Abstract
Aging is associated with a progressive decline in skeletal muscle mass. It has been hypothesized that an attenuated muscle protein synthetic response to the main anabolic stimuli may contribute to the age-related loss of muscle tissue. The aim of the present study was to compare the muscle protein synthetic response following ingestion of a meal-like amount of dietary protein plus carbohydrate between healthy young and older men. Twelve young (21 ± 1 years) and 12 older (75 ± 1 years) men consumed 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein with 40 g of carbohydrate. Ingestion of specifically produced intrinsically l-[1-13C]phenylalanine-labeled protein allowed us to assess the subsequent incorporation of casein-derived amino acids into muscle protein. Blood samples were collected at regular intervals, with muscle biopsies obtained prior to and 2 and 6 h after protein plus carbohydrate ingestion. The acute post-prandial rise in plasma glucose and insulin concentrations was significantly greater in the older compared with the younger males. Plasma amino acid concentrations increased rapidly following drink ingestion in both groups. However, plasma leucine concentrations were significantly lower at t = 90 min in the older when compared with the young group (P < 0.05). Muscle protein-bound l-[1-13C]phenylalanine enrichments increased to 0.0071 ± 0.0016 and 0.0072 ± 0.0013 mole percent excess (MPE) at 2 h and 0.0229 ± 0.0016 and 0.0213 ± 0.0024 MPE at 6 h following ingestion of the intrinsically labeled protein in the young and older males, respectively, with no differences between groups (P > 0.05). We conclude that the use of dietary protein-derived amino acids for muscle protein synthesis is not impaired in healthy older men following intake of protein plus carbohydrate.
Keywords: Skeletal muscle, Aging, Sarcopenia, Amino acids, Anabolic resistance
Introduction
Aging is associated with a gradual loss of skeletal muscle mass, often referred to as sarcopenia (Evans 1995). The loss of muscle mass is accompanied by a reduction in muscle strength, loss of functional capacity, and an increased risk of developing chronic metabolic diseases, like obesity and type 2 diabetes (Evans 1995). The age-related loss of skeletal muscle mass is facilitated by a combination of factors, which include a sedentary lifestyle and a less-than-optimal diet. The decline in muscle mass in the elderly is mainly attributed to a disruption in the regulation of skeletal muscle protein turnover, leading to an imbalance between muscle protein synthesis and degradation (Nair 2005). Interestingly, basal (fasting) muscle protein synthesis rates do not seem to differ substantially between the young and elderly (Cuthbertson et al. 2005; Volpi et al. 2001; Burd et al. 2012a). Therefore, research groups have started to focus on the muscle protein synthetic response to the main anabolic stimuli, i.e., food intake and physical activity (Cuthbertson et al. 2005; Kumar et al. 2009; Katsanos et al. 2005).
It has been well established that protein turnover in skeletal muscle tissue is highly responsive to nutrient intake in healthy, young individuals (Koopman et al. 2007). Recent reports suggest that in healthy elderly subjects, the post-prandial muscle protein synthetic response to amino acid and/or protein administration is attenuated when compared to younger controls, a phenomenon that has been coined “anabolic resistance” (Cuthbertson et al. 2005; Katsanos et al. 2005; Volpi et al. 2000). This blunted post-prandial muscle protein synthetic response is now generally thought to represent a key factor responsible for the age-related decline in skeletal muscle mass. However, there is an ongoing debate about the existence and extent of anabolic resistance in a healthy elderly population (Burd et al. 2012a, b), since various studies have failed to confirm the presence of anabolic resistance in a physiological setting where post-prandial muscle protein synthesis rates were assessed following ingestion of a single meal-like bolus of protein (Koopman et al. 2009b; Paddon-Jones et al. 2004; Pennings et al. 2011b; Symons et al. 2007, 2011).
It has been suggested that differences in post-prandial muscle protein synthetic rates between the young and elderly may become more apparent when protein is ingested in combination with carbohydrate (Volpi et al. 2000). A classic work by Volpi et al. (2000) has shown that muscle protein anabolism under conditions of hyperaminoacidemia with endogenous hyperinsulinemia is less responsive in the healthy elderly when compared to younger subjects. Consequently, we speculated that the muscle protein synthetic response to a single meal-like bolus of protein plus carbohydrate is impaired in the elderly population. This impaired response may be attributed to the insulin resistance of post-prandial muscle perfusion (Timmerman et al. 2010a, b), which reduces the capacity to utilize dietary protein-derived amino acids for de novo muscle protein synthesis. Consequently, we hypothesized that the post-prandial muscle protein synthetic response to the combined ingestion of a meal-like amount of protein plus carbohydrate is reduced in healthy older men when compared with younger males.
