Abstract
Calorie restriction (CR) is the most widely studied intervention protecting from the adverse effects of aging. Almost all prior studies have examined the effects of CR initiated in young animals. Studies examining the effects of CR on development of aging cardiomyopathy found only partial prevention. The major goal of this study was to determine whether CR initiated after aging cardiomyopathy developed could reverse the cardiomyopathy. Aging cardiomyopathy in 2-year-old mice was characterized by reduced left ventricular (LV) function, cardiac hypertrophy, and increased cardiac apoptosis and fibrosis. When short-term (2 months) CR was initiated after aging cardiomyopathy developed in 20-month-old mice, the decrease in cardiac function, and increases in LV weight, myocardial fibrosis and apoptosis were reversed, such that the aging hearts in these mice were indistinguishable from those of young mice or mice where CR was initiated in young mice. If apoptosis was the mechanism for protecting against aging cardiomyopathy, then total myocyte numbers should have reverted to normal with CR, but did not. However, the alterations in cytoskeletal proteins, which contribute to aging cardiomyopathy, were no longer observed with CR. This is the first study to demonstrate complete prevention of aging cardiomyopathy by CR and, more importantly, that instituting this intervention even later in life can rapidly correct aging cardiomyopathy, which could have important therapeutic implications.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9508-5) contains supplementary material, which is available to authorized users.
Keywords: Calorie restriction, Aging, Cardiomyopathy, Apoptosis, Myocyte loss
The effects of aging on the heart has been referred to as aging cardiomyopathy, which occurs in humans as well as in most mammals (Boyle et al. 2011) and is characterized by reduced cardiac function, increased left ventricular (LV) weight, myocardial fibrosis and apoptosis. Calorie restriction (CR) is the most frequently studied mechanism for increasing longevity, protecting against stress and retarding the onset of age-associated diseases. Almost all of these studies have initiated CR in young animals to see the extent to which the effects of aging are protected. While CR has not been studied extensively in aging cardiomyopathy, there are a few studies in rodents and humans suggesting that CR did not prevent, but at least partially ameliorated, aging cardiomyopathy (Niemann et al. 2010; Shinmura et al. 2011; Weiss and Fontana 2011). Again in these studies, CR was initiated in young animals. However, this is the first study to examine the extent to which CR, initiated after aging cardiomyopathy develops, can reverse its adverse effects on the heart. This is important from a clinical perspective, as well as from a basic scientific perspective, because of difficulties in patient compliance, it is virtually impossible to keep the majority of humans on a normal diet for their entire lives to prevent obesity, such that the possibility of maintaining a CR diet for their entire lives is essentially nonexistent. Accordingly, there were two goals of the current investigation: (1) to determine if CR instituted in young animals prevented the development of aging cardiomyopathy in mice 26 months of age, which, on a standard diet, had developed cardiomyopathy months earlier; and (2) the major goal was to determine if instituting CR late in life after aging cardiomyopathy developed could actually reverse it. If so, this could be translated to clinical therapy more readily. For the second goal, we selected an earlier time point, but still when aging cardiomyopathy had been established (20 months), reasoning that demonstrating the principle of reversing aging cardiomyopathy with short-term CR would be most feasible at the earlier time point. If the novel concept of the reversal of aging cardiomyopathy could be demonstrated, a later, less novel goal would be to determine how long after aging cardiomyopathy can be rescued.
