Abstract
The musculoskeletal system (muscle–tendon–bone) demonstrates numerous age-related changes, with modifications in tendons the least well studied, although increased predisposition to tendinopathy and rupture have been reported. In order to gain insights into the basis of age-associated increase in tendon injuries, we compared Achilles and tibialis anterior tendons and myotendinous junctions (MTJs) from 3- to 5- and 22- to 25-month-old rats for underlying structure and composition. Significant decreases were observed by qRT-PCR for collagen I, III, and V mRNA expression in tendons of old rats, but immunostaining detected no apparent differences in collagen I and V expression on the protein level. Tendons of old compared with young rats had decreased mRNA expression levels of proteoglycan 4 (PRG4) and elastin (Eln), but no differences in the mRNA expression of connective tissue growth factor, TGF-beta 1, or stromal cell-derived factor 1. For PRG4, immunostaining showed good correlation with qRT-PCR results. This is the first study to show reductions in PRG4 in tendons and MTJs of old rats. Decreased PRG4 expression in tendons could result in increased tendon stiffness and may be associated with decreased activity in the elderly. The diminished collagen mRNA expression in combination with decreased PRG4 and Eln mRNA expression may be associated with increased risk of tendon injury with aging.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-013-9514-2) contains supplementary material, which is available to authorized users.
Keywords: Aging, PRG4, Elastin, Tendon, Myotendinous junctions
Introduction
The musculoskeletal system is well known to undergo age-related changes that represent major contributors to physical frailty. With aging, skeletal muscle develops progressive denervation and loss of muscle fibers (reviewed in Augustin and Partridge 2009), and bones undergo progressive loss of mineralization and increased risk of fracture (reviewed in Smith and Rennie 2007). Less widely appreciated are marked increases in the occurrence of tendinopathy and rupture with age (Jarvinen et al. 2005). Some estimates place the incidence as high as 50 % of people in their 70s and 80 % of those in their 80s suffer from rotator cuff injuries (Milgrom et al. 1995). Tendons transmit muscle forces to the skeleton to produce joint torques and generate movement. Thus, dysfunction of tendon has serious ramifications for mobility and susceptibility to injury.
Despite its prevalence and debilitating nature, the basis for the age-associated increase in tendinopathy is unknown, although tendon injuries are thought to be preceded by changes in the underlying structure and mechanical properties (Riley 2008). We have shown that tendons stiffen considerably with age (Wood et al. 2011), but the associated changes in underlying structure have not been fully elucidated. The mechanical properties of tendons are determined by their underlying structural composition which reflects their physiological demands and capabilities (reviewed in Voleti et al. 2012). Usually, larger/stronger tendons are attached to larger/stronger muscles, while smaller and weaker muscles have smaller/weaker tendons (Narici and Maganaris 2007). Regular physical exercise leads to stronger muscle and correspondingly stronger tendon (reviewed in Kjaer et al. 2009), while age-related decrease in physical activity and changes in structural composition could be related to weakened tendons and increased risk factor for tendinopathy in the elderly (reviewed in Magnusson et al. 2008; Hess 2010). Tendons are composed primarily of collagens that contribute to the ability of the tissue to withstand high tensile forces. Collagen molecules in tendons are organized into structures that progressively increase in size from collagen fibrils to collagen fibers and fascicles of collagen fibers, all of which are enclosed by the endotenon, with the entire tendon covered by epitenon (Banos et al. 2008). Approximately 75 % of tendon dry mass is collagen type I. Lesser amounts of collagen types II, III, and V and small amounts of collagen types XII and XIV are also present in most tendons (reviewed in Banos et al. 2008). In addition to collagens, tendons contain a diverse group of glycoproteins, including proteoglycan 4 (PRG4), fibromodulin, elastin, tenascin, decorin, biglycan, and lumican (reviewed in Rees et al. 2009; Parkinson et al. 2011). Finally, expression of collagens and glycoproteins in tendons is regulated by numerous growth (transforming growth factor (TGF)-beta, connective tissue growth factor (CTGF), stromal cell-derived factor 1 (SDF1), IGF, FGF, GDF, etc.; reviewed in Oliva et al. 2012) and transcription factors (scleraxis, Krüppel-like family, etc.; reviewed in Banos et al. 2008; Spittau and Krieglstein 2012). The effect of aging on the expression and localization of most of these major tendon constituents has not been established.
Based on the considerable changes with aging in tendon mechanical properties, along with the importance of the underlying structure to determine function, the purpose of the current study was to determine the age-related changes in the expression levels and localization of proteins known to be critical for tendon function. Our approach was to analyze both Achilles and tibialis anterior (TBA) tendons of young (3–5 months) and old (22–25 months) Sprague–Dawley rats by quantitative RT-PCR and immunostaining for comparison of important tendon components. Clarification of the effect of aging on tendon composition and structure will further our understanding of the mechanisms underlying the loss of elasticity and increased stiffness of old tendons as well as the high incidence of tendinopathy and injury.
