Abstract
The complex mixture of phytochemicals in fruits and vegetables provides protective health benefits, mainly through additive and/or synergistic effects. The presence of several bioactive compounds, such as polyphenols and caffeine, implicates coffee as a potential nutritional therapeutic in aging. Moderate (three to five cups a day) coffee consumption in humans is associated with a significant decrease in the risk of developing certain chronic diseases. However, the ability of coffee supplementation to improve cognitive function in aged individuals and the effect of the individual components in coffee, such as caffeine, have not been fully evaluated. We fed aged rats (19 months) one of five coffee-supplemented diets (0, 0.165, 0.275, 0.55, and 0.825 % of the diet) for 8 weeks prior to motor and cognitive behavior assessment. Aged rats supplemented with a 0.55 % coffee diet, equivalent to ten cups of coffee, performed better in psychomotor testing (rotarod) and in a working memory task (Morris water maze) compared to aged rats fed a control diet. A diet with 0.55 % coffee appeared to be optimal. The 0.165 % coffee-supplemented group (three cups) showed some improvement in reference memory performance in the Morris water maze. In a subsequent study, the effects of caffeine alone did not account for the performance improvements, showing that the neuroprotective benefits of coffee are not due to caffeine alone, but rather to other bioactive compounds in coffee. Therefore, coffee, in achievable amounts, may reduce both motor and cognitive deficits in aging.
Keywords: Antioxidant, Anti-inflammatory, Spatial learning and memory, Chlorogenic acid
Introduction
As the US population ages, increases in age-associated diseases will occur, and there is a high probability that this population will exhibit the most common correlative motor and cognitive behavioral changes that occur with aging. A growing body of literature supports the idea that nutrition contributes to healthy aging and plays a key role in determining neurological outcomes in aging. Recent epidemiological evidence suggests that coffee consumption reduces the risk of cognitive decline, dementia, and AD. Eskelinen et al. (2009) reported a 65 % risk reduction for late-life dementia and AD among drinkers of three to five cups of coffee per day during their middle life, compared with nondrinkers. The caffeine in coffee has been implicated as the active component associated with risk reduction of cognitive decline and dementia among coffee drinkers (Maia and de Mendonca 2002; van Gelder et al. 2007), particularly among women (Ritchie et al. 2007; Santos et al. 2010). However, coffee also contains a variety of other bioavailable and potentially therapeutic phytochemicals.
Like all plant-based foods and beverages, coffee is chemically complex, consisting of more than 1,000 different compounds. Among these are caffeine, the coffee-specific lipidic diterpenes cafestol and kahweol, and polyphenols, predominantly chlorogenic acids (Urgert et al. 1997; Herrmann 1989). Due to the presence of these phytochemicals, coffee has high antioxidant activity, as measured by the oxygen radical absorbance capacity assay (Chu et al. 2009). In fact, coffee is the primary source of dietary antioxidants in the American diet (Arendash et al. 2006). Because age-related deficits in cognitive, motor, and neuronal function arise, in part, as a result of an increasing inability of the aging organism to protect itself against inflammation and oxidative stress (Shukitt-Hale 1999; Shukitt-Hale et al. 2008), coffee may provide protection against these declines.
To date, long-term coffee consumption has not been thoroughly examined for its protective effect on cognition and motor behaviors. Furthermore, the overall contribution of individual components of coffee to the health benefits of coffee consumption has not been explored. In a series of studies using AD transgenic mice, Arendash and colleagues found that caffeine can protect against, and even reverse, memory loss and AD pathology (Arendash and Cao 2010). In addition, caffeine and/or caffeinated coffee (but not decaffeinated coffee) affected plasma levels of amyloid-beta in mice and humans with AD (Arendash et al. 2006; Arendash et al. 2009; Cao et al. 2009). They concluded that clinical trials are warranted to test the use of caffeine and caffeinated coffee as safe, inexpensive, and effective therapeutics against AD (Arendash and Cao 2010). In another study, four coffees with varying degrees of caffeine and pyroglutamate, an amino acid found in coffee known for its cognitive enhancing activities, were assessed for their effects on learning in mice (Maeso et al. 2006). One coffee that was high in pyroglutamate but low in caffeine produced a partial reversal of scopolamine-induced amnesia in the passive avoidance paradigm after oral administration, suggesting an additional mechanism of action for coffee.
Therefore, in the present studies, we assessed the neuroprotective benefits of coffee in rats. Study I examined the effects of graded dietary doses of coffee on cognitive and motor deficits in aged rats following 8 weeks of coffee-supplemented diets. Study II focused on the neuroprotective effects of caffeine independent of coffee and its other components. We chose not to use decaffeinated coffee for these studies because it contains 60 % less chlorogenic acids and 70 % less chlorogenic acid lactones than caffeinated coffee (Chu et al. 2009). In addition, in contrast to the results seen with caffeine and caffeinated coffee, decaffeinated coffee was previously unable to decrease plasma amyloid-beta when provided to AD mice (Arendash and Cao 2010).
