Abstract
Calorie restriction decreases skeletal muscle apoptosis, and this phenomenon has been mechanistically linked to its protective action against sarcopenia of aging. Alterations in lipid composition of membranes have been related with the beneficial effects of calorie restriction. However, no study has been designed to date to elucidate if different dietary fat sources with calorie restriction modify apoptotic signaling in skeletal muscle. We show that a 6-month calorie restriction decreased the activity of the plasma membrane neutral sphingomyelinase, although caspase-8/10 activity was not altered, in young adult mice. Lipid hydroperoxides, Bax levels, and cytochrome c and AIF release/accumulation into the cytosol were also decreased, although caspase-9 activity was unchanged. No alterations in caspase-3 and apoptotic index (DNA fragmentation) were observed, but calorie restriction improved structural features of gastrocnemius fibers by increasing cross-sectional area and decreasing circularity of fibers in cross sections. Changing dietary fat with calorie restriction produced substantial alterations of apoptotic signaling. Fish oil augmented the protective effect of calorie restriction decreasing plasma membrane neutral sphingomyelinase, Bax levels, caspase-8/10, and −9 activities, while increasing levels of the antioxidant coenzyme Q at the plasma membrane, and potentiating the increase of cross-sectional area and the decrease of fiber circularity in cross sections. Many of these changes were not found when we used lard. Our data support that dietary fish oil with calorie restriction produces a cellular anti-apoptotic environment in skeletal muscle with a downregulation of components involved in the initial stages of apoptosis engagement, both at the plasma membrane and the mitochondria.
Keywords: Apoptotic signaling, Calorie restriction, Dietary fat, Sarcopenia, Skeletal muscle
Introduction
Aging is associated with sarcopenia, a loss of skeletal muscle mass and function. Sarcopenia of aging is of great importance for the individual’s health because of its association with weakness and frailty, resulting in the increased incidence of falls, disability, and all-cause mortality in humans (Wohlgemuth et al. 2010). Morphological changes in skeletal muscle with aging include a reduction of fiber cross-sectional area, loss of fiber number, and increases in extramyocyte space and connective tissue (Phillips and Leeuwenburgh 2005; Kim et al. 2008).
Several systems participate in the degradation of cellular constituents of skeletal muscle fibers and thus may play a role in muscle loss that occurs with disuse, denervation, or aging. These systems include apoptosis, the autophagy–lysosomal system, the calpain system, and protein degradation through the ubiquitin–proteasome system (Dirks and Leeuwenburgh 2002, 2004; Phillips and Leeuwenburgh 2005; Marzetti et al. 2008a; Wohlgemuth et al. 2010; Romanello et al. 2010). During the last years, it has been firmly established that the increase of pro-apoptotic processes in the skeletal muscle fiber is tightly associated with the loss of muscle that occurs with aging (Dirks and Leeuwenburgh 2002, 2004; Phillips and Leeuwenburgh 2005; Chung and Ng 2006; Marzetti et al. 2008a, b, 2009; Seo et al. 2008; Wohlgemuth et al. 2010), although the detailed mechanisms that are involved, as well as the importance of apoptotic contributions to sarcopenia, have not been fully unraveled (Siu et al. 2005). Elevated apoptosis with age has been found in the predominately slow fiber-containing soleus muscle (Leeuwenburgh et al. 2005), in the fast fiber-containing plantaris muscle (Pistilli et al. 2006; Wohlgemuth et al. 2010), and in the mixed fiber-containing gastrocnemius muscle (Dirks and Leeuwenburgh 2004; Siu et al. 2005), suggesting that activation of apoptotic programs is conserved across muscles of differing fiber type and activity patterns.
Mitochondria play a central role in the regulation of apoptosis in the skeletal muscle and are generally regarded as key players in the pathogenesis of myocyte loss during aging and other atrophying conditions (Jeong and Seol 2008; Seo et al. 2008; Marzetti et al. 2009; Wohlgemuth et al. 2010). Iron accumulation and biochemical markers of mitochondrial dysfunction increased in the skeletal muscle mitochondria isolated from aged rats, and the presence of dysfunctional mitochondria may increase the susceptibility to mitochondrial apoptosis (Hofer et al. 2008; Seo et al. 2008; Wohlgemuth et al. 2010). In accordance, mitochondrial apoptotic markers increase with aging in the skeletal muscle of various types (Chung and Ng 2006; Pistilli et al. 2006). In addition to mitochondrial apoptosis, death receptor apoptotic signaling is also activated with aging in rat gastrocnemius, superficial vastus lateralis, and soleus muscles (Marzetti et al. 2009; Phillips and Leeuwenburgh 2005), supporting an important role for the plasma membrane in the regulation of skeletal muscle apoptosis with age. In this way, levels of several components in the extrinsic apoptotic pathway, such as Fas-associated death domain and cleaved caspase-8, were elevated downstream of TNF-α in aged rats (Phillips and Leeuwenburgh 2005).
Calorie restriction (CR) without malnutrition delays the onset of age-related diseases and prolongs mean and maximum life spans in a variety of species (Weindruch and Sohal 1997). CR prevented the aging-associated increase of protein oxidation in the skeletal muscle (Leeuwenburgh et al. 1997), optimized the proteasome pathway with aging in the rat plantaris muscle (Hepple et al. 2008), and reversed age-related increases of mitochondrial and death receptor-activated apoptotic markers in the skeletal muscles of several types (Selman et al. 2003; Dirks and Leeuwenburgh 2004; Phillips and Leeuwenburgh 2005; Kim et al. 2008; Marzetti et al. 2008a, 2009; Wohlgemuth et al. 2010).
Alterations in membrane composition with CR may play a central role in the retardation of aging (Yu 2005) because CR has been reported to decrease long chain n-3 polyunsaturated fatty acid (PUFA) content and to increase the degree of membrane saturation (Faulks et al. 2006; Laganiere and Yu 1993). This change is hypothesized to beneficially affect aging by protecting membranes against lipid peroxidation and preventing oxidative stress (Pamplona et al. 2002; Yu et al. 2002). However, alterations in membrane lipid composition can also influence membrane proteins and functions. In accordance, we reported recently that fat-1 transgenic mice, which exhibit an increase in n-3 fatty acids and a decrease in the n-6/n-3 ratio compared to control mice, displayed lower rates of complex I-derived H2O2 production by the liver mitochondria (Hagopian et al. 2010). Another strategy to determine the role that membrane lipids play in the actions of CR is the manipulation of membrane fatty acid composition by feeding CR animals diets that differ in lipid composition. By following this approach, we have recently demonstrated that changes in mitochondrial phospholipid fatty acid in the CR mice reflected the polyunsaturated fatty acid profile of the dietary lipid sources. An increased degree of saturation was not required for reduced ROS production with CR in the skeletal muscle mitochondria, although dietary lipids influenced mitochondrial proton leak with CR (Chen et al. 2012).
The purpose of our study was to determine if dietary lipid source (fish oil, soybean oil, or lard) with CR produces alterations of mitochondrial and plasma membrane-dependent apoptotic signaling in skeletal muscle. Our data show that dietary lipid source strongly affects apoptotic signaling in skeletal muscle of young mice fed under CR for 6 months, with a marked reduction being produced by fish oil.
Methods
Chemicals
Unless otherwise stated, chemicals and reagents were purchased from Sigma-Aldrich (Madrid, Spain).
Animals and diets
A cohort of 64 male 10-week-old C57BL/6 mice was used (Charles River Laboratories, Spain). Mice were bred and raised in a vivarium at the Centro Andaluz de Biología del Desarrollo (CABD, Sevilla, Spain) under a 12-h light/dark cycle (8:00 a.m.–8:00 p.m.) under controlled conditions of temperature (22 ± 3 °C) and humidity. After habituation to a commercial rodent chow diet (Harlan Teklad #7012, Madison, WI) for 14 days, the mice were randomly assigned into four dietary groups and were fed a modified AIN-93G purified diet. The control group was fed 95 % of a pre-determined ad libitum intake (12.5 kcal). This slight restriction in food intake was initiated to prevent excessive weight gain during the study. The three CR dietary groups were maintained on 60 % of the daily allowance of the ad libitum intake (8.6 kcal), and these diets were identical except for dietary lipid sources. The diets (percent total kilo calories per day) contained 20.3 % protein, 63.9 % carbohydrate, and 15.8 % fat. The dietary fat for the control group was soybean oil. Dietary fats for the three CR groups were soybean oil (high in n-6 PUFAs, Super Store Industries, Lathrop, CA), fish oil (high in n-3 PUFAs: 18 % EPA, 12 % DHA, Jedwards International, Inc. Quincy, MA), or lard (high in saturated and monounsaturated fatty acids, ConAgra Foods, Omaha, NE). To insure adequate linoleic acid levels, the CR-fish group was supplemented with soybean oil. Fatty acid composition of dietary lipids has been detailed in a separate publication (Chen et al. 2012). All mice were housed individually and were fed the control or CR diets for 6 months. Filtered and acidified water was available ad libitum for all groups and food was replaced every day between 8:00 and 9:00 a.m.
At the end of the 6-month intervention period, the animals were sacrificed by cervical dislocation after an 18-h fast. Muscle from the hind limb was rapidly dissected, washed and trimmed of connective tissue and fat, frozen by immersion in liquid nitrogen in a buffered medium containing 10 % DMSO as cryoprotectant, and then stored at −80 °C for later analysis. Handling of animals and all experimental procedures were in accordance with the Pablo de Olavide University ethical committee rules, and the 86/609/EEC directive on the protection of animals used for experimental and other scientific purposes.
