Abstract
Chronic treatment with β-adrenergic receptor (βAR) agonists increases mortality and morbidity while βAR antagonists (β-blockers) decrease all-cause mortality for those at risk of cardiac disease. Levels of sympathetic nervous system βAR agonists and βAR activity increase with age, and this increase may hasten the development of age-related mortality. Here, we show that β-blockers extend the life span of healthy metazoans. The β-blockers metoprolol and nebivolol, administered in food daily beginning at 12 months of age, significantly increase the mean and median life span of isocalorically fed, male C3B6F1 mice, by 10 and 6.4 %, respectively (P < 0.05). Neither drug affected the weight or food intake of the mice, indicating that induced CR is not responsible for these effects, and that energy absorption and utilization are not altered by the drugs. Both β-blockers were investigated to control for their idiosyncratic, off-target effects. Metoprolol and nebivolol extended Drosophila life span, without affecting food intake or locomotion. Thus, βAR antagonists are capable of directly extending the life span of two widely divergent metazoans, suggesting that these effects are phylogenetically highly conserved. Thus, long-term use of β-blockers, which are generally well-tolerated, may enhance the longevity of healthy humans.
Keywords: Adrenoceptor, β-blocker, Longevity, Life span, Intracellular signaling, Epinephrine, Norepinephrine, Octopamine, Catecholamine
Introduction
Sympathomimetic amines such as epinephrine, norepinephrine, and dopamine are agonists for the β-adrenergic receptors (βARs) (Johnson and Terra 2002). Chronic administration of β-adrenergic receptor agonists increases mortality and morbidity in humans and other mammals (reviewed in Ho et al. 2010). In humans, enhanced production of β2-adrenergic receptors resulting from specific genetic variants is inversely associated with life span (Zhao et al. 2012). In contrast, βAR blockade with metoprolol or other β-blockers decreases mortality after acute myocardial infarct by 23 to 39 %, improves the health of individuals with heart failure, and may reduce the development of atherosclerosis (reviewed in Bristow 2000; Ellison and Gandhi 2005). Nebivolol, which is more selective for the β1 versus the β2 receptor than metoprolol, produces similar effects to metoprolol on the heart (Grassi et al. 2003; Rozec et al. 2006). Nebivolol also improves oxidative stress, insulin sensitivity, and plasma soluble P-selectin and adiponectin levels in hypertensive patients (Celik et al. 2006; Rozec et al. 2006). These effects may be due to off-target activation of the α1-adrenergic receptor (Celik et al. 2006; Rozec et al. 2006).
There has been no information regarding the effects of βAR inhibitors on the longevity of healthy animals. As part of a compound screening program designed to identify potential longevity therapeutics, we studied the effects of metoprolol and nebivolol on the life span of Drosophila melanogaster and an F1 hybrid mouse, C3B6F1. This dual screening approach was utilized to increase the likelihood that identified therapeutics would be capable generally of increasing the life span of metazoans, including humans (see discussion in Spindler 2012). The mouse study utilized an unbalanced statistical design to compare the life span of multiple treatment groups to that of one larger control group (Jeske et al. 2012).
The studies found that β1AR receptor blockade extends the median life span of mice and Drosophila without changing energy intake or utilization, suggesting that these effects are phylogenetically widespread, and therefore may extend to humans.
Materials and methods
Mouse life span and food consumption quantification
The studies used male B6C3F1 mice (Harlan Breeders; Indianapolis) randomly assigned to treatment groups. At 12 months of age, 297 mice were shifted from ad libitum chow feeding (diet no. 5001, Purina Mills, Richmond, IN) to daily feeding with 13.3 kcal/day/mouse of control diet (AIN-93M, diet no. F05312; Bio-Serv, Frenchtown, NJ). Thirty-six mice were shifted to daily feeding with an identical quantity of control diet supplemented with either metoprolol or nebivolol at the dosages indicated in the text. The 40 % CR group (36 mice) was shifted from ad libitum chow feeding to 11 kcal/day/mouse of AIN-93M 20 % restricted diet for 2 weeks, and thereafter to 7.46 kcal/day of AIN-93M 40 % restricted diet (diet no. F05314, Bio-Serv). The 40 % calorically restricted diet was fortified so the mice received fewer calories in the form of carbohydrate than the other groups but approximately equal amounts of fat, protein, vitamins, and minerals. Carbohydrate restriction was utilized because good experimental design specifies that to the degree possible only one parameter should be varied at a time to simplify the interpretation of results. Fifty-seven other groups were fed chemical agents in their diets. All mice were fed daily, beginning at 9 a.m. and ending at approximately 2 p.m. Feeding concluded sooner as the number of mice progressively declined as the study progressed. Food consumption and mouse health was monitored at the time of feeding, and any uneaten food was noted. With few exceptions, all food was eaten each day. The drugs were mixed with powdered diet and cold-pressed into 1-g pellets by Bio-Serv. The food was stored moisture free at 4 °C until used. The mice drank acidified (pH 4.0) tap water ad libitum. Acidification greatly reduces Pseudomonas colonization of water bottles and sippers, and the nasopharynx and intestines of mice (National Institutes of Health 1994; National Research 1991). The mice were weighed bimonthly. The mice were maintained in shoebox cages with 0.22-μm covers, under barrier conditions, four per cage, on a 12-h light/dark cycle at 22 °C, and 18 % humidity. Bedding was radiation sterilized. The health of the mice was examined twice daily by laboratory staff and weekly by a veterinarian. Dead mice were stored at −20 °C until necropsy. This study was approved by the Institutional Animal Care and Use Committee at the University of California, Riverside. Kaplan–Meier survival curves were compared using the Breslow–Wilcoxon test implemented in GraphPad Prism 5.01. The tests were not adjusted for multiple testing. The probability of detecting a false positive was less than 1 % (α ≤ 0.01) (Jeske et al. 2012).
