Abstract
Although aging is commonly linked to a reduction in joint range of motion, it is unclear if all body joints behave similarly. To address this issue, the main purpose of this study was to compare age-related loss of mobility of seven body joints. A total of 6,000 participants (3,835 men and 2,165 women) aged 5 to 92 years took part in this study. The maximal passive range of motion of 20 movements was evaluated by Flexitest, and each movement was scored from 0 to 4. Composite scores were obtained for each of seven joints and for overall flexibility (Flexindex (FLX)) by adding individual movement scores. Confirming previous findings, FLX systematically decreased with aging (p < .001), with female participants being more flexible for all ages (p < 0.001) and having a more gradual, 0.6 % vs. 0.8 %/year, age reduction (p < .001). Starting at 30 and 40 years, respectively, for male and female participants, the relative contribution of each composite joint score to FLX dramatically changed. Shoulder contribution to FLX male’s score went from 13.9 % at 28 years of age to only 5.2 % at 85 years of age. In general, proportionally, shoulder and trunk became less flexible, while elbow and knee mobility was preserved to a greater extent. Our findings indicated that age-related loss of mobility is rather joint-specific, which could be related to distinct routine usage patterns of the major body joints along life.
Keywords: Aging, Flexibility, Range of motion, Hypermobility, Musculoskeletal fitness, Exercise
Introduction
Progressive aging of the human population has triggered a growing interest in the study of physical fitness and age. Since the 1940s, flexibility has been recognized as part of physical fitness (Cureton 1941), and several classic studies (Beighton et al. 1973; Boone and Azen 1979; Kendall and Kendall 1948; Leighton 1955; Roaas and Andersson 1982; Roach and Miles 1991; Wells and Dillon 1952) have included a flexibility assessment as part of a functional evaluation of subjects in selected age groups.
Although stretching exercises are routinely included in about all health and performance-oriented exercise programs (Garber et al. 2011), flexibility has been much less studied than other fitness variables, which, at least in part, may reflect the limited number of evaluation methods. While the sit-and-reach test, proposed about 60 years ago (ACSM 2009; Wells and Dillon 1952), is still the most widely used, most of the simple composite (multijoints or multimovements) flexibility tests have been historically criticized and seem to portend limited utility in clinical and athletic settings (Bozic et al. 2010; Harris 1967; Holland 1968; Jackson et al. 1998). However, it is possible that many of these previous limitations or constraints of older flexibility assessment protocols were overcome by the Flexitest (Araújo 1986, 2003), which comprises passive measurements of maximal range of motion in 20 body movements and generates an overall flexibility score called Flexindex (FLX).
In a scientific context, flexibility may be defined as the maximum physiological passive range of joint (Araújo 2003). On the other hand, range of motion is specific to a given joint and a movement (Corbin 1984; Cureton 1941; Dickinson 1968; Harris 1969). And so, it is possible to be quite flexible in one movement at a given joint and, at the same time, presenting with a very poor mobility in other joint movements (Dickinson 1968; Harris 1969). While there is no question that range of motion tends to diminish with aging (Araújo 2008; Barnes et al. 2001; Beighton et al. 1973; Doriot and Wang 2006; Intolo et al. 2009; Roach and Miles 1991), it is still unclear if all major joints behave similarly and if so, what is the rate of loss along the years. In this context, using a large set of Flexitest data, the main objective of this study was to assess if the rate of age-related mobility loss differs among several major body joints.
Methods
Sample
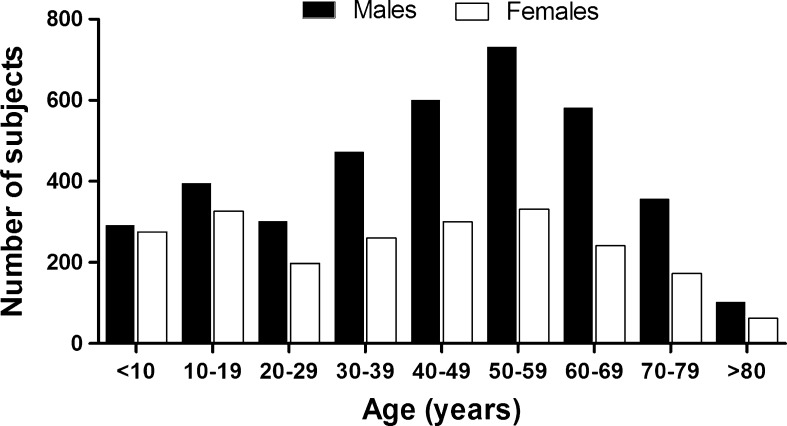
Data obtained from 1978 to 2011 (33 years) in our research labs were retrospectively analyzed. The subjects were evaluated either at their own request (or legal guardians, when under 18 years of age) or by referral of their attending physicians. The vast majority of them lived in Rio de Janeiro city, were white, and belonged to the upper socioeconomic and educational/cultural strata from the Brazilian population. Starting from all 7,850 subjects that had their flexibility assessed, we carefully excluded those formally engaged in sports competitions or professional dancing, physically handicapped and/or having a registry of abnormal joint motion related to specific acute or chronic diseases. Data from a single evaluation of the remaining 6,000 subjects (3,835 males/2,165 females and hereafter named participants, between 5 and 92 years of age (42.6 ± 22 years of age; mean ± standard deviation) were studied. The age of the participants was normally distributed for both sexes (Fig. 1).
Fig. 1.