Isolation of cytosolic and plasma membrane fractions from skeletal muscle
Because of the low yield of plasma membrane preparation methods, we chose to utilize the hind limb skeletal muscle instead of using individual muscles. Hind limb skeletal muscles were homogenized at 4 °C in ice-cold buffer containing 20 mM Tris–HCl pH 7.6, 40 mM KCl, 0.2 M sucrose, 1 mM phenylmethylsulfonyl fluoride, 10 mM EDTA, and 20 μg/μL each chymostatin, leupeptin, antipain, and pepstatin A in a Teflon glass tissue homogenizer. Samples were centrifuged at 350×g for 10 min to discard cell debris and nuclei, and the supernatant was centrifuged at 80,000×g for 45 min to separate a cytosolic and a crude membrane fraction. Cytosolic fractions were stored frozen at −80 °C until further analysis, and plasma membranes were extracted from the membranous crude fraction by two-phase partition. Briefly, the resuspended pellet was combined with a mixture of 6 % (w/w) dextran and 6 % (w/w) polyethyleneglycol in 0.1 M sucrose and 5 mM potassium phosphate, pH 7.2. The mixture was inverted vigorously 40 times at 4 °C and then centrifuged at 350×g for 5 min to separate the phases. The upper polyethylene glycol phase, containing the purified plasma membrane, was withdrawn, diluted with 25 mL of 1 mM sodium bicarbonate, and then centrifuged at 80,000×g for 45 min to recover the purified fraction. After this last centrifugation step, plasma membranes were resuspended in 25 mM Tris–HCl buffer pH 7.6, containing 10 % glycerol, 0.1 mM DTT, 1 mM EDTA and 1 mM PMSF, and then stored at −80 °C until use.
Coenzyme Q determinations
Membranes were disrupted with 1 % SDS and then, two volumes of 95 % ethanol–5 % isopropanol was added. Coenzyme Q isoforms (CoQ9 and CoQ10) were recovered from SDS alcoholic solution by extraction with five volumes of hexane. After hexane evaporation, the lipid extract was dissolved in methanol and was used for quantification of CoQ9 and CoQ10 by reversed phase HPLC separation with a C18 column (25 × 0.45 cm, 5-μm particle size). Chromatographic separation was accomplished at 1 mL/min with a mobile phase composed of a 53:45:2 mixture of methanol, n-propanol, and 1 M ammonium acetate, pH 4.4. Monitoring was carried out with a Coulochem II electrochemical detector (ESA, Chelmsford, MA) fitted with a Model 5010 analytical cell, with the electrodes set at potentials of −500 mV and +300 mV. CoQ isoforms were detected from the signal obtained at the second electrode. Concentrations were then calculated by integration of peak areas and comparison with external standards (Sigma, Madrid, Spain).
Measurement of lipid hydroperoxides
Lipid hydroperoxides were determined according to the method of Jiang et al. (1991), based on the measurement of hydroperoxide-mediated oxidation of Fe2+ to Fe3+ in the presence of xylenol orange under acidic conditions. Lipid hydroperoxides were determined by direct incubation of membranes (100 μg protein) with the xylenol orange reagent for 75 min at room temperature in the dark. Absorbance of Fe3+–xylenol orange complex was recorded at 560 nm (extinction coefficient, 43 mM−1 cm−1). Linearity of reaction was tested by constructing a standard plot with tert-butyl hydroperoxide.
Mg2+-dependent neutral sphingomyelinase activity
Mg2+-dependent neutral sphingomyelinase (nSMase) activity was assayed with purified plasma membranes as previously described (Martín et al. 2001). Assay buffer was 50 mM Tris–HCl, pH 7.4, containing 0.05 % Triton X-100, and 10 mM MgCl2. Samples (10 μg plasma membrane protein in 5–10 μL) were mixed with assay buffer plus 10 nmol of a mixture of cold SM and [methyl-14C]-SM (specific radioactivity 10,000 cpm/nmol) (Amersham, Spain). After incubation for 30 min at 37 °C, the reaction was stopped by adding 1.5 mL chloroform/methanol (2:1) and 200 μL of distilled water. Tubes were vortexed and then centrifuged at 1,500×g for 5 min to achieve separation of phases. [14C]-Phosphorylcholine present in the aqueous phase was quantified using a liquid scintillation counter (Beckman, USA). nSMase activity was expressed as CPM per minute per milligram.
Caspase activities assays
Caspase-3, -8/10, and −9 activities were determined in cytosolic fractions. Proteolytic activity of each caspase was determined by fluorimetry in an assay medium containing 25 mM HEPES–KOH buffer (pH 7.4), 10 % sucrose, 1 % NP-40, 1 mM EDTA, 1 mM PMSF, 5 mM DTT, and 200 μg of cytosolic protein in a final volume of 200 μL. Samples were preincubated for 25 min at 37 °C in assay medium and specific fluorogenic substrates were then added to a final concentration of 40 μM. Thereafter, incubation was continued for 1 h at 37 °C in the dark. The following substrates (obtained from Alexis Corporation, San Diego, CA, USA) were used: Ac-DMQD-AMC (caspase-3), Ac-IETD-AMC (caspase-8/10), and Ac-LEHD-AMC (caspase-9). After incubation with the corresponding substrate, the reaction was stopped by adding 20 μL of 1 N HCl. The mixture was diluted with 1.5 mL of water, and fluorescence signal was then recorded with an Aminco-Bowman Series 2 Luminiscence Spectrometer set at wavelengths of 380 nm (excitation) and 460 nm (emission). Assays were carried out both in the absence and in the presence of the corresponding specific inhibitor for each caspase. The following inhibitors (obtained from Alexis Corporation, San Diego, CA, USA) were used at a final concentration of 83 μM: Ac-DMQD-CHO (caspase-3); Ac-IETD-CHO (caspase-8/10); Ac-LEHD-CHO (caspase-9). Caspase activities were calculated form the difference of fluorescence measurements obtained in the presence and in the absence of the corresponding inhibitor. Activities were then expressed as arbitrary units per milligram protein.
Quantification of DNA fragmentation (apoptotic index)
The extent of DNA fragmentation was quantified in the hind limb skeletal muscle by measuring the amount of cytosolic mono- and oligonucleosomes using the ELISA kit developed by Roche Diagnostics (Mannheim, Germany) following the manufacturer’s recommendations. Absorbance was determined at 405 nm using a Flex Station 3 (Molecular Devices, Sunnyvale, CA, USA), and data were reported as arbitrary OD units per milligram cytosolic protein.
Polyacrylamide gel electrophoresis and Western blot immunodetection
About 50 μg of protein was denatured by heating in SDS–dithiothreitol loading buffer [10 % sucrose, 2 mM EDTA, 1.5 % (w/v) SDS, 20 mM dithiothreitol, 0.01 % (w/v) bromophenol blue, and 60 mM Tris–HCl, pH 6.8], separated by SDS-PAGE (12.5 % acrylamide) and then blotted onto nitrocellulose sheets. Blots were stained with Ponceau S for visualization of protein lanes. Bcl-2 and Bax polypeptides were measured in whole homogenates. Polypeptide detection was carried out by immunostaining of Western blots respectively with a rabbit anti-Bcl-2 antiserum (Santa Cruz Biotechnology, Inc.) diluted 1:200 and a mouse monoclonal anti-Bax antibody (Santa Cruz Biotechnology, Inc.) diluted at 1:200. X-linked inhibitor of apoptotic protease (XIAP) polypeptide in total homogenate was detected with a goat antiserum (Santa Cruz Biotechnology, Inc.) diluted at 1:200. Apoptosis-inducing factor (AIF) and cytochrome c were measured in cytosolic fractions and detected respectively with a goat antiserum (Santa Cruz Biotechnology, Inc.) diluted at 1:2,000 and a mouse antiserum (BD Biosciences Pharmingen) diluted at 1:2,000. The corresponding secondary IgG antibodies coupled to horseradish peroxidase (Sigma) were used to reveal binding sites by enhanced chemiluminescence (ECL-Plus, GE Healthcare Life Sciences).
Photographic films and Ponceau S-stained blots were scanned in a GS-800 calibrated densitometer (Bio-Rad) to obtain digital images. Quantification of intensity reaction was carried out using Quantity One software (Bio-Rad). Data obtained from the quantification of the stained bands (in arbitrary units) were normalized to those of the corresponding lane stained with Ponceau S in order to correct any difference in protein loading between samples, as previously validated by our group (Bello et al. 2003). In order to have an accurate estimation of changes produced by CR per se and by alterations of dietary fat in CR animals, the effects of these two dietary manipulations were assessed in separate electrophoresis gels and blots carried out under optimized conditions for each case. Thus, results of protein levels measured by Western blotting were also represented in separate plots: one for CR effect (AL-Soy vs. CR-Soy) and the other one for dietary fat effects in CR animals (CR-Lard, CR-Soy, CR-Fish).
Structural analysis of skeletal muscle fibers
Samples of the gastrocnemius muscle from at least five animals per diet were fixed in a mixture of 2.5 % glutaraldehyde–2 % paraformaldehyde in sodium 0.1 M cacodylate buffer pH 7 for 4 h and post fixed in 1 % osmium tetroxide for 1 h at 4 °C in the same buffer. After dehydration in an ascendant series of ethanol, the pieces were transferred to propylene oxide and sequentially infiltrated in EMbed 812 resin. We used the sequence propylene oxide–resin 2:1, 1:1, and 1:2 throughout 24 h. Afterwards, samples were transferred to pure resin for 24 h. Then, blocks were performed in silicon molds with fresh resin for 48 h at 65 °C. We placed the samples into the molds in order to obtain cross sections of the tissues. After sculpting, blocks were sectioned in an Ultracut Reicher ultramicrotome and semi-thin sections (0.5-μm thick) were mounted on glass slides. Then, sections were stained for 2–5 min with an aqueous solution of 1 % toluidine blue/1 % borax. From this material, we obtained pictures in a photomicroscope. Cell sizes as well as circularity coefficients were obtained using the ImageJ 1.45 software (NIH; USA). Only those pictures showing unequivocal transversal sections of muscle fibers were considered in this work.