Materials and methods
Animals
Male Fischer 344 rats (19 months old; n = 150), obtained from the NIA colony (Taconic Farms, Hudson, NY, USA), were used in two separate studies of 75 rats each. The rats were individually housed in stainless steel mesh suspended cages, provided food (NIH-31 diet) and water ad libitum, and maintained on a 12-h light/dark cycle. Following a 6-day acclimation period to the facility, the rats were weight-matched and randomly assigned to a diet group until euthanasia. The rats were fed one of the diets prior to experimental testing at 21 months of age. The rats were tested on a battery of motor tests during week 6 of the diet and on the Morris water maze (MWM) during weeks 7–8 of the diet (see below). The rats were killed during week 10 following behavioral testing, and serum and tissue were harvested. We measured food intake and body weight several times throughout the study. All rats were observed daily for clinical signs of disease.
In Study I, rats were assigned to one of five dietary groups (n = 15). Each group was provided access to a diet containing 0 (control), 0.165, 0.275, 0.55, or 0.825 % coffee extract. These doses are equivalent to 0, 3, 5, 10, and 15 cups/day, respectively, for humans (approximate body weight 75 kg), based on coffee solid/kilogram, assuming a daily dietary intake of 26 g/rat (average weight 425 g). In Study II, rats were assigned to one of five dietary groups (n = 15). Each group was provided access to a diet containing no supplements (control), 0.387 % coffee, 0.0181 % caffeine, 0.55 % coffee, or 0.0258 % caffeine. The coffee doses are equivalent to seven and ten cups of coffee per day, respectively, for humans, and the caffeine amounts are the equivalents of those found in these coffee doses. At the conclusion of Study I, there were 12 rats in the 0.165 % coffee group and 14 rats in all the other groups. At the conclusion of Study II, there were 10 rats in the control group, 14 in the 0.553 % coffee group, and 13 in all the other groups.
Animals were treated in compliance with all applicable laws and regulations as well as the principles expressed in the National Institutes of Health United States Public Health Service Guide for the Care and Use of Laboratory Animals. Both studies were approved by the Animal Care and Use Committee of the United States Department of Agriculture, Human Nutrition Research Center on Aging at Tufts University.
Diets
The diets were prepared at Harlan Teklad (Madison, WI, USA) by adding freeze-dried coffee powder or caffeine to the control diet, which was a modification of the NIH-31 diet (20 g/kg diet, 2 % w/w). The amount of corn in the control diet was adjusted to compensate for the added volume of the coffee powder or caffeine. The control NIH-31 diet was the same as that used in earlier studies in which other foods were found to be beneficial for mitigating brain aging (Youdim et al. 2000; Joseph et al. 2003; Casadesus et al. 2004; Goyarzu et al. 2004; Shukitt-Hale et al. 2005). The coffee powder, supplied by Kraft Foods Global, Inc. (Northfield, IL, USA), was prepared by lyophilizing brewed coffee. The pure caffeine was purchased from Sigma Aldrich (cat. #27600, St. Louis, MO, USA).
Psychomotor tests
A battery of age- and diet-sensitive tests of psychomotor behavior (Joseph et al. 1983; Shukitt-Hale et al. 1998; Joseph et al. 1999; Youdim et al. 2000; Shukitt-Hale et al. 2005; Ingram et al. 1994) was administered in a random order to the animals during week 6 of treatment. Each test was performed once, separated by a break between tasks. The rats were tested in random order, with the restriction that one rat from each diet group be tested in succession. Briefly, the tests included the following: (1) rod walking, which measures psychomotor coordination and the integrity of the vestibular system by requiring the animal to balance on a stationary, horizontal rod; (2) wire suspension, which measures muscle strength and the prehensile reflex or the animal’s ability to grasp a horizontal wire with its forepaws and remain suspended; (3) plank walking, which measures balance and coordination by exposing the animals to three different sizes of horizontal planks; (4) inclined screen, which measures muscle tone, strength, stamina, and balance by placing the animal on a wire mesh screen tilted 60° to the horizontal plane of the floor; and (5) accelerating rotarod, which measures fine motor coordination, balance, and resistance to fatigue by assessing the duration that the animal can remain standing/walking on a rotating, slowly accelerating rod. For a more detailed description of these tests, see Shukitt-Hale et al. (1998).