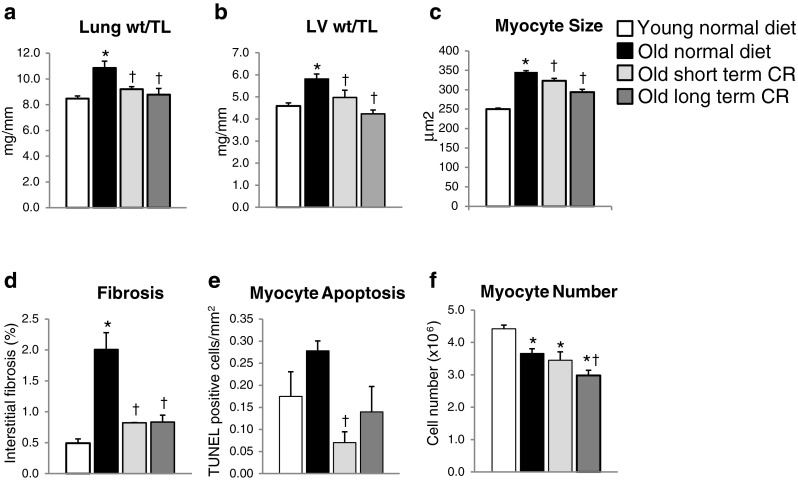
The most significant finding of this investigation was the accomplishment of the second goal, i.e., that CR induced for only 2 months after aging cardiomyopathy had already developed completely rescued the adverse effects of aging on the heart. As shown in Table 1 (A) and Fig. 1a, LV function (LV ejection fraction) and increased lung weight/tibial length (lung wt/TL) (an index of heart failure) was completely rescued and was no longer different from values in young normal mice. Long-term CR showed similar results (Fig. 1a and Table 1 (B)). Interestingly, the aging-associated reduction in LV contractile function was not accompanied by increased end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) (Table 1), which we observed previously in aging rats (Lieber et al. 2008). This point has been controversial in other rodent models with some studies showing LV dilation with aging cardiomyopathy in rodents (Iemitsu et al. 2002; Luptak et al. 2007; Niemann et al. 2010; Boyle et al. 2011), whereas others did not (Afilalo et al. 2007). Importantly, the majority of studies in aging humans did not show increased LV volume (Gardin et al. 1979; Rodeheffer et al. 1984; Pavlik et al. 2001). However, CR reduced LVEDD and LVESD significantly. Potential causes of the cardiomyopathy of aging, namely increased LV hypertrophy, as reflected as increased LV weight/tibial length (LV wt/TL) (Fig. 1b) and increased myocyte size (Fig. 1c), fibrosis (Fig. 1d), and apoptosis (Fig. 1e), were also completely rescued by short-term CR, and there was no difference from the values in young, normal mice, similar to long-term CR (Fig. 1b–e). CR is the most widely studied model protecting from the effects of aging. Almost all studies on CR involve initiating the CR in young animal models and demonstrating that the adverse effects of aging are not observed. Our finding that short-term CR was as effective as long-term CR is important for several reasons, not the least of which is that if clinical therapy is to be designed on the basis of CR, it would be difficult for patients to comply with CR for life, but would be much more feasible if the therapy could be instituted in old patients, already afflicted with the adverse effects of aging. Other studies have noted that gene changes and vascular changes of aging can be effective with CR instituted in older animals (Jung et al. 2009; Rippe et al. 2010), but this is the first study to show that functional effects of aging on a major organ, i.e., aging cardiomyopathy can be completely rescued by CR after the cardiomyopathy developed.
Table 1.
LV function
| A: Short-term calorie restriction | Prior to ST CR and ND | Old ST ND | Old ST CR |
| Number | 8 | 8 | 6 |
| Age (months) | 20 | 22 | 22 |
| BW (g) | 27 ± 1 | 27 ± 1 | 23 ± 1*† |
| LVEF (%) | 63 ± 1 | 65 ± 1 | 74 ± 1*† |
| Fractional shortening (%) | 28 ± 2 | 29 ± 1 | 36 ± 1*† |
| Heart rate (bpm) | 438 ± 20 | 492 ± 12* | 505 ± 28* |
| LVEDD (mm) | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.0 ± 0.2*† |
| LVESD (mm) | 2.6 ± 0.1 | 2.6 ± 0.1 | 1.9 ± 0.1*† |
| EDWT (mm) | 0.74 ± 0.04 | 0.89 ± 0.04 | 0.85 ± 0.06 |
| B: Long-term calorie restriction | Young ND | Old LT ND | Old LT CR |
| Number | 8 | 6 | 6 |
| Age (months) | 4 | 26 | 26 |
| BW (g) | 25 ± 1 | 26 ± 1 | 18 ±0.4**†† |
| LVEF (%) | 71 ± 1 | 53 ± 6** | 71 ± 2†† |
| Fractional shortening (%) | 34 ± 1 | 23 ± 3** | 34 ± 1†† |
| Heart rate (bpm) | 422 ± 19 | 453 ± 10 | 482 ± 26** |
| LVEDD (mm) | 3.7 ± 0.1 | 3.1 ± 0.1** | 3.0 ± 0.2** |
| LVESD (mm) | 2.4 ± 0.1 | 2.3 ± 0.2 | 2.1 ± 0.1** |
| EDWT (mm) | 0.82 ± 0.03 | 0.97 ± 0.02** | 0.77 ± 0.04†† |
ST short term, ND normal diet, CR calorie restriction, LT long term, LVEF left ventricular ejection fraction, LVEDD left ventricular end-diastolic diameter, LVESD left ventricular end-systolic diameter, EDWT end-diastolic wall thickness
*p < 0.05 vs. prior to ST CR; †p < 0.05 vs. old ST ND; **p < 0.05 vs. young ND; ††p < 0.05 vs. old LT ND
Fig. 1.