Drug treatment and Western blotting
Ten-month old male C3B6F1 mice were treated with metoprolol (1.1 g/kg diet) or nebivolol (0.27 g/kg diet) in their diets as described above, for 2 months. Tissues were collected from drug-treated and control mice, snap frozen, and stored in liquid nitrogen. Protein extracts were prepared by sonication of approximately 40 mg of heart or brain tissues in 350 μL of SDS buffer (50 mM Tris-HCl, pH 6.8, 2 % SDS, and 10 % glycerol) containing 5 μL protease inhibitor cocktail (Sigma #P8340) and 5 μL phosphatase inhibitor cocktails 2 and 3 (Sigma #P5726 and P0044) for 20 s at a setting of 7 (Branson Sonifier Analog Cell Disruptor, Model S-450A), and centrifugation at 16,000×g for 20 min. Proteins (4 % of the total protein extract) were separated by SDS-PAGE, transferred to a PVDF membrane, probed with antibodies, and developed with ECL chemiluminescence (Amersham, #RPN2135, RPN2232, or RPN2235) according to standard procedures. The antibodies were directed against phospho-MEK1/2 (Ser217/221) (Cell Signaling, #9121), phospho-p44/42 MAPK (ERK1/2; Thr202/Tyr204) (Cell Signaling, #9101), protein kinase A (PKA) C-α (Cell Signaling, #5842), and phospho-PKA C (Thr197) (Cell Signaling, #5661). Secondary antibody conjugated to horseradish peroxidase was goat anti-rabbit IgG (Abcam, #ab6721). Band intensity was quantified with NIH ImageJ. Protein loading was determined using Ponceau S staining of PVDF membranes, and the data plotted using GraphPad Prism (version 5.01, www.graphpad.com). Statistical significance between control and drug-treated was determined with a two-sample t test, assuming equal variances.
Drosophila life span measurements
Longevity studies were conducted as described previously (Spindler et al. 2012a, b). Briefly, only male flies were used to avoid the confounds associated with reproductive behavior. Wild-type stocks of Oregon-R-C D. melanogaster were obtained every 6 months from Bloomington Drosophila Stock Center (Department of Biology, Indiana University, Bloomington, IN). Freshly enclosed flies were sorted and treated as described (Spindler et al. 2012a, b). A total of 200 flies per control and treatment groups were used for each study. The bottles were incubated at 25 °C, 60 % humidity, and with a 12:12-h light/dark cycle. The food was changed twice weekly. The number of flies living at each time point was determined by visual inspection. CO2 anesthesia was not used after the initial sorting of males. Life span differences between vehicle and drug-treated groups were determined using survival analysis followed by the log rank test.
Quantification of Drosophila food consumption and locomotion
Food consumption was quantified using modified capillary feeder (CAFE) (Ja et al. 2007) and fecal plaque assays (FPAs) (Driver et al. 1986; Min and Tatar 2006) as described (Spindler et al. 2012b), and the effects of the drugs on plaque size quantified as described (Spindler et al. 2012b). Locomotion was quantified as described previously, except that a LAM 32 activity monitor (TriKenetics) was utilized.
Results
Life span results
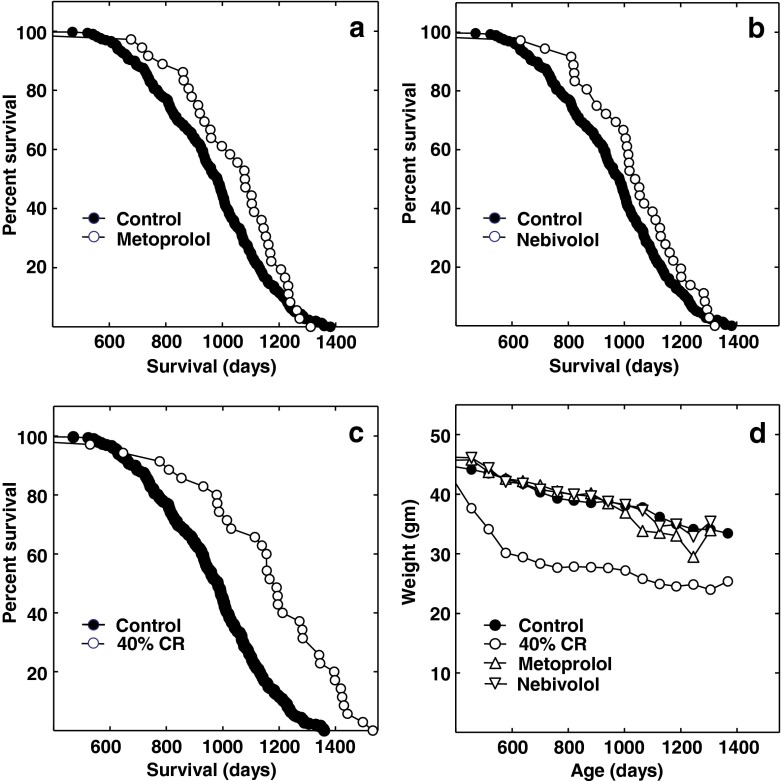
Daily, oral metoprolol treatment of male, C3B6F1 mice, begun at 12 months of age, significantly extended median life span by approximately 10 %, from 983 to 1,080 days (P = 0.016; Fig. 1a). Similarly, nebivolol treatment significantly increased their life span by 6.4 %, to 1,046 days (P = 0.023; Fig. 1b). Considering the data together, βAR antagonists increased median life span by 8.4 % (P = 0.0014). By comparison, 40 % CR begun at 12 months of age-extended median life span of the mice in this study by 23 %, to 1,191 days (Fig. 1c; P < 0.001). CR also increased maximum life span, while the βAR antagonists did not.
Fig. 1.