Experimental procedures
Animals
Male and female Sprague–Dawley rats were obtained from Fisher or from the breeding colony at Indiana University. At 3–5 months (eight males and one female) or 22–25 months of age (three males and four females), rats were euthanized with CO2 after which the Achilles tendons (tendo calcaneus) and tibialis anterior tendons were dissected from each leg, frozen in liquid nitrogen (for RNA isolation) or placed into a TBS tissue freezing medium (Triangle Biological Sciences, Durham, NC, USA), frozen in cold isopentane (for sectioning), and stored at −80 °C. All animal care and animal surgeries were performed in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85-23); the experimental protocol was approved by the Indiana University Committee for the Use and Care of Animals (IUSM-NW-19).
Immunohistochemical analysis
For the analysis, unfixed TBA tendon samples were placed into a TBS tissue freezing medium (Triangle Biological Sciences), frozen in cold isopentane, and stored at −80 °C until needed. Samples were sliced with a cryostat at a thickness of approximately 12 μm, adhered to Superfrost Plus microscopy slides, and used for staining. For immunostaining, frozen sections were fixed with ice-cold methanol for 10 min and rinsed three times with phosphate buffered saline (PBS). Sections were blocked for 30 min with PBS–0.05 % Tween20 (PBST) containing 20 % calf serum (PBST-S) at room temperature. Sections were incubated overnight at 4 °C with the primary antibodies in PBST-S. The following primary antibodies were used: rabbit anti-PRG4, rabbit anti-collagen type I, and rabbit anti-elastin were obtained from Millipore (Temecula, CA, USA); rabbit anti-collagen type III, rabbit anti-collagen type V, rabbit anti-CTGF, and rabbit anti-lysyl oxidase (LOX) were obtained from Abcam (Cambridge, MA, USA); rabbit anti-paxillin (Epitomics, Burlingame, CA, USA); mouse anti-talin and rabbit anti-collagen XXIIA1 were both from Sigma (St. Louis, MO, USA); mouse anti-SDF1 (R&D Systems, Minneapolis, MN, USA); and chicken anti-TGFb1 (Thermo Scientific, Rockford, IL, USA). One-hour room temperature incubation with Cy2 or Cy3-conjugated anti-mouse, anti-chicken, or anti-rabbit antibody (Jackson ImmunoResearch Lab., West Grove, PA, USA) was used for visualization. Sections incubated only with Cy3-conjugated anti-mouse, anti-chicken, or anti-rabbit antibody were used as negative controls. Co-staining of sections with fluorescein-conjugated wheat germ agglutinin (green, WGA-fluorescein; 1 μg/ml; Molecular Probes, Eugene, OR, USA) was used for visualization of the connective tissue as previously described (Kostrominova 2011). Nuclei were stained by 5-min incubation with a DAPI solution (Sigma, St. Louis, MO, USA) in PBST. The sections were examined and photographed with a Leica microscope. Quantification of relative intensity of immunostaining was performed using ImageJ software (http://imagej.nih.gov/ij/index.html; Schneider et al. 2012).
RNA isolation, reverse transcription, and quantitative PCR analysis
Total RNA was isolated by homogenizing samples in Trizol (GIBCO BRL, Grand Island, NY, USA) followed by the single-step purification method described by the manufacturer’s protocol. DNA contamination was removed by digestion with RNase-free DNase I using the DNA-free kit (Ambion, Austin, TX, USA). RNA concentrations were estimated with a spectrophotometer. Quantitative reverse transcription and PCR analysis (qRT-PCR) was performed as follows: 1 μg of total RNA was reverse-transcribed using a ThermoScript RT kit (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed with a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Amplifications were performed in a 20-μl total volume. Nucleotides, Taq DNA polymerase, and buffer were included in the Master SYBR Green mix (Qiagen, Valencia, CA, USA). An amplification protocol incorporated an initial incubation at 95 °C for 10 min for the activation of FastStartTaq DNA polymerase followed by 40 cycles, with a 94 °C denaturation for 15 s, 58–60 °C annealing for 30 s, and 72 °C extension for 30 s. Detection of the fluorescent product was performed at the end of the 72 °C extension period. To confirm the amplification specificity, the PCR products from each primer pair were subjected to a melting curve analysis. Relative quantification was performed based on the threshold cycle (CT value) for each of the PCR samples (Livak and Schmittgen 2001). Initially, several housekeeping genes were evaluated for the normalization of qRT-PCR data: GAPDH, cyclophilin, beta-2-microglobulin, 18S RNA, and large ribosomal protein P0 (RPL0). Normalization to the level of expression of cyclophilin, beta-2-microglobulin, and RPL0 showed similar results (data not shown). Thus, based on the preliminary findings as well as the recent study by Heinemeier et al. (2013), RPL0 was chosen for the normalization of all qRT-PCR experiments in the current study. The sequences of the primers used for the qRT-PCR analysis were designed using Primer3 software (Untergasser et al. 2012) and are summarized in Table 1. There was no difference in mRNA expression between samples from male and female rats; therefore, the data for male and female rats were combined.
Table 1.
