Abstract
Behavioral studies suggest that postural control requires increased cognitive control and visuospatial processing with aging. Consequently, performance can decline when concurrently performing a postural and a demanding cognitive task. We aimed to identify the neural substrate underlying this effect. A demanding cognitive task, requiring visuospatial transformations, was performed with varying postural loads. More specifically, old and young subjects performed mental rotations of abstract figures in a seated position and when standing on a force platform. Additionally, functional magnetic resonance imaging (fMRI) was used to identify brain regions associated with mental rotation performance. Old as compared to young subjects showed increased blood oxygenation level-dependent (BOLD) responses in a frontoparietal network as well as activations in additional areas. Despite this overall increased activation, they could still modulate BOLD responses with increasing task complexity. Importantly, activity in left lingual gyrus was highly predictive (r = −0.83, adjusted R2 = 0.65) of the older subjects' degree of success in mental rotation performance when shifting from a sitting to a standing position. More specifically, increased activation in this area was associated with better performance, once postural load increased.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9499-2) contains supplementary material, which is available to authorized users.
Keywords: Aging, Postural control, Mental rotation, Dual-tasking, fMRI
Introduction
Even though postural control is a well-automated task, it requires increased top-down control in old subjects (Doumas et al. 2008, 2009; Huxhold et al. 2006; Redfern et al. 2001). In dual-task paradigms, altered performance on cognitive tasks has been observed when shifting from a sitting to a standing position (Andersson et al. 2002; Doumas et al. 2008; Kerr et al. 1985; Redfern et al. 2001). The interference between postural and cognitive control seems to be greater for spatial than nonspatial memory tasks (Kerr et al. 1985; Sturnieks et al. 2008; VanderVelde et al. 2005; Woollacott and Vander Velde 2008). This underscores the importance of visuospatial processing in postural control, an idea that is corroborated by the postural instability of patients suffering from spatial neglect as a result of parietal damage (Perennou 2006). Furthermore, these patient-based observations suggest that (spatial) cognition and postural control have a common neural substrate.
Dual-task paradigms are commonly used to study interactions between cognitive and postural control (Woollacott and Shumway-Cook 2002). It is often hypothesized that capacity limitations in attentional resources prevent subjects from maintaining the same level of performance when dividing attention between two tasks (Tombu and Jolicoeur 2003). However, this theory cannot explain why, under some dual-task conditions, improved postural or cognitive control is observed (Andersson et al. 1998; Dault et al. 2001; Swan et al. 2004; Vuillerme et al. 2000). Moreover, tasks requiring continuous interlimb coordination show evidence of compensatory age-related brain activation when sensorimotor demands increase (Goble et al. 2010; Heuninckx et al. 2008), suggesting that reduced brain resources may not be a limiting factor for older adults. Rather, task prioritization, a strategic trade-off mechanism used to optimize either postural or cognitive demands may be a valid explanation for performance changes (Lacour et al. 2008). Indeed, whereas some have shown decreased cognitive performance of old subjects in order to maintain posture (Brown et al. 2002; Lajoie et al. 1993), others have shown increased postural instability to meet cognitive task demands (Doumas et al. 2008; Shumway-Cook et al. 1997).
Although many behavioral studies have examined the interaction between postural and cognitive performance, the neural signature of such concurrent performance remains unknown. Previous research has attempted to visualize brain regions involved in postural control by using, for example, functional magnetic resonance imaging (fMRI) (Jahn et al. 2004; Zwergal et al. 2012). Contrasting visual imagery of standing with that of lying has resulted in activations in the superior frontal gyrus, insula, middle temporal gyrus, thalamus, and cerebellum in young subjects and an age-related increase in activation across the frontal, temporal, and occipital cortices in old subjects (Zwergal et al. 2012). These activations indirectly suggest that visuospatial processing in older adults during postural control is more effortful (Jeka et al. 2010). In the present study, we focused on the brain activation underlying visuospatial processing and its relationship to postural control. Blood oxygenation level-dependent (BOLD) responses were measured, while subjects performed a mental rotation task, but mental rotation performance was also tested outside the scanner during both sitting and standing conditions. We aimed to determine whether altered visuospatial processing in the old subjects would be associated with performance changes as a result of increased postural control requirements.
Mental rotation implies the rotation of objects in the absence of a physical action (Shepard and Metzler 1971). Apart from visuospatial processing and decision making, it relies on motor processes (Wexler et al. 1998; Wohlschlager 2001; Wohlschlager and Wohlschlager 1998) and also requires intact vestibular information (Hanes and McCollum 2006; Mast et al. 2006; Peruch et al. 2011). As such, it is a demanding cognitive task that is well suited for examining the interaction between postural control and visuospatial processing. Behavioral research has established that older adults are impaired on mental rotation tasks (Cerella et al. 1981; Gaylord and Marsh 1975; Hertzog et al. 1993). However, there is a lack of brain imaging literature corroborating these results. In young subjects, activation of dorsal and ventral visual streams is observed during mental rotation (Zacks 2008). Additionally, the BOLD response in premotor and posterior parietal cortices is parametrically modulated, with increasing rotation angles (de Lange et al. 2005; Gogos et al. 2010). Based on previous aging research, we anticipated that old subjects would show an overall increase in frontoparietal activation as a result of visual processing (Madden et al. 2007) but that they would still be able to modulate their BOLD response (Goble et al. 2010; Ward et al. 2008a). In addition, in view of the brain activations observed previously for postural and visuospatial processing, we hypothesized that performance decrements in mental rotation, when comparing the standing to the seated positions, would be associated with the degree of activity in the posterior parietal and/or occipital cortex.
