Abstract
Little is known about tendons and tenocyte biological behaviour during aging and, especially, oestrogen deficiency. The aim of this study was to evaluate in vitro the proliferation and metabolism of tenocytes isolated from the Achilles tendons of ovariectomised (OVX), middle-aged (OLD) and young (YOUNG) rats. An in vitro model of micro-wound healing was also used to assess age and oestrogen deficiency differences in tendon healing. In standard culture condition, OLD and OVX tenocytes showed a significantly lower proliferation rate, collagen I, aggrecan and elastin than YOUNG ones. In OVX group, fibronectin and elastin significantly decreased in comparison to YOUNG and OLD groups, respectively, whereas vascular endothelial growth factor and metalloproteinases-13 increased than those of both YOUNG and OLD groups. In the micro-wound healing model, tenocytes from both OVX and OLD showed a significantly lower healing rate, proliferation rate, collagen I and nitrix oxide in comparison to YOUNG. OVX elastin value was significantly lower than YOUNG one and OVX healing rate and cell migration speed, proliferation rate and fibronectin results were lower, whereas collagen III and metalloproteinase-13 higher in comparison to both YOUNG and OLD groups. These results highlighted how aging and, more significantly, oestrogen deficiency negatively affect tendon metabolism and healing. Our work improves the body of knowledge on the effects of senescence and oestrogen deficiency on tenocyte behaviour and allows further studies to find solution for the prevention of tendon injuries in aging and menopause.
Keywords: Achilles tendons, Tenocytes, Aging, Oestrogen deficiency, Tendon healing
Introduction
Tenocytes play a central role in maintaining the integrity of tendon tissue and transmitting signals through their environment with proteins (Dudhia et al. 2007). Tenocytes are also responsible for tendon characteristics and repair processes due to their migration ability and their capacity to synthesize and remodel the extracellular matrix (ECM) (Arnesen and Lawson 2006). Therefore, the function, mechanics and homeostasis of tendon tissue depend on the orchestrated synthetic and degradative processes of tenocytes.
Tendons are commonly injured by young athletes and older adults, and management options are limited, expensive and sometimes ineffective, with prolonged recovery times and a reduced quality of life. Among Achilles tendon lesions, rupture is a relatively common one (Cretnik and Frank 2004; De Jonge et al. 2011) with a multifactorial etiopathology (Maffulli et al. 2003; Cook et al. 2007).
In middle age, people used to playing sports regularly do not change their habits, even if changes begin to occur in the physiology and function of connective tissues. This leads to more frequent injury. The structure of bone, skeletal muscle and tendons is altered with aging, resulting in a loss of their material properties (Chen et al. 2006).
Few studies have focused on the effect of aging on tendon, and most of them have investigated the mechanical features of tendons. Couppè et al. did not find differences in the dimensions or mechanical properties of tendons between young and old men, even though collagen content was significantly lower in older individuals, and the advanced glycation end marker was more accumulated in the tendons of older subjects (Couppè et al. 2009). In an equine tendon explant model, Dudhia et al. observed a reduction in mechanical strength, a higher susceptibility to weakening, matrix metalloproteinases (MMPs) production and disruption of the ECM in older horses compared to healthy young (Dudhia et al. 2007). In in vitro experimental studies, tenocytes from the Achilles tendons of older individuals proliferated significantly less, with a higher expression of senescence-associated beta-gal, a universal marker of aging, downregulation of cellular senescence-inhibited gene and upregulation of p27 (a CDK inhibitor that arrest cell cycle), in comparison with younger ones (Arnesen and Lawson 2006; Tsai et al. 2011). Moreover, in a wound-healing assay study, tenocytes from old mice showed lower proliferation and migration properties in comparison to those from younger mice, from lower focal adhesion proteins and higher expression of GADD 153, a growth arrest and DNA damage inducible gene (Arnesen and Lawson 2006). In in vivo evaluations, Thorpe et al. observed a higher concentration of collagen products, from micro-damages during aging, without replacement with new collagen. The higher amount of degraded collagen may explain the increased tendon injuries during aging (Thorpe et al. 2010).
In addition to aging, oestrogen levels might also play a key role, as observed in women that show a lower risk of tendinopathies during pre-menopausal years, whereas, after menopause, this risk increases (Tsai et al. 2011). Oestrogen levels (Irie et al. 2010) may influence tendon metabolism, and, in addition to several growth factors (i.e. platelet-derived growth factor, transforming growth factor-β, insulin growth factor-1, vascular endothelial growth factor, bone morphogenetic proteins, growth differentiation factors), oestrogens are important for the behaviour of musculoskeletal tissues and may affect tendon properties; postmenopausal oestrogen deficiency seems to downregulate collagen turnover and to decrease elasticity in tendon (Circi et al. 2009).
Most studies on tendons and oestrogen deficiency are clinical. Some differences in Achilles tendon structure in post-menopausal in comparison to young women indicate that hormone replacement therapy (HRT) with exogenous oestrogen may ameliorate tendon structure by preserving collagen fibre diameter rather than collagen production and thus preventing tendon ruptures. Furthermore, oestrogen positively influences tendon morphology and biomechanical properties in postmenopausal women (Bryant et al. 2008; Hansen et al. 2009). The combination of HRT and physical activity has positive effects on Achilles tendon characteristics, but exogenous oestrogen or physical activity separately does not induce tendon modification; in active subjects, positive effects of oestrogen were observed (Finni et al. 2009; Cook et al. 2007). In contrast with the previous results, collagen synthesis, during a one-legged resistance exercise, was negatively related to estradiol concentration (Hansen et al. 2009). In vivo and in vitro experiments confirmed that normal rats that were subjected to an Achilles tendon injury showed higher Achilles tendon healing rate, with higher tenocyte proliferation and density in comparison to oestrogen-deficient rats (Circi et al. 2009). In rat Achilles tenocytes in culture, a decrease in oestrogen levels downregulated the collagen turnover and reduced the elasticity of tendons (Irie et al. 2010). In an in vivo model of senescence-accelerated mouse (SAMP6), the number of tenocytes and the diameter of collagen fibres of Achilles tendons decreased after 8 months of age (Chen et al. 2006).