Age and gender distribution of the study’s sample
Protocol
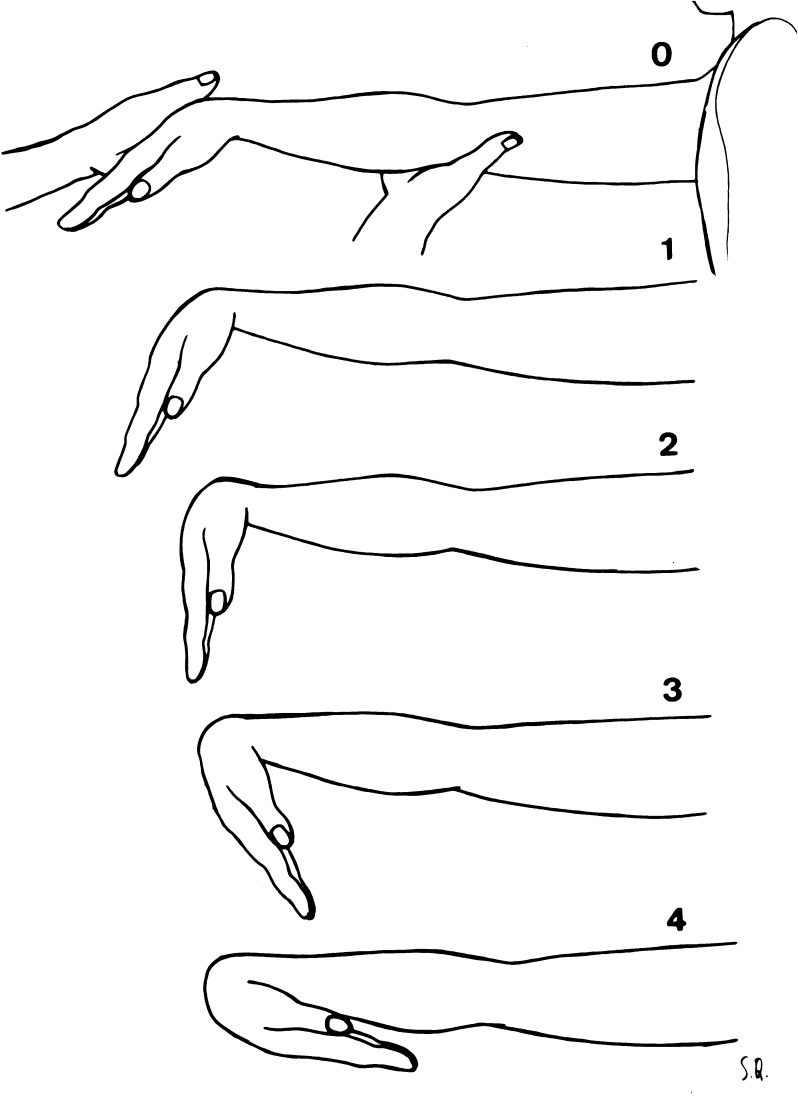
Passive range of motion in 20 body movements was assessed by Flexitest protocol that is described in detail elsewhere (Araújo 2003; Araújo and Chaves 2005; Brito et al. 2013; Chaves et al. 2002), including age and sex-specific percentile curves (Araújo 2008). Briefly, results obtained from the Flexitest are dimensionless and presented as points, with no linear or angular unit featured. It consists of the assessment of the maximum passive range of motion of 20 body joint movements, encompassing the ankle: dorsal and plantar flexion (two); knee: flexion and extension (two); hip: flexion, extension, adduction, and abduction (four); “trunk” (for simplicity, considered as a single joint): anterior and lateral flexion and extension (three); wrist: flexion and extension (two); elbow: flexion and extension (two); and shoulder: posterior abduction from a 180° of adduction, posterior adduction, posterior extension, and lateral and medial rotations (five) joints. Eight assessed movements are in the lower limbs, three are related to the trunk, and the remaining nine are in the upper limbs. For each of the movements, there are five possible scores to rate flexibility, graded from 0 to 4 in a crescent mobility order, meaning that a wider range of motion is scored higher. Once the maximum tolerable amplitude is reached, it is compared with the evaluation maps (see example in Fig. 2). A numerical score is ascribed whenever the amplitude reached equals that in the standard evaluation map. For instance, when the amplitude reaches position 2 of the map, score 2 is given until the maximum range of motion reaches the level of score 3. There is no fractional or intermediate score neither does the scoring take into account the closest grade of the scale. For bilateral movements, only the measurements for the right side of the body are used. A modified sequence of movements is used minimizing changes in body posture. In some instances, especially for older subjects or those that were more unfit, there was a partial adaptation in the original Flexitest protocol, avoiding the “lying down in the floor” position, which was replaced with movements either at the orthostatic position or lying down in an examining bed. It is relevant to consider that, for the sake of standardization, no previous warm-up is allowed. The time taken to apply the test varies according to the evaluator’s experience and the condition of the subject being assessed, but it typically lasts 3 to 5 min.
Fig. 2.