Primers used for qRT-PCR study
| Accession no. (GenBank) | Gene description | Forward primer | Reverse primer |
|---|---|---|---|
| AY596447.1 | Connective tissue growth factor (CTGF) | TGGCGAGATCATGAAAAAGA | GGACTCAAAGATGTCATTGTCC |
| NM_012722 | Elastin (Eln) | GCAGCCTGGCGTCTTG | GGATAATAGACTCCACCGGGA |
| U11038.1 | Lysyl oxidase (LOX) | GCTGCGGAAGAAAACTGC | AATCGTAGCAGTACCCTGTGG |
| Z78279 | Procollagen type I, alpha 1 (Col1a1) | AACCTGGATGCCATCAAGG | AACTGGAATCCATCGGTCAT |
| NM_032085.1 | Procollagen type III (Col3a1) | TCCTGATCAAGAATTTGGTGTG | TTTTGTTTTGCTGGGGTTTC |
| NM_134452.1 | Procollagen type V (Col5a1) | TCCCTGACAAGAAGTCTGAGG | CGCTTGAATTCACTGAACCAG |
| NM_001105962.2 | Proteoglycan 4 (Prg4) | CAGGGAAGATAGTGGCTGCT | ATGTTGCCACCTCTCTTGAA |
| NM_022402.2 | Ribosomal subunit large P0 (RPLP0) | GCGACCTGGAAGTCCAACTA | TGTCTGCTCCCACAATGAAG |
| NM_022177.3 | SDF1 (Cxcl12) | AGCCAGTCAGCCTGAGCTAC | GGCACAGTTTGGAGTGTTGA |
| NM_021578.2 | Transforming growth factor beta 1 (TGFb1) | CCACTCCCGTGGCTTCTAGT | AGCTCCATGTCGATGGTCTT |
Statistical analysis
Statistical significance of the results was assessed using SigmaStat software (Systat Software, Inc.; Point Richmond, CA, USA) to determine Student’s t test values. Differences in values with p < 0.05 were considered statistically significant.
Results
Expression levels of extracellular matrix (ECM) proteins controlling lubricating and elastic properties of tendons and growth factors that regulate expression of ECM proteins in tendons
Analysis of gene expression showed 2.6- and 3.8-fold decrease in the expression of PRG4 and Eln mRNA, respectively, in tendons of old when compared with young rats (Fig. 1a). There was no statistically significant difference in the expression of CTGF and TGFb1 mRNA between tendons of old and young rats (Fig. 1a). Expression of SDF1 in tendons of old rats showed a trend to the decreased mRNA expression without reaching statistical significance (Fig. 1a).
Fig. 1.
Evaluation of PRG4, elastin, SDF1, CTGF, and TGF-beta1 expression in tendons and MTJs of young and old rats. a mRNA expression of PRG4, Eln, SDF1, CTGF, and TGFb1 in Achilles tendons of young (N = 9) and old (N = 7) rats. Values are normalized to RPL0 expression in each sample. *p < 0.05 indicates a significant difference between tendons of young and old rats. b Immunostaining of proximal TBA tendons from young and old rats with antibodies against PRG4, elastin, SDF1, CTGF, and TGF-beta1 (red in A–J). DAPI (blue) was used to co-stain the nuclei. Arrows indicate location of the fascicular sheets. Arrowheads indicate location of the tenocyte nuclei. c Relative levels of protein expression based on intensity of immunostaining of proximal TBA tendons from young and old rats with antibodies against PRG4, elastin, SDF1, CTGF, and TGF beta 1. *p < 0.05 indicates a significant difference between tendons of young and old rats. d Immunostaining of MTJ regions of TBA tendons from young and old rats with antibodies against PRG4, elastin, SDF1, CTGF, and TGF-beta1 (red in A–T). DAPI (blue) was used to co-stain the nuclei. WGA-fluorescein (green in A–T) was used to visualize connective tissue. Arrows indicate increased protein accumulation at the muscle/tendon border. Arrowheads indicate skeletal muscle fibers
Consistent with the decreased PRG4 and Eln mRNA expression (Fig. 1a), the immunostaining of PRG4 and Eln proteins in longitudinal sections of TBA tendons of old rats was decreased when compared with young rats (Fig. 1b, c). PRG4 was localized to the nuclei (arrows in Fig. 1b: A and F) as well as to the fascicular sheets (arrowheads in Fig. 1b: A and F). Eln was organized into fibers oriented longitudinally in the same directions as the entire tendon (arrows in Fig. 1b: B and G) sometimes co-localized with flat longitudinally oriented tenocytes (arrowheads in Fig. 1b: B and G). In visual observation, it seemed that tendons from old rats had smaller number of Eln fibers. It appeared that thinner Eln fibers were only present in tendons from young but not old rats (Fig. 1b: B and G). This observation was corroborated by ~20 % decrease in relative intensity of elastin immunostaining in tendons of old rats (Fig. 1c). SDF1 protein expression was also decreased by ~20 % in TBA tendons of old rats when compared with young rats (Fig. 1b: C and H, c), whereas immunostaining with antibodies for CTGF and TGFb1 did not detect differences in the pattern of expression in tendons of young and old rats (Fig. 1b: D, E, I, and J). Relative intensity of CTGF and TGFb1 immunostaining appeared to be slightly increased in tendons of old rats (Fig. 1c). Immunostaining for CTGF and TGFb1 was localized to the fascicular sheets (arrows in Fig. 1b: D, E, I, and J) and to the nuclei (arrowheads in Fig. 1b: D, E, I, and J).