In the present study, we selected 12 healthy young (21 ± 1 years) and 12 healthy older (75 ± 1 years) men to determine post-prandial muscle protein accretion following ingestion of a single meal-like bolus of protein plus carbohydrate. As presented in previous works from our laboratory (Pennings et al. 2011a, b; Pennings et al. 2012; Koopman et al. 2009a), we applied specifically produced intrinsically l-[1-13C]phenylalanine-labeled casein protein with a high enrichment level (37.4 mole percent excess (MPE)) to assess post-prandial incorporation of dietary protein-derived l-[1-13C]phenylalanine into muscle protein. Labeled casein protein was obtained by infusing cows with large quantities of l-[1-13C]phenylalanine, collecting milk, and purifying the casein fraction (van Loon et al. 2009a). As such, we were able to assess both the short-term (2 h) and long-term (6 h) anabolic response following protein plus carbohydrate ingestion in young and older males. The present study shows that the anabolic response following ingestion of a mixed meal containing 20 g of protein and 40 g of carbohydrate is not impaired in healthy older men.
Methods
Subjects
Twelve healthy young (21 ± 1 years) and twelve healthy older (75 ± 1 years) men were selected to participate in this study. Subjects' characteristics are presented in Table 1. Exclusion criteria were body mass index (BMI) > 30 kg m−2, diabetes, all co-morbidities interacting with mobility and muscle metabolism of the lower limbs (e.g., arthrosis, arthritis, spasticity/rigidity, all neurological disorders and paralysis), use of anticoagulants, blood diseases, phenylketonuria, allergy for lidocaine, and participation in any regular exercise program. All subjects were informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained. This study was approved by the Medical Ethics Committee of the Maastricht University Medical Centre.
Table 1.
Subjects' characteristics
| Young | Older | P value | |
|---|---|---|---|
| n | 12 | 12 | |
| Age (years) | 21 ± 1 | 75 ± 1* | <0.001 |
| Weight (kg) | 74.4 ± 2.2 | 78.4 ± 2.1 | 0.195 |
| BMI (kg m−2) | 22.1 ± 0.6 | 25.8 ± 0.7* | 0.001 |
| Systolic blood pressure (mmHg) | 121 ± 2 | 145 ± 5* | <0.001 |
| Diastolic blood pressure (mmHg) | 61 ± 3 | 71 ± 4 | 0.051 |
| Fat (% body weight) | 14.4 ± 0.7 | 23.2 ± 1.1* | <0.001 |
| ALM (% body weight) | 37.4 ± 0.8 | 30.8 ± 0.7* | <0.001 |
| SMMI (kg m−2) | 8.5 ± 0.2 | 7.9 ± 0.2* | 0.024 |
| Basal plasma glucose (mmol L−1) | 5.0 ± 0.1 | 5.4 ± 0.1* | 0.012 |
| Plasma glucose OGTT t = 120 min (mmol L−1) | 5.0 ± 0.3 | 6.0 ± 0.6 | 0.177 |
| Basal plasma insulin (mU L−1) | 18 ± 3 | 21 ± 2 | 0.357 |
| Plasma insulin OGTT t = 120 min (mU L−1) | 51 ± 11 | 94 ± 16* | 0.033 |
| HbA1c (%) | 5.1 ± 0.1 | 5.7 ± 0.1* | <0.001 |
| HOMA | 4.3 ± 0.5 | 5.1 ± 0.6 | 0.357 |
| OGIS (mL min m−1) | 417 ± 18 | 346 ± 16* | 0.010 |
Values represent means ± SEMs. Data were analyzed with an unpaired, two-tailed Student's t test
BMI body mass index, ALM appendicular lean mass, SMMI skeletal muscle mass index, OGTT oral glucose tolerance test, HbA 1c glycated hemoglobin, HOMA homeostasis model assessment, OGIS oral glucose insulin sensitivity
*Significantly different from Young group, P < 0.05 (unpaired t test)
Pretesting
Prior to selection, all subjects participated in a routine medical screening. Subjects completed a health and activity questionnaire and underwent an oral glucose tolerance test (OGTT) to screen for type 2 diabetes according to the criteria set by the World Health Organization (Alberti and Zimmet 1998). Venous plasma glucose and insulin concentrations determined during the OGTT were used to determine the oral glucose insulin sensitivity (OGIS) index (Mari et al. 2001) and the homeostasis model assessment (HOMA) index (Matthews et al. 1985). Prior to the OGTT, body weight and height were measured, and body composition was determined by dual-energy X-ray absorptiometry (Discovery A, Hologic, Bedford, USA). Appendicular lean mass (ALM) was calculated from the sum of the arm and leg lean tissue masses, and the skeletal muscle mass index (SMMI) was calculated by dividing the appendicular lean mass by body height squared.
Diet and activity before testing
All subjects consumed a standardized meal (33 ± 2 kJ kg body weight−1, providing 44 % energy (En%) carbohydrate, 22 En% protein, and 34 En% fat) the evening prior to the experiment. All volunteers refrained from strenuous physical activity and maintained their habitual diet for at least 2 days prior to the experiment.
