogenesis in the adult DG (Muller et al. 2009). On the other hand, Shh is a soluble extracellular signaling protein that was first discovered to have a role in cell differentiation in the developing brain (Ruiz et al. 2002; Faigle and Song 2012). In the DG, Shh gene expression is very low and appears to be restricted to a small fraction of cells in the SGZ-GCL region (based on illustrations in Allen Brain Atlas). The cell types that release Shh in the SGZ-GCL neurogenic niche are believed to be astrocytes (Jiao and Chen 2008). Pertaining to adult hippocampal neurogenesis, studies have shown that overexpression of Shh enhances SGZ-NSC proliferation and inhibition of Shh signaling decreases SGZ-NSC proliferation in the postnatal hippocampus (Lai et al. 2003). Additional studies suggest that both quiescent NSCs and transit amplifying progenitor cells in the SGZ respond to Shh signaling and contribute to ongoing neurogenesis (Ahn and Joyner 2005; Jiao and Chen 2008). Considering the above-mentioned results, it is likely that increased expression of Stat3 and Shh genes in the aged DG is likely a compensatory reaction against greatly waned hippocampal neurogenesis seen with aging. However, it remains to be examined in future studies whether increased Stat3 and Shh expression translates to increased Stat3 and Shh proteins in the aged DG.
Genes encoding growth factors, cytokines, synaptic functions, apoptosis, cell adhesion, and cell signaling are mostly unaltered in the aged DG
The expression of multiple genes encoding growth factors and cytokines (Artn, Bdnf, Bmps, Egf, Fgf-2, Gdnf, IL-3, Inhba, Mdk, Ndk, Ndp,Nrg1, Ptn, Vegfa, etc.), synaptic functions (Ache, Apoe, Chrm2, Dlg4, Drd2, Drd5, Grin1, Nptx1, Nrcam, Pou4f1, S100b), apoptosis (Adora2a, Apoe, Ep300, Gdnf, Inhba, Notch2, Ntn1, Pax3, Rtn4, S100b, Sema4d, Vegfa, Ywhah), and cell adhesion (Ache, Bai1, Dll1, Efnb1, Fez1, Nrcam, Nrp1, Nrp1, Pard3, Rac1, Robo1, Sem4d, Slit2, Tnr) was unchanged in the aging DG, implying that any age-related changes in these proteins are not linked to changes in their gene expression. However, the expression of Adora1 was significantly increased in the aged DG. Adora1 encodes the adenosine A1 receptor that binds to adenosine, which is an endogenous neuromodulator and anticonvulsant (Boison 2008; Townsend-Nicholson et al. 1995). The expression of Adora1 is robust in pyramidal cell layers of CA3a and CA3b subregions. However, in the DG, the expression of Adora1 is low but is seen in isolated cells localized to the SGZ-GCL, the CA3c subregion, and the dentate hilus (based on Allen Brain Atlas). Increased expression of Adora1 in the aged DG may represent a compensatory reaction because aging is associated with increased hippocampal excitability (Koh et al. 2010) and activation of adenosine A1 receptors can reduce epileptiform activity and seizure-induced hippocampal damage (Hamil et al. 2012).
Conclusions
Our study provides novel evidence that age-related decline in hippocampal neurogenesis is not associated with decreased expression of selected genes that are known to mediate NSC proliferation and neurogenesis in the DG. This study also suggests that the expression of a few genes important for NSC proliferation and neurogenesis (Stat3 and Shh), DNA damage response and NF-kappaB signaling (Cdk5rap3), neuromodulation (Adora1), and neuronal differentiation (HeyL) is altered with aging in the DG. However, as this investigation used the entire DG (comprising NSCs, dentate granule cells, CA3c pyramidal neurons, and glia) for qRT-PCR analyses, studies that quantify gene expression in distinct cell populations of the SGZ are required in the future to ascertain specific age-related changes in gene expression within SGZ-NSCs and their niche cells.
Acknowledgments
This research was supported by Emerging Technology Funds (from the Texas A&M Health Science Center to A.K.S.) and grants from the National Institutes of Health (NS054780 and AG20924 to A.K.S.) and Department of Veterans Affairs (VA Merit Review Award to A.K.S.). We thank Dr. Bing Shuai for his excellent contribution to tissue harvesting.