Immunostaining with antibodies against PRG4 showed accumulation of PRG4-positive nuclei at the tendon side of the MTJs (arrows in Fig. 1d: A, F, K, and P). Interestingly, myonuclei located in the close proximity to the MTJ were PRG4-negative (Fig. 1d: F). MTJs from old rats appeared to have less PRG4-positive nuclei at the tendon side of the MTJs (arrows in Fig. 1d: K and P). The concentration of the Eln fibers also appeared to be increased at the tendon side of the MTJs (arrows in Fig. 1d: B, G, L, and Q). When compared with MTJs of young rats (arrows in Fig. 1c: B and G), MTJs of old rats appeared to have fewer Eln fibers (arrows in Fig. 1d: L and Q). Likewise, SDF1 immunostaining was increased at the MTJs and was localized to the tendon side (arrows in Fig. 1d: C, H, M, and R). MTJs from old rats appeared to have smaller SDF1-positive area (arrows in Fig. 1d: M and R). Immunostaining for CTGF and TGFb1 was also increased at the MTJs (arrows in Fig. 1d: D, E, I, J, N, O, S, and T), although there were no obvious differences between MTJs from young and old rats. TGFb1 immunostaining was notably higher in skeletal muscle fibers (arrowheads in Fig. 1d: E and O) than in the adjacent areas of tendon. Interestingly, contrary to the findings for PRG4, Eln, SDF1, and TGFb1, which all were increased on the tendon side of the MTJs (arrows in Fig. 1d: F, P, G, Q, H, R, J, and T), immunostaining for CTGF was primarily localized to the muscle side of the MTJs (arrows in Fig. 1d: I and S). Paxillin, talin, and collagen XXII also showed localization to the muscle side of the MTJs (arrows in Supplemental Fig. 1) consistent with previous reports (Turner et al. 1991; Frenette and Tidball 1998; Koch et al. 2004).
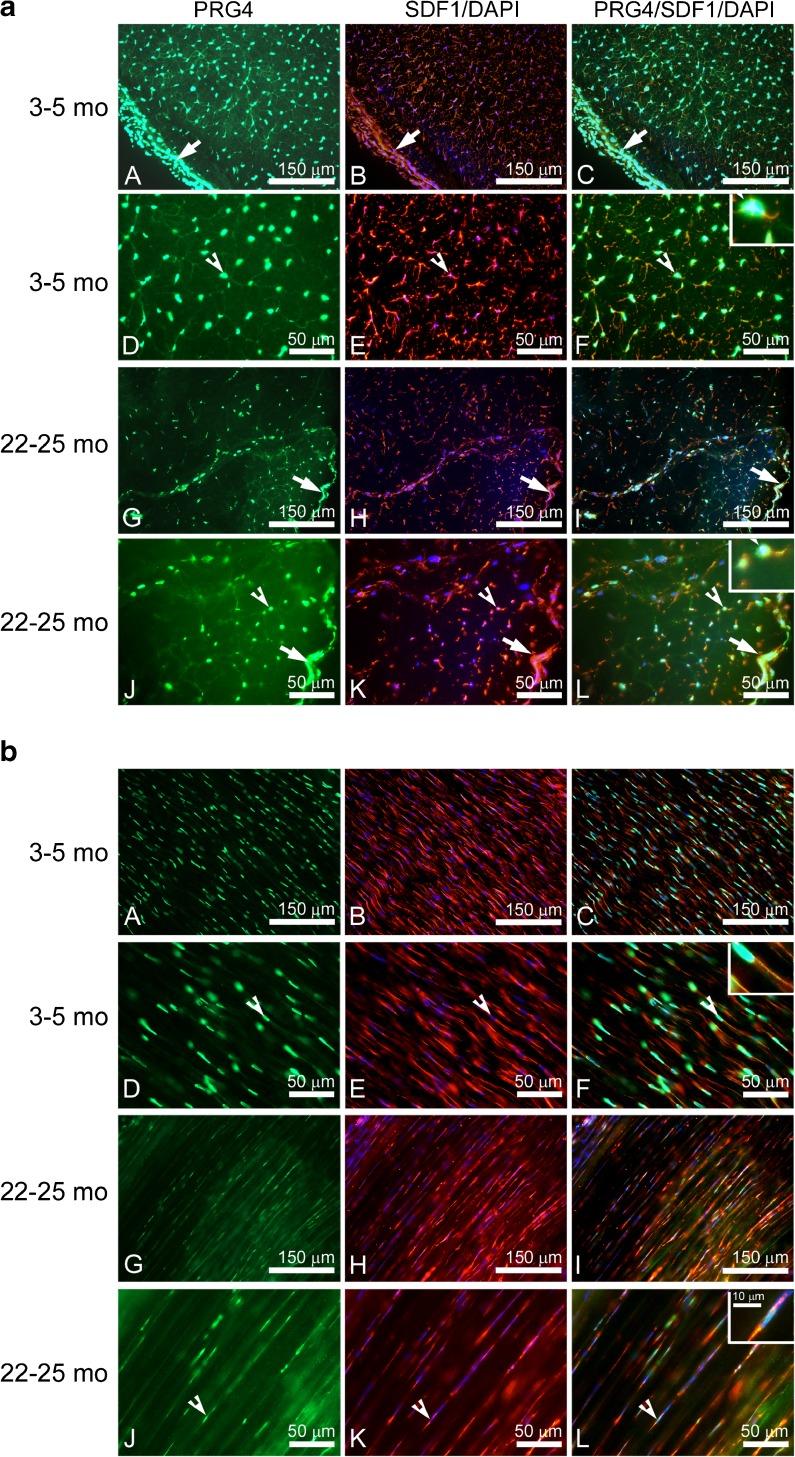
Co-localization of PRG4 and SDF1 in tendons
PRG4 and SDF1 were co-localized in the nuclear and endotenon regions in cross-sections of tendons from young and old rats (Fig. 2a). Both nuclear and endotenon regions appeared to have decreased PRG4 and SDF1 protein expression in tendons of old rats (Fig. 2a: G–L), with the SDF1 immunostaining at the endotenon region especially reduced in tendons from old rats (Fig. 2a: K). Compared with the other regions of the tendons, the epitenon region had the largest density of tenocytes with the highest levels of PRG4 and SDF1 protein co-localization (arrows in Fig. 2a). Immunostaining of longitudinal sections of tendons from young and old rats showed a similar pattern to Fig. 2a of the expression for PRG4 and SDF1 (Fig. 2b).
Fig. 2.
Co-localization of PRG4 and SDF1 in tendons of young and old rats. Immunostaining of the cross (a) and longitudinal (b) sections of proximal TBA tendons from young and old rats with antibodies against PRG4 (green) and SDF1 (red). DAPI (blue) was used to co-stain the nuclei. Arrows indicate location of the epitenon. Arrowheads indicate location of the PRG4-positive tenocyte nuclei. Inserts show higher magnification of the area at the arrow in a F and L, and b F and L
Expression of collagens and LOX in tendons