Life span of male C3B6F1 mice administered with βAR antagonists in their food daily: a Life span of mice receiving metoprolol. Shown are the life span of control (filled circles) and metoprolol (open circles) treated mice. The survival data for the same control group are shown in a, b and c. b Life span of mice administered nebivolol in their food. Shown are the life span of control (filled circles) and nebivolol- (open circles) treated mice. c The effects of 40 % CR on life span. Shown are the life span of the control (filled circles) and 40 % CR-treated (open circles) mice. d Body weight of the mice shown in panels a–c. Shown is the body weight for the control (closed circles), 40 % CR- (open circles), metoprolol- (open, upright triangles), and nebivolol- (open downward pointing triangles) treated mice. The only value that was significantly different than control was the mean weight of the metoprolol-treated mice at 1,245 days of age (P ≤ 0.01)
We used the Breslow–Wilcoxon test to calculate the statistics reported above because it gives more weight to deaths at early time points. The survival curves of the treated and untreated mice tended to converge at extreme old age (e.g., after 1,250 days, when only about 10 % of the control mice remained). Reduced or altered drug metabolism late in life may have produced mild drug toxicity at these times. The liver becomes less effective at detoxification and more sensitive to genotoxicity with age (Dhahbi and Spindler 2003). If the data are censored after 1,250 days, the effects of both metoprolol and nebivolol become significant according to both the Breslow–Wilcoxon (P = 0.016 and P = 0.027) and the Mantel–Cox tests (P = 0.047 and P = 0.046, respectively). If the censored data for both βAR antagonists are considered together, the differences from control become highly or very highly significant (P = 0.006 and P = 0.001 for the Mantel–Cox and Breslow–Wilcoxon tests, respectively).
Food consumption and body weight
Weight and food consumption data for each group are shown in Fig. 1d and Table 1, respectively. The mice were fed daily, and uneaten food from the previous day was noted, and the next allotment of food adjusted accordingly. Uneaten food was identifiable by shape, color, and texture. Per mouse food consumption in the metoprolol and nebivolol groups was not different from that of the control mice (Table 1).
Table 1.
Food consumption of βAR-treated mice relative to controls
| Age (days) | Controla | Metoprolol | Nebivolol |
|---|---|---|---|
| 395 | 112.7 | 98.9 | 97.7 |
| 426 | 100.0 | 100.0 | 99.6 |
| 456 | 110.9 | 98.9 | 98.4 |
| 487 | 102.9 | 99.7 | 99.3 |
| 517 | 98.9 | 100.1 | 100.0 |
| 547 | 99.0 | 100.0 | 100.0 |
| 578 | 101.0 | 100.0 | 100.0 |
| 608 | 105.0 | 100.0 | 100.0 |
| 639 | 97.9 | 100.1 | 100.1 |
| 669 | 97.9 | 100.1 | 100.1 |
| 700 | 93.9 | 100.0 | 100.1 |
| 730 | 96.9 | 100.1 | 100.1 |
| 760 | 98.0 | 100.0 | 100.0 |
| 791 | 88.0 | 100.0 | 100.0 |
| 821 | 101.0 | 100.0 | 99.8 |
| 852 | 97.9 | 100.1 | 99.7 |
| 882 | 100.0 | 100.0 | 100.0 |
| 912 | 105.0 | 100.0 | 100.0 |
| 943 | 105.8 | 99.8 | 100.2 |
| 973 | 105.3 | 100.6 | 100.4 |
| 1,004 | 102.5 | 100.5 | 100.5 |
| 1,034 | 105.4 | 100.6 | 100.6 |
| 1,065 | 100.9 | 102.1 | 102.1 |
| 1,095 | 103.3 | 102.6 | 102.6 |
| 1,125 | 98.9 | 105.3 | 106.0 |
| 1,156 | 90.5 | 103.8 | 106.0 |
| 1,186 | 104.2 | 100.6 | 102.6 |
| 1,217 | 100.8 | 101.5 | 102.2 |
| 1,247 | 103.3 | 101.4 | 102.7 |
| 1,277 | 102.2 | 96.1 | 100.8 |
| 1,308 | 105.7 | 100.3 | 100.3 |
| 1,338 | 106.0 | 99.1 | 99.1 |
| Statistics | |||
| Mean | 101.3 | 100.4 | 100.7 |
| SD | 5.1 | 1.5 | 1.8 |
| P valueb | 0.334 | 0.501 |
aAverage grams of diet eaten/mouse/month
bCompared to control
The mice were weighed bimonthly. The control and drug-treated mice gradually lost weight after 12 months of age when they were shifted from ad libitum chow feeding to the defined diets (Fig. 1d). Part of this decrease may have been due to the slight underfeeding of the mice to insure that they consumed all their food. Another part can be attributed to the age-related loss of weight found for even ad libitum-fed B6C3F1 mice (Sheldon et al. 1995).
Because the ratio of food consumption to body weight was not different between the control and treated groups at most time points, βARs are unlikely to alter rates of energy absorption and utilization. In extreme old age, the metoprolol-treated mice briefly dropped below the weight of the controls and nebivolol-treated mice (Fig. 1d). However, just four mice remained at this time, and three of these were underweight due to pathologies. Together, these results suggest that βAR blockers do not extend life span by reducing food consumption, absorption, or metabolism.
Causes of death
Necropsy results are summarized in Table 2. Both metoprolol and nebivolol approximately doubled the number of liver tumors, suggesting that they may be somewhat hepatotoxic at the treatment levels used, even though our dosages were low by comparison to those routinely used in mouse studies (see below). However, metoprolol appeared to reduce the growth rate of liver tumors by half, since the tumors from this group were approximately half the volume of those from the control and nebivolol-treated mice. This may be due to the effects of metoprolol on the β2AR (Rozec et al. 2006). Nebivolol is more selective for the β1AR (Rozec et al. 2006). We found no further differences in the pathologies of the mice.
Table 2.