Methods
Subjects
Fifteen healthy young adults (eight female, M = 22.4 years, range 18.6–30.4 years) and 25 healthy older adults (ten females, M = 70.9 years, range 62.3–82.9 years) participated in the study. All participants had normal or corrected to normal vision and were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield 1971). The Montreal Cognitive Assessment Test was used to determine general cognitive function in the old subjects. All old subjects scored within normal limits (score ≥26 out of 30). In order to score subjects' visuospatial memory the Rey–Osterrieth Figure Test (Osterrieth 1944) was applied and the immediate retention score was calculated (old: M = −37.7 %, SD = 18.4 %, young: M = −20.7 %, SD = 15.9 %). Immediate retention was significantly lower (p < 0.01) in the old as compared to the young group, indicating worse short-term visual memory. Participants were screened for possible contraindications and medication use before entering the scanner. They were informed about the experimental procedures and provided written informed consent. The study was approved by the local ethics committee of KU Leuven and was performed in accordance with the 1964 Declaration of Helsinki.
Experimental design
Experiment 1: postural control task
The interaction between mental rotation and postural control was tested by having subjects perform a mental rotation task while sitting down and while standing upright on a balance platform (NeuroCom International, Inc., Clackamas, OR, USA) (Woollacott and Shumway-Cook 2002). This platform samples center of pressure (COP) data (100 samples/s). It consists of a surface with dual force plates (23 × 46 cm) and a visual surround, in which the monitor showing the visual stimuli was mounted. Safety straps were used to prevent falls.
A custom-written Labview program (National Instruments, Austin, TX, USA) was used for stimulus presentation and registration of responses on the mental rotation task. Response times (RTs) were recorded with 1 ms accuracy. Subjects were presented with separate blocks of the mental rotation task and a control task with equivalent visual input and motor response requirements, but no mental rotation component. During mental rotation trials, either one of two abstract figures was shown together with its copy or its mirror image. The angular disparity between the two stimuli was 45°, 90°, 135°, or 170°. This resulted in 16 different stimulus pairs, i.e., 2 (basis figures) × 4 (rotation angles) × 2 (same versus mirror image) combinations. Stimuli were visible for 2 s, unless a motor response was given earlier. After stimulus presentation, a fixation cross was shown until the next stimulus pair (see Fig. 1). The left button of a two button computer mouse held in the right hand had to be pushed with the index finger when the stimuli were the same and the right button with the middle finger when the stimuli were mirror images. The computer mouse was taped to the subject's right hand to avoid additional muscular activity related to holding the mouse. Subjects were instructed to give a correct response as fast as possible. During the control task, subjects viewed a scrambled image of the stimuli of the mental rotation task and were instructed to make a response with either their index or middle finger as fast as possible.
Fig. 1.
Schematic representation of Experiment 1. Subjects either performed paradigm A or B, the order being counterbalanced across subjects. CT control task, MRT mental rotation task
Subjects started by performing a few practice trials to get familiarized with the stimuli, after which a block of trials (48 trials for the mental rotation task, 32 trials for the control task) in the seated position was performed. They, then, performed either three blocks (eight trials per block) of the control task or three blocks (eight trials per block) of the mental rotation task while standing on the force platform. The order of the tasks was counterbalanced across subjects (see Fig. 1). In between blocks, short rest periods were provided to allow equilibration of postural control. The two tasks were then once more performed while being seated, followed by another six blocks on the force platform. The experiment ended by performing the tasks one last time in a seated position.
No specific instructions were given to prioritize either posture or mental rotation. To discard possible task order effects, only the data from the middle seated condition were used for further analyses.
Experiment 2: brain activations associated with the mental rotation task
Within a week after participation in Experiment 1, subjects underwent a scanning session during which brain activation associated with performing the mental rotation task was assessed. Subjects lay supine in the scanner, looking at an MRI-compatible monitor at the rear end of the scanner via a mirror that was fixed to the head coil. All visual inputs were presented in the participants' foveal view. Response registration was accomplished by a scanner-compatible two-button computer mouse, lying underneath subjects' right hand. Participants performed two runs of the task, each run consisting of 11 mental rotation task blocks alternated with 11 control task blocks. Each block consisted of eight events with block lengths ranging between 21 s and 30 s. Optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/) was used to randomize the order of the mental rotation task events and to obtain a sufficient amount of jitter in order to optimize the estimation of the event-related BOLD signal to increasing rotation angles. This resulted in a variable interstimulus interval (range 2.5–4.5 s), assuring that the off-task periods and the on-task periods were not correlated. The same temporal jitter was applied to the control task events. At the beginning of each block, an instruction cue was presented for 2 s. The number of the same and mirror stimuli was equal across a scan run. Subjects were not provided with any feedback concerning their performance.
Image acquisition protocol
All scanning was performed on a Philips 3T Achieva MRI scanner (Philips, Best, The Netherlands) with a 32-channel matrix head coil using an echoplanar imaging (EPI) sequence for T2*-weighted images (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, 43 oblique slices each 2.5 mm thick, interslice gap = 0.25 mm, in-plane resolution 2.5 × 2.5 mm, 128 × 128 matrix). The field of view covered the entire cortex and the majority of the cerebellum. At the end of each scanning run, one extra T2*-weighted image containing the entire brain was acquired for registration purposes. Additionally, a field map (TR = 750 ms, TE = 5.75 ms, flip angle = 90°, 35 transverse slices each 4 mm thick, interslice gap = 0.19 mm, in-plane resolution = 2 × 2, 192 × 192 matrix) and a 3D MPRAGE high resolution T1-weighted image (TR = “shortest”, TE = 4.60 ms, flip angle = 8°, 230 sagittal slices each 1 mm thick, in-plane resolution = 0.97 × 0.98, 384 × 384 matrix) were acquired.
Data analysis
Experiment 1: postural control task
The recorded COP data, reflecting subjects' body sway, were analyzed using custom-written MATLAB scripts (MATLAB 7.4, MathWorks, Natick, MA, USA). The anterior–posterior and medial–lateral components of the COP trajectory were first low-pass filtered, using a fourth-order Butterworth dual-pass filter (cutoff frequency: 10 Hz). The path length was calculated, i.e., the entire COP excursion during a 20-s trial, and averaged across trials of the same condition. Average path lengths were log-normalized to account for a skewed distribution across subjects. The log-normalized path length was then subjected to a repeated measures Analysis of Variance (ANOVA), with between-subjects factor ‘age group’ (young, old) and within-subjects factor ‘task’ (control task or mental rotation task).