Analysis of the major collagens found in tendons showed 28.5-fold decrease in COL1a1 mRNA expression, a 7.3-fold decrease in COL3a1 mRNA, and a 4.5-fold decrease in COL5a1 mRNA expression in tendons of old compared with young rats (Fig. 3a). LOX mRNA expression was not statistically different in tendons of old and young rats (Fig. 3a).
Fig. 3.
Evaluation of collagens I, III, and V and LOX expression in tendons of young and old rats. a mRNA expression of collagens I, III, and V and LOX in Achilles tendons of young (N = 9) and old (N = 7) rats. Values are normalized to RPL0 expression in each sample. *p < 0.05 indicates a significant difference between young and old tendons. b Immunostaining of proximal TBA tendons from young and old rats with antibodies against collagens I, III, and V and LOX. c Relative levels of protein expression based on intensity of immunostaining of proximal TBA tendons from young and old rats with antibodies against collagens I, III, and V and LOX. *p < 0.05 indicates a significant difference between tendons of young and old rats
Despite significant decreases in collagen I, III, and V mRNA expression in Achilles tendons of old rats (Fig. 3a), immunostaining of the TBA tendons has not detected substantial decrease in collagen expression on the protein level (Fig. 3b). In fact, the relative intensity of collagen III immunostaining appeared to be increased in tendons of old rats (Fig. 3c). Collagen fibers were mostly oriented longitudinally in parallel with the direction of the applied force (arrows in Fig. 3b). As expected, there were fewer collagen type V fibers in tendons than type I and type III fibers (Fig. 3b). While LOX mRNA expression levels were similar in tendons of old and young rats (Fig. 3a), immunostaining of TBA tendons with antibodies against LOX detected ~2-fold increase in relative intensity in tendons of old rats (Fig. 3b, c). LOX was often localized to tenocyte nuclei (arrowheads in Fig. 3b).
Discussion
Aging in tendons is characterized by an increased susceptibility to injury likely resulting from changes in mechanical properties (Milgrom et al. 1995; Dressler et al. 2002; Jarvinen et al. 2005; Wood et al. 2011). In order to gain insights into the basis for the age-related changes in tendon mechanical properties, the current study evaluated the changes in the expression levels and localization of proteins known to be critical components of tendon function (collagens, Eln, PRG4) as well as growth factors that regulate expression of ECM proteins in tendons (TGFb1, CTGF, SDF1). Our findings suggest that aging could have a considerable effect on structural composition of tendons and MTJs which could lead to the modifications in their functional properties. To the best of our knowledge, this is the first study to show decreases with aging in PRG4 and Eln mRNA and protein expression in tendons that correlate with declines in mRNA levels for Col1a1 in tendons of old animals. We also for the first time showed increased accumulation of PRG4 protein at the MTJs of young rats and age-related decrease at MTJs of old rats.