Protocol
At 0800 h, after an overnight fast, subjects arrived at the laboratory by car or public transport. A polytetrafluoroethylene catheter was inserted into a heated dorsal hand vein, after which the hand was placed in a hot box (60 °C) to allow arterialized venous blood sampling (Abumrad et al. 1981). After a basal arterialized blood sample was collected, a fasting muscle biopsy sample was obtained from the vastus lateralis muscle. Subjects then ingested a single bolus of test drink containing 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein and 40 g of carbohydrate (50 % dextrose monohydrate, Avebe Food, Veendam, the Netherlands; 50 % maltodextrin, AppliChem GmbH, Darmstadt, Germany). The consumption of the test drink signified the beginning (t = 0 min) of a 6-h post-prandial assessment period. Arterialized blood samples were subsequently collected at t = 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 min. A second muscle biopsy sample was taken from the same limb through a new incision at t = 120 min. A third muscle biopsy sample was collected from the contralateral leg at t = 360 min. Arterialized blood samples were collected into pre-chilled EDTA-containing tubes and centrifuged at 1,000×g for 10 min at 4 °C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80 °C until further analysis. Muscle biopsy samples were obtained from the middle region of the vastus lateralis, 15 cm above the patella and ~3 cm below entry through the fascia using the percutaneous needle biopsy technique (Bergstrom 1975). Muscle biopsy samples were carefully dissected and freed from any visible non-muscle material and then immediately frozen in liquid nitrogen and stored at −80 °C until further analysis.
Preparation of intrinsically labeled protein
Intrinsically l-[1-13C]phenylalanine-labeled casein protein was obtained by infusing a Holstein cow with large quantities of l-[1-13C]phenylalanine, collecting milk, and purifying the casein fraction as described previously (van Loon et al. 2009a). The l-[1-13C]phenylalanine enrichment in the casein fraction averaged 37.4 MPE. The casein protein met all chemical and bacteriological specifications for human consumption. Subjects received a total beverage volume of 450 mL, which provided 20 g of casein protein. Drinks were flavored by adding 2 mL of vanilla flavor (Givaudan, Naarden, the Netherlands) per liter of beverage.
Plasma analyses
Plasma glucose concentrations were analyzed with an automatic analyzer ABX Pentra 400 (Horiba ABX Diagnostics, Nijmegen, the Netherlands) using an ABX Pentra Glucose HK CP Kit (A11A01667, Horiba ABX Diagnostics). Insulin was analyzed by radioimmunoassay using a commercially available kit (Insulin RIA kit HI-14K, Millipore, Billerica, USA). Plasma amino acid concentrations were determined using ultra-performance liquid chromatography tandem mass spectrometry as described previously (Waterval et al. 2009). For plasma phenylalanine enrichment measurements, plasma phenylalanine was derivatized to its t-butyldimethyl-silyl (TBDMS) derivative, and the [1-13C]phenylalanine enrichment was determined by electron ionization gas chromatography–mass spectrometry (GC–MS; model 7890AN GC/5975C MSD, Agilent, Little Falls, USA) by using selected ion monitoring of masses 336 and 337 for unlabeled and labeled [1-13C]phenylalanine, respectively (Wolfe and Chinkes 2004). Standard regression curves were applied in all isotopic enrichment analyses to assess the linearity of the mass spectrometer and to control for the loss of tracer. Enrichments (MPE) were corrected for the natural abundance of 13C phenylalanine (Biolo et al. 1992).
Muscle tissue analyses
For the measurement of l-[1-13C]phenylalanine enrichments in the tissue-free amino acid pool and mixed muscle protein, 40–60 mg of wet muscle was freeze-dried. Collagen, blood, and other non-muscle fiber material were removed from the muscle fibers under a light microscope. The isolated muscle fiber mass was weighed, and 35 volumes (7 × dry weight of isolated muscle fibers × wet/dry ratio) of ice-cold 2 % perchloric acid were added. The tissue was then homogenized by sonification (3 × 10 s, with 10 s between time intervals) and centrifuged (at 1,160×g for 20 min at 4 °C). The supernatant was collected and processed in the same manner as the plasma samples, such that tissue-free l-[1-13C]phenylalanine enrichments could be measured by using their TBDMS derivative on a GC–MS. The protein pellet was washed three times with 2 % perchloric acid and hydrolyzed with 6 mol/L HCl at 120 °C for 15 to 18 h. The hydrolyzed protein fraction was dried under a nitrogen stream while heated to 120 °C. A 50 % acetic acid solution was then added, and the hydrolyzed protein was passed over a Dowex exchange resin (AG 50 W-X8, 100–200 mesh hydrogen form, Bio-Rad, Hercules, USA) by using 1 mol/L HCl, H2O, and 2 mol/L NH4OH as eluents. The NH4OH fraction was collected and used for further analysis. l-[1-13C]phenylalanine enrichment was determined by derivatization to its N(O,S)-ethoxycarbonyl ethyl ester. The enrichment of the derivative was measured by GC-C-IRMS (MAT 253, Thermo Scientific, Bremen, Germany) by using an HP-Ultra 1 GC column (no. 19091A-112, Hewlett Packard, USA), GC Isolink, and monitoring of ion masses 44, 45, and 46. By establishing the relationship between the enrichment of a series of l-[1-13C]phenylalanine standards of variable enrichment and the enrichment of the N(O,S)-ethoxycarbonyl ethyl esters of these standards, the muscle protein-bound enrichment of phenylalanine was determined. Standard regression curves were applied to assess the linearity of the mass spectrometer and to control for the loss of tracer. The CV for the measurement of l-[1-13C]phenylalanine enrichment in mixed muscle protein averaged 0.46 ± 0.02 %.
