Abstract
Store-operated Ca2+ entry (SOCE) is a common and ubiquitous mechanism regulating Ca2+ influx into cells and participates in numerous biological processes including cell proliferation. Glomerular mesangial cells (GMCs) play a role in the regulation of the glomerular filtration rate. From a clinical point of view, many physiological functions alter with age. In the present study, we used angiotensin II, glucagon, and the sarco/endoplasmic reticulum membrane Ca2+ pump inhibitor thapsigargin to deplete the internal Ca2+ stores for the activation of SOCE. We found that SOCE was significantly attenuated in GMCs from aged (22-month-old) rats. The expression of SOCE-related components, stromal interaction molecule 1 (STIM 1) and Orai 1, in freshly isolated glomeruli notably decreased, and STIM 1 and Orai 1 puncta formation significantly reduced in primary-cultured GMCs in aged rats. Moreover, specific knockdown of STIM 1 and Orai 1 by small interfering RNA markedly suppressed SOCE and cell proliferation of GMCs isolated from young (3-month-old) rats. We conclude that the attenuation of GMCs proliferation can be attributed to the decreased SOCE partially caused by reduced expression of STIM 1 and Orai 1.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-013-9511-5) contains supplementary material, which is available to authorized users.
Keywords: Aging, STIM 1, Orai 1, Glomerular mesangial cell, Proliferation
Introduction
Store-operated Ca2+ entry (SOCE) is mediated via specific plasma membrane channels in response to the depletion of Ca2+ from intracellular Ca2+ stores. This Ca2+ entry pathway is a common and ubiquitous mechanism regulating Ca2+ influx into cells (Putney 2011). In both excitable and non-excitable cells, SOCE regulates diverse cellular functions ranging from cell proliferation and gene expression to cell contraction and secretion (Berridge et al. 2003; Parekh and Putney 2005; Venkatachalam et al. 2002). Over the past decade, we (Ma et al. 2000, 2002) and others (Mene et al. 1994) have found that SOCE plays a role in Ca2+ influx stimulated by agonists in human glomerular mesangial cells (GMCs).
The transient receptor potential (TRPC) family contains seven subfamilies that form Ca2+-selective and Ca2+-nonselective cation channels in a variety of mammalian cells. TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 proteins were identified in GMCs (Facemire et al. 2004; Sours-Brothers et al. 2009). Previous studies demonstrated that TRPC1, 4, and 6 contributed to SOCE in GMCs (Sours-Brothers et al. 2009; Wang et al. 2004, 2007). However, accumulating evidence suggests that the stromal interaction molecule 1 (STIM 1) and Orai 1 are key components of SOCE. STIM 1 is a single transmembrane protein. The N-terminus of STIM 1 is located in the lumen of the endoplasmic reticulum (ER) and functions as a Ca2+ sensor. The C-terminus of STIM 1 is located in the cytosol and activates SOCE upon store depletion by interacting with the plasma membrane Orai 1 (Zhang et al. 2005; Liou et al. 2005; Roos et al. 2005). Knockdown of STIM 1 expression using specific small interfering RNA (siRNA) significantly reduced SOCE in a number of cell types, whereas STIM 1 overexpression modestly enhanced SOCE (Feske et al. 2006; Roos et al. 2005; Williams et al. 2002; Liou et al. 2005). Orai 1 is a four-transmembrane-domain protein. Knockdown of Orai 1 notably decreased SOCE (Peinelt et al. 2006; Soboloff et al. 2006). Indeed, it is widely accepted that Orai 1 and STIM 1 concertedly determine SOCE in a variety of cells. SOCE plays a fundamental role in controlling renal microcirculation and glomerular hemodynamics. Growing evidence shows that perturbations in SOCE function in particular regions of the kidney cause pathologic outcomes, such as diabetic nephropathy. Renal senescence induces a series of physiological and structural alterations that impair renal function in aged individuals. The best documented physiological change is that of the glomerular filtration rate (GFR) in aged animals and humans. However, there is no evidence on age-dependent changes in the proliferation and SOCE of GMCs. In the present study, we investigated whether SOCE was impaired in aged rat GMCs. We further tested whether the expression levels of STIM 1 and Orai 1 were reduced in aged GMCs.