Cognitive testing
The MWM, an accepted method for testing spatial learning and memory, is an age- (Ingram et al. 1994; Brandeis et al. 1989; Shukitt-Hale et al. 1998) and diet-sensitive (Joseph et al. 1999; Youdim et al. 2000; Shukitt-Hale et al. 2005; Shukitt-Hale et al. 2009a) learning paradigm that requires the rat to use spatial cues to find a hidden platform (10 cm in diameter) submerged 2 cm below the surface of the water in a circular pool (134 cm in diameter × 50 cm in height; maintained at 23 °C) and remember its location from the previous trial. Accurate navigation is rewarded with escape from the water onto the platform, which the rat uses distal and spatial cues to efficiently locate. The working memory version of the MWM (Morris 1984; Brandeis et al. 1989) was performed daily for four consecutive days during weeks 7 and 8 of treatment, with a morning and an afternoon session, two trials per session, and a 10-min intertrial interval between the two trials. The platform was moved at the beginning of each session to one of four locations; these locations were chosen to frustrate a number of non-place learning strategies that rats may adopt (Whishaw 1985). The rats were tested in random order, with the restriction that one rat from each group be tested in succession. At the beginning of each Trial 1 (the reference memory or acquisition trial), the rat was gently immersed in the water at one of four random start locations. Each rat was allowed 120 s to escape onto the platform. If the rat failed to escape within this time, it was guided to the platform. After the rat reached the platform, it remained there for 15 s. The rat was returned to its home cage between trials (~10 min). Trial 2 (the working memory or retrieval trial) used the same platform location and start position as Trial 1. Performances were videotaped and analyzed with image tracking software (HVS Image, Hampton, UK), which allows measurements of latency to find the platform (s), path length (cm), and swimming speed (cm/s; path length/latency). For a more detailed description of the maze and the paradigm used, see Shukitt-Hale et al. (1998; Shukitt-Hale 1999).
Concentrations of caffeine and hydroxycinnamic acid metabolites in the brain and serum
Twelve days after behavioral testing, the rats were anesthetized with pentobarbital (100 mg/kg intraperitoneally). Blood was then excised from the heart, and the rats were perfused with 0.1 M phosphate-buffered saline. The brains were removed and sagittally bisected. From one half of the brain, hippocampal and cortical sections were dissected out and frozen at −80 °C. Concentrations of coffee constituents in the brain (caffeine, hippuric acid, and ferulic acid for rats in Study I in the control, three-cup, and ten-cup groups) and serum (caffeine, caffeic acid, hippuric acid, and ferulic acid for all rats in Study I) were then measured with liquid chromatography coupled with tandem mass spectrometry. The analytes were detected using an Applied Biosystems API-4000 Qtrap mass spectrometer equipped with a Turbo-Ion Spray (Electrospray, ESI) source operating in multiple reaction monitoring mode under negative ion mode. Briefly, the brain samples were homogenized in a 1:1 (v/v) MeOH/water solution at a ratio of 30 mg tissue to 1.0 mL solvent. Serum or an aliquot of 40 μL of brain homogenate was extracted with acetonitrile and methanol, vortexed, and filtered with 96-well filter plates. The filtrate was subjected to reversed-phase liquid chromatography using a Develosil 3u Combi-RP C30 140A, 50 × 4.6-mm analytical column at a flow rate of 0.6 mL/min with an analysis time of 6 min. For a more detailed description of the chromatography methods used, see Ptolemy et al. (2010).
Statistical analyses
For each measure, between-subjects analysis of variance (ANOVA) models comparing the diet groups were performed using Systat (SPSS, Inc., Chicago, IL, USA) to test for statistical significance at a level of p < 0.05. Days or trials, when appropriate, were included in the model as a within-subjects variable. To determine differences between the diet groups, post hoc comparisons were performed using Fisher’s least significant difference (LSD) post hoc analysis. To analyze working memory, separate t tests were conducted for each group between the Trial 1 and Trial 2 latencies. Correlations between behavior and brain/serum measures were carried out using Pearson’s r correlation.
Results
Study I
There were no differences in weight between any of the groups at any time during the course of the study (p > 0.05). Average weights (±standard error of the mean (SEM)) for the groups were as follows: control diet, 456.1 ± 9.4 g; 0.165 % coffee, 470.0 ± 7.4 g; 0.275 % coffee, 462.3 ± 8.6 g; 0.55 % coffee, 465.7 ± 7.0 g; and 0.825 % coffee, 469.2 ± 5.4 g. There were also no differences in food intake among the groups over the course of the study (p > 0.05): control diet, 23.10 ± 0.58 g; 0.165 % coffee, 23.25 ± 0.49 g; 0.275 % coffee, 25.12 ± 1.02 g; 0.55 % coffee, 24.39 ± 0.73 g; and 0.825 % coffee, 24.35 ± 0.41 g.
Motor testing showed that the group fed 0.55 % coffee (equivalent to ten cups) performed significantly better (p < 0.05) on the rotarod test compared to the control rats (Fig. 1). The 0.55 % coffee group also stayed on the rotarod longer than the rats in the other coffee groups (p < 0.05), with the exception of the 0.165 % coffee group (three cups), which was not different from the 0.55 % coffee or control group (Fig. 1). There were no differences (p > 0.05) between any of the coffee groups and the control rats on any of the other motor tests, meaning that coffee-fed rats did not show improvement on the remaining motor tasks when compared to controls.