Necropsy results from the mouse longevity studies shown in Fig 1. The number of mice necropsied in each group was 36. The necropsied control mice were chosen to match the ages at death of the treated mice
| Necropsy finding | Controla | Metoprolol | Nebivolol | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Enlarged spleen | 23 | 63.9 | 22 | 61.6 | 23 | 63.9 |
| Mice with liver tumors | 11 | 25.0 | 19 | 52.8 | 18 | 50.0 |
| Total mass of tumors in group (g)b | 12.33 | 17.59 | 41.45 | |||
| Mean mass of individual tumors (g) | 1.12 ± 0.89 A | 0.52 ± 0.75 B | 1.06 ± 1.47 A | |||
| Mean tumor mass per mouse (g) | 1.23 ± 0.99 | 0.926 ± 1.15 | 2.24 ± 3.03 | |||
| Liver hemangiomas | 4 | 11.1 | 4 | 11.1 | 2 | 5.6 |
| Intestinal tumors | 5 | 13.9 | 6 | 16.7 | 5 | 13.9 |
| Bladder distended | 6 | 16.7 | 5 | 13.9 | 7 | 22.2 |
aLiver tumor volume is the mean plus or minus the standard deviation of the number of tumors indicated in the table. Row values with the same capital superscript letters are not significantly different, as determined by Mann–Whitney test. Row values with different capital superscript letters are significantly different (P ≤ 0.05)
bTotal tumor mass was calculated as (π/6) × l × w × h × 1.0 g, where l is length, w is width, and h is the height of each tumor
Drugs effects on intracellular signaling
The effects of metoprolol and nebivolol on downstream βAR signaling in the heart and brain were investigated using Western blots. Agonist binding to the βARs triggers changes in stimulatory guanine nucleotide-binding proteins, which activate adenylyl cyclases, leading to increased intracellular cyclic adenosine monophosphate (cAMP) levels, and thereby, to increased PKA activity (Farfel et al. 1999). PKA phosphorylates proteins in the heart which alter intracellular calcium handling and the calcium sensitivity of myofilaments (reviewed in Sharma and McNeill 2011). βAR activation also inhibits Raf activity, which activates MEK/ERK signaling, thereby suppressing apoptosis in cardiomyocytes (Ho et al. 2010).
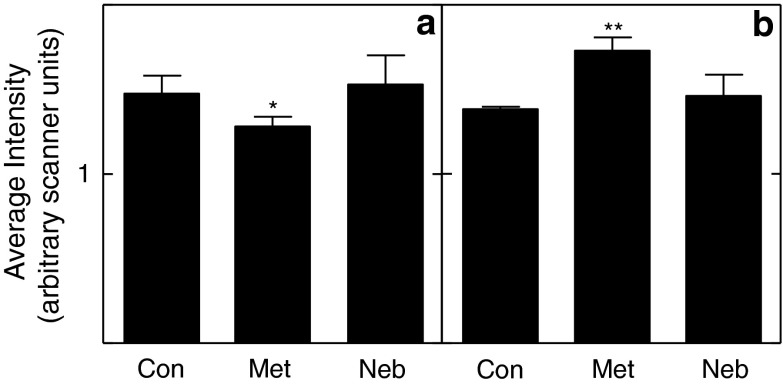
We found an effect of metoprolol on PKA protein levels in the heart and brain. Metoprolol significantly reduced PKA in the heart (Fig. 3a), while it increased levels in the brain (Fig. 3b). However, we could not detect a significant effect on the levels of phospho-PKA, phospho-ERK, or phospho-MEK in either heart or brain.
Fig. 3.
Effects of βAR antagonists on PKA protein levels. a Metoprolol (Met) treatment significantly reduced PKA levels in heart relative to control treated mice (Con). Nebivolol (Neb) had no effect. b Metoprolol treatment significantly increased PKA levels in brain relative to control
Phylogenic conservation of the response
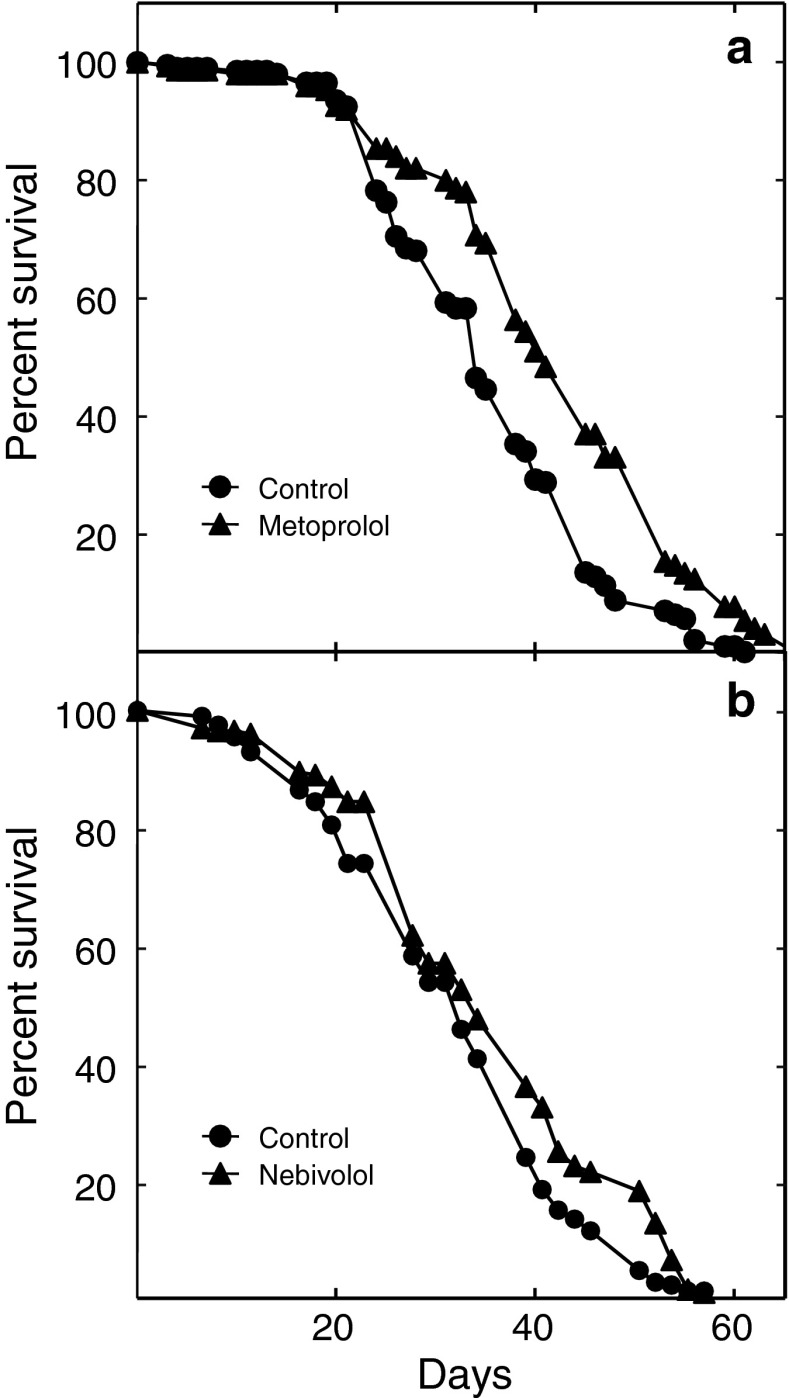
To further investigate the longevity effects of the βAR antagonists, we determined their effects on the life span of Drosophila (Fig. 2). Drosophila express a family of G protein-coupled receptors structurally and functionally related to the mammalian βARs (Maqueira et al. 2005). Metoprolol increased the median life span of the treated flies by about 23 % (Fig. 2a; P ≤ 0.0001). It also appeared to extend their maximum life span. Nebivolol increased median life span by about 15 % without extending the maximum life span (Fig. 2b; P ≤ 0.001).
