Abstract
Socioeconomic status (SES) is an important reserve variable which has been shown to benefit the aging brain’s macrostructure. However, it remains unknown whether SES affects age-related changes in the brain’s white matter (WM) microstructure. Here, we used diffusion tensor imaging to explore the relationship between SES and three components of the diffusion tensor [fractional anisotropy (FA), axial diffusivity, and radial diffusivity (DR)]. Participants were 40 (16 male) cognitively normal young adults (mean age = 33.3 years, SD = 4.27) and 44 (19 male) cognitively normal community dwelling seniors (mean age = 66.2 years, SD = 7.5). Age-related FA declines were observed across a large portion of the WM skeleton. However, seniors with high SES showed lower age-related WM integrity declines in three frontal tracts: the right anterior corona radiata and bilateral portions of WM underlying the superior frontal gyri (SFG–WM). Positive SES–FA correlations were primarily driven by negative DR–SES correlations, suggesting that SES may buffer age-related declines in myelin. The functional significance of high SES in these frontal tracts was demonstrated through positive correlations with working memory performance. Possible mechanisms through which SES may attenuate the effects of age on frontal WM integrity are discussed.
Keywords: Socioeconomic status, Diffusion tensor imaging, White matter, Brain imaging, Aging, Reserve
Introduction
Human aging results in well-established declines in the brain’s macrostructure (Good et al. 2001; Smith et al. 2007). However, at least some age-related macrostructural declines can be attenuated by positive lifestyle variables. For example, a number of neuroimaging studies have demonstrated that specific lifestyle variables such as exercise, leisure activities, education, and occupation can have a positive impact on cortical structure in aging (Brayne et al. 2010; Colcombe et al. 2006; Sole-Padulles et al. 2009). These anatomically based structural benefits of positive lifestyle variables may contribute to individual differences in clinically manifested cognitive deficits (Stern et al. 1995a, b). For example, at the simplest level, larger brains can sustain more age-related pathology (Satz 1993). This phenomenon is commonly referred to as brain reserve and reflects quantitative differences in brain structure (i.e., brain volume, neuronal, or synaptic density) that allow some people to cope with neurodegeneration better than others (Stern 2009).
In recent years, it has become increasingly apparent that socioeconomic status (SES) is an important reserve variable in aging (Marengoni et al. 2011; Singh-Manoux et al. 2011). Variations in SES have been linked with marked differences in cognition and behavior across the life span (Hackman and Farah 2009; Hackman et al. 2010). High SES has been linked with increased exposure to cognitively stimulating environments compared to low SES (Hackman et al. 2010) and is associated with a lower risk of developing dementia (Stern et al. 1994; Valenzuela and Sachdev 2006). In contrast, low SES has been linked with greater exposure to life stressors and reduced brain volume in aging (Bartres-Faz et al. 2009; Sole-Padulles et al. 2009). SES is of strong theoretical interest to life span approaches to aging because it most likely continues to affect individuals across the duration of their employment career (into the sixth decade of life in most cases). As a result, it has now become standard practice to control for SES in studies of aging and dementia.
However, little is known about the potential relationship between SES and the brain’s white matter (WM) microstructure. A greater understanding of this potential relationship is important because microstructural integrity of cerebral WM is required for proper transmission of information between cortical regions. Diffusion tensor imaging (DTI) can be used to explore potential SES–WM integrity relationships by evaluating the microstructural integrity of the brain’s WM tracts in vivo (Basser et al. 2000; Basser and Pierpaoli 1996; Le Bihan 2003). DTI is sensitive to the random motion of water molecules as they interact within tissues, thus reflecting characteristics of their immediate structural surroundings at a nominal 50-μ scale. Diffusion anisotropy can be measured by DTI by means of fractional anisotropy (FA), an index of overall tissue microstructural integrity (Pierpaoli et al. 1996).
