Abstract
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase implicated in several age-related biological mechanisms such as telomere shortening and cell senescence. We tested the hypothesis that ADMA blood level is an independent predictor of mortality in elderly. This is a longitudinal population-based cohort study. Participants are a representative cohort of 1,025 men and women (age range 65–102 years) living in Chianti area, Tuscany, Italy. The plasma ADMA was measured by liquid chromatography–tandem mass spectrometry. During the follow-up (95 ± 32 months), 384 individuals died, of whom 141 (37 %) died of cardiovascular (CV) causes. In adjusted analyses, the plasma ADMA was the strongest predictor of all-cause mortality (HR (0.1 μMol/L) 1.26, 95 % CI 1.10–1.44, P < 0.001) with a non-significant trend for CV mortality (HR 1.22, P = 0.07). The predictive effect of the ADMA level on mortality was statistically significant among participants with low to low-normal l-arginine levels (≤60 μMol/L), but not in those with l-arginine >60 μMol/L. Notwithstanding the association of ADMA with all-cause mortality was robust, this biomarker failed to add predictive power to a simple model based on the risk factors in the elderly (area under the ROC curve 0.85 ± 0.01 vs. 0.84 ± 0.01). ADMA is a strong independent predictor of mortality in the older population, and l-arginine modifies the effect of ADMA on survival. The mechanisms for this association should be targeted by future studies.
Keywords: ADMA, Elderly, Cardiovascular risk factor, Survival, Population study
Introduction
The power of classical factors to accurately predict the risk of death and clinical outcomes diminishes with advancing age (Kannel 2002; de Ruijter et al. 2009), a phenomenon which may depend on the fact that measurement of these factors at a late stage of life may not reflect the actual lifetime exposure to the same factors and/or that elderly people represent a highly selected population with a risk factor profile different from that of the general population (Mukamal et al. 2004). Because the peculiar epidemiologic characteristic of the elderly population, identification of risk factors specific to this population, is fundamental to put in place effective preventive strategies at this late stage of life. Furthermore, alternative risk markers are required for risk stratification in the very old.
Over the last three decades, major efforts have been made to identify new risk factors for death and cardiovascular (CV) disease, particularly inflammation markers (Helfand et al. 2009; de Ruijter et al. 2009; Mukamal et al. 2004); however, the vast majority of these studies focused almost exclusively in middle age populations, and information on risk factors in the elderly and very old is more limited.
The problem is of relevance because recent data indicate that in very old people, novel biomarkers like plasma homocysteine may largely outperform classical risk markers assessed by the Framingham score in the prediction of CV death (de Ruijter et al. 2009). The endogenous inhibitor of nitric oxide synthase, asymmetric dimethylarginine (ADMA), is one of the most investigated novel risk factors both in the general population and in various disease states (Zoccali 2006; Boger et al. 2009a; Brenner et al. 2012). Pre-clinical studies suggest that ADMA may be implicated in biological processes relevant to aging, such as telomerase activity, endothelial senescence (Bode-Boger et al. 2005), and endothelial dysfunction (Perticone et al. 2010; Juonala et al. 2007). In the Framingham offspring study, a community-based study on middle-aged individuals, higher ADMA was a strong predictor of death (Boger et al. 2009b). Similarly, studies have found that ADMA was a strong predictor of cardiovascular events and mortality in patient groups affected by a variety of pathological conditions from chronic kidney disease to coronary heart disease, peripheral vascular disease, and diabetes (Zoccali 2006; Boger et al. 2009a). Observational studies have reported that plasma ADMA levels rise with age (Boger et al. 2009b; Schulze et al. 2005). However, no major population-based cohort study addressed the hypothesis that ADMA level is an important risk factor for all-cause and cardiovascular mortality in the older population. We investigated the predictive power of ADMA for all-cause and cardiovascular mortality using data from the Invecchiare in Chianti study cohort which enrolled a random sample of people older than 65 years in two towns of Tuscany, Italy.
Methods
Study design and population
The “Invecchiare in Chianti Study” (InCHIANTI; aging in the Chianti area) is a longitudinal population-based study of people living in Greve in Chianti and Bagno a Ripoli in Tuscany Region of Italy (http://www.inchiantistudy.net/bindex.html). This study was planned by the Laboratory of Clinical Epidemiology of the Italian National Research Council on Aging (INRCA, Florence, Italy) in collaboration with the Laboratory of Demography and Biometry at the National Institute on Aging. The rationale, design, and data collection of the InCHIANTI study have been described elsewhere (Ferrucci et al. 2000). Briefly, the elderly InCHIANTI population consisted of 1,155 participants aged 65 to 102 randomly selected using a multistage stratified sampling method. Once enrolled, the subjects underwent an extensive baseline data set collection between September 1998 and March 2000; thereafter, the subjects were fully evaluated every 3 years, and the follow-up is still in progress. The INRCA ethics committee approved the InCHIANTI Study protocol which met the criteria outlined in the Declaration of Helsinki. The participants in the study provided written informed consent.