Fig. 2.
βAR blockers extend the life span of male Drosophila melanogaster. a Survival data for Drosophila fed with diets either containing 5 mg/mL metoprolol (filled triangles) or vehicle alone (closed circles). b Survival data for treatment with 100 μg/mL nebivolol (filled triangles) or vehicle alone (closed circles)
These increases are not due to reduced calorie consumption or altered locomotion. Food intake was quantified by two methods: FPAs (Driver et al. 1986; Min and Tatar 2006; Spindler et al. 2012a; Spindler et al. 2012b) and CAFE assays (Ja et al. 2007). Both were used because CAFE assays are widely used but FPAs recapitulate more closely the conditions of our life span studies. Results obtained with the assays are highly correlated (Spindler et al. 2012a, b). We found no effects on food consumption using either assay (Table 3). The drugs produced no change in the number or size of the fecal plaques, nor the consumption of food. Thus, CR-like effects do not explain the longevity effects of drugs. Further, neither drug changed the level of locomotor activity of the flies (Table 4). Thus, changes in activity cannot explain the increases in lifespan induced by the drugs. Instead, they must arise from alterations in the physiology of the flies.
Table 3.
Drosophila food consumption does not change in response to chemical additions to the diet
| FPAsa | CAFEb assays | ||
|---|---|---|---|
| Treatmentc | Plaque number/cm2/fly (mean ± SD)d | Plaque diameter (mm) (mean ± SD)d | μL consumed/fly/h (mean ± SD)d |
| Control | 0.1988 ± 0.0222c | 0.089 ± 0.003 | 0.3275 ± 0.0240 |
| Metoprolol | 0.1532 ± 0.0231 | 0.093 ± 0.003 | 0.2854 ± 0.0489 |
| Nebivolol | 0.1636 ± 0.0326 | 0.086 ± 0.003 | 0.3650 ± 0.0378 |
aFecal plaque assays (FPAs)
bCapillary feeder (CAFE)
cSee experimental procedures for specifics regarding the methods. The medium contained either an equal volume of DMSO vehicle, 5 mg/mL metoprolol, or 100 μg/mL nebivolol
dColumn values are not significantly different, as determined by one-way ANOVA followed by Tukey’s multiple comparison test
Table 4.
Locomotor activity after drug treatment
| Treatmenta | N | Mean±SEMb | P valuec |
|---|---|---|---|
| Vehicle | 6 | 2678 ± 450.7 | |
| Metoprolol | 6 | 2438 ± 339.2 | P = 0.68 |
| Nebivolol | 6 | 1995 ± 274.5 | P = 0.23 |
aFlies were treated with 5 mg/mL metoprolol or 100 μg/mL nebivolol for 2 days and monitored for 3 additional days at 25 °C in a LAM 32 Activity Monitor (TriKenetics)
bAverage daily infrared beam breaks per day. Ten flies were monitored in each of 6 vials (N) per treatment
cTwo-tailed unpaired T tests were used to determine the significance of the differences between the vehicle and treatment groups
Discussion
The work presented here shows for the first time that pharmacological blockade of the β1-adrenergic receptor with metoprolol or nebivolol can extend the life span of mice and files. The stimulation of life span in such phylogenically distant organisms suggests that the βAR signaling pathway is a fundamental mechanism regulating the life span of metazoans.
Possible mechanism for the longevity benefits of βAR blockers in mammals
βARs transduce signaling by the sympathetic nervous system or circulating catecholamines to a cellular response in target organs. The heart and brain are often regarded as the major targets of βAR signaling because they have the highest density of these receptors (Daly and McGrath 2011). Seventy to 80 % of the β1ARs and 20–30 % of the β2ARs in mammals are located in the heart (Reviewed in Ho et al. 2010). Activation of these receptor subtypes in cardiomyocytes increases cardiac contractile force and contraction rate, increases conduction velocity and automaticity, and increases blood pressure (reviewed in Ho et al. 2010; Taylor and Bristow 2004). The receptors signal by increasing the activity of stimulatory guanine nucleotide-binding proteins (G proteins), thereby increasing the activity of PKA (Farfel et al. 1999). Chronic or overstimulation of the adrenergic pathways adversely affects myocyte growth and function, produces hypertension (Port and Bristow 2001; Taylor and Bristow 2004), and is a prognostic indicator in heart failure (Esler et al. 1997). Mouse models of chronically enhanced β1AR, G protein, and protein kinase PKA activity suffer from increased mortality and decreased resistance to stress (see discussion in Yan et al. 2007).
Sympathetic nervous system and βAR activity increase with age, and this increase may hasten the development of age-related cardiomyopathies (Lakatta 1993; Swynghedauw et al. 1995). Mouse models of chronic βAR stimulation at the receptor (Du et al. 2000; Engelhardt et al. 1999; Liggett et al. 2000), G protein (Iwase et al. 1996), or PKA (Antos et al. 2001; Yan et al. 2007) levels increase mortality and decrease stress resistance.
In concordance with these results, desensitization of the βAR signaling systems in a transgenic Gsα overexpressing mouse decreased the development of age-related cardiomyopathy (Asai et al. 1999). Knockout of the AC5 gene (AC5-KO) increases median mouse life span by ∼30 % and maximum life span by 4 months (Yan et al. 2007). The AC5-KO mice were protected from age-related development of cardiac hypertrophy, apoptosis, fibrosis, and decreased cardiac function (Yan et al. 2007). These health-related effects may be related to activation of Raf/MEK/ERK signaling and to the upregulation of superoxide dismutase (Yan et al. 2007).