To date, only few studies have focused on the in vitro behaviour of tenocytes during aging, and even fewer in vitro studies have focused on oestrogen deficiency. Moreover, existing studies describe differences in tenocyte proliferation, but few data are available on in vitro ECM production and tenocyte metabolism. Moreover, no studies have taken into account tenocyte behaviour in the presence of both aging and oestrogen deficiency.
Therefore, the present study comparatively investigated how aging and oestrogen deficiency may affect tenocyte behaviour, by evaluating in vitro proliferation and ECM components, remodelling enzymes, growth factors (GFs), catabolic enzymes and nitric oxide (NO) of tenocytes isolated from the Achilles tendon of ovariectomised, middle-aged and young rats. An in vitro model of micro-wounds was also used to assess differences in tendon healing in aging or oestrogen deficiency.
Materials and methods
In the ambit of other projects on bone and bone metabolism (approved by the Ethical Committee of Rizzoli Orthopaedic Institute), tendons were explanted from rats immediately after euthanasia.
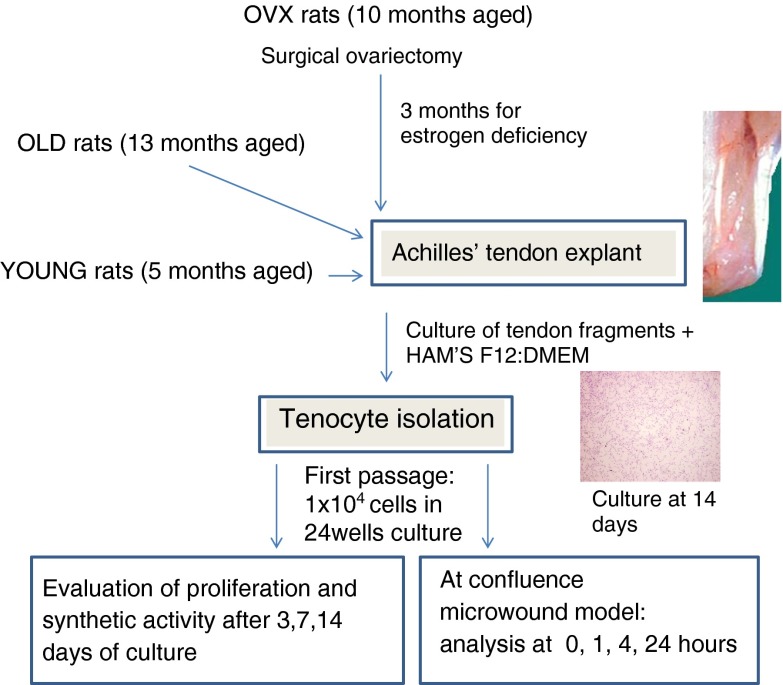
Tendon cell isolation and culture
The Achilles tendons of young (5 months old), middle-aged (13 months old) and ovariectomised (10 months at ovariectomy and euthanized 3 months later at the age of 13 months) Sprague Dawley rats were dissected under sterile conditions, rinsed in sterile physiological saline solution, and the whole tendons were cut into small pieces. Tendon fragments were transferred to 25-cm2 flasks, and growth medium HAM’S F12/ DMEM (50:50), containing 10 % foetal calf serum, 1 % penicillin-streptomycin solution and 25 μg/ml ascorbic acid, was added. Then, culture flasks were incubated at standard conditions (at 37 °C, in 95 % air/5 % CO2-humidified atmosphere), and the medium was replaced every 3 days. After 7 days, tenocytes were observed in the cultures, which were maintained under standard conditions until the flasks reached 80 % confluence (Fig. 1). The cells were then subcultured, and tenocytes were used for the experimental study after the first passage.
Fig. 1.
Experimental design of tenocyte isolation from tendon of YOUNG, OLD and OVX rats. Cells migrated from tissue and adhered to flask after the first days of culture and proliferated till 2 weeks (magnification, ×4)
Tenocytes derived from tendons of young (YOUNG), middle-aged (OLD) and ovariectomised (OVX) rats were detached by trypsin solution, counted by Trypan blue viable dye (Sigma, UK) and seeded onto 24-well plates at a concentration of 1 × 104 cells per millilitre for the experiments. Cultures were maintained at standard conditions for up to 14 days.
Tenocytes proliferation and synthetic activity
The Cell Proliferation Reagent WST-1 test (WST1, Roche Diagnostics GmbH, Manheim, Germany) was performed to assess cell viability (1, 3, 7 and 14 days); the assay is based on the reduction of tetrazolium salt to a soluble formazan salt by a reductase of the mitochondrial respiratory chain, active only in viable cells. Fifty microlitres of WST1 solution and 450 μl of medium (final dilution, 1:10) were added to the cell monolayer, and the multi-well plates were incubated at 37 °C for a further 4 h. Supernatants were quantified spectrophotometrically at 450 nm with a reference wavelength of 625 nm. Results of WST1 are reported as optical density and directly correlate with the cell number.
At the end of experimental times (3, 7 and 14 days), the supernatant was collected from the wells and centrifuged to remove particulates, if any. Aliquots were dispensed in Eppendorf tubes for storage at −70 °C and assayed with immunoenzymatic tests for collagen I (Coll I rat, USCN Life Science, China), collagen III (Coll III rat, USCN Life Science), aggrecan (aggrecan rat, USCN Life Science), nitric oxide (NO, Abnova, Taiwan), fibronectin (FBN rat, CUSABIO, USA), elastin (elastin rat, USCN Life Science), matrix metalloproteinase-13 (MMP-13 rat, CUSABIO), tissue inhibitor of metalloproteinases-1 (TIMP-1 rat, R&D Systems, MN, USA) and vascular endothelial growth factor (VEGF, R&D Systems). The concentrations measured were normalized by DNA content.