Histology in young mice on a normal diet (n = 8), old mice on a normal diet (n = 14), mice on short-term CR (n = 6), and mice on long-term CR (n = 6). In this figure, the data for the 26-month and 22-month normal diet groups were similar, so they were averaged to compare with the data from both the long-term CR and short-term CR. Short-term CR rescued the aging cardiomyopathy, which was characterized by increased lung wt/TL (a), increased LV wt/TL (b), increased myocyte size (c), increased cardiac fibrosis (d), and also increased myocyte apoptosis (e). However, the myocyte number (f) was significantly less in both normal diet and CR mice compared to that in young mice. Long-term CR induced similar beneficial results as short-term CR (asterisk, p < 0.05 vs. young mice on normal diet; dagger, p < 0.05 vs. old mice on normal diet). Data are expressed as mean ± SEM
Another novel finding is that, although CR protects against cardiac apoptosis (Fig. 1e), this mechanism is not involved in the rescue of aging cardiomyopathy by CR. This conclusion contrasts with those of prior studies suggesting that apoptosis is the mechanism for aging cardiomyopathy (Bernecker et al. 2003; Goldspink et al. 2003). This concept is based on the finding that apoptosis increases with age, resulting in reduced numbers of contractile units (myocytes) in the heart. However, extrapolating from increased total numbers of apoptotic cells in the heart to loss of myocytes is not generally possible, since 70 % of cells in the heart are not myocytes, and, accordingly, this conclusion cannot be made without specific myocyte staining as it was done in the current investigation. Thus, the current results, which found very little myocyte apoptosis and reversal with CR, do not support this concept. Our data show that total myocyte numbers did not increase with CR (Fig. 1f), even though LV function was restored. It is much more likely that the LV hypertrophy and fibrosis are much more important mechanisms.
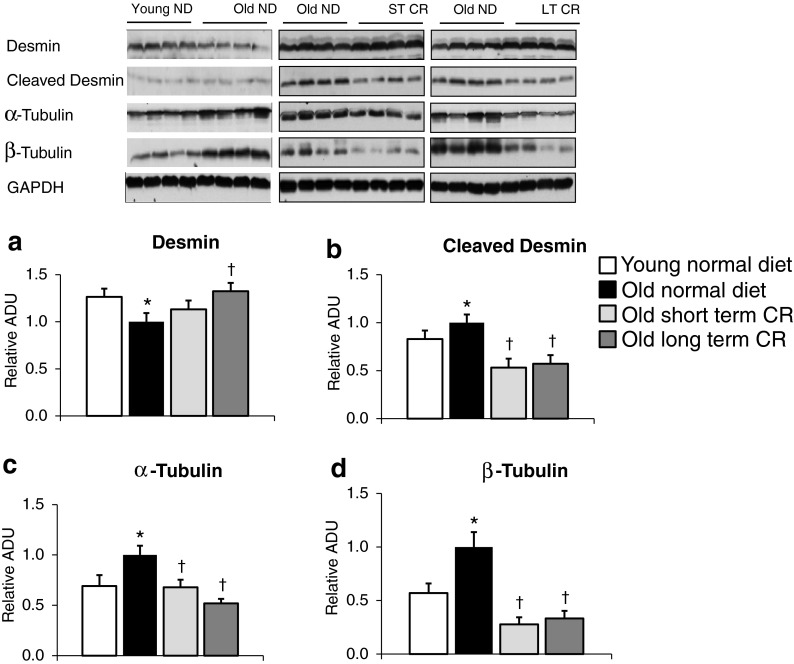
Since prevention of myocyte apoptosis is not likely the mechanism for the protection of aging cardiomyopathy by CR, other mechanisms must be considered. Previous studies demonstrated that alterations in cytoskeletal proteins can induce cardiomyopathy (Heling et al. 2000) and that these alterations also occur in aging cardiomyopathy (Heling et al. 2000; Lieber et al. 2008). We found that cardiac cytoskeletal proteins in old normal mice were increased, e.g., α-tubulin, β-tubulin, and cleaved desmin, whereas intact desmin was decreased. Short-term CR significantly reduced α-tubulin (33 %), β-tubulin (72 %), and cleaved desmin (47 %) and restored intact desmin, similar to the effects of long-term CR (Fig. 2a–d), which likely contributed to the preservation of LV function with CR. There is a direct evidence that desmin is involved in the development of cardiomyopathy, e.g., desmin knockout mice develop severe cardiomyopathy (Milner et al. 1996; Thornell et al. 1997). In addition, alterations in tubulin have been reported as the cause of LV dysfunction in hypertrophic hearts (Tagawa et al. 1997; Tsutsui et al. 1993). Therefore, it is conceivable that CR decreased the expression levels of α-tubulin, β-tubulin, and cleaved desmin and restored intact desmin in aged mice, which could be one potential mechanism that prevents the development of aging cardiomyopathy.
Fig. 2.