Fig. 1.
Latency to fall (mean ± SEM, seconds) in the rotarod test for the control and coffee groups. Means with different letters are significantly different from each other (p < 0.05; Fisher’s LSD)
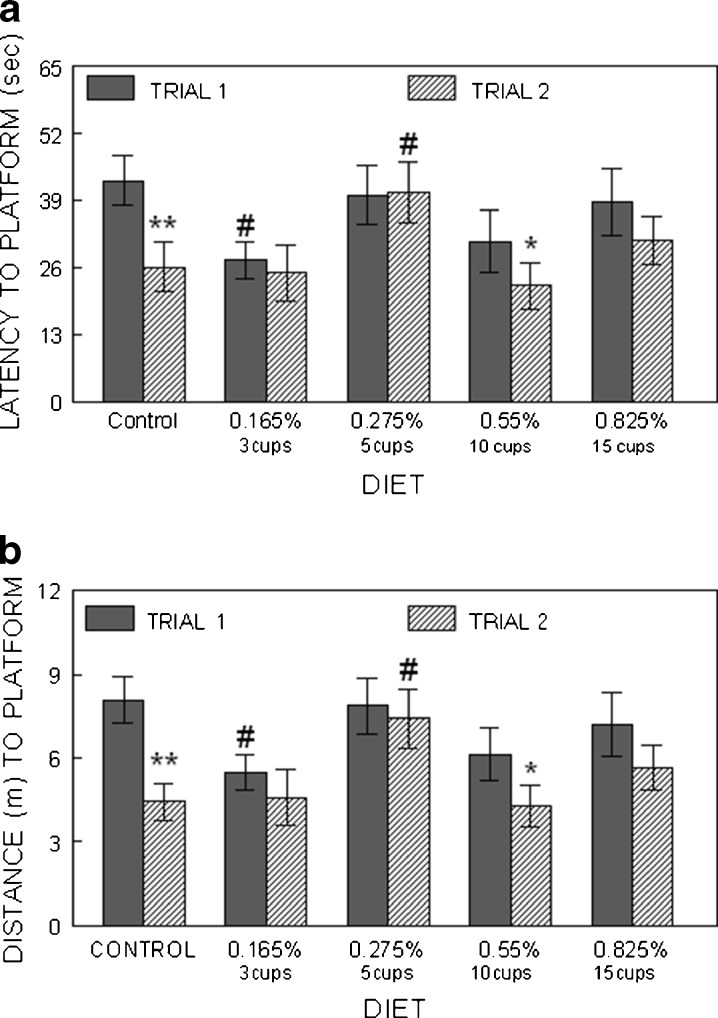
To examine cognitive performance, we performed separate t tests between the two trial latencies for each group to see if the different coffee groups showed significant improvement in their performance from Trial 1 to Trial 2, which would indicate improved working memory. We found that when rats were tested on days 3 and 4, both the control and the 0.55 % coffee groups showed significant (p < 0.05) differences between Trial 1 and Trial 2 (i.e., Trial 2 latencies were significantly less than Trial 1), showing that these rats demonstrated one-trial learning, even with the 10-min retention interval (Fig. 2a). However, a between-subjects ANOVA showed that Trial 1 performance in the control rats was significantly (p < 0.05) worse than that of the 0.165 % coffee (equivalent to three cups) group, but neither was different from the 0.55 % group. In addition, Trial 2 performance in the 0.275 % group (equivalent to five cups) was significantly (p < 0.05) worse than that of the control group and all other coffee groups, except for the highest coffee dose, 0.825 % (15 cups). Data were similar for the distance parameter (Fig. 2b). These differences were not due to swimming speed as there were no differences between the groups in that parameter (data not shown).
Fig. 2.
Morris water maze performance assessed as latency in seconds (a; mean ± SEM) and distance in meters (b) to find the hidden platform on days 3 and 4 of testing animals in the control and coffee groups. The asterisks indicate a difference (i.e., an improvement) between Trial 1 and Trial 2 performances (one asterisk indicates p < 0.05, two asterisks p < 0.01), indicating improved working memory. Number sign indicates p < 0.05, compared to control
There were no changes in cortical levels of ferulic acid, hippuric acid, or caffeine between the control, 0.165 % coffee, and 0.55 % coffee groups (data not shown). There were increases in serum levels of caffeic acid, hippuric acid, and caffeine with increasing coffee consumption; serum levels of ferulic acid were not significantly different (Table 1). However, there were no significant correlations between serum or cortical levels of caffeine or these metabolites and cognitive performance.
Table 1.