Statistic analysis
All values are expressed as mean ± SEM. Variables were tested for normality by using D’Agostino–Pearson test. The effects of CR were assessed by Student’s t test (vs. corresponding AL controls). In case data did not pass the normality test, the nonparametric Mann–Whitney test was followed. The effects of dietary fat in calorie-restricted animals were assessed by one-way ANOVA followed by post hoc analysis of significant differences with Tukey’s test for multiple comparisons. Post hoc analysis of linear trend was also performed to investigate putative alterations of tested parameters among CR diets ordered as CR-Lard → CR-Soy → CR-Fish, which resulted in a progressive decrease of n-6/n-3 ratio in phospholipids HUFA (Chen et al. 2012). In case data did not pass the normality test, the nonparametric Kruskal–Wallis test was followed. Means were considered statistically different when p < 0.05. All statistical analyses were performed using Graphpad Prism 5.03 (Graphpad Software Inc., San Diego, CA, USA).
Results and discussion
Body weight of mice fed experimental diets
Six months of CR produced a 28.9 % decrease (p < 0.0001) in body weight of young adult mice (26.96 ± 0.56 g in the CR-Soy group compared with 37.93 ± 0.68 g in the AL-Soy control group). No significant differences in body weight were observed between the CR-Soy and the remaining CR groups (26.83 ± 0.40 g in CR-Lard and 28.28 ± 0.61 g in CR-Fish). These results are in accordance with a previous report published by our group focused on a separate colony of young mice fed the same experimental diets for 1 month (Chen et al. 2012).
Structural changes of skeletal muscle fibers with CR and dietary fat
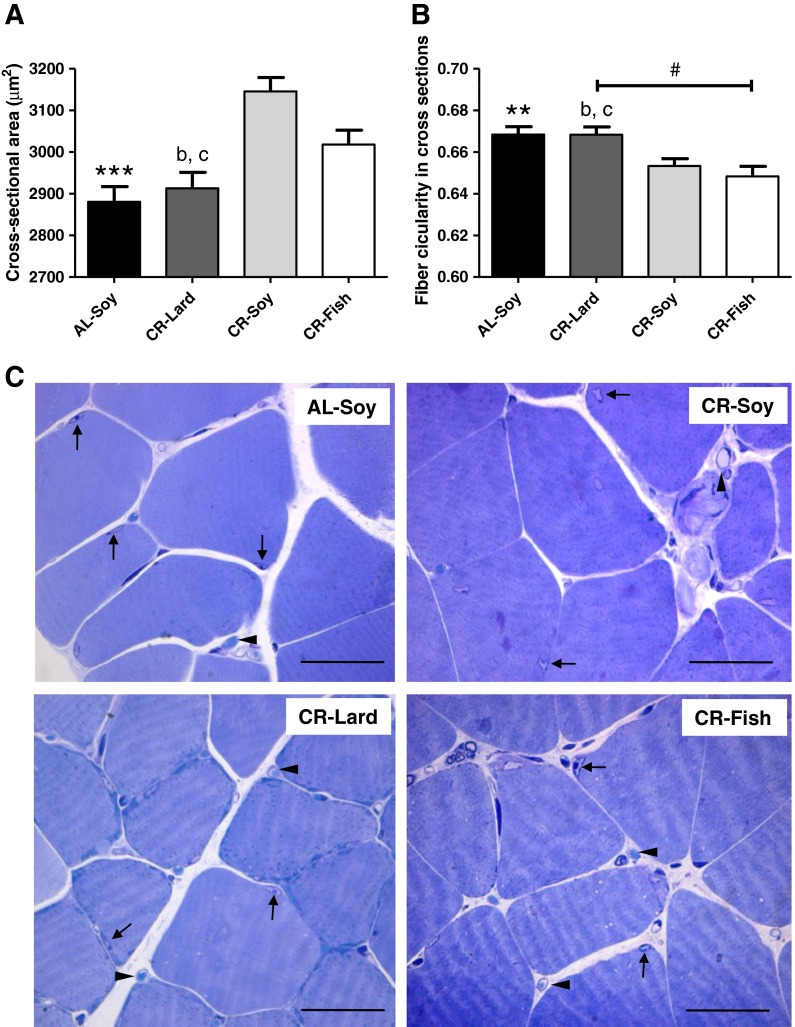
Cross-sectional area of muscle fibers is critical in the identification of muscular atrophy and a decrease of cross-sectional area of fibers with age and its attenuation by CR have been reported. In addition, the fiber shape in cross sections is also altered with age towards a more rounded shape, this structural modification being prevented by CR as well (Phillips and Leeuwenburgh 2005; Kim et al. 2008). Thus, we studied how CR and dietary fat affected the cross-sectional area and shape of skeletal muscle fibers. For these structural studies, we chose the gastrocnemius muscle because it is a mixed fiber-containing muscle (Dirks and Leeuwenburgh 2004), and it was thus considered as a good model to study whether or not changes highlighted in our biochemical studies carried out with whole hind limb skeletal muscle (see below) were translated into actual alterations of fiber size and shape.
Mean cross-sectional area of gastrocnemius fibers in AL-Soy animals was 2,881 ± 36.71 μm2, a value which is in agreement with previous studies (Kim et al. 2008; Wong et al. 2009). As depicted in Fig. 1a, fiber cross-sectional area was significantly increased by about 9 % in CR-Soy animals (3,146 ± 33.40 μm2). The increase of cross-sectional area by CR also agrees with previous data obtained with old rats (24 months) fed lifelong on an 8 % CR regime or on an 8 % CR regime plus voluntary wheel running (Kim et al. 2008). When we studied how dietary fat affected cross-sectional area, no significant differences were found between CR-Soy and CR-Fish (3,018 ± 34.59 μm2 in the latter group). However, the increase of cross-sectional area observed in CR-Soy and CR-Fish groups was no observed in the CR-Lard group (2,913 ± 38.14 μm2).
Fig. 1.
Structural changes of skeletal muscle fibers. a Calorie restriction increased fiber cross-sectional area in gastrocnemius (AL-Soy vs. CR-Soy, ***p < 0.001). Cross-sectional area was significantly lower in the CR-Lard group compared with both CR-Soy (b p < 0.001) and CR-Fish (c p < 0.001) groups. b Fiber circularity in cross-sectioned gastrocnemius muscle was decreased as a result of CR diet (AL-Soy vs. CR-Soy, **p < 0.01). Fibers from the CR-Lard group exhibited higher circularity than those from CR-Soy (b p < 0.01) and CR-Fish (c p < 0.001) groups. Additionally, a linear trend was observed as a function of dietary fat source (# p < 0.01). Between 1,000 and 1,400 fibers from five different animals were measured for calculating the cross-sectional area and circularity values. c Representative light microscopy images showing the gastrocnemius fibers from the four dietary groups. Fibers appear surrounded by connective tissues in which capillaries are frequently found (arrow heads). Arrows denote the presence of muscle fiber nuclei. Bars are equivalent to 50 μm
Using ImageJ software (NIH, USA), we determined circularity coefficients of fibers in the cross sections to yield a quantitative estimate of the fiber shape. Fiber circularity in cross sections was also affected by CR in a way that was consistent with changes in the cross-sectional area. As depicted in Fig. 1b, circularity of the fibers was significantly decreased in CR-Soy animals compared with their AL-Soy counterparts. This observation fully agrees with data published by the group of Leeuwenburgh, who reported that muscle fibers of rat plantaris muscle were smaller and less angular in the old (24 months) AL group compared with young AL, and CR reversed this change in old animals (Kim et al. 2008). We demonstrate here that CR also produces structural improvements of skeletal muscle fibers in young mice, which agrees with the recent demonstration that CR significantly enhanced stem cell availability and activity, improving regeneration and enhancing stem cell transplant efficiency in skeletal muscle of both young and old animals (Cerletti et al. 2012). Of note, dietary fat also affected circularity of fibers in cross sections, and the observed changes were consistent with modifications of cross-sectional area, i.e., no significant differences were observed between CR-Soy and CR-Fish, but the decrease of circularity provided by CR in these two groups was not observed in the CR-Lard group. Furthermore, a statistical analysis of linear trend showed a significant decrease of fiber circularity as a function of fat source (Fig. 1b), supporting that n-6/n-3 ratio may be an important factor that modulates structural alterations of skeletal muscle fibers by CR. Representative images of stained sections of gastrocnemius muscle from the four dietary groups are shown in Fig. 1c.
In summary, our structural studies are indicative that 6-month CR produced a more healthy state of skeletal muscle fibers, represented by increased cross-sectional area and decreased circularity in cross sections. In addition, we demonstrate for the first time that a fish oil-enriched CR diet results in an additional protection of the fibers, while this protective effect is not present when lard is used as the main source of dietary fat. Since the increase of pro-apoptotic processes in the skeletal muscle fiber is tightly associated with the loss of muscle (see “Introduction”), we next studied how CR and dietary fat affected apoptotic signaling initiated both at the plasma membrane and at the mitochondria.