For the present study, we excluded 130 participants because of missing plasma samples. Hence, we analyzed 1,025 subjects out of the 1,155 original cohort. The primary study outcomes were all-cause and cardiovascular mortality during follow-up from the baseline examination through December 2008. Information about the designated outcomes was obtained from the Mortality General Registry maintained by the Tuscany Region and the death certificates of the Municipality of residence. We classified the cardiovascular deaths with ICD-9-CM codes from 410 to 438.
Comorbidity and other variables
All participants were examined by an experienced clinician. Diseases were ascertained according to pre-established criteria that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations, and previous blood tests.
Smoking history was determined as self-reported and dichotomized in the analysis as “current smoking” versus “ever smoked” and “never smoked”. Weight and height were measured with participants wearing light clothes and no shoes and were used to compute the body mass index (BMI). Blood pressure was first assessed on both arms with the patient supine for at least 5 min. Then, measurements were repeated twice on the arm with the highest value of systolic blood pressure, and the mean of these two values was used to define the systolic and diastolic blood pressure.
Laboratory methods
Blood samples were obtained after a 12-h fast and after the participants had been resting for at least 15 min. The participants received detailed instructions for 24-h urine collection, including advice to take note of start and end time. Subjects with incomplete times of collection were excluded. Aliquots of serum and 24-h urine samples were stored at −80 °C and were not thawed until analyzed.
Plasma concentrations of ADMA and l-arginine were measured by tandem liquid chromatography tandem mass spectrometry MS/MS (Schwedhelm et al. 2007). Serum and urine creatinine were measured using a compensated modified Jaffe method. Renal function was assessed by creatinine clearance (CrCl) calculated according to the formula: 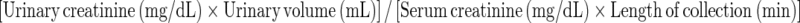 .
.
Urinary albumin excretion was assessed in an early morning spot sample by an automated urine test strip analyser (Aution Max AX-4280, A. Menarini Diagnostics, Florence, Italy) with a minimum detecting sensitivity of 5 mg/dL. Serum high sensitivity C-reactive protein (CRP) was measured in duplicate using an enzyme-linked immunosorbent assay (ELISA) and colorimetric competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies; the minimum detectable threshold was 0.03 mg/L, and the inter-assay coefficient of variation was 5 %.
Plasma homocysteine concentration was measured by a fluorometric polarized immunoassay method (IMX, Abbott Laboratories) and insulin by a commercially available radioimmunoassay kit (Sorin Biomedical, Milan, Italy). Homeostasis model assessment of insulin resistance (HOMA index) was calculated with the formula: 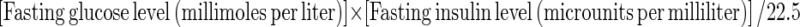 Other variables, cholesterol, uric acid, and Hb were determined by standard methods.
Other variables, cholesterol, uric acid, and Hb were determined by standard methods.
Statistical analysis
The data are expressed as mean ± standard deviation, median, and inter-quartile range or as percent frequency, as appropriate. In comparing the continuous variables across age quartiles, we used one-way ANOVA. We specifically looked at the weighted P value (P for trend) which provides the statistical significance of the underlying, if any, linear trend between the variables and age quartiles. For binary variables, the comparisons were done by a standard chi-square for linear association. The correlation analyses between ADMA and continuous variables were performed by calculating the Pearson product moment correlation coefficient, and the association between ADMA and binary variables was carried out by calculating the point bi-serial correlation coefficient.
The relationship between ADMA and all-cause and CV mortality was investigated by Kaplan–Meier analysis and by Cox regression analyses. The initial predictive model included plasma ADMA alone, and adjusted models included other covariates. Age, gender, smoking, diabetes, serum glucose and HOMA index, total cholesterol, systolic and diastolic blood pressure, and treatment with anti-hypertensive drugs, BMI, heart rate, calcium, hemoglobin, CV comorbidities, homocysteine, creatinine clearance, albuminuria, CRP and l-arginine were considered as potential covariates. In the final Cox regression model, we introduced plasma ADMA as well as variables that met the criteria to be confounders (Tripepi et al. 2008), namely variables (a) correlated with both exposure (plasma ADMA) and the study outcomes with a P < 0.20, (b) which were not an effect of exposure, and (c) not in the causal pathway between the exposure and the study outcome. The effect modification of plasma l-arginine levels on the relationship between plasma ADMA and study outcomes (all-cause and CV mortality) was investigated by adding into the multiple Cox regression models a multiplicative term of these two variables (ADMA × l-arginine). The hazard ratios of ADMA (0.1 μMol/L increase) across the whole range of plasma l-arginine levels were calculated by the linear combination method (see “Appendix”). By this approach, we constructed a model of adequate statistical power (at least 13 events for each variable into the final models). A P value <0.05 was considered as statistically significant. The additional prognostic value of ADMA beyond and above that provided by the risk model in the elderly included in this study for predicting mortality was evaluated by calculating the area under the ROC curve (AUC, a measure of discrimination) and by assessing the net reclassification index (NRI). The proportionality assumption was tested by the analysis of Schoenfeld residuals, and no violation was found. The data are expressed as hazard ratios (HR), 95 % confidence intervals (CI), and P values. All calculations were made using standard statistical packages (SPSS for Windows Version 19, Chicago, Illinois, US and STATA 9, Stata Corp, LP, TX, US).