The effect of dietary supplementation with coffee on serum levels of caffeine and hydroxycinnamic acid metabolites in aged rats
| Percentage of coffee in diet (human equivalent in cups) | Serum level (ng/mL) | |||
|---|---|---|---|---|
| Caffeine | Caffeic acid | Ferulic acid | Hippuric acid | |
| 0.000 (0) | 0.173 ± 0.03a | 0.00 ± 0.00a | 1.04 ± 0.79a | 94.84 ± 23.07a |
| 0.165 (3) | 0.274 ± 0.10a | 0.00 ± 0.00a | 13.77 ± 9.34a | 267.28 ± 102.11a,b |
| 0.275 (5) | 0.122 ± 0.03a | 0.34 ± 0.34a | 16.62 ± 12.06a | 361.53 ± 95.05b,c |
| 0.550 (10) | 14.55 ± 8.30a | 3.64 ± 1.14b | 3.20 ± 0.73a | 502.20 ± 97.94b,c |
| 0.825 (15) | 112.44 ± 56.63b | 8.16 ± 1.77c | 6.13 ± 2.77a | 552.58 ± 93.32c |
Data are presented as means ± SEMs. Values in each column with different superscript letters are significantly different (Fisher’s LSD; p < 0.05)
Study II
There were no differences in weight between any of the groups at any time during the course of the study (p > 0.05). Average weights (±SEM) for the groups were as follows: control diet, 474.0 ± 9.1 g; 0.55 % coffee, 477.9 ± 7.8 g; 0.0258 % caffeine, 482.3 ± 7.1 g; 0.387 % coffee, 478.4 ± 9.5 g; and 0.0181 % caffeine, 482.4 ± 9.0 g. There were also no differences in food intake among the groups over the course of the study (p > 0.05): control diet, 20.37 ± 0.39 g; 0.55 % coffee, 21.50 ± 0.36 g; 0.0258 % caffeine, 21.62 ± 0.44 g; 0.387 % coffee, 20.46 ± 0.66 g; and 0.0181 % caffeine, 21.17 ± 0.89 g.
Results for the motor testing showed that the group fed 0.553 % coffee (equivalent to ten cups) performed significantly better (p < 0.05) on the inclined screen test compared to the control rats (Fig. 3). In addition, the group that received 0.0181 % caffeine, the amount in seven cups of coffee, remained on the inclined screen significantly longer (p < 0.05) than the control rats and the group that received 0.0258 % caffeine (the amount in ten cups of coffee; Fig. 3). There were no additional coffee or caffeine improvements seen in any of the other motor tasks.
Fig. 3.
Latency to fall (mean ± SEM, seconds) in the inclined screen test for the control, coffee, and caffeine groups. Means with different letters are significantly different from each other (p < 0.05; Fisher’s LSD)
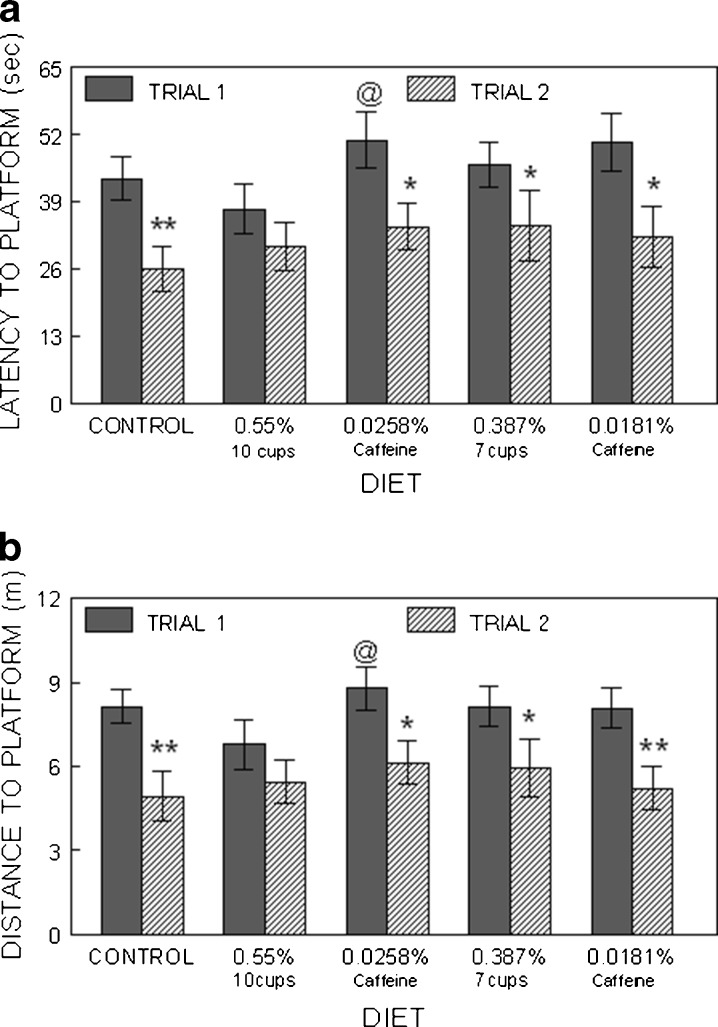
To examine cognitive performance, we performed separate t tests between the two trial latencies for each group to see if the different coffee and caffeine groups significantly improved their performance from Trial 1 to Trial 2, which would indicate improved working memory. When rats were tested on days 3 and 4, all groups except the 0.55 % coffee group showed significant differences (p < 0.05) between Trial 1 and Trial 2, i.e., Trial 2 latencies were significantly less than Trial 1, showing that these rats demonstrated one-trial learning, even with the 10-min retention interval (Fig. 4a). However, a between-subjects ANOVA showed that Trial 1 performance in the 0.55 % coffee group (equivalent to ten cups of coffee) was significantly better than that of the 0.0258 % caffeine group and tended to be better than the 0.0181 % (p = 0.06) caffeine group, but was not different from the control group. In addition, Trial 2 performance in the two caffeine groups was worse than that of the control group when rats were examined on days 1–4 (data not shown). Data were similar for the distance parameter (Fig. 4b), except that Trial 1 performance in the 0.0258 % caffeine group tended to be worse (p = 0.06) than the 0.55 % coffee group, whereas the 0.0181 % caffeine group showed some improvement and was not different than the other groups. These differences were not due to swimming speed as there were no differences between the groups in this parameter (data not shown).