Calculation of mixed muscle protein fractional synthetic rates from the ingested casein
The mixed muscle protein fractional synthetic rates (FSR) from the ingested casein over the 6-h post-prandial period were calculated using the standard precursor–product relationship (Koopman et al. 2005). Muscle FSR was calculated as follows:
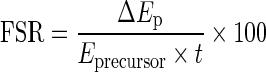 |
where ΔEp is the delta increment of muscle protein-bound l-[1-13C]phenylalanine enrichment (MPE) during the incorporation period, Eprecursor is the enrichment (MPE) of the precursor during the incorporation period, and t is the time interval (h) between biopsy samples. To adjust for non-steady state plasma tracer enrichments, precursor enrichments were calculated as the integral of the plasma l-[1-13C]phenylalanine enrichment over the time period for determination of amino acid incorporation (Zilversmit 1960).
Statistics
All data are expressed as means ± standard error of the means (SEMs). The plasma insulin and glucose responses were calculated as the positive incremental area under the curve (iAUC) above baseline values. Differences in baseline values (i.e., age, weight, BMI, body composition, blood pressure, glucose tolerance) were determined using an unpaired, two-tailed Student's t test. Two-way ANOVA with time as within-subjects factor and group as between-subjects factor was used to compare differences between groups over time in plasma glucose and insulin concentrations, plasma amino acid concentrations, and plasma l-[1-13C]phenylalanine enrichments. In case of a significant interaction between time and treatment, Bonferroni post-tests were applied to locate the differences. Differences between groups in iAUC of plasma glucose and insulin, muscle free and protein-bound l-[1-13C]phenylalanine enrichments, and mixed muscle FSR were analyzed with an unpaired, two-tailed Student's t test. Statistical significance was set at P < 0.05.
Results
Plasma glucose and insulin
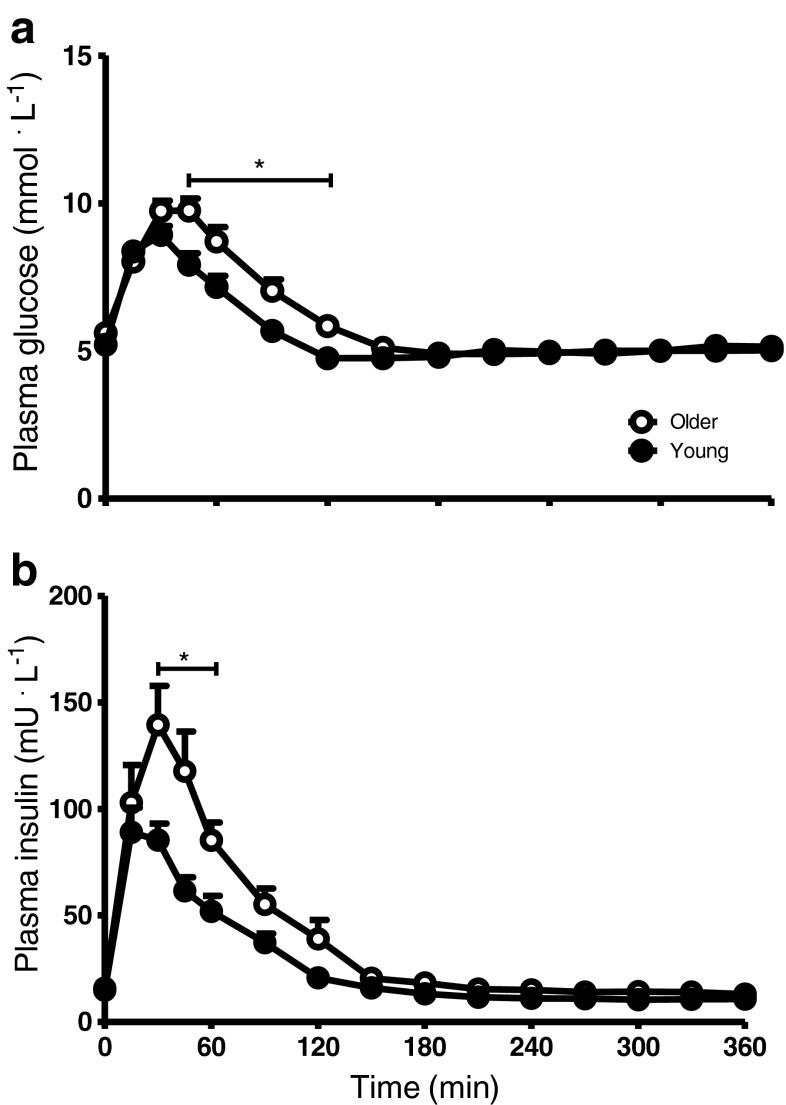
Plasma glucose and insulin concentrations following protein plus carbohydrate ingestion in young and older men are shown in Fig. 1 Plasma glucose concentrations significantly increased after drink ingestion in both groups. Post-prandial plasma glucose concentrations were significantly higher from t = 45 to t = 120 min in the older compared with the young men (significant time (P < 0.0001), group (P < 0.0001), and interaction (P < 0.0001) effect; Fig. 1a). The iAUC above fasting plasma glucose level was 206 ± 22 and 288 ± 34 mmol L−1 6 h−1 in the young and older group, respectively (P = 0.057). Plasma insulin concentrations (Fig. 1b) showed a rapid increase in both groups (significant time (P < 0.0001), group (P < 0.0001), and interaction (P < 0.0001) effect). Plasma insulin concentrations were significantly higher from t = 30 to t = 60 min in the older compared with the young group (P < 0.05). In agreement, the iAUC above the fasting plasma insulin concentration was significantly higher in the older compared with the young men (8,421 ± 1,054 vs 4,635 ± 440 mU L−1 6 h−1, respectively; P < 0.005).