While no studies have yet explored the relationship between WM microstructural integrity and SES per se, several recent studies have explored relationships between WM integrity and other reserve variables reflective of cognitive achievement (Arenaza-Urquijo et al. 2011; Teipel et al. 2009). For example, Teipel et al. (2009) explored the relationship between WM integrity and education in both healthy seniors and patients with clinically probable Alzheimer’s disease (AD) using a statistical parametric mapping approach. Interestingly, healthy seniors demonstrated a positive relationship between FA and education in WM tracts that showed an inverse relationship in AD participants. These findings suggest a relationship between reserve variables and WM integrity in healthy seniors.
However, there exist several important knowledge gaps in this area of study. The first concerns the relationship between the specific reserve variable of SES and WM integrity in healthy seniors. In particular, it is unknown whether SES is positively associated with WM integrity in regions that undergo age-related declines. WM regions that show significant age-related declines but are positively correlated with SES may reflect neuroprotective effects of SES in aging. In particular, those WM regions showing stronger SES–FA relationships in older adults than young adults may in part reflect the beneficial effects of continued exposure to high SES in aging.
If SES does offset age-related WM integrity declines in some regions, then it would be important to explore the potential biological mechanisms and functional significance of SES–WM integrity relationships in aging. An increased understanding of the neurobiological bases for potential SES–WM integrity relationships can be gained from the joint consideration of FA, radial diffusivity (DR), and axial diffusivity (DA) (Assaf and Pasternak 2008; Burzynska et al. 2010; Gold et al. 2012). For example, an SES–DR relationship would suggest that SES may help to preserve myelin integrity (Song et al. 2002, 2005), whereas an SES–DA relationship would suggest a relationship between SES and gross tissue characteristics (Sen and Basser 2005).
In terms of functional significance, it would be relevant to determine if SES-preserved WM integrity is associated with high performance on cognitive processes known to undergo marked age-related decline. One cognitive domain known to undergo significant age-related declines is working memory (Babcock and Salthouse 1990; Dobbs and Rule 1989; Park et al. 1997; Wiegersma and Meertse 1990), yet age-related declines in working memory may be attenuated by the relative preservation of WM integrity in individuals with high SES. The digit backwards (DB) span test was used to explore this possible relationship because it assesses the fundamental working memory function of maintaining information online (Groeger et al. 1999), a function which declines significantly with age (Baddeley 2000).
To address these issues, we first identified WM regions demonstrating significantly lower FA in seniors compared to young adults. We then identified SES–FA correlations within regions showing age-related declines. The FA metric represents a weighted average of DA and DR components of the diffusion tensor. We thus further characterized the neurobiological bases of significant SES–FA relationships by assessing if these relationships were primarily driven by SES–DR or SES–DA associations. Finally, we assessed the functional significance of maintained WM integrity by exploring the relationship between FA and working memory in regions where age-related WM integrity declines were offset by SES.
Methods
Participants
A total of 84 right-handed subjects participated in the present study. Participants were 40 (16 male) cognitively normal young adults (mean age = 33.3 years, SD = 4.27) and 44 (19 male) cognitively normal community dwelling seniors (mean age = 66.2 years, SD = 7.5). The young adult and senior groups did not differ in sex distribution [χ2 (1) = 0.087, P = 0.768]. Informed consent was obtained from each participant under an approved University of Kentucky Institutional Review Board protocol. All participants were recruited from the local community through posted flyers and newspaper advertisements. Exclusionary criteria for the study included the following: a major head injury and/or concussion, stroke, a neurological or psychiatric disorder, untreated high blood pressure, hypercholesterolemia or diabetes, heart disease, the use of psychotropic medications (e.g., antidepressants), or the presence of metal fragments and/or metallic implants contraindicated for magnetic resonance imaging (MRI).
Demographic and cognitive tests
The Hollingshead two-factor index of social position.
The Hollingshead two-factor index of social position (ISP) was used as a measure of SES (Hollingshead 1958). The ISP is based on an individual’s occupation and highest level of formal education. It is calculated by assigning numeric values, from 1 to 7, to an individual’s occupation and education. Scores are then weighted by multiplying by 7 (occupation) and 4 (education). Values are then summed to produce a social index. For example, an individual with a Bachelor of Arts degree (numeric value = 2) who works as an administrative assistant (numeric value = 3) would have an ISP score of 29 ([2 × 4] + [3 × 7] = 29). Lower values represent higher earning occupations and more years of education. The two groups did not differ in mean SES (young adult group: M = 29.4, SD = 12.4; senior group: M = 28.4, SD = 11.0) [t(82) = 0.378, P < 0.707], and Levene’s test of homogeneity indicated that the two groups did not differ in SES variance [F(82) = 0.193, P = 0.662].