Results
The main demographic and clinical characteristics of the study subjects as grouped according to age quartiles are summarized in Table 1. Individuals in the fourth age quartile (age >79 years) had higher ADMA, systolic pressure, plasma total homocysteine, CRP, and l-arginine, and lower BMI, calcium, hemoglobin, total cholesterol, HOMA index, and creatinine clearance as compared to those in the remaining age quartiles (Table 1). The proportion of males and smokers decreased from the first age quartile onward, whereas the frequency of individuals with detectable albuminuria, CV comorbidities, and on treatment with anti-hypertensive drugs increased in parallel with age (Table 1).
Table 1.
Demographic, somatometric, and clinical data of study subjects grouped according to age quartiles
| Whole group (n = 1,025) | Age quartiles | P for trend | ||||
|---|---|---|---|---|---|---|
| First (<69 years) (n = 288) | Second (69–74 years) (n = 270) | Third (74–79 years) (n = 217) | Fourth (>79 years) (n = 250) | |||
| Age (years) | 75 ± 7 | 67 ± 1 | 72 ± 1 | 77 ± 1 | 86 ± 4 | <0.001 |
| BMI (kg/m2) | 27.5 ± 3.9 | 28 ± 4 | 28 ± 4 | 27 ± 4 | 26 ± 4 | <0.001 |
| Male sex, n (%) | 452 (44) | 137 (48) | 129 (48) | 96 (44) | 90 (36) | 0.01 |
| Smokers, n (%) | 419 (41) | 132 (46) | 133 (49) | 90 (41) | 64 (26) | <0.001 |
| Diabetics, n (%) | 130 (13) | 34 (12) | 40 (15) | 24 (11) | 32 (13) | 0.97 |
| Systolic BP (mmHg) | 151 ± 20 | 147 ± 19 | 148 ± 19 | 154 ± 18 | 156 ± 20 | <0.001 |
| Diastolic BP (mmHg) | 84 ± 8 | 84 ± 9 | 84 ± 8 | 84 ± 8.0 | 84 ± 8 | 0.34 |
| Heart rate (beats/min) | 70 ± 12 | 69 ± 12 | 70 ± 12 | 69 ± 11 | 71 ± 12 | 0.09 |
| Calcium (mg/dL) | 9.4 ± 0.5 | 9.5 ± 0.4 | 9.5 ± 0.5 | 9.4 ± 0.4 | 9.3 ± 0.5 | <0.001 |
| Glucose (mg/dL) | 89 (81–100) | 89 (82–99) | 90 (83–103) | 81 (88–99) | 87 (80–100) | 0.68 |
| HOMA index (UI/L)/(mmol/L) | 2.27 (1.56–3.31) | 2.33(1.61–3.22) | 2.44 (1.71–3.39) | 2.34(1.64–3.46) | 1.99 (1.34–2.97) | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 1.4 | 14.1 ± 1.2 | 14.0 ± 1.3 | 13.7 ± 1.3 | 12.9 ± 1.5 | <0.001 |
| Total cholesterol (mg/dL) | 217 ± 40 | 225 ± 40 | 215 ± 35 | 216 ± 41 | 211 ± 43 | <0.001 |
| Triglycerides (mg/dL) | 128 ± 71 | 130 ± 72 | 126 ± 73 | 129 ± 68 | 126 ± 68 | 0.61 |
| Homocysteine (μMol/L) | 16.1 ± 7.1 | 13.9 ± 4.6 | 14.9 ± 6.6 | 16.6 ± 6.0 | 19.6 ± 9.3 | <0.001 |
| ADMA (μMol/L) | 0.50 ± 0.07 | 0.48 ± 0.07 | 0.49 ± 0.07 | 0.50 ± 0.07 | 0.52 ± 0.08 | <0.001 |
| l-arginine (μMol/L) | 40.4 ± 16.1 | 38.4 ± 16.8 | 41.0 ± 15.7 | 40.3 ± 15.7 | 42.1 ± 15.7 | 0.02 |
| CRP (mg/L) | 2.8 (1.3–5.9) | 2.3 (1.2–5.1) | 2.7 (1.1–5.8) | 2.8 (1.5–5.4) | 3.4 (1.5–8.9) | <0.001 |
| Creatinine clearance (mL/min/1.73 m2) | 76 ± 26 | 88 ± 24 | 79 ± 24 | 74 ± 24 | 57 ± 23 | <0.001 |
| Albuminuria, >5 mg/dL (%) | 69 (7) | 13 (5) | 13 (5) | 19 (10) | 24 (12) | 0.001 |
| Cardiovascular comorbidities, n (%) | 444 (43) | 78 (27) | 94 (35) | 112 (52) | 160 (64) | <0.001 |
| On anti-hypertensive therapy, n (%) | 438 (43) | 101 (35) | 104 (38) | 97 (45) | 136 (54) | <0.001 |
Data are expressed as mean ± SD, median, and inter-quartile range or as percent frequency, as appropriate; comparisons among groups were made by P for trend
Clinical and functional correlates of ADMA
Plasma levels of ADMA were on average 0.50 ± 0.07 μMol/L. In univariate analyses, age was the strongest correlate of circulating levels of ADMA (r = 0.26) followed by plasma total homocysteine (r = 0.19). Plasma ADMA was also positively correlated with l-arginine (r = 0.14), albuminuria (r = 0.07), CV comorbidities (r = 0.14), BMI (r = 0.08), and anti-hypertensive treatment (r = 0.07), and inversely with total cholesterol (r = −0.18), hemoglobin (r = −0.12), calcium (r = −0.10), and creatinine clearance (r = −0.14). Despite highly significant (P ranging from 0.02 to 0.001), the level of all these correlations did not exceed 0.3 and therefore should be considered weak.