Fig. 1.
a Mean (±SEM) plasma glucose and b insulin concentrations following ingestion of 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein plus 40 g of carbohydrate in healthy young (n = 12) and older (n = 12) men. a Significant time (P < 0.0001), group (P < 0.0001), and time*group interaction (P < 0.0001) effect. b Significant time (P < 0.0001), group (P < 0.0001), and time*group interaction (P < 0.0001) effect. * = P < 0.05 compared with corresponding time point in the Young group
Plasma amino acids
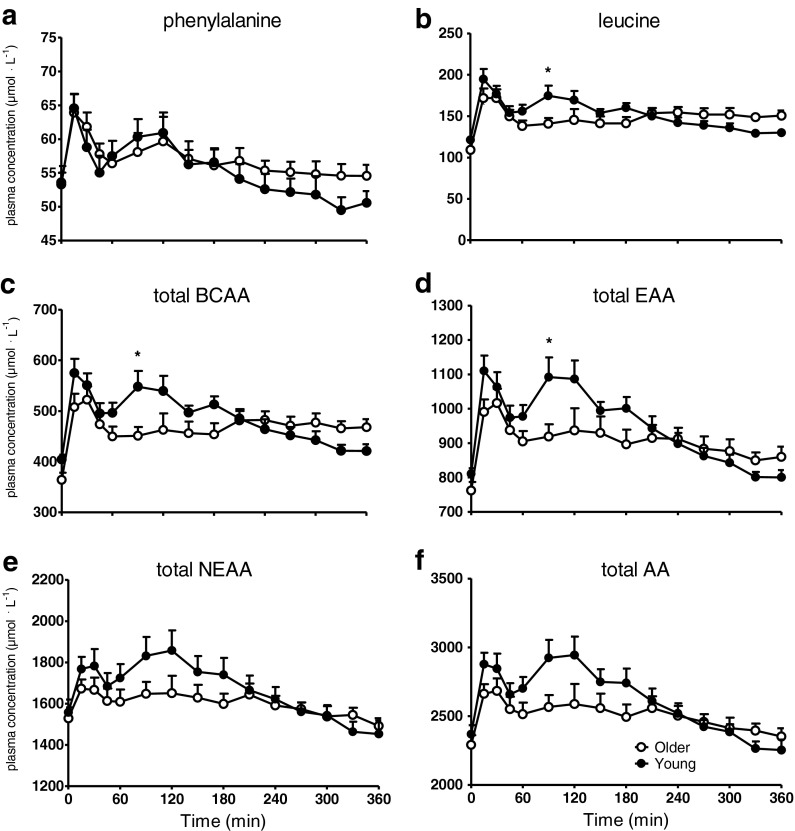
Plasma phenylalanine, leucine, total branched chain amino acid (BCAA), total essential amino acid (EAA), total non-essential amino acid (NEAA), and total amino acid concentrations over time are illustrated in Fig. 2a–f, respectively. Following protein plus carbohydrate ingestion, a rapid rise in plasma amino acid concentration was observed in both groups. Plasma phenylalanine, total plasma NEAA, and total plasma amino acid concentrations did not differ between groups, whereas plasma leucine, total plasma BCAA, and total plasma EAA concentrations were significantly lower at t = 90 min in the older when compared with the young group (P < 0.05).
Fig. 2.