In vitro tendon micro-wounding
An in vitro tendon micro-wound healing model was used (Maffulli et al. 2000). Briefly, once a confluent monolayer of tenocytes was grown in 24-well plates, a sterile Eppendorf tip was used to produce an artificial wound by scraping the cell layer. At time zero (T0), wounds were approximately 650 μm wide. All the experiments were conducted in triplicate. After changing the medium, the multiwell plates were incubated in standard conditions for 1, 4 and 24 h (T1, T4 and T24, respectively) to evaluate wound healing. At each experimental time, cell proliferation was evaluated by the WST1 test, and supernatant was recovered and stored at −70 °C for further tests for Coll I, Coll III, FBN, elastin, NO, MMP-13 and VEGF. The wounded cell cultures were observed by an inverted microscope (IX70, Olympus), and three images for each well were photographed at ×10 magnification to analyse the whole length of wound. Image analysis was performed using cellB imaging software (Olympus, Munster, Germany); the width of the artificial wounds was measured (10 measures for each image) by a blind investigator, and the average migration speed, at T24, was calculated dividing the average width values by 24 h.
Statistical analysis of cell culture data
Statistical evaluation of data was performed using the software package SPSS/PC + StatisticsTM 10.1 (SPSS Inc., Chicago, IL, USA). The results presented are the mean of the triplicate values. Data are reported as mean ± standard deviations (SD) at a significance level of p < 0.05. After having verified the normal distribution and the homogeneity of the variance, the non-parametric Kruskal–Wallis, followed by the Monte Carlo methods to compute probability, was used to analyse data. Then, the Mann–Whitney U with Bonferroni’s correction was performed to detect significant differences between groups within each experimental time.
Results
Tenocyte proliferation and synthetic activity
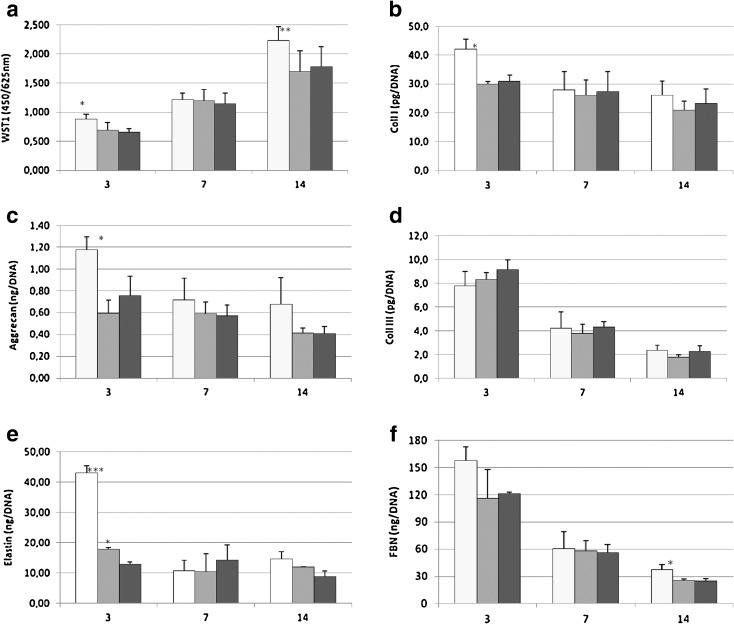
Tenocyte cultures grew regularly up to 14 days, and the results of the in vitro study on proliferation rate and synthetic activity of tenocytes isolated from OLD, OVX and YOUNG rats are summarized in Fig. 2. The proliferation rate showed no differences among the three groups at 7 days, but the YOUNG group proliferation was significantly higher than that of the OLD and OVX groups at 3 days (p < 0.05) and at 14 days (p < 0.005) (Fig. 2a).
Fig. 2.
YOUNG (white bar), OLD (grey bar) and OVX (black bar) tenocyte proliferation (a) and activity (b–f) after 3, 7 and 14 days of culture. Mean ± SD, n = 3 replicates; p value modified according to Bonferroni’s correction: * = p < 0.05, ** = p < 0.005, *** = p < 0.0005. a WST-1 test: 3 days—(single asterisk;*) YOUNG vs OLD, OVX; 14 days—(double asterisk; **) YOUNG vs OLD, OVX. b Coll I: 3 days—(single asterisk;*) YOUNG vs OLD, OVX. c Aggrecan: 3 days—(single asterisk; *) YOUNG vs OLD, OVX. e Elastin: 3 days—(triple asterisk; ***) YOUNG vs OLD, OVX; (single asterisk;*) OLD vs OVX. f FBN: 14 days—(single asterisk; *) YOUNG vs OVX
The production of components of tendon ECM, such as Coll I, aggrecan, Coll III, elastin and FBN, were tested in cell culture supernatant at 3, 7 and 14 days (Fig. 2b–f). Only at 3 days of culture, Coll I (Fig. 2b) in the YOUNG group was significantly higher when compared to the OLD and OVX groups (p < 0.05). Aggrecan production (Fig. 2c) was significantly higher, only at 3 days, in the YOUNG group when compared to the OLD and OVX groups (p < 0.05). No differences among the groups were found for Coll III (Fig. 2d) at 3, 7 and 14 days, but the Coll I/III ratio was significantly lower at 3 days (p < 0.005) in the OLD (−32.6 %) and OVX (−37.6 %) groups than that of the YOUNG group (data not shown). Elastin (Fig. 2e) showed significantly higher values in YOUNG than OLD and OVX rats (p < 0.0005), but elastin in the OLD group was significantly higher in comparison with OVX only at 3 days (p < 0.05). In the YOUNG group, FBN (Fig. 2f) deposition, only at 14 days, was significantly higher when compared to the OVX group (p < 0.05). NO, MMP-13, TIMP-1 and VEGF were also evaluated as representative of enzymatic activity and GFs involved in tendon ECM metabolism (Fig. 3 a–d).