Fig. 4.
Morris water maze performance assessed as latency in seconds (a; mean ± SEM) and distance in meters (b) to find the hidden platform on days 3 and 4 of testing animals in the control, coffee, and caffeine groups. The asterisks indicate a difference (i.e., an improvement) between Trial 1 and Trial 2 performances (one asterisk indicates p < 0.05, two asterisks p < 0.01), indicating improved working memory. Commercial at indicates p < 0.05 compared to the ten-cup group
Discussion
These results show that coffee, in easily achievable amounts, may reduce both motor and cognitive deficits in aging, similar to prior studies with berries (Joseph et al. 1999; Youdim et al. 2000; Shukitt-Hale et al. 2005; Shukitt-Hale et al. 2009a), nuts (Willis et al. 2009), and other fruits and vegetables (Shukitt-Hale et al. 2006; Shukitt-Hale et al. 2009b; Joseph et al. 1999). Furthermore, the neuroprotective benefits seen with coffee in these studies appear to be not due to caffeine alone but instead to the polyphenols and other bioactive compounds in coffee. These compounds may complement or synergize with caffeine to produce their beneficial actions (Cao et al. 2011). Furthermore, because dietary polyphenols such as chlorogenic and caffeic acid in coffee have antioxidant (Higdon and Frei 2006) and anti-inflammatory (Kempf et al. 2010) properties, they may function independently of caffeine to produce positive effects on motor and memory function during aging.
In these studies, diets supplemented with the equivalent of ten cups of coffee improved performance in aged rats, compared to age-matched control rats, on motor tests that rely on balance and fine motor coordination, including the rotarod and the inclined screen. Interestingly, inclined screen performance was also enhanced in the caffeine group equivalent to seven cups of coffee, and this was the only group in which performance was enhanced by caffeine alone in these studies. When measuring cognitive performance, caffeine equivalent to that in seven or ten cups of coffee impaired reference memory (which contributed to the improvement seen in working memory), but no impairment was seen at the highest dose of coffee (0.825 %, equivalent to 15 cups a day). These results are in contrast to those of other studies that found that caffeine improves measures of learning and memory in AD transgenic mice (Arendash et al. 2006; Arendash et al. 2009). However, when normal wild-type mice were treated long-term with caffeine from adulthood through old age, no cognitive benefit was found (Arendash et al. 2009). Given that the rats used in our current study do not exhibit AD pathology but were rather a model of normal aging, this previous result is more in agreement with that of our current study in that it is not the caffeine in coffee that contributes to the improvements observed in performance. However, another study did show that 1 mg/mL of caffeine administered to male albino CF1 mice during adulthood for 12 months prevented age-associated decline in short-term recognition memory (Costa et al. 2008), while Prediger and colleagues found that age-related deficits in olfactory discrimination and social recognition memory in rats were reversed by acute administration of caffeine (10.0 or 30.0 mg/kg, i.p.), although this was just a single injection (Prediger et al. 2005). Interestingly, one consensus point of a recent meeting was that caffeine seems particularly effective to normalize, rather than bolster, memory performance (de Mendonca and Cunha 2010b; Cunha and Agostinho 2010). In addition, de Mendonca and Cunha also stress that evidence from some, but not all, epidemiological studies may suggest a beneficial effect of moderate doses of caffeine but a possible deleterious effect of larger doses (de Mendonca and Cunha 2010a).