Relationship between ADMA and all-cause and cardiovascular mortality
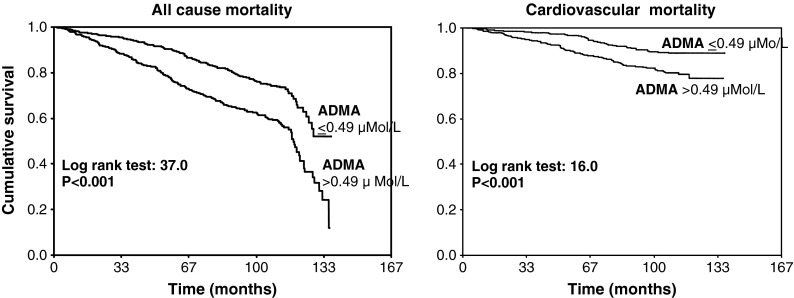
During the follow-up period (median 110 months, range 2–137 months), 384 individuals died, 141 of them (37 %) of cardiovascular causes. In a Kaplan–Meier survival analysis, patients with ADMA above the median level (>0.49 μMol/L) had a relative risk of all-cause and CV mortality that was about two times higher (all-cause death, HR 1.88, 95 % CI 1.53–2.30; CV death, HR 1.97, 95 % CI 1.40–2.79) than those with ADMA below this threshold (P < 0.001) (Fig. 1). In univariate Cox regression analyses, 0.1 μMol/L increase in plasma ADMA entailed a 73 % and 81 % increase, respectively, in the hazard ratios of all-cause (HR 1.73, 95 % CI 1.52–1.97, P < 0.001) and CV mortality (HR 1.81, 95 % CI 1.47–2.23, P < 0.001) (Table 2). The incidence rate of all-cause and CV death was also directly related with age, systolic pressure, plasma total homocysteine, CRP, CV comorbidities, and anti-hypertensive treatment and inversely associated with calcium, hemoglobin, total cholesterol, spot urinary albumin, and creatinine clearance (Table 2). BMI, male sex, diastolic pressure, heart rate, glucose, and l-arginine correlated only with all-cause mortality but failed to significantly predict CV death. In a multiple Cox regression model adjusting for potential confounders (see “Statistical analysis” in the “Methods” section) plasma ADMA resulted to be the first factor in rank, explaining the incidence rate of all-cause mortality and 0.1 μMol/L increase in ADMA engendered a 26 % increase in the hazard ratio of this outcome (Table 3). Also forcing gender, diabetes (or serum glucose), and CrCl into the model did not change the strength of the ADMA–mortality relationship (data not shown). Plasma ADMA tended to be related to CV mortality (+22 % excess risk for each 0.1 μMol/L increase in ADMA), but this difference did not attain statistical significance (P = 0.07) (Table 3).
Fig. 1.
Kaplan–Meier survival curves of ADMA and l-arginine (as re-codified according to the corresponding median value) for all-cause and CV mortality
Table 2.
Univariate Cox regression analyses of all-cause and cardiovascular mortalities
| Units of increase | Hazard ratio, 95 CI, and P value | ||
|---|---|---|---|
| All-cause mortality | CV mortality | ||
| Age | 1 year | 1.14 (1.12–1.15), P < 0.001 | 1.17 (1.14–1.19), P < 0.001 |
| BMI | 1 kg/m2 | 0.97 (0.95–1.00), P = 0.05 | 0.98 (0.93–1.02), P = 0.36 |
| Male sex | 0.78 (0.64–0.95), P = 0.02 | 0.84 (0.60–1.17), P = 0.30 | |
| Smoking | 0 = no; 1 = yes | 1.13 (0.92–1.38), P = 0.24 | 0.79 (0.56–1.13), P = 0.18 |
| Diabetes | 0 = no; 1 = yes | 1.26 (0.95–1.67), P = 0.11 | 1.22 (0.76–1.96), P = 0.41 |
| Systolic pressure | 5 mmHg | 1.06 (1.04–1.09), P < 0.001 | 1.09 (1.05–1.14), P < 0.001 |
| Diastolic pressure | 5 mmHg | 1.07 (1.01–1.13), P = 0.04 | 1.10 (0.99–1.21), P = 0.06 |
| Heart rate | 5 beats/min | 1.07 (1.02–1.12), P = 0.002 | 1.06 (0.99–1.14), P = 0.12 |
| Calcium | 1 mg/dL | 0.60 (0.47–0.77), P < 0.001 | 0.63 (0.42–0.95), P = 0.03 |
| Glucose | 5 mg/dL | 1.02 (1.01–1.04), P = 0.02 | 1.02 (0.99–1.06), P = 0.11 |
| HOMA index | 1 UI/L/mmol/L | 1.01 (0.95–1.07), P = 0.80 | 1.05 (0.97–1.14), P = 0.24 |
| Hemoglobin | 1 g/dL | 0.79 (0.73–0.85), P < 0.001 | 0.77 (0.69–0.87), P < 0.001 |
| Total cholesterol | 20 mg/dL | 0.85 (0.81–0.90), P < 0.001 | 0.91 (0.84–0.99), P = 0.04 |
| Triglycerides | 20 mg/dL | 1.02 (0.99–1.04), P = 0.27 | 1.03 (0.99–1.07), P = 0.17 |