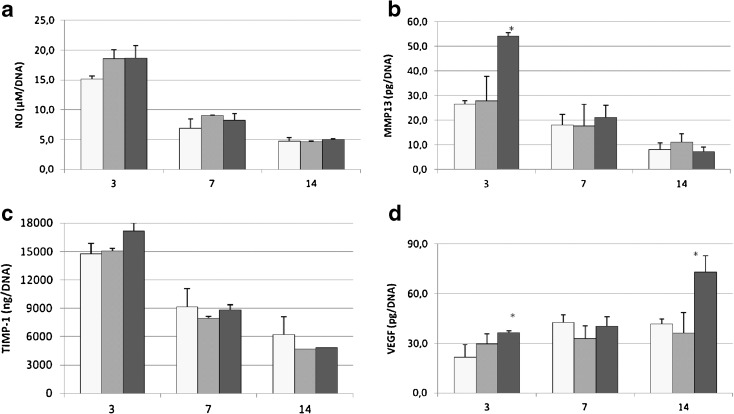
Fig. 3.
YOUNG (white bar), OLD (grey bar) and OVX (black bar) tenocyte activity (a–d) after 3, 7 and 14 days of culture. Mean ± SD, n = 3 replicates; p value modified according to Bonferroni’s correction: * = p < 0.05, ** = p < 0.005, *** =p < 0.0005. b MMP-13: 3 days—(single asterisk; *) OVX vs YOUNG, OLD. d VEGF: 3 days—(single asterisk; *) OVX vs YOUNG, 14 days—(single asterisk; *) OVX vs YOUNG, OLD
In the OVX group, at 3 days, a significantly higher level of MMP-13 (Fig. 3b) was observed in comparison to YOUNG and OLD groups (p < 0.05). VEGF level (Fig. 3d) had significantly higher values in the OVX group compared to the YOUNG group at 3 days (p < 0.05) and in comparison to the OLD and YOUNG groups at 14 days (p < 0.05). NO and TIMP-1 did not show significant differences between groups in all experimental times (Fig. 3a, c).
In vitro tendon micro-wounding
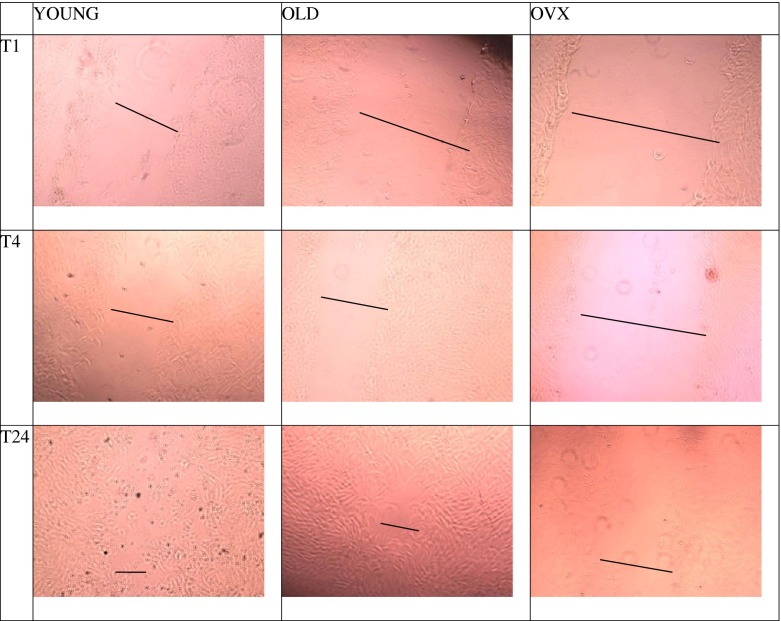
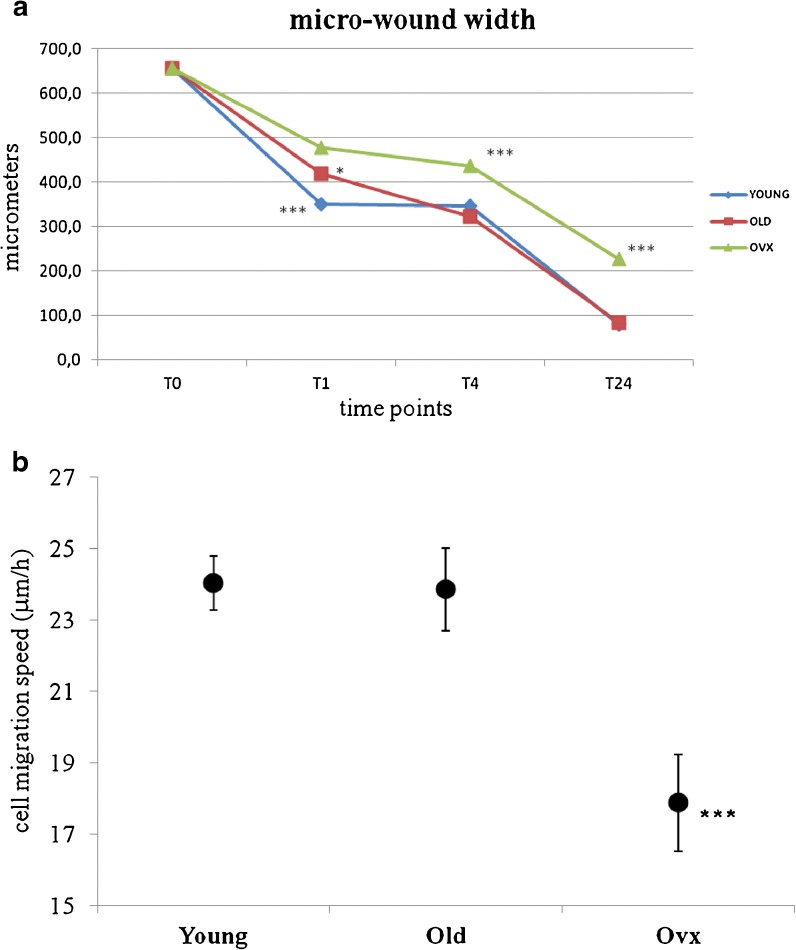
The width of the micro-wounding in vitro model was calculated at the end of each time point. In Fig. 4, examples of different groups and experimental time measures of micro-wounds are shown. The results of healing, as a mean of measures in micrometres of wound width, and the results of average cell migration speed from T0 to T24 are reported in Fig. 5 (a, b). At T1, significant differences were found among all groups; the recovery rate of YOUNG rats was 47 %, whereas the healing of OLD and OVX rats were 36 and 27 % (p < 0.0005), respectively. Moreover, significant differences were found between OLD and OVX rats (p < 0.05). After 3 h (T4), tenocytes from OVX rats showed 33.4 % of healing, significantly differing from both OLD (51 %) and YOUNG (47 %) rats (p < 0.0005). At