Example of a Flexitest evaluation map—movement XII: wrist flexion
According to the scale of measurement and the way that each one of the 20 evaluation maps were designed, data following a Gaussian distribution centering on score 2, with scores 1 and 3 less common and the extreme scores, 0 and 4, quite rare. So it is valid to add together the results of each of the 20 movements to obtain an overall flexibility or joint mobility index, called the Flexindex (FLX), representing a major advantage over other methods such as goniometry, where this is not possible. Additionally, there are no so-called ceiling and floor effects for FLX often seen with other flexibility tests, as, for example, the Beighton–Horan joint laxity test [9], since the maximum extreme values (0 and 80) have never actually obtained. Intraclass correlation coefficients were used to assess reliability in different settings: in summary, intra-observer r values ranged from .78 to .99 (median of .93 for the 20 movements) and went up to .99 for FLX scores. Considering interobserver reliability, slightly although highly significant r values of .95 were found to FLX scores (Araújo 2003). Long-term clinical practice has shown that experienced evaluators tend to agree in scoring 18 or 19 of the 20 movements, and the difference between judgments is never above one point. Concurrent validity of the Flexitest was assessed against sit-and-reach and Beighton–Horan tests by using 30 subjects from 2 to 54 years of age with FLX scores ranging from 35 to 68 and r values were .40 and .91, respectively. Corroborating the recognized limitations of the sit-and-reach test for overall assessment of body flexibility, there were no significant association (r = .26) between the results of Beighton–Horan and sit-and-reach (Araújo 2003; Silva et al. 2000).
For this study, besides the calculation of FLX, two other approaches were undertaken, allowing a direct comparison of flexibility scores amongst different joints. Initially, the Flexitest scores for each individual movement in a given joint were summed and then standardized by dividing this sum by the number of movements for each individual joint (e.g., four to hip, five to shoulder, etc.). In sequence, the individual contribution of each one of these seven standardized joint scores to the FLX was expressed as a percent of total and analyzed according to age and separately by sex. Using these approaches, we were able to directly compare the flexibility profile of each joint despite their differences in the number of movements assessed.
All flexibility measurements included in this 33 years of retrospective analysis were carried out by just four exercise and sports medicine physicians and three physical educators highly experienced in using the Flexitest. Following the Flexitest’s protocol, no warm-up exercise was provided before the actual measurement. For practical reasons, it was not possible to standardize the time of the day for the evaluation of the 6,000 participants; however, previous data from our laboratory has indicated a quite small circadian variability (Araújo 2003).
For the Flexitest’s evaluations carried out between 1978 and 1993, there were no obligations to obtain informed consent according to the country’s federal laws. Starting in 1994, all subjects (if minors, the legal guardians) read and signed an informed consent form and agreed in being submitted to the Flexitest. All testing procedures and retrospective data analyses were formally approved by an institutional research and ethics committee.
Statistical analysis
Descriptive statistics were calculated for the Flexitest data, 20 individual movements and FLX, separated by sex. The frequency of two specific combinations of scores, 0 and 4, in the assessment of the flexibility of the 20 movements, was obtained for all participants. Both visual inspection of FLX’s data plotting and best curve-fitting modeling directed us to perform linear regression analysis between FLX and age for both sexes. Subsequently, the results of the joint motion profile with aging were also studied by linear regression analysis, by relating the corresponding joint standardized percent contribution to the FLX (sum of the seven standardized joint scores equal to 100 %) to age expressed in years, separately for both sexes, and their slopes compared. For this, the mean percent of the FLX standardized score for each one of the seven joints was calculated at specific age groups, at 5-year intervals (except for a 7-year grouping in the oldest group, 85–91 years old), comprising a total of 17 age groups. Linear regression slopes were compared, and the Runs test was used to assess the adequacy of linearity of the models. The chi-square statistic was computed for comparing frequencies of presence or absence of extreme scores in participants of both sexes. The relationship between variables was assessed by Pearson correlation. The significance level was set at 5 % of probability. All calculations were performed and figures prepared by Prism 5.04 (GraphPad Software, Inc., USA).
Results
In this sample of 6,000 participants (36 % women) aged 5 to 98 years, FLX scores ranged from 6 to 76 points, being 6 to 71 points and 11 to 76 points, respectively, for male and female participants. The highest sex-specific FLX scores were found, respectively, in a 6-year-old boy and a 7-year-old girl. The interquartile range of FLX (third quartile minus first quartile) increased from approximately 6 points at childhood to 10 to 12 points at early adulthood, and then tended to plateau. For the whole sample, FLX scores followed a Gaussian distribution and both the mean and median were 41 points. In separate assessments according to sex, means and medians were 36 for male subjects and 47 for female subjects.