Mg2+-dependent neutral sphingomyelinase activity and coenzyme Q levels in plasma membrane
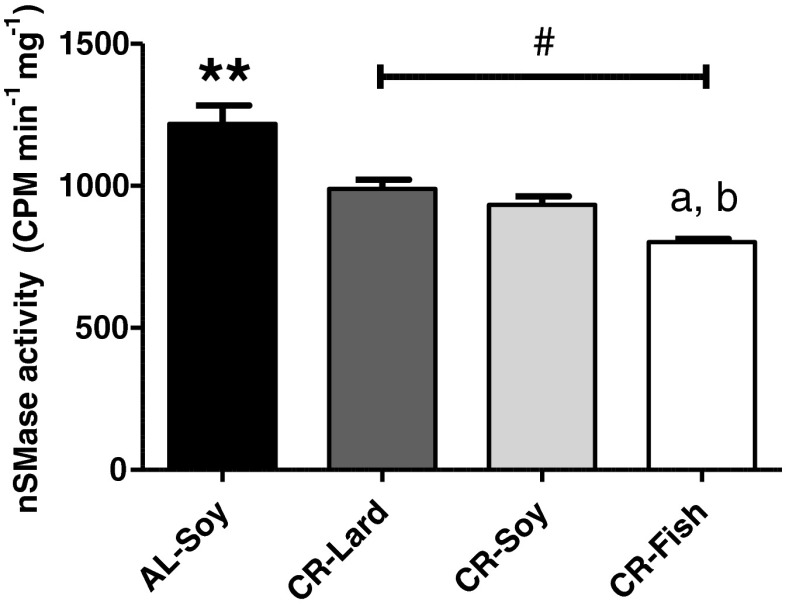
Previous reports have documented that activation of TNF-α-dependent apoptotic signaling is a major contributor to sarcopenia of aging (Phillips and Leeuwenburgh 2005; Marzetti et al. 2009), underscoring the importance of the plasma membrane in the regulation of this process. Pro-apoptotic responses to TNF-α are mediated by activation of the plasma membrane nSMase (Kim et al. 1991; Adam-Klages et al. 1996). Previous data from our group have supported a role for nSMase activation in aging liver and its modulation by dietary fat (Bello et al. 2006). However, to our knowledge, no study has been set to elucidate if CR and fat source produce alterations of the nSMase activity in skeletal muscle plasma membrane.
As shown in Fig. 2, CR produced a significant decrease of nSMase activity in the skeletal muscle plasma membrane, which fully agrees with the inhibitory action of CR on the apoptotic extrinsic pathway in this tissue (Phillips and Leeuwenburgh 2005; Marzetti et al. 2008a, 2009). Interestingly, when we studied the effects of dietary fat on calorie-restricted mice, we found that nSMase activity was further decreased in the CR-Fish group compared to both CR-Lard and CR-Soy groups. In addition, statistical analysis of linear trend showed a significant decrease of nSMase activity as a function of fat source. This finding supports that the n-6/n-3 ratio may be an important factor that modulates nSMase inhibition by CR in the skeletal muscle, as previously observed for structural changes of the fibers.
Fig. 2.
Sarcolemmal Mg2+-dependent neutral sphingomyelinase activity. A significant decrease of nSMase activity by CR was observed (AL-Soy vs. CR-Soy, **p < 0.01). Dietary fish oil further decreased this enzyme activity, both compared with CR-Lard (a p < 0.01) and with CR-Soy (b p < 0.05). In addition, post hoc analysis of linear trend among CR diets ordered as CR-Lard → CR-Soy → CR-Fish (number sign) was statistically significant with p < 0.01. Data are mean ± SEM (n = 4)
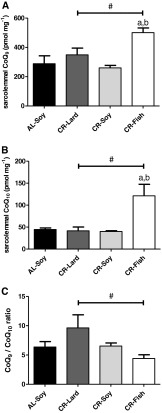
We have previously demonstrated that plasma membrane nSMase activity is regulated by the endogenous antioxidant coenzyme Q (CoQ, ubiquinone), which behaves as a noncompetitive inhibitor of the enzyme, thus protecting cells against cell death (Martín et al. 2001; Navas and Villalba 2004; Navas et al. 2005, 2007). Our previous investigations demonstrated that dietary supplementation with CoQ abolished age-related increase of nSMase activity in the rat liver plasma membrane (Bello et al. 2005), and CR increased CoQ levels and activated the CoQ-dependent antioxidant system in the liver and brain plasma membranes obtained from old rats, attenuating age-related oxidative damage (de Cabo et al. 2004; Hyun et al. 2006). Since no information is available about the role of sarcolemmal CoQ and the effects of CR and dietary fat, we measured the levels of CoQ9 and CoQ10 in purified sarcolemmal fractions, the two CoQ isoforms of the mouse (Turunen et al. 2004). No significant differences between the AL-Soy and CR-Soy groups were observed for both CoQ isoforms (Fig. 3a, b). This is consistent with previous studies carried out with rat liver and brain plasma membranes (de Cabo et al. 2004; Hyun et al. 2006) and with mouse liver plasma membrane (López-Lluch et al. 2005), that showed no alterations of CoQ levels by CR in young animals, although significant increases of the CoQ10 isoform by CR were indeed observed in the respective old groups. Despite a lack of CR effect per se, the predominant fat source of CR diets had a profound impact on CoQ levels in skeletal muscle plasma membrane. Both CoQ isoforms, but particularly CoQ10, were significantly increased in the CR-Fish group compared with the CR-Lard and CR-Soy groups. Of note, post hoc analysis of linear trend also demonstrated a significant effect of dietary fat (Fig. 3a, b). CoQ9/CoQ10 ratio also exhibited a statistically significant linear trend towards lower values in the CR-Fish group (Fig. 3c), indicating a predominant increase of the CoQ10 isoform in the skeletal muscle plasma membrane. Preferential increase of the CoQ10 isoform compared with CoQ9 has been also observed in the liver and brain plasma membranes from old rats fed a CR diet (de Cabo et al. 2004; Hyun et al. 2006). We can conclude that the decrease of nSMase activity in the skeletal muscle plasma membrane after a 6-month CR takes place without a concomitant increase of CoQ levels. However, the significant increase of both CoQ9 and CoQ10 is consistent with minimal nSMase activity in the CR-Fish group.
Fig. 3.
Sarcolemmal coenzyme Q9 and Q10 levels. a CoQ9 levels were not affected by CR, whereas a significant increase was observed in the CR-Fish group compared to CR-Lard (a p < 0.05) and to CR-Soy (b p < 0.01). Linear trend among the three CR groups was statistically significant with p < 0.05 (number sign). b Changes of CoQ10 levels were similar to those of CoQ9. No changes by CR itself (AL-Soy vs. CR-Soy) was observed. However, CoQ10 levels were dramatically increased in the CR-Fish group compared with both CR-Lard (a p < 0.01) and CR-Soy (b p < 0.01) groups. A significant linear trend among the three CR groups was also observed (number sign, p < 0.05). c CoQ9/CoQ10 ratio was not affected by CR itself (AL-Soy vs. CR-Soy), but this ratio was significantly decreased as a function of dietary fat source, with CR-Lard diet exhibiting the highest and CR-Fish the lowest ratio (number sign, p < 0.01). Data are mean ± SEM (n = 4)
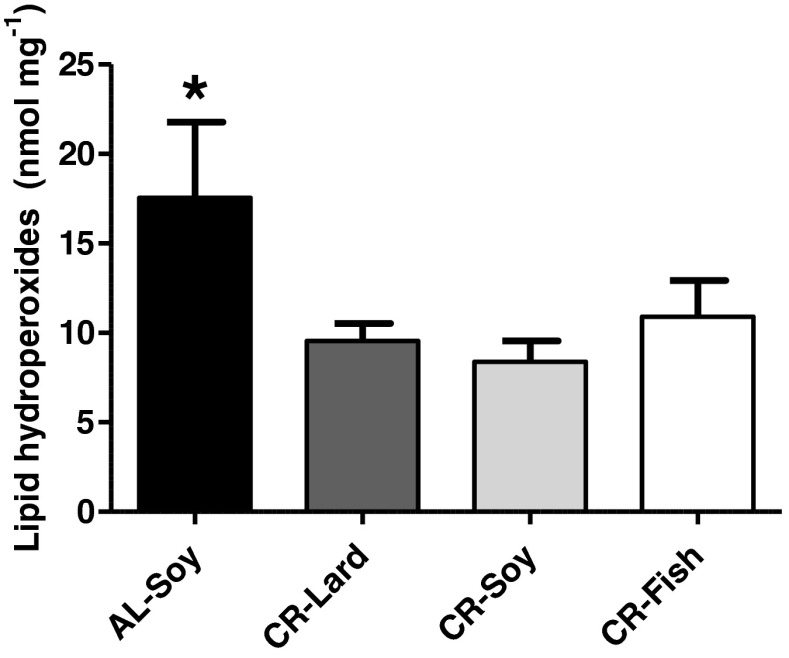
Lipid hydroperoxides
Due to the higher susceptibility of PUFA towards peroxidation (Bello et al. 2006), elevated levels of sarcolemmal CoQ in the CR-Fish group could be representative of a cellular response against oxidative stress because it is long known that CoQ levels can be regulated according to the prevailing oxidative status (Ernster and Dallner 1995). In agreement with this oxidative stress-dependent regulation, CoQ levels in the liver plasma membrane were increased when rats were fed a vitamin E and selenium deficient pro-oxidant diet (Navarro et al. 1998). We thus measured lipid hydroperoxides in the four groups of mice. As expected, levels of lipid hydroperoxides were significantly decreased by CR in the skeletal muscle (Fig. 4), which agrees with the generally accepted idea that CR decreases oxidative stress in tissues. Of note, when we compared the three CR groups, we found no significant differences of lipid hydroperoxides levels as a function of fat source, indicating that CR can decrease oxidative stress regardless of the composition of dietary fat. This is apparently in contrast with our recent observation that lipid peroxidation was increased in skeletal muscle mitochondria isolated from mice fed the same CR-Fish diet for 1 month, compared with both the AL-Soy and CR-Lard groups (Chen et al. 2012). However, data reported here fully agree with our previous study focused on rats fed the same experimental diets for 6 months, that showed no increase of lipid peroxidation in the CR-Fish group (Ramsey et al. 2005). Furthermore, we also observed no differences in basal levels of lipid hydroperoxides between groups of rats fed lifelong with diets enriched in sunflower oil, olive oil, or sunflower oil plus coenzyme Q10, although membranes from the sunflower group were indeed more susceptible to peroxidation initiated by an azo compound ex vivo (Bello et al. 2005, 2006). Taken together, our data may be indicative that long-term CR in the CR-Fish group results in an adaptation of membranes towards decreased levels of oxidative stress. This could be also due to the amount of the antioxidant t-butylhydroquinone contained in the CR-Fish diet compared with the other groups (Chen et al. 2012). Whatever the case, increase of CoQ9 and CoQ10 levels in the skeletal muscle plasma membranes of the CR-Fish group is unlikely a response to enhanced lipid peroxidation, but it could represent the existence of a different way of regulation of CoQ biosynthesis in the skeletal muscle which is not linked to increased oxidative stress (Parrado-Fernández et al. 2011).