a Mean (±SEM) plasma phenylalanine, b leucine, c total branched chain amino acid (BCAA), d total essential amino acid (EAA), e total non-essential amino acid (NEAA), and f total amino acid (AA) concentrations following ingestion of 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein plus 40 g of carbohydrate in healthy young (n = 12) and older (n = 12) men. a Significant time (P < 0.0001) effect. b Significant time (P < 0.0001) and time*group interaction (P = 0.0018) effect. c Significant time (P < 0.0001), group (P = 0.0050), and time*group interaction (P < 0.0001) effect. d Significant time (P < 0.0001) and time*group interaction (P = 0.0032) effect. e Significant time (P < 0.0001) and group (P = 0.0032) effect. f Significant time (P < 0.0001) and group (P = 0.0005) effect. * = P < 0.05 compared to corresponding time point in the Young group
Plasma l-[1-13C]phenylalanine enrichments
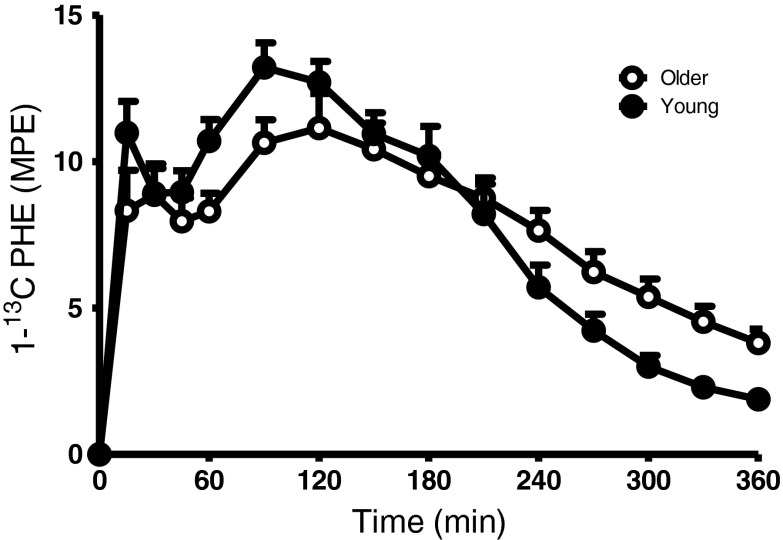
The time course of the increase in plasma l-[1-13C]phenylalanine enrichment (MPE) is shown in Fig. 3. Following ingestion of 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein plus 40 g of carbohydrate, plasma l-[1-13C]phenylalanine enrichments increased rapidly. Peak plasma l-[1-13C]phenylalanine enrichments averaged 13.2 ± 0.8 and 11.1 ± 1.2 MPE in the young and older group, respectively. There was a significant time (P < 0.0001) and time*group interaction (P = 0.0006) effect; however, there were no significant differences in plasma l-[1-13C]phenylalanine enrichment between groups at individual time points.
Fig. 3.
Mean (±SEM) plasma l-[1-13C]phenylalanine enrichments (MPE) following ingestion of 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein plus 40 g of carbohydrate in healthy young (n = 12) and older (n = 12) men. Significant time (P < 0.0001) and time*group interaction (P = 0.0006) effect. No significant differences between groups at individual time points
Muscle tracer analysis
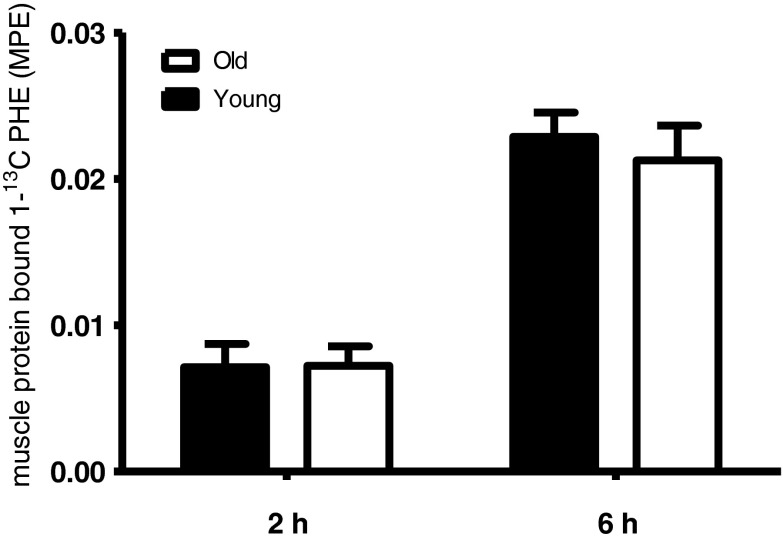
Muscle free l-[1-13C]phenylalanine enrichments did not differ between groups in the muscle biopsy taken 2 h following drink ingestion (6.6 ± 0.6 and 6.1 ± 0.7 MPE in the young and older group, respectively; P = 0.6). In the biopsy taken at 6 h, muscle free l-[1-13C]phenylalanine enrichments were significantly greater in the older compared with the young men (2.7 ± 0.3 and 1.7 ± 0.2 MPE, respectively; P < 0.05). Muscle protein-bound l-[1-13C]phenylalanine enrichments at 2 and 6 h into the post-prandial period are shown in Fig. 4. Muscle protein-bound l-[1-13C]phenylalanine enrichments averaged 0.0071 ± 0.0016 and 0.0072 ± 0.0013 MPE at 2 h and 0.0229 ± 0.0016 and 0.0213 ± 0.0024 MPE at 6 h in the young and older group, respectively, with no significant differences between groups (P = 0.97 and 0.58, respectively).
Fig. 4.
Mean (±SEM) muscle protein-bound l-[1-13C]phenylalanine enrichments (MPE) at 2 and 6 h following the ingestion of 20 g of intrinsically l-[1-13C]phenylalanine-labeled protein plus 40 g of carbohydrate in healthy young (n = 12) and older (n = 12) men. No significant differences were observed between groups
Mixed muscle protein fractional synthetic rates calculated over the entire 6-h post-prandial period, with the plasma tracer enrichments used as the precursor pool, did not differ between groups (0.048 ± 0.003 and 0.044 ± 0.005 % h−1 in the young and older group, respectively (P = 0.44)).
Discussion
In the present study, we observed no differences in the use of the dietary protein-derived amino acids for muscle protein synthesis following the combined ingestion of a meal-like amount of protein plus carbohydrate between young and older males. Similar post-prandial incorporation of dietary protein-derived amino acids in young and older subjects was observed during both the acute (2 h) and more prolonged (6 h) post-prandial period.