For mental rotation trials, the mean error rate (percent wrong) and median correct and incorrect RTs were determined for each condition. The weighted mean of the median RT was calculated according to the number of correct and incorrect responses. RT and the error rate were determined for each subject and subjected to a repeated measures ANOVA, with between-subjects factor ‘age group’ (young, old) and within-subjects factor ‘body position’ (sitting, standing). For all statistical tests, the level of significance was set to α = 0.05, and post-hoc Tukey tests were applied when appropriate.
Experiment 2: brain activations associated with the mental rotation task
Behavioral analysis
For the control task, the median RT was calculated for each subject. The data were subjected to a one-sided two-sample t test to determine whether the older group responded slower during the control task. For mental rotation trials, the mean error rate and median correct and incorrect RTs were determined for each rotation angle across the two scan runs. The weighted mean of the median RT were calculated according to the number of correct and incorrect responses. These data were subjected to a repeated measures ANOVA to confirm that subjects were indeed showing a linear parametric modulation with increasing task difficulty (i.e., rotation angle). There was one between-subjects factor ‘age group’ (young, old) and one within-subjects factor rotation angle (45°, 90°, 135°, and 170°).
fMRI analysis
Processing of fMRI data was done using Feat, part of the FMRIB software Library (Oxford University College, Oxford, UK) (Smith et al. 2004). For each subject, all EPI scans were realigned to the middle scan of that time series. To account for geometric distortion as a consequence of field inhomogeneities, EPI unwarping was performed based on the acquired field map. Slice timing correction was applied to correct for the delay in the acquisition of different slices in EPI scans. Images were smoothed with an isotropic Gaussian kernel of 5 mm full width at half maximum and a 1/125 Hz high pass filter was applied to remove low frequency noise. Scans were then registered to a common space. First, the mean functional image was registered to a T2*-weighted scan comprising the entire brain using linear registration (Flirt). Second, the anatomical scan was registered to a representative template image (MNI152_T1_1mm_brain) in Montreal Neurological Institute (MNI) space using nonlinear transformations (Fnirt) with a 10-mm warp resolution. Next, the derived normalization parameters from the previous two steps were combined and applied to all EPI scans.
For each subject, a design matrix was set up for each time series according to the general linear model framework (Beckmann et al. 2003). For each condition (control task, 45°, 90°, 135°, and 170° rotation), a regressor containing the stimulus onsets was entered in the model. Additionally, a number of confound regressors for the onsets of the instruction cues and for the motion parameters were included. The hemodynamic responses generated by the events were modeled by convolving delta stick functions with a standard double gamma hemodynamic response function and its first temporal derivative. Parameter estimates for the t-contrasts ‘mental rotation task > control task’ (block comparison) and ‘linear parametric modulation’ (within mental rotation task block comparison) were obtained.
In the next step, the results from both runs were combined for each subject (fixed effects analysis). Mixed effects models (FLAME 1, (Beckmann et al. 2003)) were then used to run a group analysis for the contrast ‘mental rotation task > control task’. In order to explain age-related activation differences that were related to the older subjects' increased error rates, individual error rates were included in the model as a covariate. The following t-contrasts were calculated: ‘effect of mental rotation across groups,’ ‘effect of mental rotation in the old group,’ ‘effect of mental rotation in the young group,’ age-related overactivation, i.e., ‘old > young,’ and age-related underactivation, i.e., ‘old < young’.
In addition, a mixed effects model was run for the contrast ‘linear parametric modulation’. For this model, an inclusive mask was used, representing the positive effect of mental rotation across both groups (thresholded at z = 3.1, p < 0.01). This was done to constrain the search to only those voxels involved in the mental rotation operation. In other words, given that a voxel was more active during mental rotation than during the control task, we tested whether its activation scaled as a function of rotation angle. Individual error rates were again included in the model as a covariate. The following t-contrasts were calculated: ‘effect of parametric modulation across groups,’ ‘effect of parametric modulation in the old group,’ ‘effect of parametric modulation in the young group,’ age-related increased modulation, i.e., ‘old > young’ and age-related reduced modulation, i.e., ‘old < young’.
Finally, to determine the effect brain activation might have had on the altered performance in the standing as opposed to the sitting position, a regression model was created, containing subjects' parameter estimates for the contrast ‘mental rotation > control task’. For both groups, a covariate reflecting the performance change (error ratestanding − error ratesitting) was entered and positive and negative correlations were modeled. Positive correlations indicate that a greater decrement in mental rotation performance while standing is associated with higher BOLD responses and vice versa for negative correlations.
All activation maps were thresholded at z = 3.1 (p < 0.001 at the voxel level) and a cluster significance threshold of p < 0.01. Anatomical labels were ascribed to activated clusters by using the Julich histological atlas. For visualization purposes and in order to obtain correlation coefficients, percent signal change for each rotation angle was extracted from each activated cluster (http://mumford.bol.ucla.edu/).
Results
Experiment 1: postural control task
Effect of mental rotation on postural control
Path lengths of subjects' body sway showed a significant main effect of group (F(1, 38) = 17.560, p < 0.001) and of task (F(1, 38) = 11.765, p = 0.001) and a trend towards significance for the group × task interaction (F(1, 38) = 3.057, p = 0.088). Old as compared to young subjects showed increased body sway, and this effect tended to be increased when performing the mental rotation task as opposed to a simple control task. Since in both conditions subjects had to press buttons, the increased body sway observed in both groups was not a simple consequence of the motor output related to these button presses. The increased body sway rather implies an interaction between the cognitive processing of the mental rotation task and postural control.
Effect of postural control on mental rotation
During standing as well as sitting conditions, old as compared to young subjects, had higher RTs (main effect of group (F(1, 38) = 35.3, p < 0.001) and made more errors when performing the mental rotation task (main effect of group (F(1, 38) = 12.9, p < 0.001). RTs showed a marginally significant effect of body position (main effect of body position (F(1, 38) = 3.99, p = 0.052)) (see Fig. 2).
Fig. 2.