T24, both OLD and YOUNG rats showed an almost complete recovery of the wound with 87.3 and 87.8 % of healing, respectively, whereas healing of OVX rats was significantly lower (p < 0.0005) at 65.4 %. According to the recovery rate data, the average of cell migration speed of OVX was 17.88 μ/h (16.53–19.24 μ/h), significantly lower than both YOUNG 24.02 μ/h (23.26–24.77 μ/h) and OLD 23.85 μ/h (22.69–25.02 μ/h) (p < 0.0005).
Fig 4.
Light inverted microscopic images of in vitro wounded tenocytes by YOUNG, OLD and OVX groups at T1, T4 and T24 experimental times, respectively. The black bar shows the gap between the margins of the cell wound. Original magnification, ×10
Fig. 5.
a Diagram showing the wound closure at different culture time points, (1, 4 and 24 h) of artificial wounds created in rat primary tenocyte cultures from YOUNG, OLD and OVX groups. Mean ± SD, n = 30 measures for three replicates; p value modified according to Bonferroni’s correction: * = p < 0.05; ** = p < 0.005; *** = p < 0.0005. T1: (triple asterisk; ***) YOUNG vs OVX, OLD; (single asterisk; *) OLD vs OVX T4; T24: (triple asterisk; ***) OLD, YOUNG vs OVX. b Average cell migration speed measured as microns per hour, after 24 h of migration to recovery the artificial wound. (Mean ± SD, n = 30 measures for three replicates; p value modified according to Bonferroni’s correction: * = p < 0.05, ** = p < 0.005, *** = p < 0.0005). (Triple asterisk; ***) YOUNG, OLD vs OVX
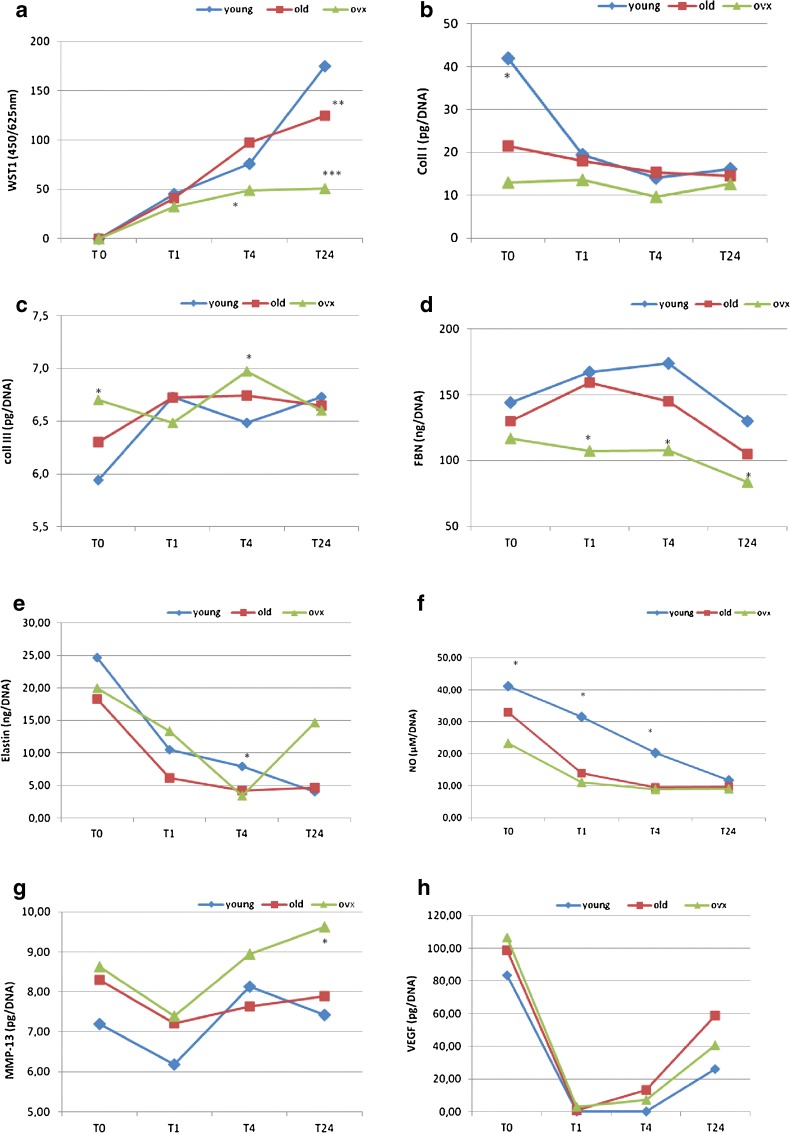
Cell proliferation measure (Fig. 6a) showed that at T4 and T24, the OVX group tenocyte proliferation was significantly lower when compared to both YOUNG and OLD (T4 p < 0.05, T24 p < 0.0005) groups. A significant difference was also found between OLD and YOUNG groups at T24 (p < 0.005).