The primary constituent of tendon is collagen, and we showed marked reductions in mRNA for all major collagen types found in tendons. We observed age-related reductions of mRNA expression levels of collagen types I, III, and V. Although immunostaining is generally considered to be a qualitative rather than quantitative technique, we have not detected apparent changes in protein expression of collagens I and V in tendons during visual observation and quantification of relative intensity of staining when this technique was used for the evaluation. Our observations correlate with reported previously decrease in collagen turnover in old tendons (Dressler et al. 2002; reviewed in Kjaer et al. 2006). Slower turnover of collagen with aging could lead to the accumulation of micro-damage and partially degraded collagen molecules predisposing old tendons to mechanical stress-related injury (Thorpe et al. 2010).
Relative to type I collagen, the expression of type V collagen in tendons is much less studied. An age-related increase in type V collagen was previously suggested to explain the altered tendon biomechanical properties in rabbit patellar tendons with aging (Dressler et al. 2002). In that study, type V collagen was practically undetectable in tendons of 1-year-old rabbits but clearly observed in tendons of 4-year-old rabbits using SDS-polyacrylamide gel electrophoresis (Dressler et al. 2002). Contrary to the findings of Dressler et al. (2002), in the present study, type V collagen was readily detectable in tendons of young rats and mRNA expression was decreased in old rats, although the decrease was less pronounced than the decrease of type I collagen mRNA expression in tendons of old rats. In a separate study, increased expression of type V collagen correlated with accumulation of the smaller diameter collagen fibrils after ligament injury (Niyibizi et al. 2000), and a study by Lu et al. (2011) showed that collagen type V expression was increased in injured tendons. Thus, understanding of the role of type V collagen in tendon and ligament function and its role in aging and injured tendons warrants further evaluation.
While collagens constitute 65–80 % of the dry mass, Eln is an additional fibrillar protein that constitutes a minor component of tendon structure, contributing ~1–2 % to the dry mass of tendons (reviewed in Kannus 2000). The main property of Eln is its ability to deform reversibly with little force and without loss of energy. Eln also has low stiffness and can undergo large strains. Our experiments showed that Eln was easily visualized by immunostaining in tendons of young rats. Eln expression was enriched at the proximal end of the TBA tendon on the tendon side of the MTJs. We hypothesize that high Eln expression at MTJs helps to provide a mechanical link between the highly compliant skeletal muscle fibers and the stiffer tendons. Decreased Eln expression at the MTJs and in tendons of old animals could lead to diminished elasticity and predispose tendons to mechanical stress related injury, especially within the MTJ. Our observation that the most significant age-related change in tendon mechanical properties was stiffening of the proximal region of the tendon near the MTJ (Wood et al. 2011) correlates well with an age-related decrease in Eln expression in this region.
We also compared LOX mRNA and protein expression, a major player in collagen type I maturation, in tendons of young and old rats. LOX is a copper-dependent metalloenzyme that participates in maturation of collagen and elastin by oxidation of hydroxylysine residues and allowing formation of covalent crosslinks (reviewed by Maki 2009). It is essential for the stabilization of the ECM. LOX is very sensitive to mechanical loading and is often used as a marker of the ECM response to mechanical stimulation. For example, mechanical loading of tendons increased expression of LOX by ~37-folds while Col1a1 expression was up-regulated only ~5-fold (Heinemeier et al. 2007). Our expectation was that an age-associated decrease in physical activity would be associated with decreased LOX expression in tendons of old rats, but we detected no noticeable changes in LOX mRNA expression and increased LOX protein expression in tendons of old when compared with young rats. Other functions of LOX not related to collagen and elastin crosslinking could be involved in the regulation of LOX expression in tendons. For example, LOX crosslinking of lysyl residues in histones H1 and H2 is essential for stabilization of cell nuclei and this function of LOX is critical for cancer prevention (reviewed in Li et al. 2011). Our experiments also demonstrated localization of LOX to the nuclei of tenocytes. We hypothesize that LOX could play a role in nuclear stabilization in tendons and this role might be extremely important during aging as a response mechanism to increased oxidative stress and hypoxia. It was previously reported that LOX promoter contains hypoxia response element and antioxidant response element (reviewed in Li et al. 2011) and, therefore, could be potentially regulated by age-related oxidative stress and hypoxia in tendons.
PRG4 is a glycoprotein with anti-adhesive and lubricating properties that is also known as lubricin or superficial zone protein. Funakoshi et al. (2008) showed that PRG4 was localized to fascicular sheets in tendons where it facilitates inter-fascicular movement. PRG4 knockout mice had significantly higher peak gliding resistance of the collagen fascicles in tails than the wild-type mice (Kohrs et al. 2011). The decreased expression with aging of PRG4 in tendons and MTJs reported in the current study may result in decreased gliding properties of fascicular sheets in tendons of old rats and predispose tendons to the mechanical stress-related injury. Another previously unreported finding of our study is co-localization of PRG4 and SDF1 in tendons, although the significance of this finding is unclear. SDF1 is a chemo-attractant reported to be up-regulated in both murine and human dystrophic muscle and in other sites of tissue damage and is important for tissue regeneration (Perez et al. 2009). Our experiments showed a trend toward a decrease with aging in SDF1 mRNA and immunostaining also demonstrated decreased SDF1 protein expression in tendons of old compared with young rats. The lower SDF1 expression correlated well with decreased PRG4 expression in tendons of old rats. It was previously reported that SDF1 mRNA expression was decreased in bone marrow of PRG4 knockout mice (Novince et al. 2011), suggesting that PRG4 could be involved in the regulation of SDF1 expression. Decreased SDF1 expression in tendons of old rats could impact the function of tendon-derived stem/progenitor cells and/or tendon regenerative capacity.