Behavioral results of Experiment 1 for postural (left-hand side) and mental rotation (right-hand side) performance. Error bars denote means ± standard errors. CT control task, MRT mental rotation task, PL path length (cm), RT median response time. *p < 0.05, post-hoc Tukey corrected; **p < 0.001, post-hoc Tukey corrected
The error rate increased in both groups in the standing as compared to the sitting position (main effect of body position (F(1, 38) = 4.58, p = 0.038)) but more so in the old group as shown in Fig. 2. Nevertheless, there was no significant interaction (group × body position interaction (F(1, 38) = 2.305, p = 0.13). Extracorrelational analysis showed that neither in the sitting nor in the standing condition, a speed accuracy trade-off occurred (see Supplementary Material S1 for more detail).
Experiment 2: brain activations associated with the mental rotation task
Behavioral data
For the RTs, the main effect of group was significant (F(1, 38) = 35.66, p < 0.001), meaning that, in general, the older subjects were slower at responding than their younger counterparts. There was also a main effect of rotation angle (F(3, 114) = 82.72, p < 0.001) and a marginally significant group × rotation angle interaction (F(3, 114) = 2.59, p = 0.056). Both groups showed a comparable increase in RTs as rotation angles increased (see Fig. 3). Furthermore, the difference between the RTs of both groups on the control task was only marginally significant (Mold = 352 ms, Myoung = 320 ms, p = 0.085), suggesting that both groups had a comparable baseline.
Fig. 3.
Median response time (RT) and mean error rate during the scanning experiment. Error bars represent means ± standard errors
For the error rates, there was a significant main effect of group (F(1, 38) = 9.76, p < 0.01), implying that older adults made more errors than young adults. There was also a main effect of rotation angle (F(3, 114) = 22.950, p < 0.001) but no group × rotation angle interaction (F(3, 114) = 0.330, p = 0.80). Both groups, thus, showed a similar increase in error rates as the task became more difficult.
Brain activation during the mental rotation task
Areas showing brain activation differences between young and older adults
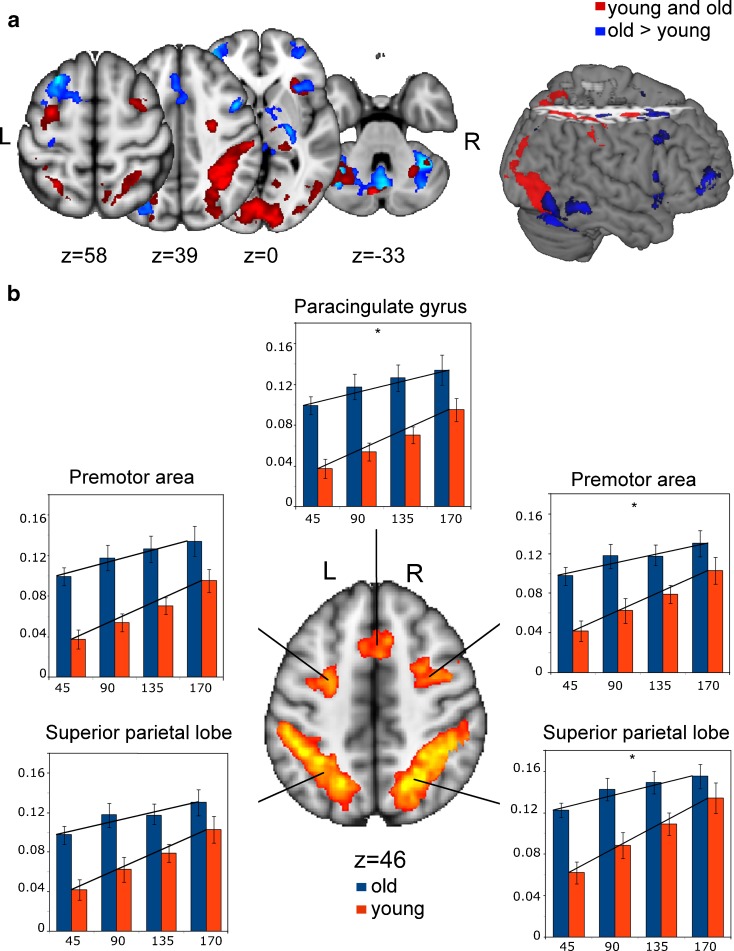
Brain activation maps showed that both groups activated a network comprising the bilateral dorsal premotor cortex (PMd), the bilateral superior parietal lobe extending to the intraparietal sulcus, the right insula, left thalamus, and bilateral cerebellum (lobe VI), extending to the lingual and fusiform gyrus (see Fig. 4a and Table 1), when performing the mental rotation as opposed to the control task. Compared to young subjects, older subjects showed increased BOLD responses in most of these regions. Table 2 shows peak activations of regions for which older subjects showed increased BOLD responses and more extended activation patterns. Additionally, older subjects activated brain regions that were not significant when looking at the young group separately and, therefore, reflect the recruitment of unique brain regions to support task performance. These additional activations were situated in the bilateral frontal pole, the paracingulate, globus pallidus, and left insula stretching to the frontal operculum. No regions were found to be more active in the young than in the old group. Clusters for which a linear increase in the BOLD response with increasing rotation angle was found in both groups were situated on the border of the paracingulate gyrus and presupplementary motor area (pre-SMA), the bilateral premotor cortices and the intraparietal sulci, extending to the superior parietal lobes. For each rotation angle, the percent signal change was extracted from each cluster. t tests performed on the slopes of these data showed that for the paracingulate gyrus, the right premotor cortex, and the right superior parietal lobe, the slope of the old group was significantly smaller (p < 0.05, Bonferroni corrected for the number of clusters) than that of the young group (see Fig. 4b). This was due to greater activation in the old group at smaller rotation angles.
Fig. 4.
Activation maps representing a regions that were active in both groups for the contrast ‘mental rotation task > control task’ (red) and regions that were overactivated by the old group (blue) and b regions of linear parametric modulation (p < 0.01, cluster-corrected for multiple comparisons). Bar plots show means ± standard errors of the % signal change (y-abcis), according to the four rotation angles (x-abcis). * Significantly (p < 0.05, Bonferroni-corrected for the five clusters) smaller slope in the old as compared to the young group. A color reproduction of the figure can be found online on www.springer.com
Table 1.