Fig. 6.
YOUNG, OLD and OVX tenocyte proliferation (a) and activity (b–h) from T0 to T24 of artificial wound. Mean ± SD, n = 3 replicates; p value modified according to Bonferroni’s correction: * = p < 0.05, ** = p < 0.005, *** = p < 0.0005). WST-1—T4: (single asterisk; *) YOUNG, OLD vs OVX; T24: (double asterisk; **) YOUNG vs OLD; (triple asterisk; ***) YOUNG, OLD vs OVX. Coll I—T0: (single asterisk; *) YOUNG vs OLD, OVX. Coll III—T0: (single asterisk; *) OVX vs YOUNG, OLD; T4: (single asterisk; *) OVX vs YOUNG. FBN—T1, T4: (single asterisk; *) YOUNG, OLD vs OVX; T24: (single asterisk; *) YOUNG vs OVX. ELASTIN—T4: (single asterisk; *) YOUNG vs OVX. NO—T0, T1: (single asterisk; *) YOUNG vs OVX; T4: (single asterisk; *) YOUNG vs OLD, OVX. MMP-13—T24: (single asterisk; *) OVX vs YOUNG, OLD
The measure of cell synthetic activity during the healing of a micro-wound showed a different behaviour of the tenocytes (Fig. 6b–h). Coll I was significantly higher in YOUNG rats when compared to OLD and OVX ones at T0 (p < 0.05) (Fig. 6b), whereas Coll III was significantly higher in OVX rats than in OLD and YOUNG ones at T0 (p < 0.05) and YOUNG ones at T4 (p < 0.05) (Fig. 6c). No differences were found for both collagens in the other experimental times, and their ratio was significantly lower in OLD (−51.6 %) and OVX (−72.4 %) groups at T0, and in OVX at T4 (−36.4 %) than in YOUNG (data not shown). FBN production was significantly lower in the OVX group in comparison with the OLD and YOUNG ones, at T1 and T4 (p < 0.05). At T24, its production was lower in the OVX group than in the YOUNG one (p < 0.05) (Fig. 6d). Elastin concentration showed higher values in YOUNG rats than in OVX ones (p < 0.05) at T4 (Fig. 6e). The amount of NO in the YOUNG group was significantly higher when compared to the OLD group at T4 (p < 0.05) and the OVX group at T0, T1 and T4 (p < 0.05) (Fig. 6f). MMP-13 production was higher in the OVX group in comparison to the YOUNG and OLD groups at T24 (p < 0.05) (Fig. 6g). VEGF concentration did not show significant differences between the groups in all experimental times (Fig.6h).
Discussion
Aging is linked to a decline in muscle mass, strength and physical function and, since tendons are considered to have a pivotal role in the transfer of muscle force to produce movement (Couppè et al. 2009), it is important to investigate the changes in tendon features and behaviour during aging.
Little is known about what happens to tendons and tenocytes during aging and oestrogen deficiency. The present investigation characterized in vitro the behaviour of tenocytes derived from young (YOUNG), middle-aged (OLD) and ovariectomised (OVX) rat Achilles tendons.
Because it was demonstrated that differences in tenocytes were generally better detected at the early culture passages (Yao et al. 2006, 2011), cells at the first passage were chosen in the study.
ECM components, remodelling enzymes, GFs, catabolic enzymes and NO were evaluated to investigate differences due to age and/or oestrogen deficiency in standard culture up to 14 days and in an in vitro model of tendon healing. Moreover, the Coll I/III ratio was evaluated because the expression Coll I/III ratio is an important value when evaluating the response of tendons to diseases. When tendons are injured, an increased proportion of type III collagen is produced during the early reparative response, and it can increase up to 15 % of total collagen content following injury. Also, in the case of degenerative tendon diseases, collagen breaks down and the tendon tries to repair itself, but the cells produce new collagen with an abnormal structure and composition. The new collagen has an abnormally low type I/III ratio. The excess type III collagen at the expense of type I collagen weakens the tendon, making it prone to further injury.
With aging, cell proliferation, Coll I, Coll I/III ratio, aggrecan and elastin production were significantly lower than in the young condition. The low rate of cell proliferation and Coll I production in aging is in accordance with other studies (Tsai et al. 2011; Arnesen and Lawson 2006; Couppè et al. 2009; Thorpe et al. 2010). To our knowledge, no previous studies have evaluated the production of aggrecan, Coll III, elastin and FBN in tenocytes derived from the tendon of older subjects.
In the second part of the study, an in vitro micro-wound healing model was used (Maffulli et al. 2000; Fini et al. 2012), and again, differences between OLD and YOUNG groups were found to be related to both cell proliferation and activity. Tenocytes from OLD and YOUNG rats exhibited similar proliferation rates and cell migration speed in the micro-wound model, even though, at 24 h, the cell proliferation rate was significantly lower in the OLD rats than in the YOUNG ones (Arnesen and Lawson 2006). In addition, during tendon healing in aging, the production of Coll III, FBN and MMP-13 was not significantly different from what was observed at a young age. A significantly lower amount of Coll I might be responsible for the decreased mechanical properties of tendons in aged subjects observed in clinical or in vivo studies (Couppè et al. 2009; Dudhia et al. 2007; Thorpe et al. 2010). Moreover, a decrease of NO release was observed in aging during tendon healing.