Experimental procedures
Materials
Fura-4/AM, Pluronic F-127, RPMI 1640 medium, fetal bovine serum (FBS), and Lipofectamine RNAiMAX reagent were purchased from Invitrogen. Small interfering RNA was synthesized by Invitrogen. Desmin antibody was purchased from Abcam. STIM 1 and Orai 1 antibodies were purchased from ProSci. β-Tubulin antibody was purchased from Santa Cruz. Bromodeoxyuridine (BrdU) antibody was purchased from Shanghai Fankel Biological Technology, Ltd. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Nonidet P-40, sodium deoxycholate, SDS, EDTA, collagenase type I, thapsigargin, angiotensin II, glucagon, BrdU, and BSA were purchased from Sigma. The enhanced chemiluminescence (ECL) western blotting detection system was purchased from Amersham Pharmacia. Protease inhibitor cocktail tablets were purchased from Roche.
Preparation, culture of rat GMCs, and siRNA transfection
All animal experiments were conducted in accordance with NIH publication no. 8523 and approved by the Animal Experimentation Ethics Committee of Anhui Medical University. Primary-cultured rat GMCs were isolated from male Sprague–Dawley rats aged 3 or 22 months old. The rats were fed with standard diet. Primary culture of GMCs was performed according to the literature with slight modifications (Singh et al. 2003). The rats were anesthetized, and the bilateral kidneys were isolated. The capsules of the kidneys were removed into phosphate-buffered saline (PBS) (140 mmol/L NaCl, 3 mmol/L KCl, 25 mmol/L Tris, pH 7.4) at 4 °C. The kidneys were longitudinally opened. The center and medulla of the kidneys were cut away, and the cortex was saved. We used two razor blades held together to mince the cortexes until they resembled a paste. The cortex paste was then passed through the center of a 120 sieve, followed by a 150 sieve and a 250 sieve. Following washout, glomeruli from the upper side of the 250 sieve were collected. The samples were centrifuged at 500 × g for 10 min at 4 °C. The pellet was resuspended in 0.3 % collagenase (type I) and incubated for 30 min at 37 °C. Subsequently, the cell suspension was seeded into a 25-cm2 flask containing RPMI 1640 medium supplemented with 10 % FBS, 100 μg/mL penicillin, and 100 U/mL streptomycin. The cells were cultured in a 37 °C incubator with 5 % CO2.
Rat STIM 1 and Orai 1 siRNA sequences were obtained from the literature. The sequence for rat STIM-1 siRNA was UAAGGGAAGACCUCAAUUA (sense strand) and UAAUUGAGGUCUUCCCUUA (antisense strand) (Potier et al. 2009). The Orai-1 siRNA sequence was CAACAGCAAUCCGGAGCUU (sense strand) and AAGCUCCGGAUUGCUGUUG (antisense strand) (Potier et al. 2009). siRNA delivery was achieved using Lipofectamine RNAiMAX reagent according to the manual.
Western blot analysis
The proteins were extracted from the lysates of glomeruli with detergent extracted buffer, which contained 1 % Nonidet P-40, 150 mmol/L NaCl, and 20 mmol/L Tris–HCl, pH 8.0, plus protease inhibitor cocktail tablets. For immunoblots, the poly (vinylidene difluoride) membrane carrying transferred proteins was incubated at 4 °C overnight with the primary antibodies: anti-STIM 1 or anti-Orai 1 (1:200). Immunodetection was accomplished using horseradish peroxidase-conjugated secondary antibody, followed by processing through an ECL detection system. The optical density of each blot was normalized to that of β-tubulin analyzed within the same lane and represented as relative optical density.
Immunofluorescence
The freshly isolated kidneys were embedded in tissue freezing medium (Leica) and placed in nitrogen to solidify the medium and tissue. Then, 5-μm-thick sections were prepared. For primary-cultured GMCs, the cells were seeded onto coverslip. After overnight growth, the cells were treated with agonists for 10 min in Ca2+-free saline solution. The sections or cells were fixed with 4 % formaldehyde for 10 min, followed by permeabilization with 0.1 % Triton X-100 in PBS. After blocking with 2 % bovine serum albumin with PBS at room temperature for 1 h, they were incubated with primary antibody at 4 °C overnight. After washing with PBS three times, the sections were incubated with donkey anti-rabbit IgG conjugated to Alexa Fluor 488 (1:200) for 1 h at room temperature. Eventually, the sections were mounted in 90 % glycerol in PBS, and the fluorescent signals were detected using a Leica TCS SP5 confocal laser system.