The proposed mechanisms for these positive health benefits of β-blockers include suppression of cardiac arrhythmias, reduction of cardiomyocyte hypertrophy, necrosis and apoptosis, improved calcium handling and force of contraction through suppressed fetal gene expression, increased cardiac βAR receptor density through increased receptor expression and decreased internalization, reduced serum C-reactive protein levels (reduced systemic inflammation), and restoration of cardiac glucose oxidation (reviewed in Sharma and McNeill 2011). Metoprolol and other βAR blockers also have been shown to induce a switch from fatty acid to glucose oxidation in the heart of nondiabetic patients with heart failure, perhaps through inhibition of carnitine palmitoyltransferase (Andersson et al. 1991; Eichhorn et al. 1994; Panchal et al. 1998; Sharma and McNeill 2011).
The longevity benefits of β-blockers reported here are likely to involve organs other than just smooth and cardiac muscle. Normal mice are somewhat resistant to the development of overt cardiovascular disease, although they do develop mild cardiomyopathy with age (Dhahbi et al. 2006). The necropsies performed in our studies did not detect cardiac-related deaths. We found no enlarged hearts. However, βARs are more widely involved in mammalian health and longevity. Polymorphisms of the βAR genes have been associated with diseases, including asthma (Chung et al. 2011; Johnson 2001), obesity (Martinez et al. 2003), and cancer (Wang et al. 2006). In cancer, they are associated with cell proliferation and apoptosis (Meinhardt et al. 1999; Sastry et al. 2007), chemotaxis and metastasis (Entschladen et al. 2002; Lang et al. 2004; Shang et al. 2009), and tumor growth (Shang et al. 2009). Consistent with these results, βARs are also localized in epithelium, endothelium, nerve axons/processes, and mast cells (Chung et al. 2011; Daly and McGrath 2011). The wide tissue distribution of the receptor and the pleiotropic effects of βAR agonists and antagonists suggest that the increases in longevity found here may involve multiple cell and organ systems.
Effects of the drugs on intracellular βAR signaling
Surprisingly, we found limited evidence of alterations in intracellular signaling downstream of the βAR receptors. Metoprolol significantly reduced PKA levels in the heart, while it increased them in the brain (Fig. 3). The absence of a similar effect of nebivolol suggests that these effects may be related to the effects of metoprolol on β2-adrenergic receptor activity (Rozec et al. 2006). Since both metoprolol and nebivolol extended life span, these results are unlikely to be related to the longevity effects of the treatments. Further, neither drug produced a significant change in the levels of phospho-PKA, pERK, or pMEK in either the heart or brain (data not shown).
The absence of a clear effect of the inhibitors on their major signaling pathways in the heart and brain may have three origins. First, inhibition of downstream βAR signaling may have been too subtle to detect by the assays employed here. Relatively low doses of the inhibitors were used, as discussed below, and thus inhibition of βAR signaling may have remained below the level of statistical significance. Second, the longevity effects of the drugs may be due to disrupted βAR signaling in a subpopulation of cells, and these effects might require assays such as immunocytochemistry for detection. A third possibility is that the major longevity targets of these βAR inhibitors are not the heart or brain. For example, metoprolol appeared to reduce the growth rate of liver tumors by approximately half, suggesting that liver might be an important target of the drug. Further studies will be required to investigate these possibilities.
Drug dosages
Metoprolol was administered at 30 mg/kg body weight/day (315 mg metoprolol/kg diet), a relatively low dose. Published mouse studies use metoprolol dosages of 20 to 165 mg/kg body weight/day (Bartholomeu et al. 2008; Baumhakel et al. 2008; Sorrentino et al. 2011). The recommended human dosage is 1.25 to 5.63 mg/kg body weight/day. Mouse drug dosages per kilogram body weight are often 8- to 12.3-fold higher than their human dosages due to pharmacokinetic and pharmacodynamic differences between mammals with very different body masses (Reagan-Shaw et al. 2008; and reviewed in Spindler 2012). Thus, the dosage used here is at the lower end of that typically used in mice. Nebivolol was used at 1 mg/kg body weight/day (11.74 mg nebivolol/kg diet). Mouse studies typically use nebivolol dosages of from 1 to 10 mg/kg body weight/day (Baumhakel et al. 2008; Sacco et al. 2011; Sorrentino et al. 2011). The recommended human dose is from 63 to 500 μg/kg body weight/day. Again, a relatively low dose of nebivolol was used for the studies reported here.
Study design
An unbalanced statistical design was employed to minimize the number of mice utilized per test group while maintaining statistical power (Jeske et al. 2012). In this design, a large group of control mice and many smaller groups of “test” mice are used to maximize the number of test groups. When comparing multiple treatments to a common control, unbalanced designs have been shown to offer economic advantages (see for example, Jeske et al. 2012 for a discussion of this in the context of Weibull analyses). Specifically, we used 297 control mice and groups of 36 mice per treatment. The sample sizes in this study are similar to those required for a Weibull survival analyses set up to have an 75 % probability of detecting an 10 % increase in mean life span with a 1 % (α ≤ 0.01) probability of a false positive across 58 test groups.
βAR mechanisms in Drosophila
In insects, octopamine, a biogenic monoamine structurally related to noradrenaline, serves the role of a neurohormone, neuromodulator, and neurotransmitter (Roeder 1999, 2005). A family of three receptors in D. melanogaster, termed DmOctb1R, DmOctb2R, and DmOctb3R, has the highest homology to the vertebrate βARs (Evans and Maqueira 2005; Maqueira et al. 2005). They are activated preferentially by octopamine, a biogenic amine structurally and functionally similar to adrenaline and noradrenaline, the major vertebrate βARs (Roeder 1999). While octopamine appears to be the primary physiological ligand of these receptors, intracellular cAMP also increases significantly in response to adrenaline and noradrenaline, suggesting they may mediate signaling in some insect nervous systems (Evans and Maqueira 2005).