| Homocysteine | 1 μMol/L | 1.05 (1.04–1.06), P < 0.001 | 1.06 (1.04–1.08), P < 0.001 |
| ADMA | 0.1 μMol/L | 1.73 (1.52–1.97), P < 0.001 | 1.81 (1.47–2.23), P < 0.001 |
| Creatinine clearance | 1 mL/min/1.73 m2 | 0.98 (0.97–0.98), P < 0.001 | 0.97 (0.96–0.98), P < 0.001 |
| l-arginine | 1 μMol/L | 1.01 (1.001–1.01), P = 0.01 | 1.01 (0.99–1.02), P = 0.06 |
| CRP | 1 mg/L | 1.02 (1.02–1.03), P < 0.001 | 1.03 (1.02–1.04), P < 0.001 |
| Albuminuria (>5 mg/dL) | 0 = no; 1 = yes | 2.65 (1.95–3.61), P < 0.001 | 2.88 (1.76–4.70), P < 0.001 |
| Cardiovascular comorbidities | 0 = no; 1 = yes | 2.76 (2.24–3.39), P < 0.001 | 4.99 (3.40–7.32), P < 0.001 |
| On anti-hypertensive therapy | 0 = no; 1 = yes | 1.53 (1.25–1.87), P < 0.001 | 2.49 (1.77–3.50), P < 0.001 |
Data are expressed as hazard ratio, 95 % confidence, and P values
Table 3.
Multiple Cox regression model of all-cause and cardiovascular mortality
| All-cause mortality | Units of increase | Hazard ratio (95 % CI) | P value |
|---|---|---|---|
| ADMA | 0.1 μMol/L | 1.26 (1.10–1.44) | <0.001 |
| Age | 1 year | 1.13 (1.11–1.14) | <0.001 |
| Smoking | 0 = no; 1 = yes | 1.40 (1.13–1.74) | <0.001 |
| Homocysteine | 1 μMol/L | 1.02 (1.01–1.03) | <0.001 |
| Albuminuria | 0 = no; 1 = yes | 2.18 (1.58–3.01) | <0.001 |
| Total cholesterol | 20 mg/dL | 0.99 (0.99–0.994) | 0.02 |
| Anti-hypertensive treatment | 0 = no; 1 = yes | 1.18 (0.96–1.45) | 0.12 |
| Systolic arterial pressure | 5 mmHg | 1.02 (0.99–1.05) | 0.20 |
| Hemoglobin | 1 g/dL | 1.06 (0.98–1.14) | 0.16 |
| l-arginine | 1 μMol/L | 1.00 (1.00–1.01) | 0.60 |
| BMI | 1 kg/m2 | 1.00 (0.98–1.03) | 0.88 |
| Calcium | 1 mg/dL | 0.96 (0.76–1.21) | 0.72 |
| CV mortality | |||
| Age | 1 year | 1.16 (1.13–1.19) | <0.001 |
| Anti-hypertensive treatment | 0 = no; 1 = yes | 1.89 (1.33–2.7) | <0.001 |
| Albuminuria | 0 = no; 1 = yes | 2.45 (1.45–4.12) | <0.001 |
| Homocysteine | 1 μMol/L | 1.03 (1.00–1.05) | 0.02 |
| Hemoglobin | 1 g/dL | 1.17 (1.03–1.34) | 0.02 |
| ADMA | 0.1 μMol/L | 1.22 (0.98–1.51) | 0.07 |
| Systolic arterial pressure | 5 mmHg | 1.03 (0.98–1.08) | 0.22 |
| l-arginine | 1 μMol/L | 1.01 (0.99–1.02) | 0.34 |
| BMI | 1 kg/m2 | 1.01 (0.97–1.05) | 0.73 |
| Calcium | 1 mg/dL | 0.94 (0.64–1.37) | 0.74 |
| Total cholesterol | 20 mg/dL | 1.00 (0.99–1.00) | 0.92 |
| Smoking | 0 = no; 1 = yes | 1.01 (0.70–1.47) | 0.94 |
Data are expressed as hazard ratio, 95 % confidence, and P values
24 h creatinine clearance did not meet criteria to be a confounder (see “Statistical analysis” in the “Methods” section), and for this reason, this biomarker was not included into the multivariate Cox models
Notwithstanding the robust association of ADMA with all-cause mortality, this biomarker failed to improve the predictive power of a simple model based on risk factors listed in Table 3 (model without ADMA, AUC 0.84 ± 0.01; model with ADMA, AUC 0.85 ± 0.01). Reclassification analysis showed that in 384 persons who died, the plasma ADMA improved classification in a model including standard risk factors and homocysteine in 12 patients (3.1 %) but worsened classification in 6 patients (1.6 %), thus providing a net gain in reclassification of 1.5 %. In 641 subjects who survived, the model including ADMA reclassified 42 of them at a lower risk category (6.6 %) and 32 of them at a higher risk category (5.0 %), thus giving a net gain in reclassification of 1.6 %. Overall, the NRI was 3.1 %, a figure that failed to reach statistical significance (P = 0.08).