[Ca2+]i measurement
Cytosolic Ca2+ ([Ca2+]i) was measured as described elsewhere (Shen et al. 2011). Briefly, GMCs were loaded with 10 μmol/L Fluo-4/AM at 37 °C for 30 min. Ca2+ stores were depleted by treating GMCs with 4 μmol/L thapsigargin for 10 min in Ca2+-free PBS (0Ca2+–PSS), which contained (in mmol/L) 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L glucose, 0.2 mmol/L EGTA, and 5 HEPES, pH 7.4. Ca2+ influx was initiated by applying 1 mmol/L extracellular Ca2+. Fluorescence was recorded using a Leica TCS SP5 confocal laser system. Changes in [Ca2+]i were displayed as the ratio of fluorescence relative to the intensity before the application of extracellular Ca2+ (F1/F0).
MTT assay
Cell proliferation was assessed via the uptake of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The rat GMCs were seeded in 96-well plates in RPMI 1640 plus 10 % FBS. On the following day, the cells were starved for 16 h. Then, 200 μL of RPMI 1640 plus 10 % FBS was added to each well, and the cells were cultured for 24 h. Then, 200 μL of fresh medium containing MTT (final concentration, 250 μg/mL) was added to each well. After incubation at 37 °C for 4 h, the cell medium was carefully removed. The trapped MTT crystals were then solubilized by adding 200 μL DMSO/well at 37 °C for 15 min. Absorbance at 450 nm was measured in a PECTRA max 190 reader (molecular device).
Bromodeoxyuridine assay
Bromodeoxyuridine, which can be incorporated into newly synthesized DNA, was also used to verify cell proliferation. Briefly, primary-cultured rat GMCs were seeded onto coverslip and synchronized in DMEM medium supplemented with 0.1 % fetal bovine serum for 3 days. The cells were labeled with BrdU for 24 h and then fixed. Then, immunocytochemistry was employed to identify BrdU-positive cells. BrdU-positive cells were counted under microscope.
Results
SOCE in GMCs of young and aged rats
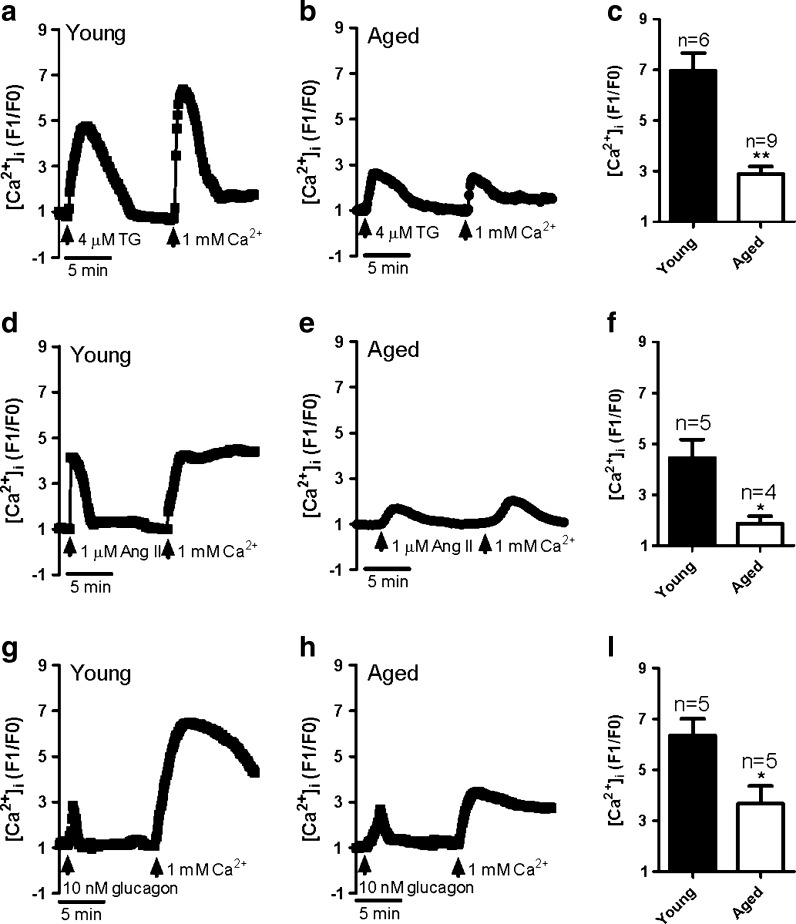
Firstly, thapsigargin (TG) was used to activate SOCE by passive depletion of internal Ca2+ stores. The GMCs were treated with 4 μM TG in Ca2+-free saline solution for 10 min after which 1 mM Ca2+ was added to the extracellular solution. TG-evoked Ca2+ release significantly reduced in aged rat GMCs comparing with young cells (supplementary Fig. 1a; Fig. 1a, b). Simultaneously, Ca2+ influx increased robustly in GMCs from young rats (Fig. 1a, c), but this response was markedly attenuated in aged rat GMCs (Fig. 1b, c). Interestingly, physiological agonists angiotensin II (Ang II, 1 μM) and glucagon (10 nM)-evoked SOCE also significantly attenuated in aged rat GMCs (Fig. 1d–i). Ang II-evoked Ca2+ release dramatically reduced in aged rat GMCs in Ca2+-free saline solution, but no significant difference was found in glucagon-evoked Ca2+ release between young and aged GMCs (supplementary Fig. 1b, c; Fig. 1d, e, g, h). Therefore, SOCE are greatly impaired in aged GMCs. On the other hand, TG and Ang II-sensitive Ca2+ stores are also impaired in aged GMCs, but glucagon-sensitive stores are not. It causes that the intensity of the store depletion induced by TG and Ang II becomes weaker. This may be the reason why the reduction of SOCE evoked by TG and Ang II is much stronger than that induced by glucagon.