MTJs are architecturally complex specialized structures that provide stable interfaces between tendon and skeletal muscle capable of withstanding significant mechanical forces (Tidball and Lin 1989). A number of proteins are reported to be specifically expressed or to show increased levels at the MTJs (Law et al. 1994; Jarvinen et al. 2003; Welser et al. 2009). Consistent with previous reports (Turner et al. 1991; Frenette and Tidball 1998; Koch et al. 2004), we confirmed paxillin, talin, and collagen XXII localization to the muscle side of the MTJs. In addition, we have shown for the first time increased accumulation of PRG4 and Eln at the MTJs of young rats, as well as accumulation of SDF1, CTGF, and TGFb1 at the MTJs. While PRG4, Eln, SDF1, and TGFb1 were clearly localized to the tendon side of the MTJs, CTGF was localized to the ends of the skeletal muscle fibers embedded into tendons. It is well known that muscle fibers secrete specialized proteins at the MTJs (Law et al. 1994; Jarvinen et al. 2003; Welser et al. 2009), but to our knowledge, the present study is the first to show that tenocytes located in close proximity to the MTJs also participate in defining structural composition of the MTJs by increased deposition of the specific proteins.
The TGF-beta family of growth factors plays an important role in all aspects of tendon function including tendon growth, repair, regulation of mechanically induced collagen synthesis, and ECM turnover (Duncan et al. 1999; Nakatani et al. 2002). Studies in fibroblasts suggest that CTGF is a down-stream mediator of TGFb1-induced collagen synthesis (Grotendorst et al. 1996; Duncan et al. 1999). It was previously reported that PRG4 expression is positively regulated by exogenously added TGF-beta1 and negatively regulated by IL-1beta in cultured cells (Lee et al. 2008; Cheng et al. 2010). Therefore, we hypothesized that decreased PRG4 mRNA and protein expression detected in our study in tendons of old rats could be mediated by the decrease in TGFb1 and CTGF expression. Contrary to our hypothesis, there were no apparent changes in mRNA expression and there was a slight increase in protein expression of TGFb1 and CTGF in tendons of old when compared with young rats.
In conclusion, the major findings of the current study are decreased PRG4 and Eln mRNA expression in tendons of old rats along with a decrease of collagen mRNA expression. Decreased PRG4 and Eln expression in tendons could be responsible for the increased tendon stiffness, in particular in the region near the muscle (Wood et al. 2011). Moreover, decreased PRG4 expression at the MTJs could lead to the disorganization of MTJ structures and increased MTJ instability predisposing to the mechanical stress-induced MTJ failure. Our future goals are to elucidate the molecular mechanisms involved in the regulation of collagen turnover and PRG4 and Eln expression in tendons and MTJs in aging animals.
Electronic supplementary material
Evaluation of paxillin, talin and collagen XXII expression at MTJs of young rats. Immunostaining of MTJ regions of TBA tendons from young rats with antibodies against paxillin, talin and collagen XXII (red in A–C). DAPI (blue) was used to co-stain the nuclei. WGA-fluorescein (green in A and C) and anti-collagen type I antibodies (green in B) were used to visualize connective tissue. Arrows indicate increased protein accumulation at the muscle/tendon border. (PDF 2212 kb)
Acknowledgments
This study was supported by NIH Grant AR-055624 (SVB) and IUSM-Northwest (TYK).