It has been hypothesized that the loss of muscle mass with aging is, at least partly, attributed to the reduced responsiveness of senescent muscle to the anabolic properties of food intake (Cuthbertson et al. 2005; Katsanos et al. 2005; Volpi et al. 2000). However, data on this topic are equivocal, and there are various reasons to suggest that anabolic resistance is not a characteristic of aging per se (Burd et al. 2012a, b). Data from our lab (Koopman et al. 2009b; Pennings et al. 2011b) as well others (Paddon-Jones et al. 2004; Symons et al. 2007, 2011) have failed to detect any age-related differences in the post-prandial muscle protein synthetic response to dietary protein ingestion. A classic work by Volpi et al. (2000) indicates that the proposed age-related blunting of the post-prandial muscle protein synthetic response to food intake may be more pronounced under conditions where both amino acids and carbohydrates are administered. Moreover, in everyday life, protein is generally consumed with carbohydrate. In general, a normal meal provides 15–30 energy% protein and 60 energy% carbohydrate. A meal typically consumed by a male adult generally contains about 20 g of protein (which would translate to approximately 100 g of steak or two eggs) and about 40 g of carbohydrate. Therefore, in the present study, we compare the muscle protein synthetic response following the ingestion of a single meal-like amount of protein plus carbohydrate between healthy young and older males.
Following ingestion of the dietary protein plus carbohydrate, a rapid rise in both plasma glucose and insulin concentrations in both the young and older subjects was observed (Figs. 1 and 2). The post-prandial rise in plasma glucose and insulin concentrations was more pronounced in the older subjects when compared with the young, which is in line with the reduced capacity for glucose tolerance in the older versus the younger subjects (Table1). In agreement, the older subjects showed higher blood HbA1C contents and lower OGIS values, without classifying as either being glucose intolerant or type 2 diabetic. Clearly, some level of compensatory hyperinsulinemia was observed in the older group to facilitate post-prandial plasma glucose and/or amino acid handling. A rapid rise in plasma amino acid concentrations was also observed following dietary protein ingestion (Fig. 2). Plasma phenylalanine (Fig. 2a) and leucine (Fig.2b) concentrations showed a rapid rise, and levels remained elevated throughout most of the 6-h post-prandial period. In the early post-prandial period, plasma amino acid levels and [1-13C]phenylalanine enrichments tended to be higher in the young versus the older group. This apparent delay in plasma amino acid concentrations and enrichments can be accounted for by either a delayed digestion and/or absorption rate (Boirie et al. 1997; Volpi et al. 1999; Koopman et al. 2009b; Pennings et al. 2011b), and/or a greater amino acid uptake secondary to the compensatory hyperinsulinemia (Hatzakorzian et al. 2011) (Fig.1b), and/or an attenuated inhibition of the post-prandial reduction in protein breakdown (Wilkes et al. 2009).
In the present study, we decided to provide casein protein, which likely provides a post-prandial response that is most representative of a mixed meal. Previous work from other labs (Tang et al. 2009) as well as ours (Pennings et al. 2011a) has shown that ingestion of whey protein results in a greater muscle protein synthetic response when compared with casein. Therefore, we expected that potential differences in the muscle protein synthetic response to protein ingestion between the young and older subjects would be most prominent after consuming casein as opposed to whey protein. However, even with casein as the protein source, we did not detect any impairments in the post-prandial muscle protein synthetic response in the elderly men (Fig.4).
Furthermore, we focussed on the metabolic fate of the ingested protein during the acute and more prolonged post-prandial phase. By using intrinsically l-[1-13C]phenylalanine-labeled protein, specifically produced by the infusion of large amounts of tracer in a lactating Holstein cow (Pennings et al. 2011c; van Loon et al. 2009b), we are able to detect the appearance of dietary protein-derived phenylalanine in the circulation (Fig.3) and assess its subsequent incorporation in skeletal muscle protein (Koopman et al. 2009a; Koopman et al. 2009b; Pennings et al. 2011a; Pennings et al. 2011b; Wall et al. 2012). Two hours after ingestion of the protein, skeletal muscle protein already showed a substantial increase in [1-13C]phenylalanine enrichment (0.0071 ± 0.0016 and 0.0072 ± 0.0013 MPE in the young and older group, respectively; P = 0.97). This clearly shows the rapid incorporation of dietary protein-derived amino acids into the muscle protein pool (Fig.4). Four hours later, the enrichment had increased further to 0.0229 ± 0.0016 and 0.0213 ± 0.0024 MPE, respectively, with no differences between groups (Fig.4). This is of particular interest as Drummond et al. presented data to support the idea that the muscle protein synthetic response to food intake may be delayed as opposed to reduced in the older population (Drummond et al. 2008). In the present study, there were no differences in the capacity of the older vs younger subjects to redirect the dietary protein-derived amino acids toward muscle protein synthesis during the acute as well as the more prolonged post-prandial period. When calculating the fractional muscle protein synthetic rate from the ingested protein, similar values were observed. Together, these results indicate that, when ingested together with carbohydrate, post-prandial incorporation of dietary protein-derived amino acids in skeletal muscle protein is neither delayed nor reduced in a healthy older population.