Fig. 1.
Store-operated Ca2+ entry in primary-cultured glomerular mesangial cells from young and aged rats. Representative traces showing 4 μM thapsigargin (TG; a, b), 1 μM angiotensin II (Ang II; d, e), or 10 nM glucagon (g, h)-evoked Ca2+ release in Ca2+-free saline solution and 1 mM Ca2+ application-induced [Ca2+]i rise in young (a, d, g) and aged (b, e, h) GMCs. c, f, i Summary of data showing changes in [Ca2+]i rise in response to extracellular Ca2+ application after TG (c), Ang II (f), or glucagon (i) treatment in young and aged GMCs. Mean ± standard error (SE). **P < 0.01, *P < 0.05. Young (3 months), n = 5–6 vs aged (22 months), n = 4–9
Expression levels of STIM 1 and Orai 1 in GMCs of young and aged rats
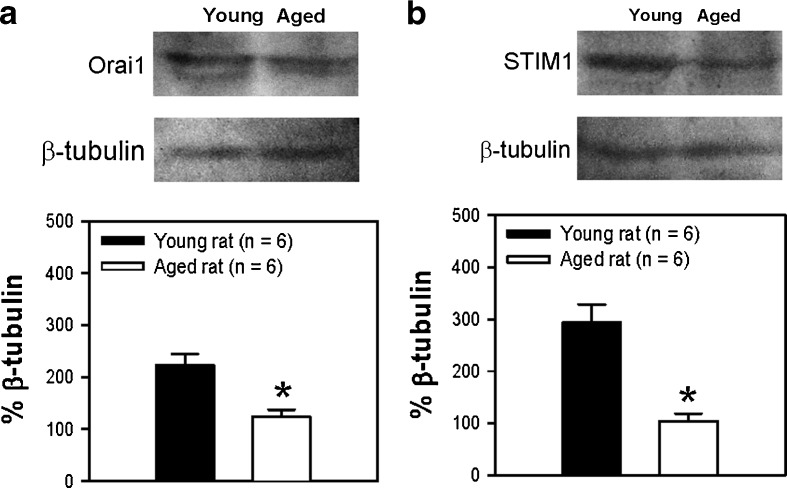
Many studies have demonstrated that STIM 1 and Orai 1 proteins are involved in SOCE. To study the mechanism for the reduced SOCE in aged rats, we examined the expression levels of the two proteins in GMCs from young and aged rats. Western blotting showed that the expression of both STIM 1 and Orai 1 proteins was reduced in aged GMCs compared with that in younger rats (Fig. 2). Immunofluorescence staining also indicated that the fluorescence signals representing STIM 1 and Orai 1 in GMCs were much less intense in aged rats compared with young rats (Fig. 3). No signal was observed in the negative control (omission of primary antibody) (Fig. 3, control).
Fig. 2.
Expression levels of STIM 1 and Orai 1 in glomeruli from young and aged rats. Representative images and summarized data showing the expression levels of Orai 1 (a) and STIM 1 (b) in fresh isolated glomeruli from young and aged rats. β-Tubulin was used as loading control. Protein levels were expressed as relative optical density. Mean ± SE; *P < 0.05. Young (3 months) vs aged (22 months), n = 6
Fig. 3.