References
- Ahn S, Joyner AL. In vivo analyses of quiescent adult neural stem cells responding to sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Terao K, Hisatsune T. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging. 2011;32:140–150. doi: 10.1016/j.neurobiolaging.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3:345–351. doi: 10.1111/j.1474-9728.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell. 2011;10:466–482. doi: 10.1111/j.1474-9726.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann R, Beyer A, Heinisch JJ, Wilhelm T. Posttranscriptional expression regulation: what determines translation rates? PLoS Comput Biol. 2007;3:e57. doi: 10.1371/journal.pcbi.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J Comp Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. doi: 10.1186/1476-4598-1-2. [DOI] [PubMed] [Google Scholar]
- Ching YP, Qi Z, Wang JH. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene. 2000;242:285–294. doi: 10.1016/S0378-1119(99)00499-0. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman GL, Barthold W, Osbaldiston GW, Foster SJ, Jonas AM. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977;32:258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/S0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Nora Abrous D. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R, Song H (2012) Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. doi:10.1016/j.bbagen.2012.09.002 [DOI] [PMC free article] [PubMed]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, Pilpel Y. Determinants of translation efficiency and accuracy. Mol Syst Biol. 2011;7:481. doi: 10.1038/msb.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analyses. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Hamil NE, Cock HR, Walker MC. Acute down-regulation of adenosine A(1) receptor activity in status epilepticus. Epilepsia. 2012;53:177–188. doi: 10.1111/j.1528-1167.2011.03340.x. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Ihle JN. The stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–217. doi: 10.1016/S0955-0674(00)00199-X. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jalali A, Bassuk AG, Kan L, Israsena N, Mukhopadhyay A, McGuire T, Kessler JA. HeyL promotes neuronal differentiation of neural progenitor cells. J Neurosci Res. 2011;89:299–309. doi: 10.1002/jnr.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23:684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wu J, He C, Yang W, Li H. Tumor suppressor protein C53 antagonizes checkpoint kinases to promote cyclin-dependent kinase 1 activation. Cell Res. 2009;19:458–468. doi: 10.1038/cr.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacol. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Larsson C, Lardelli M, White I, Lendahl U. The human NOTCH1, 2, and 3 genes are located at chromosome positions 9q34, 1p13-p11, and 19p13.2-p13.1 in regions of neoplasia-associated translocation. Genomics. 1994;24:253–258. doi: 10.1006/geno.1994.1613. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Taira T, Kaila K, Rauvala H. Activity-induced enhancement of HB-GAM expression in rat hippocampal slices. Neuroreport. 1996;7:1670–1674. doi: 10.1097/00001756-199607080-00029. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lindner AB, Demarez A. Protein aggregation as a paradigm of aging. Biochim Biophys Acta. 2009;1790:980–996. doi: 10.1016/j.bbagen.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Maroney PA, Yu Y, Nilsen TW. MicroRNAs, mRNAs, and translation. Cold Spring Harb Symp Quant Biol. 2006;71:531–535. doi: 10.1101/sqb.2006.71.043. [DOI] [PubMed] [Google Scholar]
- Mata J, Marguerat S, Bahler J. Post-transcriptional control of gene expression: a genome-wide perspective. Trends Biochem Sci. 2005;30:506–514. doi: 10.1016/j.tibs.2005.07.005. [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Chakrapani BPS, Schwegler H, Hofmann H-D, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–441. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/S0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Voikar V, Kaksonen M, Lauri SE, Hienola A, Taira T, Rauvala H. Role of heparin-binding growth-associated molecule (HB-GAM) in hippocampal LTP and spatial learning revealed by studies on overexpressing and knockout mice. Mol Cell Neurosci. 2002;20:330–342. doi: 10.1006/mcne.2002.1104. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Raghavan A, Ogilvie RL, Reilly C, Abelson ML, Raghavan S, Vasdewani J, Krathwohl M, Bohjanen PR. Genome-wide analysis of mRNA decay in resting and activated primary human T lymphocytes. Nucleic Acids Res. 2002;30:5529–5538. doi: 10.1093/nar/gkf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262:16625–16635. [PubMed] [Google Scholar]
- Ruiz A, Altaba I, Palma V, Dahmane N. Hedgehog–Gli signaling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schouten M, Buijink MR, Lucassen PJ, Fitzsimons CP. New neurons in aging brains: molecular control by small non-coding RNAs. Front Neurosci. 2012;6:25. doi: 10.3389/fnins.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Townsend-Nicholson A, Baker E, Schofield PR, Sutherland GR. Localization of the adenosine A1 receptor subtype gene (ADORA1) to chromosome 1q32.1. Genomics. 1995;26:423–425. doi: 10.1016/0888-7543(95)80236-F. [DOI] [PubMed] [Google Scholar]
- Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32:1906–1914. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Wu J, Lei G, Mei M, Tang Y, Li H. A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing Protein 1 (DDRGK1) and modulates NF-kappaB signaling. J Biol Chem. 2010;285:15126–15136. doi: 10.1074/jbc.M110.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]