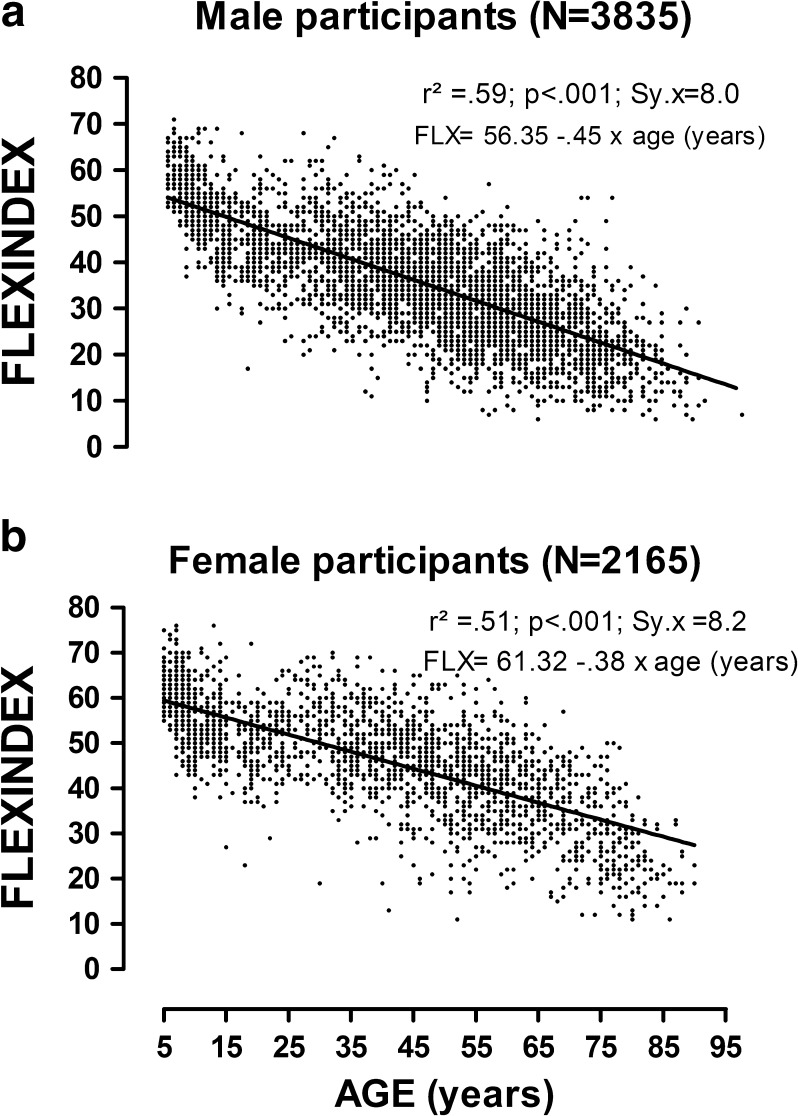
There was a clear trend for diminishing flexibility with aging (p < .001). Female participants tended to be more flexible from childhood to seniority (p < .001), and their age decay was significantly slower as compared to male participants. Specifically, the yearly average reduction in FLX score was somewhat faster, 0.6 % and 0.8 %, for female as compared to male participants (p < .001). Linear regression analysis between FLX and age (years) provided significant r2 values (p < .001), which were of 0.59 for male and 0.52 for female participants (see Fig. 3).
Fig. 3.
Linear regression equations for Flexindex (points) versus age (years): male (upper panel) and female (lower panel) participants
A mode of score 2 and all possible scores, i.e., 0 to 4, were observed for all of the 20 individual movements evaluated. The presence of 0 and 4 scores in at least one of the body movements evaluated were identified in 2,078 (34.6 %) and 4,248 (76.7 %) of the participants, respectively. There was a clear age trend for the frequency of those extreme scores, with 0 scores being more commonly found in the oldest participants and 4 scores more often seen in children of both sexes.
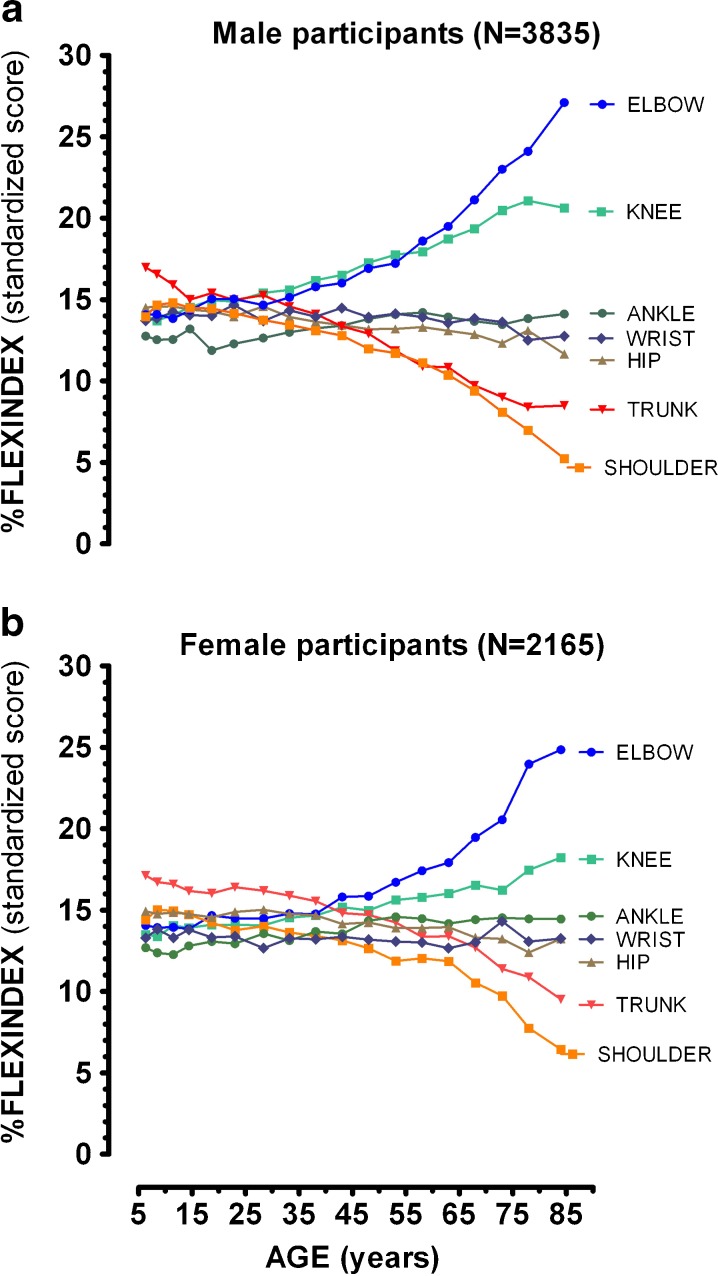
Table 1 shows the most relevant results derived from the linear regression analysis and from the Runs test used to evaluate the relationship between percent FLX standardized scores, and age. Slopes, expressed as percent of change in the FLX standardized score/year of age, demonstrated a very similar pattern for both sexes with a more pronounced decay in trunk and shoulder joint measurements, minimal changes along the years for the ankle, wrist, and hip measurements, and a proportional increase in the contribution of knee and elbow scores to overall flexibility (FLX). On the other hand, Runs test results permitted us to note that there were significant deviations from a linear model from the percent of FLX standardized score versus age expressed in years for some of the joints analyzed in this study, most notably the hip and wrist scores for participants of both sexes. A visual inspection of mean percent of standardized FLX score for each one of the seven joints at various ages shown in Fig. 4, men at the upper panel and women at the lower panel, demonstrated that values remained stable until approximately 30 and 40 years of age, respectively, for male and female participants. For example, if all the joints equally contributed to FLX, the percent of standardized FLX score would be 14.3 % (100 %/seven joints). Assessing the data for male participants, the shoulder’s percent of standardized FLX score was about 13.9 % at 6 years of age, remained about constant in 13.8 % at 28 years of age and then substantially dropped, achieving a value of 11.1 % at 58 years of age and 5.2 % at 85 years of age, with a very similar pattern for female participants.
Table 1.
Linear regression analysis and Runs test results: Percent Flexindex standardized scores for reach one of the seven joints versus age (years) for 6,000 participants
| Body joints | Ankle | Knee | Hip | Trunk | Wrist | Elbow | Shoulder |
|---|---|---|---|---|---|---|---|
| Male participants (N = 3,835) | |||||||
| Regression analysis (p value) | <.001a | <.001a | <.001a | <.001a | .001a | <.001a | <.001a |
| Slope (%/year of age) | .022 ± .003 | 0.94 ± .003 | −.031 ± .002 | −.111 ± .005 | −.012 ± .004 | .144 ± .014 | −.106 ± .009 |
| Runs test (p value) | .044a | .003a | .484 | .109 | .419 | <.001a | <.009a |