Endogenous STIM 1 and Orai 1 expression in glomeruli from young and aged rats. Kidney sections were stained with anti-STIM 1 or anti-Orai 1 antibody and mesangial cell marker anti-desmin antibody. In contrast to the control (omission of primary antibody), the staining for STIM 1 and Orai 1 was shown in glomeruli from young and aged rats
STIM 1 and Orai 1 contribute to SOCE activity in GMCs
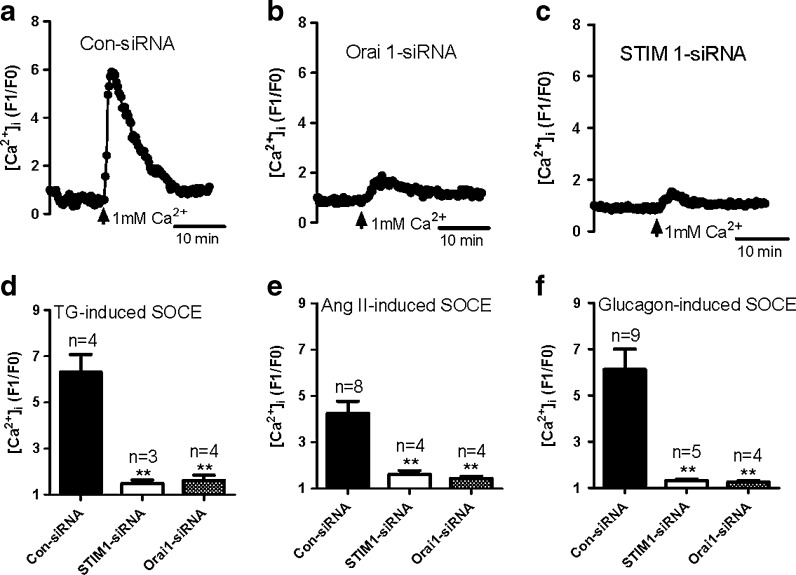
In order to determine whether STIM 1 or Orai 1 contributed to SOCE in GMCs, we transfected siRNAs against human STIM 1 or Orai 1 into primary-cultured GMCs isolated from young rats. Knockdown of either STIM 1 or Orai 1 did not affect TG-evoked Ca2+ release (supplementary Fig. 2a) but significantly reduced SOCE (Fig. 4a–d) in GMCs. These results are consistent with reports that STIM 1 contributes to SOCE in GMCs (Zhang et al. 2007; Sours-Brothers et al. 2009) and, for the first time, show that Orai 1 also mediates SOCE in GMCs. Similar results were also found in 1 μM Ang II-evoked and 10 nM glucagon-evoked SOCE (Fig. 4e, f). Ang II- and glucagon-evoked Ca2+ release were not altered in the treatment of Orai 1 siRNA but suppressed by STIM 1 siRNA significantly (supplementary Fig. 2b, c). It indicates that probably, STIM 1 is essential to maintain the Ca2+ level of Ang II and glucagon-sensitive store. However, Orai 1 siRNA did not alter agonist-evoked Ca2+ release and expectedly suppressed agonist-evoked SOCE. The result safely convinces that Orai 1 and STIM 1 involve in SOCE in GMCs.
Fig. 4.
Role of STIM 1 and Orai 1 in store-operated Ca2+ entry (SOCE) in primary-cultured glomerular mesangial cells from young rat. a–c Representative traces showing 1 mM Ca2+ application-induced [Ca2+]i rise in scrambled siRNA control (a Con-siRNA), Orai 1 (b), and STIM 1 (c) siRNA-transfected GMCs treated by 4 μM thapsigargin for 10 min in Ca2+-free saline solution. d–f Summary of data showing changes in [Ca2+]i rise in response to extracellular Ca2+ application after the treatment of TG (d), angiotensin II (e), or glucagon (f) in Ca2+-free saline solution in young and aged GMCs. Mean ± SE. **P < 0.01 compared to GMCs transfected with scrambled siRNA control, n = 3–9
It is well known that store depletion will trigger STIM 1 and Orai 1 to form puncta (Zhou et al. 2010; Liou et al. 2007). STIM 1 and Orai 1 puncta formation directly correlates to SOCE activity (Liou et al. 2005). Similar to the glomerulus, immunofluorescence images also indicate that the expression level of STIM 1 and Orai 1 decreased in aged rat GMCs (Fig. 5). In GMCs from young rats, 4 μM TG, 1 μM Ang II, and 10 nM glucagon strongly induced STIM 1 and Orai 1 puncta formation (Fig. 5). However, the intensity of STIM 1 and Orai 1 puncta formation is obviously weakened in aged rat GMCs (Fig. 5).