Brain activation during mental rotation
| Area | Side | MNI coordinates | Z value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Superior frontal sulcus (PMd) | L | −27 | −4 | 52 | 5.61 |
| R | 28 | −8 | 44 | 5.01 | |
| Insula | R | 33 | 23 | −5 | 4.85 |
| Anterior intraparietal gyrus | L | −32 | −48 | 45 | 5.57 |
| R | 43 | −43 | 45 | 5.55 | |
| Lingual gyrus | R | 6 | −85 | −6 | 4.86 |
| Lateral occipital gyrus | L | −38 | −64 | −6 | 4.84 |
| R | 50 | −65 | −6 | 4.87 | |
| Thalamus | L | −13 | −24 | 9 | 4.87 |
| R | 13 | −22 | 9 | 4.11 | |
| Cerebellum VI | L | −7 | −72 | −27 | 6.36 |
Z values and activation peaks of regions that were significantly (p < 0.01; cluster-corrected for multiple comparisons) activated in both old and young subjects, during mental rotation performance
PMd dorsal premotor area
Table 2.
Age-related increased activation during mental rotation
| Area | Side | MNI coordinates | Z value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Frontal pole | L | −40 | 56 | 6 | 4.59 |
| R | 47 | 38 | 28 | 4.3 | |
| Middle frontal gyrus | R | 46 | 10 | 39 | 4.26 |
| Superior frontal gyrus (pre-PMd) | L | −25 | 20 | 58 | 4.62 |
| Insula | L | −35 | 20 | −3 | 4.14 |
| R | 42 | 21 | −5 | 4.37 | |
| Precentral gyrus (SM1) | L | −34 | −16 | 44 | 4.01 |
| Paracingulate gyrus | R | 9 | 9 | 32 | 4.27 |
| Anterior intraparietal sulcus | L | −32 | −61 | 42 | 4.39 |
| Globus pallidus | L | −23 | −10 | 3 | 4.22 |
| R | 21 | −10 | 0 | 3.30 | |
| Thalamus | L | −7 | −2 | 5 | 4.52 |
| R | 10 | −4 | 5 | 3.51 | |
| Cerebellum lobe VI/ Crus I | L | −36 | −55 | −33 | 4.4 |
| Cerebellum lobe VI | R | 38 | −49 | −33 | 5.14 |
Z values and activation peaks showing significantly (p < 0.01; cluster-corrected for multiple comparisons) higher activation for old as opposed to young subjects, during mental rotation performance. Regions in bold were only active in the old group
PMd dorsal premotor aread to appropriate levels
Linking brain activation associated with mental rotation to performance under increased postural control requirements
In the old subjects, a cluster in the left lingual gyrus (MNI: −14, −86, −13), shown in Fig. 5, was observed for which the percent signal change was negatively correlated with the change in performance from the seated to the standing position (error ratestanding − error ratesitting, r = −0.83, p < 0.05). This may indirectly reflect the impact of postural load on visuospatial processing because the BOLD response of this region was not correlated with mental rotation performance (i.e., error rate) in the sitting position (r = 0.06, p > 0.05) but did correlate with performance in the standing position (r = −0.52, p < 0.05). In order to confirm that this relationship between brain activity and performance change was not mediated by a trade-off in postural performance or by subject's age, a stepwise regression model was run in MATLAB 7.4 (MathWorks, Natick, MA, USA). This model included the old subjects' performance change on the mental rotation task (error ratestanding − error ratesitting) as dependent variable and three predictor variables: percent signal change in left lingual gyrus, change in postural control (PLmental rotation − PLcontrol task) and age. Only the percent signal change was a significant predictor, explaining 65 % (adjusted R2 = 0.65, p < 0.001) of the variance in performance decrement on the mental rotation task. This can possibly be interpreted as follows: old subjects with a high BOLD response in this area maintained and even improved their mental rotation performance level when going from a sitting to a standing position, whereas those with a low BOLD response did not. This was not mediated by a trade-off in postural control or age. Additionally, the older subjects with high BOLD responses also performed the mental rotation task better in the standing position. In the young group, no correlations were found between brain activity and performance changes, consistent with their high overall performance on the task.
Fig. 5.
Bar plots show means ± standard errors of the percent signal change (left-hand side) of left lingual gyrus, a region that correlated negatively with performance change (error ratestanding − error ratesitting) in the old group (right-hand side). A color reproduction of the figure can be found online on www.springer.com
Discussion
In the current study, the interaction between visuospatial performance and postural control was investigated, with specific focus on the brain activations underlying mental rotation. We assumed that the effects of increased postural control requirements on visuospatial task performance would be greater in the old group. Indeed, in the old group, error rates were higher in the standing as opposed to the sitting condition. We had two main hypotheses with respect to brain activation levels. Firstly, we predicted that older as compared to young adults would show an overall increase in brain activation during mental rotation. The fMRI results confirmed that there was elaborate overactivation in the old group in a frontoparietal network. Secondly, we hypothesized that degree of brain activity in the posterior parietal or occipital cortex would be associated with performance changes in mental rotation when comparing the standing to the seated position. We found a negative correlation between the BOLD response of a region in left lingual gyrus and mental rotation performance when postural control requirements were increased from the seated to the standing position.
Age-related brain overactivation during mental rotation
When performing the mental rotation task, the dorsal visual stream was recruited by both groups, with an extensive activation observed along the bilateral intraparietal sulcus. Although some have suggested that, particularly, the right parietal lobe is involved in mental rotation (Corballis 1997; Harris et al. 2000), there are several reports of left parietal activation (Cohen et al. 1996; Seurinck et al. 2004; Zacks 2008). The activation of the old subjects also spread from the occipital to the temporal cortex, suggesting activity of the ventral visual stream involved in object recognition (Haxby et al. 1991). Both groups also activated premotor and anterior cerebellar regions. PMd and anterior cerebellum are involved in egocentric mental rotation of hands and tools and have, therefore, been linked to the storage of long-term memory for tool use (Bonda et al. 1996; Vingerhoets et al. 2002). However, mental rotation of abstract figures has been shown to interfere with the planning of rotational hand movements (Wexler et al. 1998; Wohlschlager 2001; Wohlschlager and Wohlschlager 1998). More specifically, concordant hand rotation results in faster responses on a mental rotation task, whereas disconcordant hand rotations seem to inhibit mental rotation. As such, it has been suggested that mental rotation, either of tools or abstract figures, relies equally on visuomotor as on visuospatial processes (Lamm et al. 2007). In the old group, premotor activation extended into pre-PMd, suggesting an increased cognitive task load (Heuninckx et al. 2005), possibly associated with response selection (Picard and Strick 2001).