Further evidence that coffee is superior to caffeine alone in providing beneficial effects is a study that showed that coffee increased plasma levels of several beneficial cytokines (Cao et al. 2011). Acute intraperitoneal treatment with caffeinated coffee, but not decaffeinated coffee or caffeine alone, increased plasma levels of granulocyte colony-stimulating factor (GCSF), interleukin-10, and interleukin-6 (Cao et al. 2011). Only the increase in plasma GCSF was correlated with enhanced cognitive performance following long-term treatment with either coffee or decaffeinated coffee. The authors hypothesize that another coffee component synergizes with caffeine to enhance plasma GCSF levels, resulting in the multiple therapeutic actions of coffee against AD (Cao et al. 2011). However, it is possible that this/these component(s) in coffee are lost during the decaffeination process, similar to chlorogenic acid, which is reduced by as much as 60 % in decaffeinated coffee Chu et al. (2009). Additional studies should be conducted before concluding that caffeine is the critical, active ingredient in coffee.
In our current study, coffee improved performance on measures of spatial working memory. Specifically, coffee at 0.165 % of the diet (three-cup equivalent) improved measures of reference or long-term memory, whereas 0.387 % coffee (seven cups) and 0.55 % coffee (ten cups) improved working or short-term memory. However, as seen in another study (Cao et al. 2009), caffeine levels in the blood were not significantly correlated with cognitive performance. Although control animals also showed improved working memory in the MWM, their Trial 1 performance was worse than that of the coffee-supplemented rats. This impairment may have accounted for the improvement in working memory in this group. It is possible that the control group relied on other methods to solve the MWM rather than on spatial memory navigation, whereas the coffee groups used spatial strategies to solve the maze.
Other studies have also shown positive effects of coffee on cognitive function in animals (Abreu et al. 2011) and humans (Cropley et al. 2012). Rats fed a diet supplemented with brewed coffee (3 or 6 %) or caffeine (0.04 or 0.08 %) for 7 weeks showed improved long-term memory in the object recognition test (Abreu et al. 2011). Chronic coffee and caffeine intake also modulates the antioxidant system in the rat brain by reducing lipid peroxidation of membranes, increasing glutathione, and increasing the activity of two antioxidant enzymes, glutathione reductase and superoxide dismutase (Abreu et al. 2011). Modulation of oxidative stress may be one mechanism by which coffee improved cognitive function in our current study as older rats show declines in antioxidant defenses in the brain and are more vulnerable to increased oxidative stress (Shukitt-Hale 1999).
The coffee doses used in this study are equivalent to human consumption of 0, 3, 5, 10, and 15 cups. Calculation of consumption in relation to humans was based on the equivalency of solid coffee per kilogram of body weight per day (1 cup = 0.034 g/kg/day), without adjusting for metabolic rate. If the metabolic rate (MR) is considered (MR = Mass−1/4), a factor of 3.6 would be applied to the dosing when comparing humans and rats. Thus, the coffee doses used in this study, normalized for the metabolic rate, would be equivalent to human consumption of 0, 0.2, 0.8, 1.4, 2.7, and 4.1 cups.
In summary, coffee, a beverage consumed worldwide, is a complex mixture containing not only caffeine but various bioavailable polyphenols such as chlorogenic acid that may act via numerous mechanisms to produce the beneficial effects seen in motor and cognitive behavior (Cho et al. 2009; Cropley et al. 2012). Coffee components have been shown to have antioxidant and anti-inflammatory properties, and they act directly on neuronal survival signaling cascades within the aging brain to improve behavior (Miller and Shukitt-Hale 2012). It has been well established that complex mixtures of polyphenolic phytochemicals in fruits and vegetables can provide protective health benefits through a combination of additive and/or synergistic effects. Given its global appeal, moderate coffee consumption may provide neurological benefits through similar mechanisms.
Acknowledgments
This study was supported in part by Kraft Foods, Inc. The United States Department of Agriculture is an equal opportunity provider and employer.
Footnotes
In memory of James A. Joseph, our valued colleague and friend, who passed away while this paper was being written.