Fig. 5.
Ca2+ store depletion-induced STIM 1 and Orai 1 puncta formation and co-localization. Primary-cultured mesangial cells from young and aged rats were treated by 4 μM thapsigargin, 1 μM angiotensin II, or 10 nM glucagon for 10 min in Ca2+-free saline solution and fixed. Cells were stained with anti-STIM 1 and anti-Orai 1 antibody. After subtracting background (omission of primary antibody), fluorescent signals represent STIM 1 (green) and Orai 1 (red) proteins
Decrease in proliferation in GMCs from aged rats
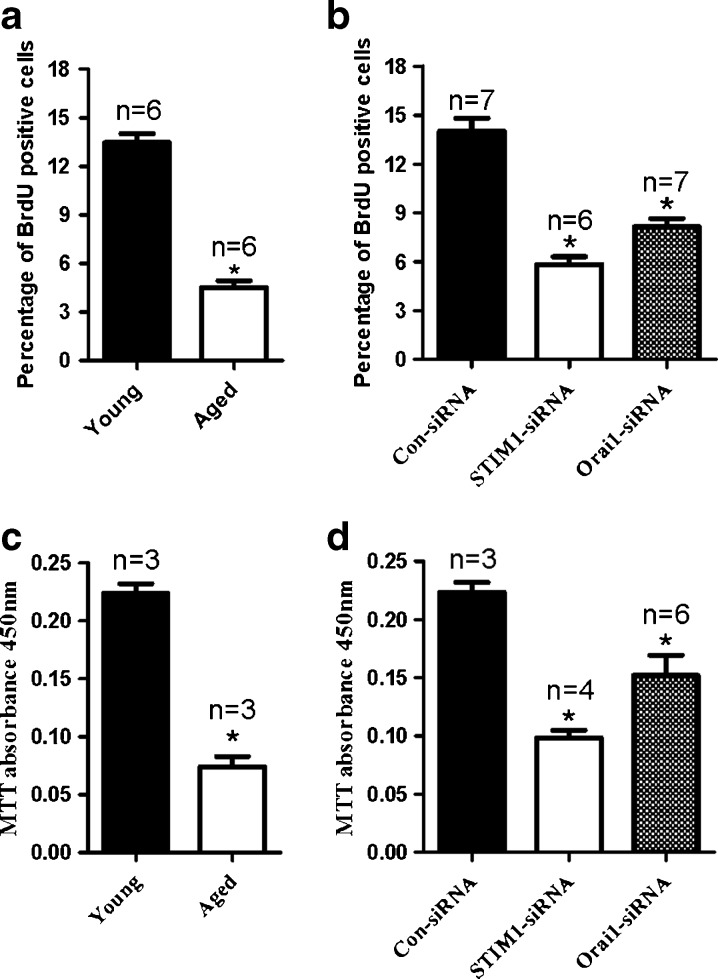
Many studies have demonstrated a role for SOCE in cell proliferation. We thus investigated whether a lower SOCE in the aged GMCs led to a lower rate of GMC proliferation. MTT and BrdU assays were used to assess GMC proliferation. As shown in Fig. 6a, c, proliferation was notably lower in aged rat GMCs compared with young rat GMCs. Furthermore, knockdown of either STIM 1 or Orai 1 suppressed the proliferation of primary-cultured GMCs from young rats (Fig. 6b, d).
Fig. 6.