Apart from the increased BOLD responses of the old group in regions activated by the young group, old subjects also showed activation in additional regions, mainly the globus pallidus and middle frontal gyrus, extending into Brodmann areas 10, 44, and 45. Activation in the anterior prefrontal cortex has been reported before in young subjects during mental rotation (Cohen et al. 1996; Kosslyn et al. 2001; Wraga et al. 2003), and this region has been related to the maintenance of intention (Burgess et al. 2001) and the monitoring of response selection (Ramnani and Owen 2004). The increased recruitment of prefrontal regions may also reflect a more generic pattern that is typical for aging, as increased prefrontal activity in older subjects is associated with better memory performance (Cabeza et al. 2002; Cabeza 2002; Grady et al. 2008; Gutchess et al. 2005; Rosen et al. 2002, 2005) as well as more accurate motor coordination performance (Heuninckx et al. 2008). Basal ganglia activation, on the other hand, has not been observed frequently. Nevertheless, studies in stroke patients show that these subcortical nuclei are of critical importance when performing mental rotation (Harris et al. 2002).
Despite their increased brain activity, old subjects were still capable of parametrically modulating their BOLD response in paracingulate, bilateral premotor, and superior parietal areas. Similar findings have been observed in the context of motor control (Goble et al. 2010; Ward et al. 2008b). Moreover, Hedden et al. (2011) have shown that failure to modulate brain activity may only become evident when substantial white matter hyperintensities, as measured by Fluid Attenuated Inversion Recovery, are present.
Finally, subjects' mental rotation performance (i.e., error rate) when lying in the scanner was entered in the fMRI model as a covariate. This showed that there were no brain regions for which the degree of activation was related to performance when lying in the scanner. Associations between brain activation and mental rotation performance when comparing the standing versus the sitting positions are discussed next.
Mental rotation and postural control
Already at low levels of task difficulty (i.e., while performing the control task), an increased body sway was observed in the old as compared to the young group, which was further enhanced by mental rotation. Additionally, error rates on the mental rotation task increased when performed in a standing as compared to a sitting position. However, when looking more closely at the individual data, we also observed individuals for which error rates were lower when standing upright, i.e., their performance improved. Previous research has suggested that old subjects prioritize their posture under challenging conditions due to fear of falling but try to optimize their cognitive performance when standing on a stable surface (Doumas et al. 2008; Shumway-Cook et al. 1997). As subjects wore safety straps in the present study, some may have chosen to optimize their mental rotation performance (as per the instruction to respond as quickly as possible). The idea of task optimization has long been proposed by psychologists as a key to successful aging (Baltes and Baltes 1990). More specifically, older adults may optimize their chances of achieving preselected goals by using a compensation strategy. At a muscular level, this may imply altered body posture to improve cognitive performance. At a neural level, increased age-related brain activation may be compensatory in nature. For example, Goble et al. (2010) and Heuninckx et al. (2008) had old and young subjects perform a motor coordination task according to two task complexity levels. Whereas no differentially activated brain regions were significantly correlated to performance for the easy coordination mode, a positive correlation was found for the more difficult coordination mode. Importantly, a number of regions for which the old subjects showed an increased BOLD response were not predictive of performance. These results suggest that not all increased neural recruitment may be efficient. Alternatively, compensatory recruitment may be revealed more successfully with higher levels of task difficulty.
Here, we obtained a comparable result. In the old group, the capability to preserve mental rotation performance from the sitting to the standing position as well as performance in the standing position were highly correlated (r = −0.83, r = −0.52, respectively) to the brain activity in left lingual gyrus. This brain–behavior relation did not hold true for the sitting condition. The observed activation cluster (MNI: −14, −86, −13) is likely to correspond to human V3 (Rottschy et al. 2007). Activation in the left lingual gyrus has been reported previously during mental rotation of hands (Seurinck et al. 2004) as well as during successive direction discrimination of a moving random dot pattern (Cornette et al. 1998). However, lingual gyrus activation may be more related to the perception and encoding of visual stimuli (Cornette et al. 1998; de Lange et al. 2005) than to the actual rotation because its activation did not increase parametrically with increasing rotation angles. Moreover, this area may also have a more direct link with motor performance: our coordinate is in the vicinity of an activation cluster (MNI: −13, −90, −10) reported by Ronsse et al. (2010) when examining the effects of visual feedback on the learning of a new bimanual coordination task. Their results imply that this area also comes into play during online movement control when visual information is a prominent source of input. The data suggest that subjects with a higher BOLD response in left lingual gyrus were able to optimize cognitive task performance with increasing postural load. However, it is unsure whether lingual gyrus activation is specifically predictive for the current dual-task combination or whether it may serve as a more generic marker for age-related dual-task deficits related to visuospatial processing.
Limitations
Brain activation was measured while subjects were lying in an MR scanner. As a consequence, possible interactions between visual, proprioceptive, and vestibular stimulation while subjects were performing mental rotations in an upright position could not be modeled. However, mental rotation performance (i.e., error rate) in the scanner and in the standing position were correlated (old: r = 0.65, p < 0.001, young: r = 0.83, p < 0.001). It appears reasonable to assume that the obtained brain activations during the former condition are, at least, partially representative for the latter. Reduced cross-modal deactivations have been detected in older subjects, with respect to visual and auditory processing (Peiffer et al. 2009) and a similar age-related increase in multisensory processing has been observed in the context of postural control (Zwergal et al. 2012). In view of this evidence for reduced inhibitory interactions between sensory systems, the brain activations we observed are possibly an underestimation of the true activation during standing.