In standard conditions, in oestrogen deficiency, tenocytes behaved in the same manner as those of OLD rats in terms of proliferation, Coll I, aggrecans and elastin synthesis compared to YOUNG rats. However, whereas aging did not cause significant differences in the production of FBN, NO, MMP-13, TIMP-1 and VEGF as compared to tenocytes derived from young animals, a decrease in FBN and an increase in MMP-13 and VEGF were observed in tenocytes from the OVX group. Moreover, we also observed significant differences between the OLD and OVX groups, and, in oestrogen deficiency situation, elastin, MMP-13 and VEGF levels significantly changed and might further negatively influence tendon conditions.
Similarly, results obtained with the micro-wound model suggested that, following an injury, oestrogen deficiency might be responsible for a significantly lower healing rate, cell migration speed and a poor quality of healed tissue in comparison not only to the healthy young rats (because of a lower proliferation rate, production of Coll I, FBN, elastin and NO and a higher amount of Coll III and MMP-13) but also to OLD rats (because of a lower proliferation rate, production of FBN and ratio of Coll I/III and a higher production of Coll III and MMP-13). Our results are in accordance with those of Circi et al. who observed a lower Achilles tendon healing rate and tenocyte proliferation rate in ovariectomized rats (Circi et al. 2009). The present study contributes to the body of knowledge on the possible effects of senescence on tenocytes and their metabolism and helps to clarify the role of oestrogen in tenocyte synthetic activity and metabolism.
The present work is an in vitro study that combines the analysis of aging and oestrogen deficiency by investigating the proliferation capabilities and synthetic activity of tenocytes from OLD and OVX rats in both standard and dynamic conditions (micro-wound model) and dosing different molecules not yet tested in other previous works, such as aggrecan, elastin, FBN, MMP-13, VEGF, TIMP-1 and NO. FBN is an important constituent of ECM that supports cell proliferation and migration at the injured site (Fini et al. 2012). FBN and aggrecans are extracellular glycoproteins that play a role in the migration and adhesion of cells and are involved in the development of multi-molecular hydrophilic complexes and protect collagen fibres, thus favouring the sliding of adjacent collagen fibres (Ilic et al. 2005; Samiric et al. 2004; Scott 2003).
MMPs are a family of zinc-dependent endopeptidases responsible for collagen degradation in rapid remodelling, e.g. inflammation or tissue damage (Birch et al. 2008). When soft tissue remodelling is active, such as during maturation and after injury, MMP-13 is highly expressed (Lo et al. 2004). TIMPs are the natural endogenous inhibitors of the MMPs; therefore, the balance between the activities of MMPs and TIMPs regulates tendon remodelling, produces collagen dysregulation and predisposes to tendinopathy and tendon tears (Del Buono et al. 2012).
VEGF plays a role in tendinopathy. It is highly expressed in injured tendons, whereas its expression is almost completely downregulated in healthy tendons; in addition, VEGF stimulates the expression of MMPs and inhibits the expression of TIMPs (Savitskaya et al. 2011).
Elastin at less than 1 % is a component of the elastic fibres present in tendons of extremities that undergo changes in length. It is also responsible for the linkage of unique, copper-dependent amino acids desmosine and isodesomosine to lysine residues (Ritty et al. 2002).
An innovative aspect of the present work is the dosage of NO in OLD, YOUNG and OVX groups and during the healing time of the micro-wound. NO is a free radical molecule which is toxic in large doses. In normal uninjured tendons, its amount and activity are low, but it plays an important role in the enhancement of tendon healing, increasing ECM synthesis. Indeed, an increase in its production is observed after tendon injuries with a peak at day 7 and a return to baseline at day 14, with an improvement in nitric oxide synthase (NOS) activity. A low NOS expression in non-injured tendon and a gradual increase after injury indicate that NO might play an important role in the healing process (Lin et al. 2001). NO administration to manage Achilles tendon injuries gives an improvement in range of motion and strength, and a higher NOS activity as a result of tendon overuse is evident in rotator cuff tendons (Bokhari and Murrell 2012; Szomor et al. 2001). The addition of NO to cultured tenocytes upregulated some genes involved in apoptosis, cell adhesion/communication, cell growth and/or maintenance, ECM regulation and oxidative stress resistance and signal transduction (Murrell 2007). In our study, in standard culture conditions, the level of NO did not show significant differences among the various groups at all experimental times, but in the micro-wound model, YOUNG group values were significantly higher than OLD ones at T4 and those of OVX rats at T0, T1 and T4. Its downregulation in OVX seems to be related in altered tendon repair processes.
The present study has some limitations. First of all, results were obtained in in vitro cultured cells no longer organized into tissues, in monolayer and static conditions. In future studies, it will be necessary to better understand the impact of the impaired biological behaviour of cultured tenocytes on in vivo tendon morphology and functional properties by means of histological, immunohistochemical and biomechanical tests. However, as previously said, the morphology and biomechanical behaviour of tendons in oestrogen deficiency or aging has been the principal issue addressed in the existing studies in this field, and a decrease in mechanical strength (Dudhia et al. 2007), elasticity (Circi et al. 2009) and biomechanical properties (Bryant et al. 2008; Hansen et al. 2009) has already been shown. Moreover, it would be interesting to study whether oestrogen supplementation, in culture conditions, allows recovery of at least some of the drawbacks produced by its deficiency.
In conclusion, proliferation and tenocyte biosynthesis are negatively and at least partially independently affected by aging and oestrogen deficiency, even though oestrogen deficiency exerts a greater negative effect than aging in culture. In micro-wound healing, the OLD group was able to recover the injury, although at a slower rate, whereas oestrogen deficiency showed higher negative effects on tendon healing because it was accompanied by lower cell proliferation, cell speed migration and an altered metabolism, by combining ECM protein loss and MMP overexpression.
Our work improves the body of knowledge on the effects of senescence and oestrogen deficiency on tenocyte behaviour and allows further studies to find solution for the prevention of tendon injuries in aging and menopause.