Fig. 4.
Lipid hydroperoxides were significantly lower in the CR-Soy group compared to its AL counterpart (*p < 0.05). No significant differences between any of the three CR groups were observed. Data are mean ± SEM (n = 4)
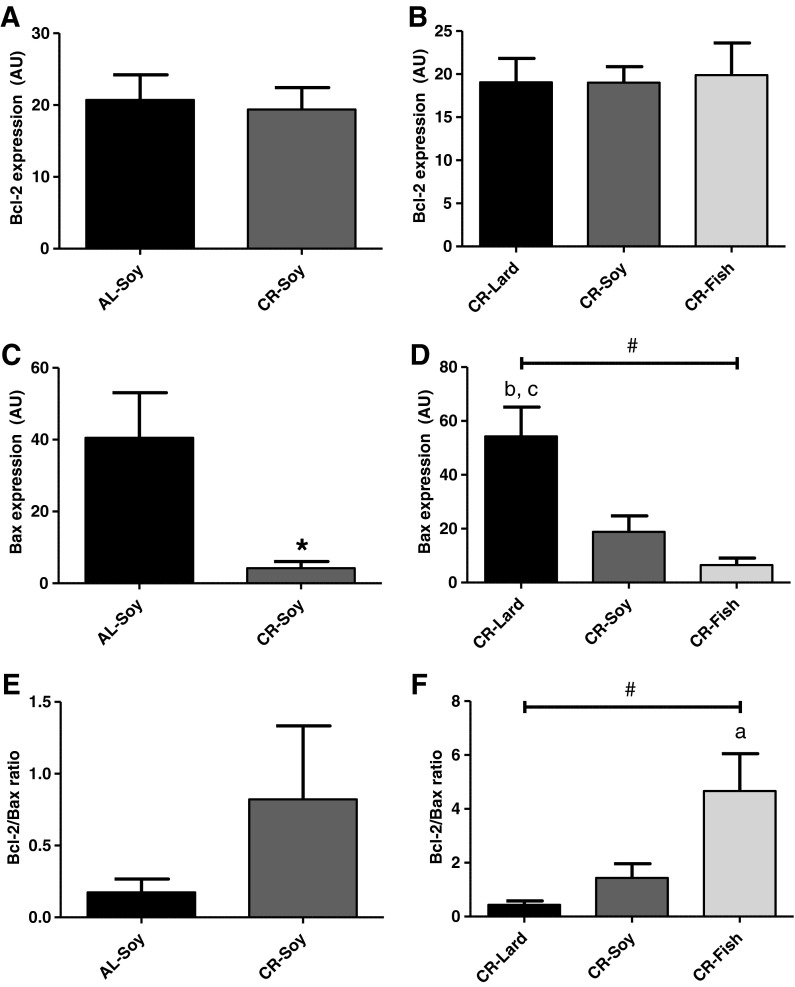
Bcl-2 and Bax polypeptides
We next measured levels of Bcl-2 and Bax polypeptides as two major regulators of mitochondrial apoptosis. Bcl-2 levels did not change by either CR per se or by dietary fat in calorie-restricted animals (Fig. 5a, b). However, a strong regulation of Bax levels by these two dietary manipulations was observed. CR produced a significant decrease of Bax (Fig. 5c), and Bcl-2/Bax ratio tended to increase in the CR-Soy group compared with AL-Soy (Fig. 5e), although differences did not reach a statistical significance in the case of Bcl-2/Bax ratio. When we compared the three CR groups as a function of dietary fat, we found no changes for Bcl-2 (Fig. 5b) but Bax levels were strongly altered by diet lipid source (Fig. 5d). Among the three CR groups, Bax levels were maximal in CR-Lard, intermediate in CR-Soy, and minimal in CR-Fish. As a consequence, Bcl-2/Bax ratio was minimal in the CR-Lard group and maximal in CR-Fish, and linear trend was statistically significant both for Bax levels and for Bcl-2/Bax ratio (Fig. 5f).
Fig. 5.
Bax and Bcl-2 polypeptides. a No changes of Bcl-2 protein levels were observed by CR (AL-Soy vs. CR-Soy). b Levels of Bcl-2 polypeptide were also unaltered as a function of dietary fat source in animals fed under CR. c Despite a lack of changes in Bcl2 levels, CR produced a dramatic decrease of Bax polypeptide (AL-Soy vs. CR-Soy, *p < 0.05). d Bax levels were also strongly modulated by lipid source in animals fed under CR (bCR-Lard vs. CR-Soy, p < 0.05; cCR-Lard vs. CR-Fish, p < 0.01). Linear trend as a function of dietary fat source was statistically significant with p < 0.01 (#). e A trend towards higher Bcl-2/Bax ratio was observed in CR-Soy compared to AL-Soy, although no significant differences were observed in this case. f Bcl-2/Bax ratio was significantly higher in the CR-Fish group compared with CR-Lard (a p < 0.05). Additionally, linear trend as a function of dietary fat source was statistically significant with p < 0.01 (#). Data are mean ± SEM (n = 4)
To our knowledge, this is the first report to demonstrate that Bax levels are strongly regulated by CR in the skeletal muscle through a mechanism dependent on dietary fat, whereas Bcl-2 levels do not respond to either CR or fat source. Available data on Bcl-2 and Bax levels in skeletal muscle are mainly focused on age-mediated changes, and it should be noted that some inconsistencies can be found. Levels of some proteins of the Bcl-2 family were shown to increase with aging in rat gastrocnemius (Siu et al. 2005; Chung and Ng 2006), and Bcl-2/Bax ratio was decreased in senescent (29-month-old) compared with adult (16-month-old) rats (Chung and Ng 2006). Bax mRNA and protein content, and Bcl-2 protein content were also higher in the plantaris muscle from aged rats when compared with their young counterparts (Pistilli et al. 2006). However, the particular contribution of changes in Bcl-2 family to sarcopenia of aging remained unclear since the alteration of Bcl-2/Bax ratio, as a function of age was not reproduced in a separate study (Marzetti et al. 2008b). Another paper reported that aging increased Bax, whereas 12 weeks of treadmill exercise training markedly reduced Bax while increasing Bcl-2 and Bcl-2/Bax ratio in the white gastrocnemius and soleus muscles of old rats (Song et al. 2006). However, no changes in mitochondrial Bcl-2 and Bax levels, and in Bcl-2/Bax ratio were detected in another study focused on the gastrocnemius muscle from old (24-month) compared with young (6-month) rats (Dirks and Leeuwenburgh 2002). It has been proposed that these discrepancies could be reasonably explained by the differences in the age and the strain of the animals being examined in the different studies, as well as by differences in the approach of measurement being adopted (Siu et al. 2005). In addition, taking into account our results that dietary fat is a major factor that determines Bax levels in animals subjected to CR, these inconsistencies could be also due, at least partially, to dietary differences among animals used by different research groups. In accordance with this idea, we have previously reported that dietary fat (sunflower vs. olive oil) also modifies Bcl-2 and Bax apoptotic signaling in aging liver (Bello et al. 2006).