The ability of cell proliferation determined by BrdU and MTT assays. a Percentage of BrdU-positive glomerular mesangial cells. b GMCs growth data determined by absorbance units at 450 nm in the MTT assay of young and aged rats. Mean ± SE. *P < 0.05, compared to young GMCs, n = 3–7. b, d Summary of data showing percentage of BrdU-positive young GMCs (b) and cell growth of young GMCs (d) treated by scrambled siRNA control (Con-siRNA), STIM 1, or Orai 1 siRNA transfection. Mean ± SE. *P < 0.05, compared to GMCs transfected with scrambled siRNA control, n = 3–7
Discussion
The present study found that the SOCE activity and proliferation of GMCs from aged rats were significantly reduced in comparison with younger rats. These changes may be attributed to the reduced expression of SOCE-related components including STIM 1 and Orai 1 in aged rat GMCs. It is known that intracellular Ca2+ is a critical second messenger linking external stimuli to cell proliferation. If the cell is not capable of maintaining an adequate level of Ca2+ in intracellular stores, many cellular functions including posttranslational modifications of recombinant proteins will be abnormal. Store depletion may elicit problematic protein trafficking, sarcoplasmic reticulum (SR) stress (Cudna and Dickson 2003), growth arrest (Short et al. 1993), and apoptosis (He et al. 1997) caused by the resulting disturbance of posttranslational protein modification. Ca2+ influx through SOCE involves keeping a sustained high Ca2+ concentration in the Ca2+ stores and refilling the SR when it releases Ca2+. SOCE was shown to be upregulated in proliferating porcine bronchial smooth muscle cells, aortic smooth muscle cells, and pulmonary artery smooth muscle cells (Sweeney et al. 2002; Lu et al. 2009; Berra-Romani et al. 2008). Our data suggest that SOCE activity contributes to mesangial cell proliferation and that decreased SOCE contributed to a lower proliferation rate in GMCs.
We and others have demonstrated that SOCE participates in Ca2+ signaling in GMCs (Mene et al. 1994; Ma et al. 2000; Mene et al. 1996; Zhang et al. 2007). The present study found that TG-, Ang II-, and glucagon-evoked activations of SOCE were significantly reduced in primary-cultured aged rat GMCs in comparison with those from younger rats. Recently, STIM 1 has been identified as a Ca2+ sensor in the ER, activating SOCE in the plasma membrane upon store depletion (Liou et al. 2005; Roos et al. 2005). STIM 1 that is diffusely distributed in the ER membrane translocates to the ER–plasma membrane junctions upon store depletion, where it triggers SOCE (Lewis 2007; Baba et al. 2006; Liou et al. 2007). Thus, SOCE can be considered as channels that are regulated by STIM 1 and require store depletion that leads to clustering of STIM 1. Several studies have shown that STIM 1 regulates SOCE in GMCs (Zhang et al. 2007; Sours-Brothers et al. 2009). Moreover, Orai 1 is critical for TRPC1-mediated SOCE in platelets and salivary glands (Braun et al. 2009; Ong et al. 2007). Dynamic assembly of a TRPC1–STIM 1–Orai 1 complex is involved in SOCE, and endogenous Orai 1, STIM 1, and TRPC1 concertedly regulate SOCE in salivary gland cells (Ong et al. 2007). However, until now, there has been no evidence that Orai 1 contributes to SOCE in GMCs. The present study demonstrates that STIM 1 and Orai 1 protein levels were significantly lower in aged rat GMCs compared with those from younger rats. This may be a mechanism for decreased mesangial cell proliferation in aging.
Physiological agonists including Ang II and glucagon activate G-protein-coupled receptor to produce IP3 via phospholipid C pathway then release store Ca2+ to evoke SOCE. In pathological condition such as renin–Ang II-dependent hypertension and type II diabetes, the concentration of Ang II or glucagon is usually elevated in local renal tissue or circulation (Kobori et al. 2007; Christensen et al. 2011). Inappropriate activation of SOCE in GMCs may enhance GMCs proliferation and contraction leading to decreased GFR. Therefore, it may be one of mechanisms for causing hypertension and diabetic kidney disease. However, recent study reported that SOCE reduced in platelets from diabetic patients (Jardin et al. 2011). Thus, the change of SOCE and its elements in GMCs of diabetic animals is still remained.
Beside STIM 1 and Orai 1, other isoforms including STIM 2, Orai 2, and Orai 3 are also widely expressed in mammalian cells. STIM 2 can weakly activate Orai and is more sensitive to small changes in ER Ca2+ concentration (Soboloff et al. 2012). Recent studies show that Orai 1, Orai 2, and Orai 3 are all expressed in the kidney (Berna-Erro et al. 2012). But the expression pattern and functional role of STIM 2, Orai 2, and Orai 3 in mesangial cells are absent in the present study and still unknown. Therefore, we cannot exclude these components to function in SOCE and proliferation in GMCs. In future, this study should be beneficial to make the whole picture clear. However, our finding safely suggests that STIM 1 and Orai 1 importantly involve in SOCE and proliferation in GMCs. In conclusion, our data suggest that the attenuated GMC proliferation in aging can be attributed to the decrease in SOCE partially caused by reduced expression of STIM 1 and Orai 1.