| Deviation from linearity | Yes | Yes | No | Yes | No | Yes | Yes |
| Female participants (N = 2,165) | |||||||
| Regression analysis (p value) | <.001a | <.001a | <.001a | <.001a | .472 | <.001a | <.001a |
| Slope (%/year of age) | .029 ± .003 | .052 ± .003 | −.026 ± .003 | −.084 ± .006 | −.002 ± .003 | .122 ± .014 | −.090 ± .009 |
| Runs test (p value) | .682 | .145 | .251 | <.001a | .975 | <.001a | <.001a |
| Deviation from linearity | Yes | No | No | Yes | No | Yes | Yes |
Slope values are expressed as mean ± standard error of the mean
aSignificant for regression analysis or significant deviation from linearity (Runs test)
Fig. 4.
Percent of Flexindex standardized score for each one of the seven joints versus age (years). Higher percentage means that the relative contribution of the joint’s results to the Flexindex (sum of the scores of the 20 individual movements) is larger. Male (upper panel) and female (lower panel) participants
Discussion
To the best of our knowledge, this is the largest and most comprehensive flexibility study ever performed, and its results demonstrate several novel findings. Most of the literature relating flexibility and age were obtained using active measurements, in which there are several confounding variables such as subject’s muscle strength and motivation to reach a true maximal range of motion. Previously applied passive techniques, such as goniometry, have been applied in relatively small samples (Soucie et al. 2011) and are very limited to assess trunk mobility. In addition to the large sample size, the exclusion of participants with locomotor disabilities and competitive athletes, the wide age range, the inclusion of participants of both sexes, and the number of body joint movements assessed makes the current investigation particularly impactful. Thus, these results may, within the limitations of sample characteristics, be extended for similar populations.
An interesting feature of this study is the use of the Flexitest, a dimensionless flexibility assessment protocol. Flexitest’s intra- and interobserver reliabilities have been thoroughly evaluated (Araújo 2003), favorably contrasted with other methods used for joint range of motion assessment (Silva et al. 2000), and it has been used in several research studies (Araújo 2008; Araújo and Araújo 2004; Araújo and Chaves 2005; Brito et al. 2013, 2012; Chaves et al. 2002; Coelho and Araújo 2000; Nobrega et al. 2005; Signorelli et al. 2012a, b; Silva et al. 2000). Flexitest also presents several positive features, such as easiness by which mastering the measurement technique is achieved, standardization of no previous warming up routine, and the relatively short time for evaluation. In addition, no equipment or large space is needed, and detailed age and sex-specific norms are easily available (Araújo 2002, 2003, 2008).
Similar to several previous studies that have used different populations and methods for joint range of motion assessment (Beighton et al. 1973; Boone and Azen 1979; Roach and Miles 1991; Salter 1955; Soucie et al. 2011), it was found that flexibility is significantly reduced with age for both sexes. However, the rate of decline is slightly but significantly slower for female as compared to male participants, which is a particularly novel finding of the current investigation. While the biological reasons for this finding are still unclear and out of the scope of the current study, in this context, the female’s flexibility advantage is quite small or minimal for pre-pubertal children and progressively increases towards seniority.