Alterations in cytoskeletal proteins during aging and CR. With aging cardiomyopathy, there were significant increases in cleaved desmin (b) but a decrease in intact desmin (a) as well as α- (c) and β-tubulin (d). Both short-term and long-term CR corrected these alterations in cytoskeletal proteins in aging (n = 4/group) (asterisk, p < 0.05 vs. young normal diet; dagger p < 0.05 vs. old normal diet). Data were normalized to old normal diet
We also examined oxidative stress, one of the most studied mechanisms mediating the adverse effects of aging (Droge 2002; Harman 1956; Ungvari et al. 2008). We examined the antioxidants, MnSOD, and catalase, in long-term CR mice. The levels of MnSOD and catalase were significantly increased in long-term CR as compared to age-matched old mice on the standard diet, supporting the concept that CR induces a protective effect against oxidative stress in aging hearts (Fig. S1a–b). In addition, the ratio of LC3-II/LC3-I, a marker of autophagy, was decreased in old mice on normal diet but increased in long-term CR mice (Fig. S1c), suggesting that autophagy declines with age but is enhanced by CR. However, MnSOD, catalase, as well as the ratio of LC3-II/LC3-I did not increase significantly in short-term CR mice, suggesting that not all mechanisms are identical for reversing aging cardiomyopathy by short-term vs. long-term CR. We recently reported that the stress resistance regulator, SIRT1, was upregulated in the hearts of CR mice and cardiac protective genes, which regulate GTPase activator activity (e.g., Rgs2) and glutathione transferase activity (e.g., Gstk1, Gstm), which were downregulated in the heart with pressure overload but were significantly upregulated with CR (Yan et al. 2012), suggesting that these genes could be involved in the cardioprotection of CR mice.
In summary, instituting CR even later in life after aging cardiomyopathy develops can completely rescue the adverse effects of aging on the heart. It will be important to determine if the effects of aging in other organs can also be rescued by initiating CR later in life. The results of these studies could provide new impetus for designing aspects of CR for therapy in aging patients.
Electronic supplementary material
(DOCX 38 kb)
Acknowledgments
We thank Serge Salganik for statistical analysis and Dr. Doru Chirieac for technical help. This work was supported by the National Institute of Health grants 5P01AG027211, 5R21HL097264, 1R01HL102472, 5R01HL033107, 5T32HL069752, 5R01HL095888, 5P01HL069020, and 5R01HL091781.
References
- Afilalo J, Sebag IA, Chalifour LE, Rivas D, Akter R, Sharma K , Duque G (2007). Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol 293:H1451–1456 [DOI] [PubMed]
- Bernecker OY, Huq F, Heist EK, Podesser BK, Hajjar RJ. Apoptosis in heart failure and the senescent heart. Cardiovasc Toxicol. 2003;3:183–190. doi: 10.1385/CT:3:3:183. [DOI] [PubMed] [Google Scholar]
- Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Henry WL, Savage DD, Ware JH, Burn C, Borer JS (1979). Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J Clin Ultrasound. 7: 439–447 [DOI] [PubMed]
- Goldspink DF, Burniston JG, Tan LB. Cardiomyocyte death and the ageing and failing heart. Exp Physiol. 2003;88:447–458. doi: 10.1113/eph8802549. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.RES.86.8.846. [DOI] [PubMed] [Google Scholar]
- Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M , Yamaguchi I (2002). Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol 283:H1750–1760 [DOI] [PubMed]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res: Official Journal of the European Histamine Research Society [et al] 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- Lieber SC, Qiu H, Chen L, Shen YT, Hong C, Hunter WC, Aubry N, Vatner SF, Vatner DE. Cardiac dysfunction in aging conscious rats: altered cardiac cytoskeletal proteins as a potential mechanism. Am J Physiol Heart Circ Physiol. 2008;295:H860–H866. doi: 10.1152/ajpheart.00146.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luptak I, Yan J, Cui L, Jain M, Liao R , Tian R (2007). Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation 116:901–909 [DOI] [PubMed]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res. 2010;88:267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- Pavlik G, Olexo Z, Osvath P, Sido Z , Frenkl R (2001). Echocardiographic characteristics of male athletes of different age. Br J Sports Med 35:95–99 [DOI] [PMC free article] [PubMed]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML , Lakatta EG (1984). Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation 69:203–213 [DOI] [PubMed]
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G., 4th Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res. 1997;80:281–289. doi: 10.1161/01.RES.80.2.281. [DOI] [PubMed] [Google Scholar]
- Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol. 1997;29:2107–2124. doi: 10.1006/jmcc.1997.0446. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Ishihara K, Cooper G., 4th Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993;260:682–687. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–H1219. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Park JY, Dillinger JG, De Lorenzo MS, Yuan C, Lai L, Wang C, Ho D, Tian B, Stanley WC, et al. Common mechanisms for calorie restriction and adenylyl cyclase type 5 knockout models of longevity. Aging Cell. 2012;11:1110–1120. doi: 10.1111/acel.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)