The novelty of the presented data lies in the observation that the post-prandial use of dietary protein-derived amino acids following ingestion of a meal-like amount of protein and carbohydrate does not differ between healthy young versus older men, despite their reduced muscle mass. Clearly, age does not necessarily impair the capacity to direct ingested protein towards muscle protein synthesis. Using this approach, we extend on a previous work from our lab (Koopman et al. 2009b; Pennings et al. 2011b) as well as from other labs (Paddon-Jones et al. 2004; Symons et al. 2007, 2011; Chevalier et al. 2011), further supporting the concept that anabolic resistance is not a characteristic of aging per se (Burd et al. 2012a). We speculate that impairments in the post-prandial muscle protein synthetic response are likely more evident in more compromised, frail, and sedentary elderly populations, especially during short periods of hospitalization, bed rest, and/or immobilization (Wall and van Loon 2012; Tieland et al. 2012; Burd et al. 2012a), as opposed to the healthy, independently living elderly included in the present study. The fact that there are no obvious impairments in the metabolic response to meal ingestion does not imply that we should not aim to develop effective strategies to further augment the post-prandial muscle protein synthetic response in the elderly population.
We conclude that healthy older men do not show impairments in the capacity to use dietary protein-derived amino acids for post-prandial muscle protein synthesis when ingested in a single meal-like bolus of dietary protein plus carbohydrate.
Footnotes
Alexandra Kiskini and Henrike M. Hamer contributed equally to this work.
References
- Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tessari P, Inchiostro S, Bruttomesso D, Fongher C, Sabadin L, Fratton MG, Valerio A, Tiengo A. Leucine and phenylalanine kinetics during mixed meal ingestion: a multiple tracer approach. Am J Physiol. 1992;262(4 Pt 1):E455–E463. doi: 10.1152/ajpendo.1992.262.4.E455. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65(2):489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- Burd NA, Wall BT, van Loon LJ. The curious case of anabolic resistance: old wives' tales or new fables? J Appl Physiol. 2012;112(7):1233–1235. doi: 10.1152/japplphysiol.01343.2011. [DOI] [PubMed] [Google Scholar]
- Burd NA, Wall BT, van Loon LJ. Last word on viewpoint: the curious case of anabolic resistance: old wives' tales or new fables? J Appl Physiol. 2012;112(7):1237. doi: 10.1152/japplphysiol.00109.2012. [DOI] [PubMed] [Google Scholar]
- Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 2011;66(6):681–688. doi: 10.1093/gerona/glr036. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ (1995) What is sarcopenia? The Journals of Gerontology Series A, Biological Sciences and Medical sciences 50 Spec No:5–8 [DOI] [PubMed]
- Hatzakorzian R, Carvalho G, Bui H, Sato T, Wykes L, Shum-Tim D, Schricker T. High-dose insulin administration is associated with hypoaminoacidemia during cardiac surgery. Metab Clin Exp. 2011;60(10):1392–1397. doi: 10.1016/j.metabol.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- Koopman R, Saris WH, Wagenmakers AJ, van Loon LJ. Nutritional interventions to promote post-exercise muscle protein synthesis. Sports Medicine. 2007;37(10):895–906. doi: 10.2165/00007256-200737100-00005. [DOI] [PubMed] [Google Scholar]
- Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288(4):E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139(9):1707–1713. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(Pt 1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81(5):953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Pennings B, Boirie Y, Senden JM, Gijsen AP, Saris WH, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than casein and casein hydrolysate in elderly men. Am J Clin Nutr. 2011;93(5):997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302(8):E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJ. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. Journal of Dairy Science. 2011;94(9):4366–4373. doi: 10.3168/jds.2011-4451. [DOI] [PubMed] [Google Scholar]
- Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86(2):451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. The Journal of Nutrition, Health & Aging. 2011;15(5):376–381. doi: 10.1007/s12603-010-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107(3):987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95(8):3848–3857. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–2771. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L, Boirie Y, Gijsen A, Fauquant J, de Roos A, Kies A, Lemosquet S, Saris W, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92(10):4812–4822. doi: 10.3168/jds.2009-2317. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92(10):4812–4822. doi: 10.3168/jds.2009-2317. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–4490. doi: 10.1210/jc.85.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Dirks ML, Verdijk LB, Snijders T, Hansen D, Vranckx P, Burd NA, Dendale P, van Loon LJ. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly, type 2 diabetic men. Am J Physiol Endocrinol Metab. 2012;303(5):E614–E623. doi: 10.1152/ajpendo.00138.2012. [DOI] [PubMed] [Google Scholar]
- Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev. 2012 doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- Waterval WA, Scheijen JL, Ortmans-Ploemen MM, Habets-van der Poel CD, Bierau J. Quantitative UPLC-MS/MS analysis of underivatised amino acids in body fluids is a reliable tool for the diagnosis and follow-up of patients with inborn errors of metabolism. Clin Chim Acta. 2009;407(1–2):36–42. doi: 10.1016/j.cca.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr. 2009;90(5):1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL (2004) Isotope tracers in metabolic research: principles and practice of kinetic analysis. John Wiley and Sons, Inc., New Jersey
- Zilversmit DB. The design and analysis of isotope experiments. Am J Med. 1960;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]