Conclusion
Visuospatial performance was associated with increased prefrontal and parietal brain activation in the old group, suggesting increased cognitive load and enhanced sensory processing and monitoring of response selection. Nevertheless, older subjects were able to modulate BOLD responses with increasing rotation angles, albeit to a lesser extent than young subjects. This suggests some degree of age-related neural recruitment modularity under more demanding performance conditions. Degree of left lingual gyrus activation was predictive of performance change on the mental rotation task as postural control demands increased. In other words, increased activation in this visual area guarded against performance loss under more compromising postural control conditions. Older subjects with a high response in the lingual gyrus may have chosen to apply an improved rotation solving strategy when standing upright. Viewed from a dual-task perspective, the data may lend support to the prediction that visual processing becomes increasingly important with aging, as postural load increases. However, it is important to underline that this interpretation is indirect and does not constitute causality. Nevertheless, this may provide an interesting working hypothesis for future investigation. From an interventional perspective, it may be worthwhile to consider the promotion of balance exercises under conditions of high cognitive and sensory demands. This may challenge the plasticity of the aging brain.
Electronic supplementary material
(DOC 27 kb)
Acknowledgments
Support for this work was provided through a grant from the Research Fund of the Katholieke Universiteit Leuven, Belgium (OT/71/11) and the Research Foundation-Flanders (G.0721.12). Van Impe A. is funded by a Ph.D. fellowship of the Research Foundation-Flanders. Coxon J.P. is funded by a postdoctoral fellowship of the Research Foundation-Flanders (1224010 N). Bruijn S.M. is funded by a visiting postdoctoral fellowship of the Research Foundation-Flanders (GP.030.10.N).
Contributor Information
A. Van Impe, Email: van_impe_annouchka@hotmail.com
S. P. Swinnen, Phone: +32-16-329071, FAX: +32-16-329197, Email: Stephan.Swinnen@faber.kuleuven.be
References
- Andersson G, Yardley L, Luxon L. A dual-task study of interference between mental activity and control of balance. Am J Otol. 1998;19:632–637. [PubMed] [Google Scholar]
- Andersson G, Hagman J, Talianzadeh R, Svedberg A, Larsen HC. Effect of cognitive load on postural control. Brain Res Bull. 2002;58:135–139. doi: 10.1016/s0361-9230(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: the model of selective optimization with compensation. In: Baltes MM, Baltes PB, editors. Successful aging: perspectives from the behavioral science. New York: Cambridge University Press; 1990. pp. 1–34. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bonda E, Frey S, Petrides M. Evidence for a dorso-medial parietal system involved in mental transformations of the body. J Neurophysiol. 1996;76:2042–2048. doi: 10.1152/jn.1996.76.3.2042. [DOI] [PubMed] [Google Scholar]
- Brown LA, Sleik RJ, Polych MA, Gage WH. Is the prioritization of postural control altered in conditions of postural threat in younger and older adults? J Gerontol A Biol Sci Med Sci. 2002;57:M785–M792. doi: 10.1093/gerona/57.12.m785. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cerella J, Poon LW, Fozard JL. Mental rotation and age reconsidered. J Gerontol. 1981;36:620–624. doi: 10.1093/geronj/36.5.620. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, Di Girolamo GJ, Thompson WL, Anderson AK, Brookheimer SY, Rosen BR, Belliveau JW. Changes in cortical activity during mental rotation: a mapping study using functional MRI. Brain. 1996;119:89–100. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Mental rotation and the right hemisphere. Brain Lang. 1997;57:100–121. doi: 10.1006/brln.1997.1835. [DOI] [PubMed] [Google Scholar]
- Cornette L, Dupont P, Rosier A, Sunaert S, Van Hecke P, Michiels J, Mortelmans L, Orban GA. Human brain regions involved in direction discrimination. J Neurophysiol. 1998;79:2749–2765. doi: 10.1152/jn.1998.79.5.2749. [DOI] [PubMed] [Google Scholar]
- Dault MC, Geurts AC, Mulder TW, Duysens J. Postural control and cognitive task performance in healthy participants while balancing on different support-surface configurations. Gait Posture. 2001;14:248–255. doi: 10.1016/s0966-6362(01)00130-8. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Hagoort P, Toni I. Neural topography and content of movement representations. J Cogn Neurosci. 2005;17:97–112. doi: 10.1162/0898929052880039. [DOI] [PubMed] [Google Scholar]
- Doumas M, Smolders C, Krampe RT. Task prioritization in aging: effects of sensory information on concurrent posture and memory performance. Exp Brain Res. 2008;187:275–281. doi: 10.1007/s00221-008-1302-3. [DOI] [PubMed] [Google Scholar]
- Doumas M, Rapp MA, Krampe RT. Working memory and postural control: adult age differences in potential for improvement task priority and dual tasking. J Gerontol B Psychol Sci Soc Sci. 2009;64:193–201. doi: 10.1093/geronb/gbp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord SA, Marsh GR. Age differences in the speed of a spatial cognitive process. J Gerontol. 1975;30:674–678. doi: 10.1093/geronj/30.6.674. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity frequency-induced neural modulation and task-specific compensatory recruitment. Hum Brain Mapp. 2010;31:1281–1295. doi: 10.1002/hbm.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, Gavrilescu M, Davison S, Searle K, Adams J, Rossell SL, Bell R, Davis SR, Egan GF. Greater superior than inferior parietal lobule activation with increasing rotation angle during mental rotation: an fMRI study. Neuropsychologia. 2010;48:529–535. doi: 10.1016/j.neuropsychologia.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex. 2008;18:189–199. doi: 10.1093/cercor/bhm045. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hanes DA, McCollum G. Cognitive–vestibular interactions: a review of patient difficulties and possible mechanisms. J Vestib Res. 2006;16:75–91. [PubMed] [Google Scholar]
- Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G, Watson JD. Selective right parietal lobe activation during mental rotation: a parametric PET study. Brain. 2000;123:65–73. doi: 10.1093/brain/123.1.65. [DOI] [PubMed] [Google Scholar]