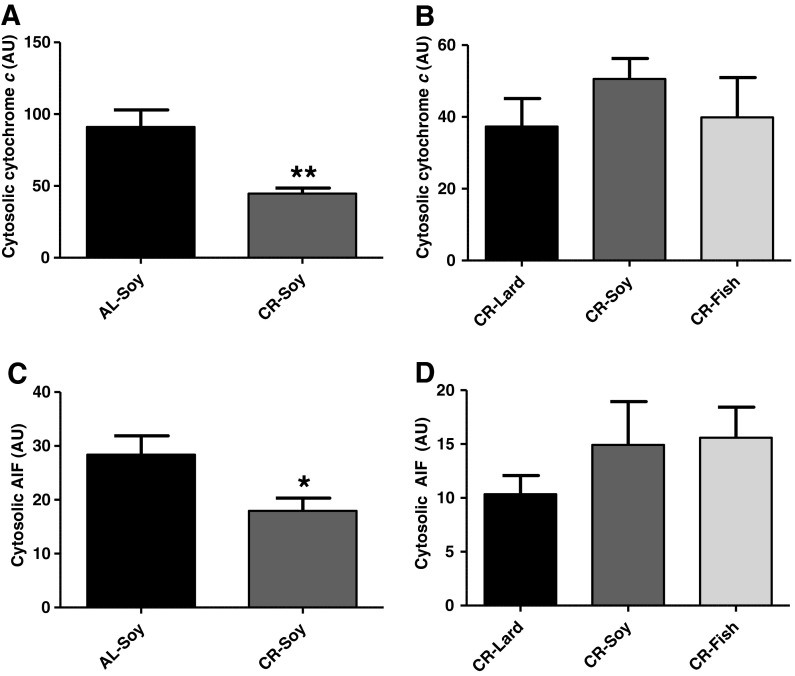
Cytosolic cytochrome c and AIF
Our demonstration that CR and dietary fat strongly regulate Bax levels and Bcl-2/Bax ratio might indicate a modulation of mitochondrial apoptotic signaling by our dietary interventions. Mitochondrial apoptosis relies on the release of pro-apoptotic factors after permeabilization of mitochondrial membranes. Release of cytochrome c to the cytosol promotes assembly of the apoptosome which triggers a caspase cascade via activation of caspase-9, whereas release of apoptosis-inducing factor (AIF) to the cytosol and its translocation to the nucleus triggers DNA fragmentation through a caspase-independent pathway in the skeletal muscle (Marzetti et al. 2008b). We found that CR decreased both cytochrome c and AIF release/accumulation into the cytosol (Fig. 6a, c). This constitutes the first demonstration that CR decreases cytosolic levels of cytochrome c and AIF in the skeletal muscle from young mice and is in accordance with the concomitant decrease of Bax levels in the CR-Soy group compared with AL-Soy. Cytosolic AIF has been shown to be increased by aging in rat plantaris (Dirks and Leeuwenburgh 2004) and gastrocnemius (Marzetti et al. 2008b) while decreased by CR in the plantaris muscle from old rats (Dirks and Leeuwenburgh 2004), which also agrees with our results. Conversely, AIF levels in cytosol increased in the gastrocnemius by hind limb suspension-induced atrophy in old but not in young rats, although no increase by aging itself was seen in another study (Siu et al. 2005). The decrease of cytosolic cytochrome c release/accumulation by CR we show here is important, since cytochrome c levels in cytosol have been reported to increase under conditions leading to skeletal muscle atrophy, as hind limb suspension, both in young and in old rats (Siu et al. 2005). On the other hand, our results are apparently in contrast with a previous report that documented no change of cytosolic cytochrome c by CR in the gastrocnemius from old rats (Dirks and Leeuwenburgh 2004). CR-mediated effects on cytochrome c release/accumulation into the cytosol could be species (rats vs. mice) or muscle type specific. Additionally, since all reported data correspond to old animals, the possibility exists that the decrease of cytosolic cytochrome c by CR is specific of young animals. The elucidation of these possibilities remains for further research. Whatever the case, it must be noted that inconsistencies can be also found regarding the effect of aging on cytochrome c release/accumulation into the cytosol, since increases (Siu et al. 2005), no change (Dirks and Leeuwenburgh 2002; Marzetti et al. 2008b), and also decreases (Dirks and Leeuwenburgh 2004) of cytosolic cytochrome c with aging have been reported. Additional factors, yet to be determined, could be involved in the regulation of cytochrome c release from mitochondria by aging and CR in the skeletal muscle.
Fig. 6.
Cytosolic content of cytochrome c and AIF. Calorie restriction decreased cytosolic levels of both cytochrome c (a, p < 0.01) and AIF (c, p < 0,05). Dietary fat source caused no alterations in cytosolic levels of either cytochrome c (b) or AIF (d). Data are presented as mean ± SEM (n = 4)
When the effect of dietary fat on cytosolic levels of AIF and cytochrome c was tested, we found no significant differences among any of the CR groups (Fig. 6b, d), despite the existence of significant changes of Bax levels and Bcl-2/Bax ratio as a function of fat source (see above). Besides Bax, other proteins mediate mitochondrial permeability transition, and their levels also change as a function of age (Marzetti et al. 2008b). The study of CR and fat source effects on components of the mitochondrial permeability transition pore remain for further investigation.
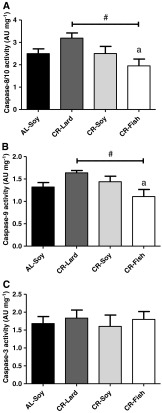
Caspases activities, XIAP levels, and apoptotic index
Our previous results indicate that both the extrinsic (plasma membrane-dependent) and the intrinsic (mitochondrial) pathways of apoptosis can be modulated by CR and dietary fat. We next analyzed how these dietary manipulations affected activities of caspase-8/10, which is activated via the extrinsic pathway, and caspase-9, which is an indicator of mitochondrial apoptosis. In addition, we also measured activity of caspase-3, the most representative executioner caspase (Boatright and Salvesen 2003).
CR per se did not produce alterations in any of the caspases measured (see AL-Soy vs. CR-Soy in Fig. 7a, b, c), but changing dietary fat in animals fed under CR had a modulating effect over the activity of the two regulatory caspases we measured here. As shown in Fig. 7a, b, activities of caspase-8/10 and −9 were lower in the CR-Fish group, and post hoc analysis of linear trend also showed a significant effect of dietary fat. However, no changes as a function of fat source were observed for caspase-3 (Fig. 7c).
Fig. 7.
Caspase-8/10, -9, and −3 activities. A similar pattern of changes was observed for caspase 8/10 (a) and caspase 9 (b). Activities of these two caspases were decreased as a function of dietary fat source (number sign, statistically significant linear trend with p < 0.05). In addition, for both caspases the activities were lower in the CR-Fish group compared with CR-Lard (a p < 0.05). c No changes in caspase-3 activity were observed due to either CR or to different dietary fat source. Data are mean ± SEM (n = 4)
The lack of changes in caspase activities by CR per se is in agreement with previous reports. One study focused on the rat plantaris muscle has documented that activities of caspases-3 and −9 were not affected by mild (8 %) CR (alone or in combination with lifelong voluntary exercise), although these two activities were increased with age (Wohlgemuth et al. 2010). Furthermore, caspase-3 and −9 activities were unchanged by either age or CR in the rat gastrocnemius muscle (Dirks and Leeuwenburgh 2002, 2004). However, another study did find a significant increase of caspase-3 protein content (cleaved caspase-3) with age and its attenuation by CR in rat gastrocnemius (Marzetti et al. 2009), and another one reported that cleaved caspase-9 content was augmented in senescent compared with adult rats, although the effect of CR was not tested (Chung and Ng 2006). On the other hand, it has been also reported that caspase-9 mRNA and activity were strongly increased by age, but caspase-3 mRNA was unchanged and caspase-3 activity only slightly increased with age in the rat gastrocnemius. Neither caspase-3 nor caspase-9 was altered by hind limb suspension in both age groups (Siu et al. 2005). Regarding caspase-8/10, a previous study found no changes in caspase-8 protein content (cleaved caspase-8) by either age or CR in the rat soleus muscle (Phillips and Leeuwenburgh 2005), but, in striking contrast, cleaved caspase-8 was significantly increased with age and this increase abolished by CR in the superficial vastus lateralis (Phillips and Leeuwenburgh 2005) and in the gastrocnemius (Marzetti et al. 2009). The possibility exists that CR decreases caspase-8/10 activity in muscles containing fast twitch fibers preferentially in aged animals.
Of note, our observations that 6 months of CR per se did not decrease the activity of any of the measured caspases are in contrast with the significant effects of CR on apoptotic signaling components acting upstream caspase activation, i.e., the decrease of nSMase (this work) and other components of the extrinsic pathway (Phillips and Leeuwenburgh 2005; Marzetti et al. 2008a, 2009), and the strong decrease of Bax and cytochrome c release/accumulation into cytosol (this work), which supports that activation of caspases and the subsequent induction of cell death may not be necessarily the result of increasing upstream elements of apoptotic signaling in skeletal muscle. Interestingly, it has been previously suggested that pro-apoptotic markers may not always induce apoptosis just because they are present. For instance, in addition to the subsequent cleavage and activation of pro-caspase-9 upon apoptosome formation, caspase-9 can also be activated via an independent additional pathway in muscle cells (Ho and Zacksenhaus 2004; Ho et al. 2004; Pistilli et al. 2006). On the other hand, the decrease of caspase-8/10 and −9 in the CR-Fish group compared with the other CR groups is in agreement with a general effect of this diet decreasing pro-apoptotic markers of both the extrinsic and the intrinsic pathways, the increase of cross-sectional area, and the decrease of circularity of muscle fibers in cross sections (see above).