References
- Abreu RV, Silva-Oliveira EM, Moraes MF, Pereira GS, Moraes-Santos T. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacol Biochem Behav. 2011;99(4):659–664. doi: 10.1016/j.pbb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 1):S117–126. doi: 10.3233/JAD-2010-091249. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimers Dis. 2009;17(3):661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142(4):941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Intern J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash GW, Holtzman DM, Potter H. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer’s disease transgenic mice. J Alzheimers Dis. 2009;17(3):681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Wang L, Lin X, Mamcarz M, Zhang C, Bai G, Nong J, Sussman S, Arendash G. Caffeine synergizes with another coffee component to increase plasma GCSF: linkage to cognitive benefits in Alzheimer’s mice. J Alzheimers Dis. 2011;25(2):323–335. doi: 10.3233/JAD-2011-110110. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7(5–6):309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- Cho ES, Jang YJ, Hwang MK, Kang NJ, Lee KW, Lee HJ. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat Res. 2009;661(1–2):18–24. doi: 10.1016/j.mrfmmm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Chu YF, Brown PH, Lyle BJ, Chen Y, Black RM, Williams CE, Lin YC, Hsu CW, Cheng IH. Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. J Agric Food Chem. 2009;57(20):9801–9808. doi: 10.1021/jf902095z. [DOI] [PubMed] [Google Scholar]
- Costa MS, Botton PH, Mioranzza S, Souza DO, Porciuncula LO. Caffeine prevents age-associated recognition memory decline and changes brain-derived neurotrophic factor and tyrosine kinase receptor (TrkB) content in mice. Neuroscience. 2008;153(4):1071–1078. doi: 10.1016/j.neuroscience.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Cropley V, Croft R, Silber B, Neale C, Scholey A, Stough C, Schmitt J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl) 2012;219(3):737–749. doi: 10.1007/s00213-011-2395-0. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis. 2010;20(Suppl 1):S95–116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Cunha RA. Putative neuroprotective effects of caffeine in clinical trials. Concluding remarks. J Alzheimers Dis. 2010;20(Suppl 1):S249–252. doi: 10.3233/JAD-2010-01411. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Cunha RA. Therapeutic opportunities for caffeine in Alzheimer’s disease and other neurodegenerative disorders. J Alzheimers Dis. 2010;20(Suppl 1):S1–2. doi: 10.3233/JAD-2010-01420. [DOI] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7(2):75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- Herrmann K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit Rev Food Sci Nutr. 1989;28(4):315–347. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46(2):101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Jucker M, Spangler E. Behavioral manifestations of aging. In: Mohr U, Cungworth DL, Capen CC, editors. Pathobiology of the aging rat. Washington: ILSI; 1994. pp. 149–170. [Google Scholar]
- Joseph JA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6(3):153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Bartus RT, Clody D, Morgan D, Finch C, Beer B, Sesack S. Psychomotor performance in the senescent rodent: reduction of deficits via striatal dopamine receptor up-regulation. Neurobiol Aging. 1983;4(4):313–319. doi: 10.1016/0197-4580(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19(18):8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Tuomilehto J. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91(4):950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- Maeso N, del Castillo C, Cornejo L, Garcia-Acicollar M, Alguacil LF, Barbas C. Capillary electrophoresis for caffeine and pyroglutamate determination in coffees study of the in vivo effect on learning and locomotor activity in mice. J Pharm Biomed Anal. 2006;41(4):1095–1100. doi: 10.1016/j.jpba.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9(4):377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- Miller MG, Shukitt-Hale B. Coffee and Alzheimer’s disease—animal and cellular evidences. In: Chu YF, editor. Coffee: emerging health effects and disease prevention. Oxford: Wiley-Blackwell; 2012. pp. 77–96. [Google Scholar]
- Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J NeurosciMeth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26(6):957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Ptolemy AS, Tzioumis E, Thomke A, Rifai S, Kellogg M. Quantification of theobromine and caffeine in saliva, plasma and urine via liquid chromatography-tandem mass spectrometry: a single analytical protocol applicable to cocoa intervention studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(3–4):409–416. doi: 10.1016/j.jchromb.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69(6):536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- Santos C, Lunet N, Azevedo A, de Mendonca A, Ritchie K, Barros H. Caffeine intake is associated with a lower risk of cognitive decline: a cohort study from Portugal. J Alzheimers Dis. 2010;20(Suppl 1):S175–185. doi: 10.3233/JAD-2010-091303. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B. The effects of aging and oxidative stress on psychomotor and cognitive behavior. Age. 1999;22:9–17. doi: 10.1007/s11357-999-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22(3):295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12(3):135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Galli R, Meterko V, Carey A, Bielinski D, McGhie T, Joseph JA. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. AGE. 2005;27:49–57. doi: 10.1007/s11357-005-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Kalt W, Carey AN, Vinqvist-Tymchuk M, McDonald J, Joseph JA. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition. 2009;25(5):567–573. doi: 10.1016/j.nut.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Lau FC, Joseph JA. Berry fruit supplementation and the aging brain. J Agric Food Chem. 2008;56(3):636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33(6):615–624. doi: 10.1016/S0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Urgert R, Essed N, van der Weg G, Kosmeijer-Schuil TG, Katan MB. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. Am J Clin Nutr. 1997;65(2):519–524. doi: 10.1093/ajcn/65.2.519. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61(2):226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. PhysiolBehav. 1985;35:139–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Willis LM, Shukitt-Hale B, Cheng V, Joseph JA. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br J Nutr. 2009;101:1140–1144. doi: 10.1017/S0007114508059369. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova N, Bickford PC, Joseph JA. Short-term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000;3:383–397. [Google Scholar]