Abbreviations
- CTGF
Connective tissue growth factor
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- Eln
Elastin
- IGF
Insulin-like growth factor
- FGF
Fibroblast growth factor
- GDF
Growth differentiation factor
- LOX
Lysyl oxidase
- MTJs
Myotendinous junctions
- PRG4
Proteoglycan 4
- SDF1
Stromal cell-derived factor 1
- TBA
Tibialis anterior muscle
- TGFb
Transforming growth factor beta
References
- Augustin H, Partridge L. Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta. 2009;1790:1084–1094. doi: 10.1016/j.bbagen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang Y, Wang Z, Yang M, Wu Y. Differential regulation of proteoglycan-4 expression by IL-1alpha and TGF-beta1 in rat condylar chondrocytes. Tohoku J Exp Med. 2010;222:211–218. doi: 10.1620/tjem.222.211. [DOI] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, et al. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res. 2002;20:1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- Frenette J, Tidball JG. Mechanical loading regulates expression of talin and its mRNA, which are concentrated at myotendinous junctions. Am J Physiol. 1998;275:C818–C825. doi: 10.1152/ajpcell.1998.275.3.C818. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Schmid T, Hsu HP, Spector M. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J Bone Joint Surg Am. 2008;90:803–814. doi: 10.2106/JBJS.G.00627. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M (2013) Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports. doi:10.1111/j.1600-0838.2011.01414.x [DOI] [PubMed]
- Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Hurme T, et al. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 2003;116:857–866. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Magnusson P, Krogsgaard M, Boysen Moller J, Olesen J, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, et al. A novel marker of tissue junctions, collagen XXII. J Biol Chem. 2004;279:22514–22521. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrs RT, Zhao C, Sun YL, Jay GD, Zhang L, et al. Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. J Orthop Res. 2011;29:384–389. doi: 10.1002/jor.21247. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY. Application of WGA lectin staining for visualization of the connective tissue in skeletal muscle, bone, and ligament/tendon studies. Microsc Res Tech. 2011;74:18–22. doi: 10.1002/jemt.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994;107(Pt 6):1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- Lee SY, Nakagawa T, Reddi AH. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376:148–153. doi: 10.1016/j.bbrc.2008.08.138. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou J, Chen L, Luo Z, Zhao Y. Lysyl oxidase, a critical intra- and extra-cellular target in the lung for cigarette smoke pathogenesis. Int J Environ Res Public Health. 2011;8:161–184. doi: 10.3390/ijerph8010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu P, Zhang GR, Song XH, Zou XH, Wang LL, et al. Col V siRNA engineered tenocytes for tendon tissue engineering. PLoS One. 2011;6:e21154. doi: 10.1371/journal.pone.0021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586(1):71–81. doi: 10.1113/jphysiol.2007.139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296–298. [PubMed] [Google Scholar]
- Nakatani T, Marui T, Hitora T, Doita M, Nishida K, et al. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002;20:1380–1386. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN. Plasticity of the muscle–tendon complex with disuse and aging. Exerc Sport Sci Rev. 2007;35(3):126–134. doi: 10.1097/jes.0b013e3180a030ec. [DOI] [PubMed] [Google Scholar]
- Niyibizi C, Kavalkovich K, Yamaji T, Woo SL. Type V collagen is increased during rabbit medial collateral ligament healing. Knee Surg Sports Traumatol Arthrosc. 2000;8:281–285. doi: 10.1007/s001670000134. [DOI] [PubMed] [Google Scholar]
- Novince CM, Koh AJ, Michalski MN, Marchesan JT, Wang J, et al. Proteoglycan 4, a novel immunomodulatory factor, regulates parathyroid hormone actions on hematopoietic cells. Am J Pathol. 2011;179:2431–2442. doi: 10.1016/j.ajpath.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva F, Gatti S, Porcellini G, Forsyth NR, Maffulli N. Growth factors and tendon healing. Med Sport Sci. 2012;57:53–64. doi: 10.1159/000328878. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Samiric T, Ilic MZ, Cook J, Handley CJ. Involvement of proteoglycans in tendinopathy. J Musculoskelet Neuronal Interact. 2011;11(2):86–93. [PubMed] [Google Scholar]
- Perez AL, Bachrach E, Illigens BM, Jun SJ, Bagden E, et al. CXCR4 enhances engraftment of muscle progenitor cells. Muscle Nerve. 2009;40:562–572. doi: 10.1002/mus.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees SG, Dent CM, Caterson B. Metabolism of proteoglycans in tendon. Scand J Med Sci Sports. 2009;19(4):470–478. doi: 10.1111/j.1600-0838.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Riley G. Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Rennie MJ. New approaches and recent results concerning human-tissue collagen synthesis. Curr Opin Clin Nutr Metab Care. 2007;10:582–590. doi: 10.1097/MCO.0b013e328285d858. [DOI] [PubMed] [Google Scholar]
- Spittau B, Krieglstein K. Klf10 and Klf11 as mediators of TGF-beta superfamily signaling. Cell Tissue Res. 2012;347(1):65–72. doi: 10.1007/s00441-011-1186-6. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, et al. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem. 2010;285:15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989;257:77–84. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- Turner CE, Kramarcy N, Sealock R, Burridge K. Localization of paxillin, a focal adhesion protein, to smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. Exp Cell Res. 1991;192:651–655. doi: 10.1016/0014-4827(91)90090-h. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- Welser JV, Rooney JE, Cohen NC, Gurpur PB, Singer CA, et al. Myotendinous junction defects and reduced force transmission in mice that lack alpha7 integrin and utrophin. Am J Pathol. 2009;175:1545–1554. doi: 10.2353/ajpath.2009.090052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011;111(4):999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of paxillin, talin and collagen XXII expression at MTJs of young rats. Immunostaining of MTJ regions of TBA tendons from young rats with antibodies against paxillin, talin and collagen XXII (red in A–C). DAPI (blue) was used to co-stain the nuclei. WGA-fluorescein (green in A and C) and anti-collagen type I antibodies (green in B) were used to visualize connective tissue. Arrows indicate increased protein accumulation at the muscle/tendon border. (PDF 2212 kb)