ADMA and clinical outcomes: effect modification by arginine
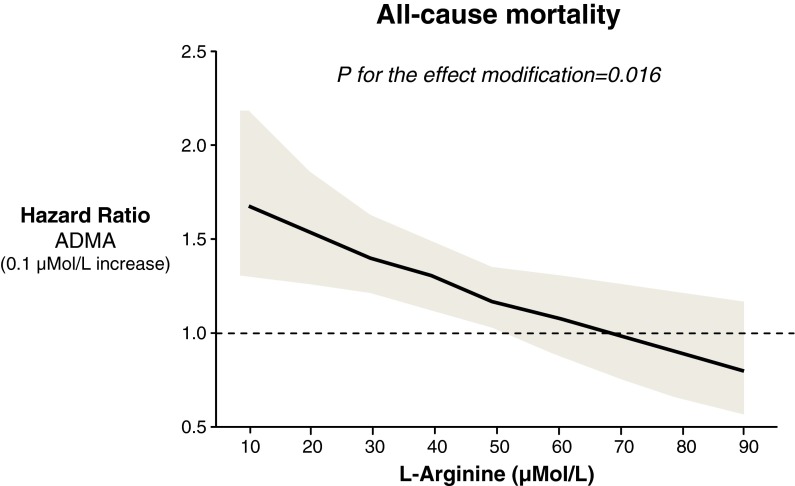
The analysis of the effect modification of ADMA by plasma l-arginine levels showed that on both univariate (P = 0.02) and multivariate Cox regression analyses, there was a significant inverse interaction between ADMA and l-arginine for predicting death, implying that the effect of ADMA on mortality increases as plasma l-arginine decreases and vice versa. Accordingly, plasma ADMA entailed a significant hazard ratio for mortality in patients with l-arginine ranging from 10 to 60 μMol/L (excess risk for death associated with a 0.1 μMol/L increase in ADMA ranging from +68 to + 16 %), while such an effect was abolished for plasma l-arginine values >60 μMol/L (Fig. 2). Other independent variables in the multiple Cox regression model of the ADMA and l-arginine interaction for all-cause mortality were age (HR 1.12, CI 1.11–1.14, P < 0.001), albuminuria (HR 2.19, CI 1.59–3.02, P < 0.001), homocysteine (HR 1.02, CI 1.01–1.04, P = 0.001), smoking (HR 1.42, CI 1.15–1.77, P = 0.001), and total cholesterol (HR 0.99, CI 0.99–0.99, P = 0.02). The same analysis showed no interaction between ADMA and l-arginine for predicting CV mortality (P = 0.12). No interaction was found between ADMA and homocysteine or diabetes for all-cause and cardiovascular mortality, indicating that these risk factors had complementary, rather than supplementary value for the prediction of these outcomes.
Fig. 2.
Hazard ratios (solid line), 95 % confidence intervals (shaded area) and reference (broken line) for all-cause mortality of a fixed (0.1 μMol/L) excess in plasma ADMA across all possible values of L-arginine. Accordingly, plasma ADMA entailed a significant hazard ratio for mortality in patients with L-arginine ranging from 10 to 60 μMol/L (excess risk for death associated with a 0.1 μMol/L increase in ADMA ranging from +68 to +16 %) while such an effect was abolished for plasma L-arginine values >60 μMol/L
Discussion
The key finding of the present study is that the predictive power of ADMA for cardiovascular and total mortality extends to the very old age, while some traditional risk factors lose their predictive power. Furthermore, the risk associated with elevated plasma ADMA is attenuated when plasma l-arginine levels are above the median. This interaction is in keeping with previous observations (Boger et al. 2009b) and the biological regulation of the NO system by these amino acids (Moncada and Higgs 1993). As in previous studies showing just minor gains in predictive power by the C-statistic with other biomarkers, we found that ADMA does not add relevant prognostic power to simple models based on conventional risk factors.
Biology of ADMA and clinical outcomes
ADMA is a powerful inhibitor of nitric oxide synthase and also competes with l-arginine for the binding site of this enzyme. A variety of clinical and cohort studies show that reduced production of endogenous NO coincides with high ADMA levels, engenders endothelial dysfunction, and that this dysfunction is reversed by l-arginine (Boger et al. 1998; Cardounel et al. 2007). ADMA is a correlate of endothelium-dependent vasodilation in the low risk, non-smoking healthy population (Ardigo et al. 2007), and in young people (Juonala et al. 2007), as well as in patients with uncomplicated hypertension (Perticone et al. 2005) or chronic kidney disease (Zoccali et al. 2001; Ravani et al. 2005). Endothelial dysfunction itself is a strong predictor for death from coronary heart disease (Schachinger et al. 2000). Intravenous infusion of ADMA increases vascular tone and arterial pressure (Kielstein et al. 2004; Achan et al. 2003), and reduces cerebral blood flow (Kielstein et al. 2006) in healthy individuals. Of note, in our cohort of elderly individuals, higher ADMA was associated both with all-cause and cardiovascular death. This finding differs from observations in the Framingham heart study where ADMA was associated with non-cardiovascular death only (Boger et al. 2009b). It may reflect simple sample size issues as well as aging-related changes in the relative contribution of risk markers to outcomes. The nitric oxide system is fundamental for the regulation of a variety of cellular and organ system functions (Moncada and Higgs 1993), particularly in old people (Gerhard et al. 1996). Our data extend to the elderly observations in middle-aged populations with coronary disease (Meinitzer et al. 2007) as well as in other high risk populations like patients with chronic kidney disease (Zoccali et al. 2001; Ravani et al. 2005). However, the causal role of ADMA in the high risk for death and cardiovascular complications in the elderly needs to be tested in intervention studies.