Of course, overall flexibility decay is not fully explained by aging in both males and females. In reality, just 50–60 % of the FLX variability was explained by age. It is highly possible that adoption of a less active lifestyle starting at the first years of adult life and increasing in prevalence to middle age and then to seniority contributes for this result. As a matter of fact, as it also occurs with other physical fitness attributes, flexibility variability substantially increases with age, which is plausibly related to large differences in regular physical activity/exercise patterns.
On the other hand, it should be emphasized that there are also some limitations in our study, including the specific demographic characteristics of the sample, the cross-sectional rather than an ideal longitudinal design, and the lack of detailed information regarding regular physical activity pattern of the participants evaluated. Notwithstanding, it is very plausible to hypothesize that these limitations have not affected the core of the interpretation of the data, particularly given the large sample size.
A recent and very comprehensive systematic review showed that flexibility can be improved by specific training; however, according to these authors, there are several constraints in the 22 studies analyzed that preclude obtaining a more definitive answer, regarding if these benefits are transformed in better functional outcomes for older adults (Stathokostas et al. 2012). Although, by reading their data, indeed, it can be identified that most studies are relatively short (few weeks long) and that flexibility was most often assessed by multicomposite tests, such as sit-and-reach, which, in our opinion, may compromise the interpretation of the information. While it is widely recognized that to have some degree of range of motion in the major body joints is needed for performing most of the daily living activities, such as sitting and rising from the floor (Brito et al. 2013), an ability that have been recently shown to be predictive of all-cause mortality in middle age and older adults (Brito et al. 2012), it is still unknown what the ideal overall flexibility level for health is (Jackson et al. 1998) or if there is a cutoff value for FLX regarding personal autonomy. There are some evidence that low levels of flexibility could be related to risk of falls in older adults, leading the authors to recommend that flexibility training be a priority for those subjects (Menz et al. 2006; Dai et al. 2012). Distinctly from aerobic fitness, for which it seems that there is an upper limit for improvement that can be considered to be harmful or unhealthy, especially for middle-aged subjects (Myers 2002), the same does not always occur with flexibility (Gleim and McHugh 1997). In reality, extremely high levels of joint mobility, such as those often associated with hypermobility, are often indicative of some connective tissue disorders (Araújo and Chaves 2005; Grahame and Hakim 2008) and seem to predispose individuals to injury (Gleim and McHugh 1997). So, it seems that the relationship between flexibility and health is more likely U-shaped, with extremely low and high values being considered adverse. Although, flexibility or stretching routines are currently recommended as part of an exercise session, surprisingly the prescription tend to be general and non-individualized. On the other hand, more often these exercises are included at the end of the session, attempting to avoid potential deleterious effects on strength performance (Costa et al. 2011, 2010; Gleim and McHugh 1997; Rubini et al. 2007).
In a clinical context, while scores of 0 and 1 in the Flexitest assessment should be selected for improvement in the range of motion in an individualized prescription of a routine of flexibility exercises, it can be suggested that those movements scored as 4 in the Flexitest should not be targeted for systematic stretching training. This is especially true for subjects that are not regularly engaged in competitive sports or dance activities, as it was the case of our participants. In practical terms, it seems that there are no benefits for health in being hypermobile in many joints or body movements.
Focusing in the main objective of this study, our data clearly show that, starting at the fourth or fifth decade of life, loss of flexibility with aging is faster in some of the major body joints as compared to others. Similar trends for both sexes, indicating that the trunk and shoulders are the ones most affected by aging. Additionally, these data further corroborate the well-established concept of specificity for flexibility and also reinforces the recommendation that for an adequate evaluation of flexibility in subjects of various age groups, the maximal range of motion should be quantified in several movements and in many different joints in order to get a more precise and unbiased overall appraisal (Araújo and Araújo 2004).
At this stage of discussion, some other points should be commented. In reality, since most of the degenerative conditions tend to occur in distal joints, especially in the knee and hand joints, missing a subclinical condition or disease in the participants would tend to bias our data for an even larger difference, since knee mobility was one of the less affected by age. Additionally, it should be recognized that the anatomical factors limiting the range of motion varied from one joint to other and even for different movements in the same joint. While our study was not aimed to determine the mechanism behind this profile, it can be speculated that extreme range of motion positions in the movements of these two most affected joints—shoulder and trunk—are rarely performed in daily living conditions, which may predispose to a more accelerated reduction in the maximal range of motion for their various movements. On the other hand, the relatively faster decay in the mobility of shoulder and trunk could be speculated to contribute negatively to the ability to recover from an abrupt loss of balance and then precipitating a fall.
Interestingly, there is some recent evidence showing that the elbow and knee joints were less prone to improve their range of motion after a 10-week strength training program in middle-aged women as compared to shoulder and trunk movements (Monteiro et al. 2008). This finding could be due to the fact that these former joints were already positioned in daily living near their maximal range of motion when compared to the shoulder and trunk joints. Notwithstanding, surely further studies are needed to determine the practical relevance of joint specificity for age-related loss of mobility, as demonstrated by our data, in order to refine rehabilitation recommendations and exercise prescription guidelines for general and, particularly, for the older population.