Acknowledgments
The research was supported by Rizzoli Orthopaedic Institute, “5 PER MILLE Project” (CUP D31J09000260001).
References
- Arnesen SM, Lawson AM. Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev. 2006;127:726–732. doi: 10.1016/j.mad.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Birch HL, Worboys S, Eissa S, Jackson B, Strassburg S, Clegg PD. Matrix metabolism rate differs in functionally distinct tendons. Matrix Biol. 2008;27(3):182–189. doi: 10.1016/j.matbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bokhari AR, Murrell GAC. The role of nitric oxide in tendon healing. J Should Elbow Surg. 2012;21:238–244. doi: 10.1016/j.jse.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, Marshall-Gradisnik S, Payne C, Crossley KM. Effects of oestrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol. 2008;105(4):1035–1043. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]
- Chen H, Yao XF, Emura S, Shoumura S. Morphological changes of skeletal muscle, tendon and periosteum in the senescence-accelerated mouse (SAMP6): A murine model for senile osteoporosis. Tissue Cell. 2006;38:325–335. doi: 10.1016/j.tice.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Circi E, Akpinar S, Akgun RC, Tunkay IC. Biomechanical and histological comparison of the influence of oestrogen deficient state on tendon healing potential in rats. Int Orthop. 2009;33:1461–1466. doi: 10.1007/s00264-009-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Bass SL, Black JE. Hormone therapy is associated with smaller Achilles tendon diameter in active post-menopausal women. Scand J Med Sci Sports. 2007;17(2):128–132. doi: 10.1111/j.1600-0838.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Couppè C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- Cretnik A, Frank A. Incidence and outcome of rupture of the Achilles tendon. Wien Klin Wochenschr. 2004;116(Suppl 2):33–38. [PubMed] [Google Scholar]
- de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45:1026–1028. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- Del Buono A, Oliva F, Longo UG, Rodeo SA, Orchard J, Denaro V, Maffulli N. Metalloproteases and rotator cuff disease. J Should Elbow Surg. 2012;21(2):200–208. doi: 10.1016/j.jse.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Dudhia J, Scott CM, Draper ERC, Heinegard D, Pitsillides AA, Smith RK. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Fini M, Bondioli E, Castagna A, Torricelli P, Giavaresi G, Rotini R, Marinelli A, Guerra E, Orlandi C, Carboni A, Aiti A, Benedettini E, Giardino R, Melandri D (2012) Decellularized human dermis to treat massive rotator cuff tears: in vitro evaluations. Connect Tissue Res. doi:10.3109/03008207.2011.649929 [Epub ahead of print] [DOI] [PubMed]
- Finni T, Kovanen V, Ronkainen HA, Pollanen E, Bashford GR, Kaprio J, Alèn M, Kujala UM, Sipila S. Combination of hormone replacement therapy and high physical activity is associated with differences in Achilles tendon size in monozygotic female twin pairs. J Appl Physiol. 2009;106:1332–1337. doi: 10.1152/japplphysiol.91439.2008. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kongsgaard M, Holm L, Skovgaard D, Magnusson SP, Qvortrup K, et al. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol. 2009;106:1385–1393. doi: 10.1152/japplphysiol.90935.2008. [DOI] [PubMed] [Google Scholar]
- Ilic MZ, Carter P, Tyndall A, Dudhia J, Handley CJ. Proteoglycans and catabolic products of proteoglycans present in ligament. Biochem J. 2005;385:381–388. doi: 10.1042/BJ20040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Takahata M, Majima T, Abe Y, Komatsu M, Iwasaki N, Minami A. Effect of selective estrogen receptor modulator/raloxifene analogue on proliferation and collagen metabolism of tendon fibroblast. Connect Tissue Res. 2010;51:179–187. doi: 10.3109/03008200903204669. [DOI] [PubMed] [Google Scholar]
- Lin JH, Wang M-X, Wei A, Ahu W, Murrell GAC. The cell specific temporal expression of nitric oxide synthase isoforms during Achilles tendon healing. Inflamm Res. 2001;50:1–8. doi: 10.1007/PL00000228. [DOI] [PubMed] [Google Scholar]
- Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32(5):1223–1229. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Murrell GAC. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41:227–231. doi: 10.1136/bjsm.2006.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritty TM, Ditsios K, Starcher BC. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002;268(4):430–440. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. Eur J Biochem. 2004;271:3612–3620. doi: 10.1111/j.0014-2956.2004.04307.x. [DOI] [PubMed] [Google Scholar]
- Savitskaya YA, Izaguirre A, Sierra L, Perez F, Cruz F, Villalobos E, Almazan A, Ibarra C. Effect of angiogenesis-related cytokines on rotator cuff disease: the search for sensitive biomarkers of early tendon degeneration. Clin Med Insights Arthritis Musculoskelet Disord. 2011;4:43–53. doi: 10.4137/CMAMD.S7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Elasticity in extracellular matrix “shape modules” of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szomor ZL, Wang MX, Kruller A, Murrell GA, Farmer KM, Kirkham BW, et al. Differential expression of cytokines and nitric oxide synthase expression in human rotator cuff bursae. Ann Rheum Dis. 2001;60:431–432. doi: 10.1136/ard.60.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem. 2010;285(21):15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WC, Chang HN, Yu TY, Chien CH, Fu LF, Liang FC, Pang JH. Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res. 2011;29(10):1598–1603. doi: 10.1002/jor.21418. [DOI] [PubMed] [Google Scholar]
- Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12(7):1843–1849. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- Yao L, Bestwick CS, Bestwick LA, Aspden RM, Maffulli N. Non-immortalized human tenocyte cultures as a vehicle for understanding cellular aspects to tendinopathy. Transl Med. 2011;1(1):173–194. [PMC free article] [PubMed] [Google Scholar]