ADMA and death: effect modification by l-arginine
l-arginine is the substrate to NO synthase and a competitive antagonist of ADMA. The l-arginine–ADMA direct link found in the present and in previous studies (Perticone et al. 2010; Boger et al. 2009b) may underlie a mechanism aimed at minimizing the adverse effect of high ADMA at organ level (Perticone et al. 2005). The so called l-arginine paradox, e.g., an ADMA-induced right shift of the NOS concentration–response curve for l-arginine, implies that only small changes in l-arginine plasma concentrations may cause marked changes in NOS activity in order to avoid a relevant lack of NO (Tsikas et al. 2000; Boger 2004). On this background, we planned specific statistical analyses to test the interaction between ADMA and this amino acid for the risk of all-cause and cardiovascular death. In line with the hypothesis that l-arginine levels can modify the effect of ADMA on health outcomes, we found that there is no association of ADMA with survival in individuals with high l-arginine levels but strong and dose dependently higher across the normal to low l-arginine range. No such interaction existed with cardiovascular mortality. Our findings in the elderly extends to this stage-of-life observations in the Framingham heart study, where the risk of death was highest in patients with ADMA in the fourth quartile, and l-arginine in the first quartile of the distribution of these amino acids in this middle-aged cohort (Boger et al. 2009b). On the other hand, it should be noted that in the face of a robust interaction between ADMA and l-arginine for all-cause death, there was no such interaction for cardiovascular death. ADMA triggers endothelial cell senescence, and l-arginine prevents this phenomenon (Scalera et al. 2006). In the absence of ADMA, long term exposure to l-arginine paradoxically accelerates senescence of endothelial cells by translational and post-translational activation of arginase II (Scalera et al. 2009) which may explain the lack of l-arginine–ADMA interaction for cardiovascular death in the elderly.
Limitations and strengths
Our study has limitations. The first limitation depends on the observational nature of our findings. Whether ADMA is causally implicated in all-cause and cardiovascular death in the elderly demands specific mechanistic studies testing the biological pathways, whereby ADMA may affect these outcomes. Furthermore, findings in our aged population of European descent need to be confirmed in coeval cohorts of other ethnicities. Strengths of our study are the good characterization and the accurate follow-up of the study cohort as well as the state-of-art measurement of plasma ADMA and l-arginine. Furthermore, the strong interaction between ADMA and l-arginine, the substrate of NO synthase, and the precursor of NO adds biological plausibility to the hypothesis that ADMA is causally implicated in the high risk of death in the elderly. However, given the numerous pathways involving l-arginine, it may constitute an oversimplification to attribute all ADMA-related pathology to impairment of NO signaling.
Conclusions
In a randomly selected, community-based sample of old and very old subjects, higher ADMA independently predicted all-cause and cardiovascular mortality. The relationship between ADMA and mortality depended on l-arginine levels, being maximal in subjects with low plasma l-arginine and minimal in those with high levels of this amino acid. Further observational and intervention studies in the elderly are needed to confirm these findings and to clarify the pathophysiological mechanisms underlying these associations.
Appendix
Calculation of the hazard ratio for death of ADMA according to l-arginine levels
Table 4.
Multiple Cox regression analysis of “ADMA × l-arginine interaction” for explaining death
| Regression coefficients | |
|---|---|
| ADMA (0.1 μMol/L increase) | 0.607 |
| l-arginine (μMol/L) | 0.0496 |
| ADMA × l-arginine (μMol2/L2) | −0.009* |
| Age (1 year) | 0.118 |
| Albuminuria >5 mg/dL (0 = no; 1 = yes) | 0.781 |
| Homocysteine (0.1 μMol/L) | 0.020 |
| Smoking (0 = no; 1 = yes) | 0.327 |
| Total cholesterol (20 mg/dL) | −0.0033 |
| Systolic pressure (5 mmHg) | 0.0216 |
| Anti-hypertensive treatment (0 = no; 1 = yes) | 0.1616 |
| Hemoglobin (1 g/dL) | 0.0441 |
| BMI (1 kg/m2) | 0.0035 |
| Calcium (1 mg/dL) | −0.034 |
For illustrative purpose, only regression coefficients are reported (the corresponding hazard ratios are given in the “Results” section in the text)
*P = 0.016
A significant interaction between ADMA and l-arginine (P = 0.016) implies that the hazard ratio for death of ADMA must be calculated at pre-specified values of l-arginine by combining the corresponding regression coefficients derived from Cox regression analysis. For example, the hazard ratios for death of ADMA at l-arginine values of 50 and 60 μMol/L are calculated as follows:
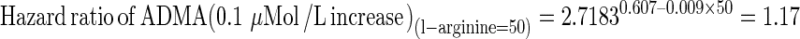 |
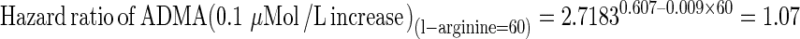 |
As a consequence of the effect modification of l-arginine on the death risk portended by ADMA, the effect of ADMA is associated to a significant risk increase (hazard ratio = 1.17, relative risk increase = 0.17 or 17 %) in patients with l-arginine of 50 μMol/L and to a modest and not significant risk increase (hazard ratio = 1.07, relative risk increase = 0.07 or 7 %) in those with l-arginine of 60 μMol/L.