Unfortunately, there is currently no other available study that is similar to ours that would allow for a direct comparison of results. Likely, the closest one is a recent paper (Soucie et al. 2011) that has reported passive goniometry of 11 movements at five different joints from 600 men and women 2 to 69 years of age (their data tables are freely available from www.cdc.gov/normaljoints). However, these authors did not run any joint comparisons with aging, precluding a more objective comparison with our data.
In summary, Flexitest assessment of 20 body movements from seven major joints in 6,000 participants of both sexes (a total of 120,000 flexibility measurements) further advances the concept that there is a significant trend for an aging loss of mobility by showing that this reduction in mobility is rather joint-specific, which is suggested to be related to different patterns of daily living usage. Considering our results, it is interesting to consider specifically age-guided prescription of stretching exercise routines for middle-aged and older subjects, emphasizing these body joints that are more susceptible to a faster age loss of mobility. Although, theoretically logical, this very practical approach should be validated by future long-term observational studies or randomized trials combining serial flexibility assessments and an age-guided stretching exercise prescription.
Acknowledgments
The authors express their gratitude to the financial support provided by Conselho Nacional de Pesquisa e Desenvolvimento Científico e Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro.
References
- ACSM's guidelines for exercise testing and prescription. 8. Baltimore: Lippincott; 2009. [Google Scholar]
- Araújo CGS. Flexiteste: uma nova versão dos mapas de avaliação. Kinesis. 1986;2(2):251–267. [Google Scholar]
- Araújo CGS. Flexitest: proposal of five variability indices for joint mobility. Rev Bras Med Esporte. 2002;8(1):13–19. doi: 10.1590/S1517-86922002000100003. [DOI] [Google Scholar]
- Araújo CGS. Flexitest: an innovative flexibility assessment method. 1. Champaign: Human Kinetics; 2003. [Google Scholar]
- Araújo CGS. Flexibility assessment: normative values for flexitest from 5 to 91 years of age. Arq Bras Cardiol. 2008;90(4):257–263. doi: 10.1590/S0066-782X2008000400008. [DOI] [PubMed] [Google Scholar]
- Araújo CGS, Araújo DSMS. Flexitest: inappropriate use of condensed versions. Rev Bras Med Esporte. 2004;10(5):381–384. doi: 10.1590/S1517-86922004000500005. [DOI] [Google Scholar]
- Araújo CGS, Chaves CP. Adult women with mitral valve prolapse are more flexible. Br J Sports Med. 2005;39(10):720–724. doi: 10.1136/bjsm.2004.014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CJ, Van Steyn SJ, Fischer RA. The effects of age, sex, and shoulder dominance on range of motion of the shoulder. J Shoulder Elbow Surg. 2001;10(3):242–246. doi: 10.1067/mse.2001.115270. [DOI] [PubMed] [Google Scholar]
- Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DC, Azen SP. Normal range of motion of joints in male subjects. J Bone Joint Surg Am. 1979;61(5):756–759. [PubMed] [Google Scholar]
- Bozic PR, Pazin NR, Berjan BB, Planic NM, Cuk ID. Evaluation of the field tests of flexibility of the lower extremity reliability and the concurrent and factorial validity. J Strength Cond Res. 2010;24(9):2523–2531. doi: 10.1519/JSC.0b013e3181def5e4. [DOI] [PubMed] [Google Scholar]
- Brito LBB, Araújo DSMS, Araújo CGS. Does flexibility influence the ability to sit and rise from the floor? Am J Phys Med Rehab. 2013;92(3):241–247. doi: 10.1097/PHM.0b013e3182744203. [DOI] [PubMed] [Google Scholar]
- Brito LBB, Ricardo DR, Araújo DSMS, Ramos PS, Araújo CGS (2012) Ability to sit and rise from the floor as a predictor of all-cause mortality. Eur J Prev Cardiol. doi:10.1177/2047487312471759 [DOI] [PubMed]
- Chaves CP, Simão R, Araújo CGS. Lack of flexibility variation during menstrual cycle in university students. Rev Bras Med Esporte. 2002;8(6):212–218. doi: 10.1590/S1517-86922002000600002. [DOI] [Google Scholar]
- Coelho CW, Araújo CGS. Relação entre aumento da flexibilidade e facilitações na execução de ações cotidianas em adultos participantes de programa de exercício supervisionado. Rev Bras Ciên Mov. 2000;2(1):31–41. doi: 10.1590/S1516-635X2000000100005. [DOI] [Google Scholar]
- Corbin CB. Flexibility. Clin Sports Med. 1984;3(1):101–117. [PubMed] [Google Scholar]
- Costa PB, Ryan ED, Herda TJ, Walter AA, Defreitas JR, Stout JR, Cramer JT. Acute effects of static stretching on peak torque and the hamstrings-to-quadriceps conventional and functional ratios. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- Costa PB, Ryan ED, Herda TJ, Walter AA, Hoge KM, Cramer JT. Acute effects of passive stretching on the electromechanical delay and evoked twitch properties. Eur J Appl Physiol. 2010;108(2):301–310. doi: 10.1007/s00421-009-1214-3. [DOI] [PubMed] [Google Scholar]
- Cureton TK. Flexibility as an aspect of physical fitness. Res Quart. 1941;12:381–390. [Google Scholar]