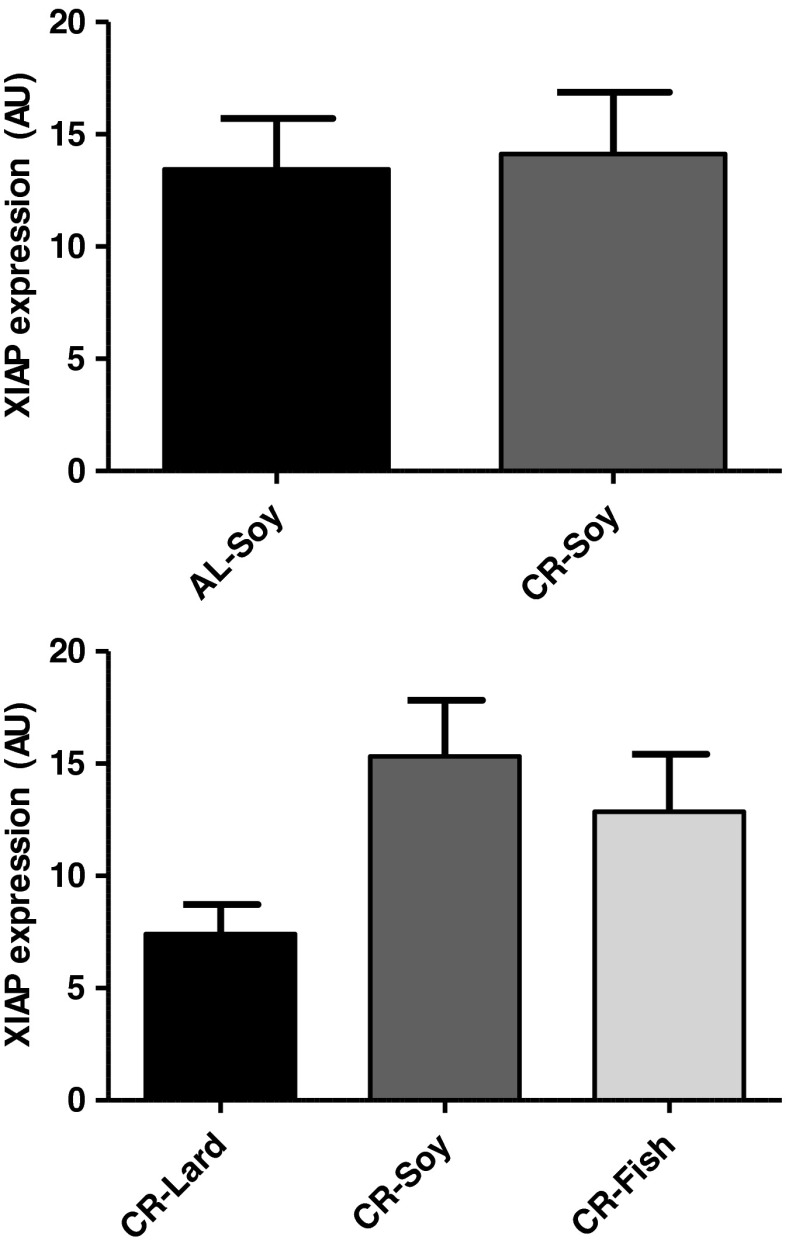
Changes of caspase polypeptide levels are not always translated into changes of caspase activity due to the existence of additional regulators of caspase activity. X-linked inhibitor of apoptosis (XIAP) can bind to caspase-3 and inhibit its protease activity. Levels of XIAP increased in rat gastrocnemius of old animals (Dirks and Leeuwenburgh 2004; Siu et al. 2005), and this increase was reversed by long-term CR (Dirks and Leeuwenburgh 2004). This could explain why caspase-3 activity was unchanged by either age or CR in the rat gastrocnemius (Dirks and Leeuwenburgh 2002, 2004) even when levels of cleaved caspase-3 increased with age and this increase was attenuated by CR (Marzetti et al. 2009). Also, increase of a caspase-3 inhibitor with age could explain why caspase-3 activity increased only slightly with age in the rat gastrocnemius, despite the strong increases of caspase-9 mRNA and activity (Siu et al. 2005). Thus, we also studied XIAP levels in our model but found no significant change with CR. Regarding the effect of fat source, XIAP levels tended to be lower in the CR-Lard group compared with CR-Soy or CR-Fish, although differences did not reach statistical significance (Fig. 8). In accordance with our results, no significant changes of XIAP levels were detected in the rat gastrocnemius when 6-month-old rats were subjected to short-term (2 months) CR (Selman et al. 2003). Taken together, our results and those from others support that the changes of this apoptosis regulator and its modulation by dietary manipulations might be specific of old animals. This agrees with the proposal that different apoptotic mechanisms may be responsible for the regulation of apoptosis in young and in aged skeletal muscles (Siu et al. 2005). It remains for further studies to elucidate whether or not fat source alters XIAP levels in skeletal muscle from old mice.
Fig. 8.
XIAP protein levels. No changes were observed due to either CR or to changes in dietary fat source in animals fed under CR. Data are mean ± SEM (n = 4)
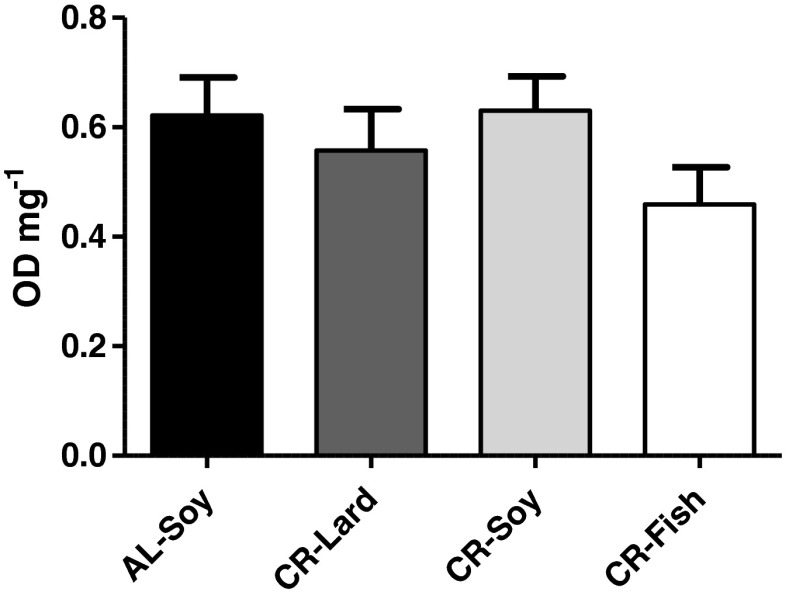
We finally measured the extent of DNA fragmentation by quantifying the amount of cytosolic mono- and oligonucleosomes to have an estimate of apoptosis levels in skeletal muscle samples. No differences in DNA fragmentation were observed between any of the mice groups of this study (Fig. 9), which is in agreement with the lack of changes of the executioner caspase-3. Caspase-3 content correlated with the apoptotic index in the rat gastrocnemius from old but not from young animals (Dirks and Leeuwenburgh 2002), although it has been later suggested that the mitochondrial caspase-independent apoptotic pathway may play a more prominent role in skeletal muscle loss than caspase-mediated apoptosis (Marzetti et al. 2008b). It is likely that we could not detect significant effects of diet on muscle caspase-3 and apoptotic index by our techniques because only very few fibers were undergoing apoptosis in young mice.
Fig. 9.
Apoptotic index. No changes in DNA fragmentation were observed due to either CR or to changes in dietary fat source in animals fed under CR. Data are mean ± SEM (n = 4)
The protective effect of CR on skeletal muscle and its augmentation by dietary fish oil is actually demonstrated by the significant improvement of fiber structural features (cross-sectional area and shape) in the gastrocnemius. Our structural study most likely reflects those subtle changes with much more sensitivity than the biochemical analyses that were carried out with a more limited number of samples. We acknowledge that the use of whole hind limb skeletal muscle for our biochemical analyses may decrease sensitivity to detect changes that might take place only in few fibers of specific muscles. However, this was necessary because of the low yield of the plasma membrane isolation procedure, which made it impossible to study individual muscles. In accordance with the idea proposed by Pistilli et al. (2006), we favor the explanation that the CR-Fish diet results in a cellular anti-apoptotic “environment” in the skeletal muscle. This environment is characterized by a downregulation of the components that trigger the initial stages of apoptosis engagement, both at the plasma membrane and the mitochondria although, according to our results, this seems not to be fully transmitted to downstream executioners. Consistent with this idea, the increase in proapoptotic markers due to hind limb suspension resulted in a cellular environment, which was poised for apoptosis to occur, although this final step was not fully engaged in aged plantaris muscles (Pistilli et al. 2006).
Why did fish oil diet potentiate the anti-apoptotic effect of CR in skeletal muscles? Eicosapentaenoic acid (EPA) is an n-3 PUFA with demonstrable anti-inflammatory activities that may have potential benefits on atrophic skeletal muscle conditions. Of note, EPA-enriched supplement attenuated the progression of cachexia in patients with advanced pancreatic cancer (Babcock et al. 2000), which agrees with the reduction of apoptotic signaling in the mouse skeletal muscle we show here. It is well-known that dietary alterations have a marked influence on gene expression (Reddy-Avula et al. 2002). PUFA regulation of gene transcription factors is an emerging area of study and differential effects have been reported, whereas n-3 PUFAs inhibit, n-6 PUFAs activate pro-inflammatory NF-κB (Jump 2002). Similarly, it was found that saturated fatty acids (palmitate), but not PUFAs, activate NF-κB in differentiated human myotubes (Weigert et al. 2004). Interestingly, several outcomes of CR might be potentiated by n-3 PUFA contained in fish oil. For instance, EPA and docosahexaenoic acid (DHA) exhibit anti-adipogenic action (Buckley and Howe 2009) which may involve a metabolic switch that includes enhancement of β-oxidation and upregulation of mitochondrial biogenesis (Flachs et al. 2005). Of note, dietary n-6 and n-3 lipids with calorie restriction inhibited apoptosis of splenic lymphocytes, prevented autoimmune disease, and prolonged life span in autoimmune-prone (NZB X NZW) F1 (B/W) female mice (Jolly et al. 2001; Reddy-Avula et al. 2002).