- Harris IM, Harris JA, Caine D. Mental-rotation deficits following damage to the right basal ganglia. Neuropsychology. 2002;16:524–537. doi: 10.1037//0894-4105.16.4.524. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb Cortex. 2011;22:1038–1051. doi: 10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Vernon MC, Rypma B. Age differences in mental rotation task performance: the influence of speed/accuracy tradeoffs. J Gerontol. 1993;48:150–156. doi: 10.1093/geronj/48.3.p150. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxhold O, Li SC, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage. 2004;22:1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav. 2010;42:197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- Kerr B, Condon SM, McDonald LA. Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform. 1985;11:617–622. doi: 10.1037//0096-1523.11.5.617. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Wraga M, Alpert NM. Imagining rotation by endogenous versus exogenous forces: distinct neural mechanisms. Neuroreport. 2001;12:2519–2525. doi: 10.1097/00001756-200108080-00046. [DOI] [PubMed] [Google Scholar]
- Lacour M, Bernard-Demanze L, Dumitrescu M. Posture control aging and attention resources: models and posture-analysis methods. Neurophysiol Clin. 2008;38:411–421. doi: 10.1016/j.neucli.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- Lamm C, Windischberger C, Moser E, Bauer H. The functional role of dorso-lateral premotor cortex during mental rotation: an event-related fMRI study separating cognitive processing steps using a novel task paradigm. NeuroImage. 2007;36:1374–1386. doi: 10.1016/j.neuroimage.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast FW, Merfeld DM, Kosslyn SM. Visual mental imagery during caloric vestibular stimulation. Neuropsychologia. 2006;44:101–109. doi: 10.1016/j.neuropsychologia.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed]
- Osterrieth P. Le teste de copie d'une figure complex: contribution à l'étude de la perception et de la memoire. Arch Psychol. 1944;30:206–356. [Google Scholar]
- Peiffer AM, Hugenschmidt CE, Maldjian JA, Casanova R, Srikanth R, Hayasaka S, Burdette JH, Kraft RA, Laurienti PJ. Aging and the interaction of sensory cortical function and structure. Hum Brain Mapp. 2009;30:228–240. doi: 10.1002/hbm.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perennou D. Postural disorders and spatial neglect in stroke patients: a strong association. Restor Neurol Neurosci. 2006;24:319–334. [PubMed] [Google Scholar]
- Peruch P, Lopez C, Redon-Zouiteni C, Escoffier G, Zeitoun A, Sanjuan M, Deveze A, Magnan J, Borel L. Vestibular information is necessary for maintaining metric properties of representational space: evidence from mental imagery. Neuropsychologia. 2011;49:3136–3144. doi: 10.1016/j.neuropsychologia.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM (2004) Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neuosci 5:184–194 [DOI] [PubMed]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture. 2001;14:211–216. doi: 10.1016/s0966-6362(01)00144-8. [DOI] [PubMed] [Google Scholar]
- Ronsse R, Puttemans V, Coxon JP, Goble DJ, Wagemans J, Wenderoth N, Swinnen SP. Motor learning with augmented feedback: modality-dependent behavioral and neural consequences. Cereb Cortex. 2010;21:1283–1294. doi: 10.1093/cercor/bhq209. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Gabrieli JD, Stoub T, Prull MW, O'Hara R, Yesavage J, Toledo-Morrell L. Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex. 2005;41:595–602. doi: 10.1016/s0010-9452(08)70199-0. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K. Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp. 2007;28:1045–1059. doi: 10.1002/hbm.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seurinck R, Vingerhoets G, de Lange FP, Achten E. Does egocentric mental rotation elicit sex differences? NeuroImage. 2004;23:1440–1449. doi: 10.1016/j.neuroimage.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci. 1997;52:M232–M240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Fitzpatrick RC, Lord SR. Effects of spatial and nonspatial memory tasks on choice stepping reaction time in older people. J Gerontol A Biol Sci Med Sci. 2008;63:1063–1068. doi: 10.1093/gerona/63.10.1063. [DOI] [PubMed] [Google Scholar]
- Swan L, Otani H, Loubert PV, Sheffert SM, Dunbar GL. Improving balance by performing a secondary cognitive task. Br J Psychol. 2004;95:31–40. doi: 10.1348/000712604322779442. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- VanderVelde TJ, Woollacott MH, Shumway-Cook A. Selective utilization of spatial working memory resources during stance posture. Neuroreport. 2005;16:773–777. doi: 10.1097/00001756-200505120-00023. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, de Lange FP, Vandemaele P, Deblaere K, Achten E. Motor imagery in mental rotation: an fMRI study. NeuroImage. 2002;17:1623–1633. doi: 10.1006/nimg.2002.1290. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Nougier V, Teasdale N. Effects of a reaction time task on postural control in humans. Neurosci Lett. 2000;291:77–80. doi: 10.1016/s0304-3940(00)01374-4. [DOI] [PubMed] [Google Scholar]
- Ward NS, Swayne OB, Newton JM. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging. 2008;29:1434–1446. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler M, Kosslyn SM, Berthoz A. Motor processes in mental rotation. Cognition. 1998;68:77–94. doi: 10.1016/s0010-0277(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Wohlschlager A. Mental object rotation and the planning of hand movements. Percept Psychophys. 2001;63:709–718. doi: 10.3758/bf03194431. [DOI] [PubMed] [Google Scholar]
- Wohlschlager A, Wohlschlager A. Mental and manual rotation. J Exp Psychol Hum Percept Perform. 1998;24:397–412. doi: 10.1037//0096-1523.24.2.397. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Vander Velde T. Non-visual spatial tasks reveal increased interactions with stance postural control. Brain Res. 2008;1208:95–102. doi: 10.1016/j.brainres.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wraga M, Thompson WL, Alpert NM, Kosslyn SM. Implicit transfer of motor strategies in mental rotation. Brain Cogn. 2003;52:135–143. doi: 10.1016/s0278-2626(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. J Cogn Neurosci. 2008;20:1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33:1073–1084. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 27 kb)