Electronic supplementary material
(PDF 67 kb)
Acknowledgments
This work was supported by grants from NSF of China (grant no. 30800384); NSF of Anhui Province Department of Education (grant no. KJ2008B292); Scientific Research of BSKY (grant nos. XJ201106 and XJ200913) and Young Prominent Investigator Supporting Program from Anhui Medical University; Anhui Provincial Natural Science Foundation (11040606M171 and 1108085J11); and National Institutes of Health (NIH) of USA (5RO1DK079968-01A2m Ma).
Contributor Information
Bing Shen, Phone: +86-551-5161132, FAX: +86-551-5161126, Email: shenbing@ahmu.edu.cn.
Juan Du, Phone: +86-551-5161132, FAX: +86-551-5161126, Email: dujuanf@yahoo.com.cn.
References
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103(45):16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Woodard GE, Rosado JA. Orais and STIMs: physiological mechanisms and disease. J Cell Mol Med. 2012;16(3):407–424. doi: 10.1111/j.1582-4934.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295(3):C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113(9):2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- Christensen M, Bagger JI, Vilsboll T, Knop FK. The alpha-cell as target for type 2 diabetes therapy. Rev Diabet Stud. 2011;8(3):369–381. doi: 10.1900/RDS.2011.8.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudna RE, Dickson AJ. Endoplasmic reticulum signaling as a determinant of recombinant protein expression. Biotechnol Bioeng. 2003;81(1):56–65. doi: 10.1002/bit.10445. [DOI] [PubMed] [Google Scholar]
- Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286(3):F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- He H, Lam M, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol. 1997;138(6):1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I, Lopez JJ, Zbidi H, Bartegi A, Salido GM, Rosado JA. Attenuated store-operated divalent cation entry and association between STIM1, Orai1, hTRPC1 and hTRPC6 in platelets from type 2 diabetic patients. Blood Cells Mol Dis. 2011;46(3):252–260. doi: 10.1016/j.bcmd.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104(22):9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L17–L25. doi: 10.1152/ajplung.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Smith S, Child A, Carmines PK, Sansom SC. Store-operated Ca(2+) channels in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2000;278(6):F954–F961. doi: 10.1152/ajprenal.2000.278.6.F954. [DOI] [PubMed] [Google Scholar]
- Ma R, Kudlacek PE, Sansom SC (2002) Protein kinase Calpha participates in activation of storeoperated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Cell Physiol 283(5):C1390–C1398. [DOI] [PubMed]
- Mene P, Teti A, Pugliese F, Cinotti GA. Calcium release-activated calcium influx in cultured human mesangial cells. Kidney Int. 1994;46(1):122–128. doi: 10.1038/ki.1994.251. [DOI] [PubMed] [Google Scholar]
- Mene P, Pugliese F, Cinotti GA. Regulation of capacitative calcium influx in cultured human mesangial cells: roles of protein kinase C and calmodulin. J Am Soc Nephrol. 1996;7(7):983–990. doi: 10.1681/ASN.V77983. [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282(12):9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8(7):771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23(8):2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. Origins of the concept of store-operated calcium entry. Front Biosci (Schol Ed) 2011;3:980–984. doi: 10.2741/202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Kwan HY, Ma X, Wong CO, Du J, Huang Y, Yao X. cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J Biol Chem. 2011;286(22):19439–19445. doi: 10.1074/jbc.M110.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short AD, Bian J, Ghosh TK, Waldron RT, Rybak SL, Gill DL. Intracellular Ca2+ pool content is linked to control of cell growth. Proc Natl Acad Sci U S A. 1993;90(11):4986–4990. doi: 10.1073/pnas.90.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol. 2003;14(4):873–880. doi: 10.1097/01.ASN.0000060804.40201.6E. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281(30):20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13(9):549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med (Maywood) 2009;234(6):673–682. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002;92(4):1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4(11):E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004;287(2):C357–C364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- Wang X, Pluznick JL, Settles DC, Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Renal Physiol. 2007;293(6):F1768–F1776. doi: 10.1152/ajprenal.00365.2007. [DOI] [PubMed] [Google Scholar]
- Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta. 2002;1596(1):131–137. doi: 10.1016/S0167-4838(02)00211-X. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Meng H, Li ZH, Shu Z, Ma X, Zhang BX. Regulation of STIM1, store-operated Ca2+ influx, and nitric oxide generation by retinoic acid in rat mesangial cells. Am J Physiol Renal Physiol. 2007;292(3):F1054–F1064. doi: 10.1152/ajprenal.00286.2006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol. 2010;17(1):112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 67 kb)