- Dai B, Ware WB, Giuliani CA. A structural equation model relating physical function, pain, impaired mobility (IM), and falls in older adults. Arch Gerontol Geriatr. 2012;55(3):645–652. doi: 10.1016/j.archger.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Dickinson RV. The specificity of flexibility. Res Quart. 1968;39:792–794. [PubMed] [Google Scholar]
- Doriot N, Wang X. Effects of age and gender on maximum voluntary range of motion of the upper body joints. Ergonomics. 2006;49(3):269–281. doi: 10.1080/00140130500489873. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gleim GW, McHugh MP. Flexibility and its effects on sports injury and performance. Sports Med. 1997;24(5):289–299. doi: 10.2165/00007256-199724050-00001. [DOI] [PubMed] [Google Scholar]
- Grahame R, Hakim AJ. Hypermobility. Curr Opin Rheumatol. 2008;20(1):106–110. doi: 10.1097/BOR.0b013e3282f31790. [DOI] [PubMed] [Google Scholar]
- Harris ML. Flexibility. Phys Ther. 1967;49(6):591–600. doi: 10.1093/ptj/49.6.591. [DOI] [PubMed] [Google Scholar]
- Harris ML. A factor analytic study of flexibility. Res Q. 1969;40(1):62–70. [PubMed] [Google Scholar]
- Holland GJ. The physiology of flexibility: a review of the literature. Kinesiol Rev. 1968;1:49–62. [Google Scholar]
- Intolo P, Milosavljevic S, Baxter DG, Carman AB, Pal P, Munn J. The effect of age on lumbar range of motion: a systematic review. Man Ther. 2009;14(6):596–604. doi: 10.1016/j.math.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Jackson AW, Morrow JR, Jr, Brill PA, Kohl HW, 3rd, Gordon NF, Blair SN. Relations of sit-up and sit-and-reach tests to low back pain in adults. J Orthop Sports Phys Ther. 1998;27(1):22–26. doi: 10.2519/jospt.1998.27.1.22. [DOI] [PubMed] [Google Scholar]
- Kendall HO, Kendall FP. Normal flexibility according to age groups. J Bone Joint Surg Am. 1948;30A(3):690–694. [PubMed] [Google Scholar]
- Leighton JR. Flexibility characteristics of males 10 to 18 years of age. Arch Phys Med Rehabil Arch Phys Med Rehabil. 1955;36(9):571–578. [Google Scholar]
- Menz HB, Morris ME, Lord SR. Foot and ankle risk factors for falls in older people: a prospective study. J Gerontol A Biol Sci Med Sci. 2006;61(8):866–870. doi: 10.1093/gerona/61.8.866. [DOI] [PubMed] [Google Scholar]
- Monteiro WD, Simao R, Polito MD, Santana CA, Chaves RB, Bezerra E, Fleck SJ. Influence of strength training on adult women's flexibility. J Strength Cond Res. 2008;22(3):672–677. doi: 10.1519/JSC.0b013e31816a5d45. [DOI] [PubMed] [Google Scholar]
- Myers J. Exercise capacity and mortality among men referred for exercise testing. New Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Paula KC, Carvalho AC. Interaction between resistance training and flexibility training in healthy young adults. J Strength Cond Res. 2005;19(4):842–846. doi: 10.1519/r-15934.1. [DOI] [PubMed] [Google Scholar]
- Roaas A, Andersson GB. Normal range of motion of the hip, knee and ankle joints in male subjects, 30–40 years of age. Acta Orthop Scand. 1982;53(2):205–208. doi: 10.3109/17453678208992202. [DOI] [PubMed] [Google Scholar]
- Roach KE, Miles TP. Normal hip and knee active range of motion: the relationship to age. Phys Ther. 1991;71(9):656–665. doi: 10.1093/ptj/71.9.656. [DOI] [PubMed] [Google Scholar]
- Rubini EC, Costa AL, Gomes PS. Effects of stretching on strength performance. Sports Med. 2007;37(3):213–224. doi: 10.2165/00007256-200737030-00003. [DOI] [PubMed] [Google Scholar]
- Salter N. Methods of measurement of muscle and joint function. J Bone Joint Surg Br. 1955;37-B(3):474–491. doi: 10.1302/0301-620X.37B3.474. [DOI] [PubMed] [Google Scholar]
- Signorelli GR, Duarte CV, Ramos PS, Araújo CGS. Melhoria da capacidade funcional excede a da condição aeróbica: dados de 144 pacientes de programa de exercício. Revista Brasileira de Cardiologia. 2012;25(4):299–308. [Google Scholar]
- Signorelli GR, Perim RR, Santos TM, Araújo CG. A pre-season comparison of aerobic fitness and flexibility of younger and older professional soccer players. Int J Sports Med. 2012;33(11):867–872. doi: 10.1055/s-0032-1311597. [DOI] [PubMed] [Google Scholar]
- Silva LPS, Palma A, Araújo CGS. Validity of perception in the flexibility evaluation of adults evaluation of adults. Rev Bras Ciên e Mov. 2000;8(3):15–20. [Google Scholar]
- Soucie JM, Wang C, Forsyth A, Funk S, Denny M, Roach KE, Boone D, Hemophilia Treatment Center N Range of motion measurements: reference values and a database for comparison studies. Haemophilia. 2011;17(3):500–507. doi: 10.1111/j.1365-2516.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- Stathokostas L, Little RM, Vandervoort AA, Paterson DH. Flexibility training and functional ability in older adults: a systematic review. J Aging Res. 2012;2012:306818. doi: 10.1155/2012/306818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KF, Dillon EK. The sit and reach—a test of back and leg flexibility. Res Quart. 1952;23:115–118. [Google Scholar]