Contributor Information
Francesco Pizzarelli, Phone: +39-55-6936223, FAX: +39-55-6936223, Email: fpizzarelli@yahoo.com.
Pietro Dattolo, Phone: +39-55-6936225, Email: pierodattolo@tin.it.
References
- Achan V, Broadhead M, Malaki M, Whitler J, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- Ardigo D, Stuehlinger M, Franzini L, Valtuena S, Piatti PM, Pachinger O, Reaven GM, Zavaroni I. ADMA is independently related to flow-mediated vasodilation in subjects at low cardiovascular risk. Eur J Clin Invest. 2007;37:263–269. doi: 10.1111/j.1365-2362.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- Bode-Boger SM, Scalera F, Martens-Lobenhoffer J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc Med. 2005;10(Suppl 1):S65–S71. doi: 10.1177/1358836X0501000110. [DOI] [PubMed] [Google Scholar]
- Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “l-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–2847S. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.CIR.98.18.1842. [DOI] [PubMed] [Google Scholar]
- Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality–an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481–487. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T, Fleming TH, Rosenhagen C, Krauser U, Mieth M, Bruckner T, Martin E, Nawroth PP, Weigand MA, Bierhaus A, Hofer S. L-arginine and asymmetric dimethylarginine are early predictors for survival in septic patients with acute liver failure. Mediat Inflamm. 2012 doi: 10.1155/2012/210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.HYP.27.4.849. [DOI] [PubMed] [Google Scholar]
- Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- Juonala M, Viikari JS, Alfthan G, Marniemi J, Kähönen M, Taittonen L, Laitinen T, Raitakari OT. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation. 2007;116:1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Coronary heart disease risk factors in the elderly. Am J Geriatr Cardiol. 2002;11:101–107. doi: 10.1111/j.1076-7460.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Impraim B, Simmel S, Bode-Boger SM, Tsikas D, Frolich JC, Hoeper MM, Haller H, Filser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Böger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–2029. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, März W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kronmal RA, Tracy RP, Cushman M, Siscovick DS. Traditional and novel risk factors in older adults: cardiovascular risk assessment late in life. Am J Geriatr Cardiol. 2004;13:69–80. doi: 10.1111/j.1076-7460.2004.02123.x. [DOI] [PubMed] [Google Scholar]
- Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–523. doi: 10.1016/j.jacc.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Perticone F, Maio R, Perticone M, Miceli S, Sciacqua A, Tassone EJ, Shehaj E, Tripepi G, Sesti G. Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol. 2010;142:236–241. doi: 10.1016/j.ijcard.2008.12.131. [DOI] [PubMed] [Google Scholar]
- Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–2455. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- Scalera F, Martens-Lobenhoffer J, Tager M, Bukowska A, Lendeckel U, Bode-Boger SM. Effect of L-arginine on asymmetric dimethylarginine (ADMA) or homocysteine-accelerated endothelial cell aging. Biochem Biophys Res Commun. 2006;345:1075–1082. doi: 10.1016/j.bbrc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Scalera F, Closs EI, Flick E, Martens-Lobenhoffer J, Boissel JP, Lendeckel U, Heimburg A, Bode-Böger SM. Paradoxical effect of L-arginine: acceleration of endothelial cell senescence. Biochem Biophys Res Commun. 2009;386:650–655. doi: 10.1016/j.bbrc.2009.06.091. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Boger RH. Determination of a reference value for N(G), N(G)-dimethyl-l-arginine in 500 subjects. Eur J Clin Invest. 2005;35:622–626. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Boger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2007;851:211–219. doi: 10.1016/j.jchromb.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Tripepi G, Jager KJ, Dekker FW, Zoccali C. Testing for causality and prognosis: etiological and prognostic models. Kidney Int. 2008;74:1512–1515. doi: 10.1038/ki.2008.416. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Boger RH, Sandmann J, Bode-Boger SM, Frölich JC. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000;478:1–3. doi: 10.1016/S0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- Zoccali C. Asymmetric dimethylarginine (ADMA): a cardiovascular and renal risk factor on the move. J Hypertens. 2006;24:611–619. doi: 10.1097/01.hjh.0000217839.26971.8d. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frolich J, Boger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/S0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]