One interesting possibility to explore in future investigations is how CR and dietary fat affect other systems known to be involved in the degradation of skeletal muscle fibers constituents in addition to apoptosis, such as the autophagy–lysosomal system, the calpain system, and protein degradation through the ubiquitin–proteasome system (Dirks and Leeuwenburgh 2002, 2004; Phillips and Leeuwenburgh 2005; Marzetti et al. 2008a; Wohlgemuth et al. 2010; Romanello et al. 2010). In this way, besides attenuating the proteolytic and apoptotic effects of a cachectic factor in fully differentiated myotubes from the murine C2C12 myogenesis model (Tisdale and Dhesi 1990), and protecting against skeletal muscle damage induced by pro-inflammatory TNF-α (Magee et al. 2008), EPA caused a reduction on the rate at which skeletal muscle was lost due to downregulation of the ubiquitin–proteasome pathway in a murine model of cachexia (Whitehouse et al. 2001).
Another important aspect that deserves further investigation is whether or not the effect of CR and dietary fat on skeletal muscle apoptotic signaling is maintained or altered in old mice. It is known that aging complicates the apoptotic regulation in models of suspension-induced muscle atrophy, and that different apoptotic mechanisms may be involved in activating apoptosis in young and aged skeletal muscles (Siu et al. 2005; Pistilli et al. 2006). How these dietary manipulations will affect longevity of mice will also warrant further research.
Acknowledgments
This work was supported by NIH grant 1R01AG028125-01A1 (to JJR, PN and JMV), Ministerio de Economía y Competitividad BFU2011-23578 (to JMV), Junta de Andalucía Proyectos de Excelencia grant P09-CVI-4887 (to JMV), Junta de Andalucía Proyectos Internacionales grant (to JMV), and BIO-276 (Junta de Andalucía and the University of Córdoba, to JMV). JALD was funded by a predoctoral fellowship of the Spanish Ministerio de Educación. HK was funded by a predoctoral fellowship of the Agencia Española de Cooperación Internacional al Desarrollo.
References
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Krönke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/S0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Babcock T, Helton WS, Espat NJ. Eicosapentaenoic acid (EPA): an antiinflammatory omega-3 fat with potential clinical applications. Nutrition. 2000;16:1116–1118. doi: 10.1016/S0899-9007(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Bello RI, Alcaín FJ, Gómez-Díaz C, López-Lluch G, Navas P, Villalba JM. Hydrogen peroxide and cell density-regulated expression of cytochrome b5 reductase in HeLa cells. J Bioenerg Biomembr. 2003;35:169–179. doi: 10.1023/A:1023702321148. [DOI] [PubMed] [Google Scholar]
- Bello RI, Gómez-Díaz C, Burón MI, Alcaín FJ, Navas P, Villalba JM. Enhanced antioxidant protection of liver membranes in long-lived rats fed on a coenzyme Q-supplemented diet. Exp Gerontol. 2005;40:694–706. doi: 10.1016/j.exger.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Bello RI, Gómez-Díaz C, Burón MI, Navas P, Villalba JM. Differential regulation of hepatic apoptotic pathways by dietary olive and sunflower oils in the aging rat. Exp Gerontol. 2006;41:1174–1184. doi: 10.1016/j.exger.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Buckley JD, Howe PRC. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obesity Rev. 2009;10:648–659. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hagopian K, McDonald RB, Bibus D, López-Lluch G, Villalba JM, Navas P, Ramsey JJ (2012) The Influence of dietary lipid composition on skeletal muscle mitochondria from mice following one month of calorie restriction. J Gerontol A Biol Sci Med Sci 67:1121–1131 [DOI] [PMC free article] [PubMed]
- Chung L, Ng Y-C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Cabello R, Rios M, López-Lluch G, Ingram DK, Lane MA, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressors, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova SJ, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Weber KL, Hwee DT, Van Eenennaam AL, López-Lluch G, Villalba JM, Burón I, Navas P, German JB, Watkins SM, Chen Y, Wei A, McDonald RB, Ramsey JJ. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS One. 2010;5:e12696. doi: 10.1371/journal.pone.0012696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Qin M, Nakamoto H, Goto S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1231–R1237. doi: 10.1152/ajpregu.90478.2008. [DOI] [PubMed] [Google Scholar]
- Ho AT, Li QH, Hakem R, Mak TW, Zacksenhaus E. Coupling of caspase-9 to Apaf-1 in response to loss of pRb or cytotoxic drugs is cell-type-specific. EMBO J. 2004;23:460–472. doi: 10.1038/sj.emboj.7600039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AT, Zacksenhaus E. Splitting the apoptosome. Cell Cycle. 2004;3:446–448. doi: 10.4161/cc.3.4.818. [DOI] [PubMed] [Google Scholar]
- Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun D-H, Emerson SS, Jo D-G, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/BMBRep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Woollard AC, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26:853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- Jolly C, Muthukumar A, Reddy-Avula C, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB X NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–2760. doi: 10.1093/jn/131.10.2753. [DOI] [PubMed] [Google Scholar]
- Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor α and γ interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- Kim J-H, Kwak H-B, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8 %) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology. 1993;39:7–18. doi: 10.1159/000213509. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuated dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- López-Lluch G, Rios M, Lane MA, Navas P, de Cabo R. Mouse liver plasma membrane redox system activity is altered by aging and modulated by calorie restriction. Age. 2005;27:153–160. doi: 10.1007/s11357-005-2726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P, Pearson S, Allen J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Disease. 2008;7:24. doi: 10.1186/1476-511X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín SF, Navarro F, Forthoffer N, Navas P, Villalba JM. Neutral magnesium-dependent sphingomyelinase from liver plasma membrane: Purification and inhibition by ubiquinol. J Bioenerg Biomembr. 2001;33:143–153. doi: 10.1023/A:1010704715979. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung H-Y, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, Quinn LS, Leeuwenburgh C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130:272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro F, Navas P, Burgess JR, Bello RI, de Cabo R, Arroyo A, Villalba JM. Vitamin E and selenium deficiency induces the expression of the ubiquinone-dependent antioxidant system at the plasma membrane. FASEB J. 1998;12:1665–1673. doi: 10.1096/fasebj.12.15.1665. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM. Regulation of ceramide signaling by plasma membrane Coenzyme Q reductases. Methods Enzymol. 2004;378:200–206. doi: 10.1016/S0076-6879(04)78016-7. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM, Lenaz G. Coenzyme Q-dependent functions of plasma membrane in the aging process. Age. 2005;27:139–146. doi: 10.1007/s11357-005-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7S:S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Parrado-Fernández C, López-Lluch G, Rodríguez-Bies E, Santa-Cruz S, Navas P, Ramsey JJ, Villalba JM. Calorie restriction modifies ubiquinone and COQ transcript levels in mouse tissues. Free Radic Biol Med. 2011;50:1728–1736. doi: 10.1016/j.freeradbiomed.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Siu PM, Always SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol Biol Sci Med Sci. 2006;61:245–255. doi: 10.1093/gerona/61.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Humble SJ, Koomson EK, Ram JJ, Bevilacqua L, Hagopian K. Influence of mitochondrial membrane fatty acid composition on proton leak and H2O2 production in liver. Comp Biochem Physiol B Biochem Mol Biol. 2005;140:99–108. doi: 10.1016/j.cbpc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Reddy-Avula CP, Lawrence RA, Zaman K, Fernandes G. Inhibition of intracellular peroxides and apoptosis of lymphocytes in lupus-prone B/W mice by dietary n-6 and n-3 lipids with calorie restriction. J Clin Immunol. 2002;22:206–219. doi: 10.1023/A:1016088708457. [DOI] [PubMed] [Google Scholar]
- Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Gredilla R, Phaneuf S, Kendaiah S, Barja G, Leeuwenburgh C. Short-term calorie restriction and regulatory proteins of apoptosis in heart, skeletal muscle and kidney of Fischer 344 rats. Biogerontology. 2003;4:141–147. doi: 10.1023/A:1024149923693. [DOI] [PubMed] [Google Scholar]
- Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, Knutson MD, Chung HY, Leeuwenburgh C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–716. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young and aged rats. Am J Physiol. 2005;289:R1015–R1026. doi: 10.1152/ajpcell.00154.2005. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ, Dhesi JK. Inhibition of weight loss by omega-3 fatty acids in an experimental cachexia model. Cancer Res. 1990;50:5022–5026. [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factorkappaB. J Biol Chem. 2004;279:23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001;61:3604–3609. [PubMed] [Google Scholar]
- Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LE, Garland T, Jr, Rowan SL, Hepple RT. Anatomic capillarization is elevated in the medial gastrocnemius muscle of mighty mini mice. J Appl Physiol. 2009;106:1660–1667. doi: 10.1152/japplphysiol.91233.2008. [DOI] [PubMed] [Google Scholar]
- Yu BP. Membrane alteration as a basis of aging and the protective effects of calorie restriction. Mech Ageing Dev. 2005;126:1003–1010. doi: 10.1016/j.mad.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Yu BP, Lim BO, Sugano M. Dietary restriction downregulates free radical and lipid peroxide production: plausible mechanism for elongation of life span. J Nutr Sci Vitaminol (Tokyo) 2002;48:257–264. doi: 10.3177/jnsv.48.257. [DOI] [PubMed] [Google Scholar]