In larvae, DmOctβ1R, DmOctβ2R, and DmOctβ3R are expressed in salivary glands and imaginal disks, while DmOctβ2R and DmOctβ3R are expressed in midgut, and DmOctβ3R is expressed in gonads (Ohhara et al. 2012). In adult flies, all three receptors are expressed in the CNS, and in ovary and testis (Ohhara et al. 2012). The rate of contraction of the adult heart in Drosophila is accelerated by octopamine and by norepinephrine (Johnson et al. 1997; Zornik et al. 1999). Together, the studies above suggest that in insects the biogenic amines recapitulate many of their key functions in mammals. Thus, the longevity effects of metoprolol and nebivolol in Drosophila may be mediated by similar pathways.
One final point is that the classical βAR agonist isoproterenol and antagonist propranolol have weak effects on the responses of all three octopamine receptors (Evans and Maqueira 2005). These observations may explain why rather high oral doses of metoprolol and nebivolol were required to produce the life span effects of the β-blockers in Drosophila. Reduced uptake, bioavailability, stability, or increased metabolism or excretion of the inhibitors in flies also may have contributed. Thus, the studies presented in this report indicate that inhibition of βAR signaling in mice and flies is capable of extending life span.
Acknowledgments
The authors thank Ms Carol Boyd for her help in feeding and monitoring the mice, and Ms Amber Graham, Karla Mabida, Sheena Tran, Tracy Nguyen, and Bianca Mabida, and Mr. Kenneth P. Ablao for their technical help with the studies. This work was funded by Alva, LLC, whose business is funding research. The funding organization and its members had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Stephen R. Spindler, Phone: +1-951-8273597, FAX: +1-951-8274294, Email: spindler@ucr.edu
Patricia L. Mote, Email: mote@ucr.edu
Rui Li, Email: ruili@ucr.edu.
Joseph M. Dhahbi, Email: jdhahbi@ucr.edu
Amy Yamakawa, Email: ayama001@ucr.edu.
James M. Flegal, Email: james.flegal@ucr.edu
Daniel R. Jeske, Email: daniel.jeske@ucr.edu
Rui Li, Email: ruili@ucr.edu.
Alex L. Lublin, Email: alex.lublin@ucr.edu
References
- Andersson B, Blomstrom-Lundqvist C, Hedner T, Waagstein F. Exercise hemodynamics and myocardial metabolism during long-term beta-adrenergic blockade in severe heart failure. J Am Coll Cardiol. 1991;18:1059–1066. doi: 10.1016/0735-1097(91)90767-4. [DOI] [PubMed] [Google Scholar]
- Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, Ishikawa Y, Shannon RP, Wagner TE, Vatner DE, Homcy CJ, Vatner SF. Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest. 1999;104:551–558. doi: 10.1172/JCI7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeu JB, Vanzelli AS, Rolim NP, Ferreira JC, Bechara LR, Tanaka LY, Rosa KT, Alves MM, Medeiros A, Mattos KC, Coelho MA, Irigoyen MC, Krieger EM, Krieger JE, Negrao CE, Ramires PR, Guatimosim S, Brum PC. Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J Mol Cell Cardiol. 2008;45:240–249. doi: 10.1016/j.yjmcc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Baumhakel M, Schlimmer N, Buyukafsar K, Arikan O, Bohm M. Nebivolol, but not metoprolol, improves endothelial function of the corpus cavernosum in apolipoprotein e-knockout mice. J Pharmacol Exp Ther. 2008;325:818–823. doi: 10.1124/jpet.107.135681. [DOI] [PubMed] [Google Scholar]
- Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.CIR.101.5.558. [DOI] [PubMed] [Google Scholar]
- Celik T, Iyisoy A, Kursaklioglu H, Kardesoglu E, Kilic S, Turhan H, Yilmaz MI, Ozcan O, Yaman H, Isik E, Fici F. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J Hypertens. 2006;24:591–596. doi: 10.1097/01.hjh.0000209993.26057.de. [DOI] [PubMed] [Google Scholar]
- Chung LP, Waterer G, Thompson PJ. Pharmacogenetics of beta2 adrenergic receptor gene polymorphisms, long-acting beta-agonists and asthma. Clin Exp Allergy. 2011;41:312–326. doi: 10.1111/j.1365-2222.2011.03696.x. [DOI] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC. Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends Pharmacol Sci. 2011;32:219–226. doi: 10.1016/j.tips.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Spindler SR (2003) Aging of the liver. In: Aspinall R (ed) Aging of the organs and systems. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 271–291
- Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL, Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- Driver CJ, Wallis R, Cosopodiotis G, Ettershank G. Is a fat metabolite the major diet dependent accelerator of aging? Exp Gerontol. 1986;21:497–507. doi: 10.1016/0531-5565(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Du XJ, Gao XM, Wang B, Jennings GL, Woodcock EA, Dart AM. Age-dependent cardiomyopathy and heart failure phenotype in mice overexpressing beta(2)-adrenergic receptors in the heart. Cardiovasc Res. 2000;48:448–454. doi: 10.1016/S0008-6363(00)00187-5. [DOI] [PubMed] [Google Scholar]
- Eichhorn EJ, Heesch CM, Barnett JH, Alvarez LG, Fass SM, Grayburn PA, Hatfield BA, Marcoux LG, Malloy CR. Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1994;24:1310–1320. doi: 10.1016/0735-1097(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Ellison KE, Gandhi G. Optimising the use of beta-adrenoceptor antagonists in coronary artery disease. Drugs. 2005;65:787–797. doi: 10.2165/00003495-200565060-00006. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entschladen F, Lang K, Drell TL, Joseph J, Zaenker KS. Neurotransmitters are regulators for the migration of tumor cells and leukocytes. Cancer Immunol Immunother. 2002;51:467–482. doi: 10.1007/s00262-002-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Kaye D, Lambert G, Esler D, Jennings G. Adrenergic nervous system in heart failure. Am J Cardiol. 1997;80:7L–14L. doi: 10.1016/S0002-9149(97)00844-8. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Farfel Z, Bourne HR, Iiri T. The expanding spectrum of G protein diseases. N Engl J Med. 1999;340:1012–1020. doi: 10.1056/NEJM199904013401306. [DOI] [PubMed] [Google Scholar]
- Grassi G, Trevano FQ, Facchini A, Toutouzas T, Chanu B, Mancia G. Efficacy and tolerability profile of nebivolol vs atenolol in mild-to-moderate essential hypertension: results of a double-blind randomized multicentre trial. Blood Press Suppl. 2003;2:35–40. doi: 10.1080/08038020310023271. [DOI] [PubMed] [Google Scholar]
- Ho D, Yan L, Iwatsubo K, Vatner DE, Vatner SF. Modulation of beta-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev. 2010;15:495–512. doi: 10.1007/s10741-010-9183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circ Res. 1996;78:517–524. doi: 10.1161/01.RES.78.4.517. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske, D. R., Flegal, J., and Spindler, S. R. (2012) Minimum size survival analysis sampling plans for comparing multiple treatment groups to a single control group. Communications in Statistics–Theory and Methods (in press)
- Johnson E, Ringo J, Dowse H. Modulation of Drosophila heartbeat by neurotransmitters. J Comp Physiol B. 1997;167:89–97. doi: 10.1007/s003600050051. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Terra SG. Beta-adrenergic receptor polymorphisms: cardiovascular disease associations and pharmacogenetics. Pharm Res. 2002;19:1779–1787. doi: 10.1023/A:1021477021102. [DOI] [PubMed] [Google Scholar]
- Johnson M. Beta2-adrenoceptors: mechanisms of action of beta2-agonists. Paediatr Respir Rev. 2001;2:57–62. doi: 10.1053/prrv.2000.0102. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Lang K, Drell TL, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, Entschladen F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer. 2004;112:231–238. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.CIR.101.14.1707. [DOI] [PubMed] [Google Scholar]
- Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Corbalan MS, Sanchez-Villegas A, Forga L, Marti A, Martinez-Gonzalez MA. Obesity risk is associated with carbohydrate intake in women carrying the Gln27Glu beta2-adrenoceptor polymorphism. J Nutr. 2003;133:2549–2554. doi: 10.1093/jn/133.8.2549. [DOI] [PubMed] [Google Scholar]
- Meinhardt G, Wendtner CM, Hallek M. Molecular pathogenesis of chronic lymphocytic leukemia: factors and signaling pathways regulating cell growth and survival. J Mol Med (Berl) 1999;77:282–293. doi: 10.1007/s001090050351. [DOI] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (1994) Manual of microbiologic monitoring of laboratory animals. NIH p. 151–154
- Council NR. Infectious diseases of mice and rats. Washington: National Academy; 1991. p. 397. [Google Scholar]
- Ohhara Y, Kayashima Y, Hayashi Y, Kobayashi S, Yamakawa-Kobayashi K. Expression of beta-adrenergic-like octopamine receptors during Drosophila development. Zoolog Sci. 2012;29:83–89. doi: 10.2108/zsj.29.83. [DOI] [PubMed] [Google Scholar]
- Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail. 1998;4:121–126. doi: 10.1016/S1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/S0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Rozec B, Quang TT, Noireaud J, Gauthier C. Mixed beta3-adrenoceptor agonist and alpha1-adrenoceptor antagonist properties of nebivolol in rat thoracic aorta. Br J Pharmacol. 2006;147:699–706. doi: 10.1038/sj.bjp.0706648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco G, Evangelista S, Manzini S, Parlani M, Bigioni M. Combined antihypertensive and cardioprotective effects of nebivolol and hydrochlorothiazide in spontaneous hypertensive rats. Future Cardiol. 2011;7:757–763. doi: 10.2217/fca.11.70. [DOI] [PubMed] [Google Scholar]
- Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- Shang ZJ, Liu K, Liang DF. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38:371–376. doi: 10.1111/j.1600-0714.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- Sharma V, McNeill JH. Parallel effects of beta-adrenoceptor blockade on cardiac function and fatty acid oxidation in the diabetic heart: confronting the maze. World J Cardiol. 2011;3:281–302. doi: 10.4330/wjc.v3.i9.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon WG, Bucci TJ, Hart RW, Turturro A. Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol Pathol. 1995;23:458–476. doi: 10.1177/019262339502300403. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C, Schaefer A, Hilfiker-Kleiner D, Luscher TF, Balligand JL, Drexler H, Landmesser U. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol. 2011;57:601–611. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Review of the literature and suggestions for the design of rodent survival studies for the identification of compounds that increase health and life span. Age (Dordr) 2012;34:111–120. doi: 10.1007/s11357-011-9224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS One. 2012;7:e39581. doi: 10.1371/journal.pone.0039581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR, Li R, Dhahbi JM, Yamakawa A, Sauer F. Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using Drosophila. PLoS One. 2012;7:e29782. doi: 10.1371/journal.pone.0029782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swynghedauw B, Besse S, Assayag P, Carre F, Chevalier B, Charlemagne D, Delcayre C, Hardouin S, Heymes C, Moalic JM. Molecular and cellular biology of the senescent hypertrophied and failing heart. Am J Cardiol. 1995;76:2D–7D. doi: 10.1016/S0002-9149(99)80484-6. [DOI] [PubMed] [Google Scholar]
- Taylor MR, Bristow MR. The emerging pharmacogenomics of the beta-adrenergic receptors. Congest Heart Fail. 2004;10:281–288. doi: 10.1111/j.1527-5299.2004.02019.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hao B, Chen X, Zhao N, Cheng G, Jiang Y, Liu Y, Lin C, Tan W, Lu D, Wei Q, Jin L, Lin D, He F. Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: a case–control study in a Chinese population. Cancer Lett. 2006;240:297–305. doi: 10.1016/j.canlet.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Zhao L, Yang FXK, Cao H, Zheng G, Zhang Y, Li J, Cui H, Chen X, Zhu Z, He H, Mo X, Kennedy BK, Suh Y, Zeng Y, Tian X (2012) Common genetic variants of the â2-adrenergic receptor affect its translational efficiency and are associated with human longevity. Aging Cell. doi:10.1111/acel.12011 [DOI] [PMC free article] [PubMed]
- Zornik E, Paisley K, Nichols R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides. 1999;20:45–51. doi: 10.1016/S0196-9781(98)00151-X. [DOI] [PubMed] [Google Scholar]