The Cattell culture fair intelligence test.
Because measures of SES are correlated with intelligence, and a relationship between intelligence quotient (IQ) and WM integrity has been reported (Chiang et al. 2011), we controlled for IQ using the Cattell culture fair (CCF) (Cattell and Cattell 1960). The CCF (scale 3) consists of 50 items and assesses inductive reasoning through the ability to perceive relationships in shapes and figures. Age-adjusted IQ scores were used because fluid intelligence has been shown to decline with age (Schretlen et al. 2000). The two groups did not differ in mean IQ (young adult group: M = 120.2, SD = 19.7; senior group: M = 123.8, SD = 21.1) [t(82) = −0.804, P < 0.424]. In addition, the CCF was used as a nuisance covariate in all analyses in order to control for within-group variance in IQ.
The digits span subtests of the Wechsler memory scale (Wechsler 1997).
Participants were read digit lists aloud at a rate of one per second with consistent tone and emphasis. Immediately after the digits were read, the participants were instructed to repeat each set of digits verbally in reverse order (digit backward; DB). Participants received two trials for each set of digits that ranged from 2 to 8 numbers. If the participant was unable to repeat all digits in a given set, the test was terminated. Totals were based on the number of trials that were accurately reported in the correct order. All scores were then adjusted for age.
Diffusion tensor imaging acquisition
Data were collected on a 3 Tesla Siemens TIM scanner at the University of Kentucky using an 8-channel head array coil. Whole-brain diffusion tensor images (40 contiguous 3-mm thick axial slices) were acquired with 36 non-collinear encoding directions (b = 1,000 s/mm2) and five images without diffusion weighting (b = 0 s/mm2, b0) using a double spin echo EPI sequence and the following parameters: repetition time = 6,900 ms, echo time (TE) = 84 ms, inversion time = 2,500 ms, flip angle = 90°, acquisition matrix = 128 × 128, field of view = 224 mm, in-plane resolution = 1.75 × 1.75 mm voxels. In addition, a double-echo gradient-echo sequence (TE1 = 5.19 ms, TE2 = 7.65 ms) with slice position and spatial resolution matching those of the EPI acquisition was used to map the spatial inhomogeneity of the B0 field.
Diffusion tensor imaging preprocessing and analysis
Diffusion tensor imaging (DTI) data were preprocessed and analyzed using the Functional MRI of the Brain (FMRIB) software library (FSL v4.1.5). Each diffusion-weighted volume was corrected for motion and residual eddy current distortion using a 12-parameter affine alignment to the corresponding b0 image, via FMRIB’s Linear Image Registration Tool (FLIRT: http://www.fmrib.ox.ac.uk/fsl). Images were then corrected for static field inhomogeneity distortions using B0 field maps. Brain masks were then generated from each b0 image using FMRIB’s brain extraction tool (BET v2.1) to exclude non-brain voxels from all subsequent processing (Smith et al. 2006). Next, FMRIB’s Diffusion Toolbox (FDT v2.0) was used to fit the diffusion tensor and calculate eigenvalues, fractional anisotropy (FA), axial diffusivity (DA), and radial diffusivity (DR).
Registration of FA images into MNI152 space and subsequent voxelwise analyses followed a series of procedures known as Tract-Based Spatial Statistics [TBSS v1.2; (Smith et al. 2006)], as described in detail in our previous work (Gold et al. 2010; Smith et al. 2010). Briefly, the initial step in this process was to remove likely outliers from the fitted tensor by eroding brain edge artifacts and zeroing the end slices. Next, all participants’ FA images were aligned to the FMRIB58_FA_1mm template using a nonlinear registration approach based on free-form deformations and B-Splines (Rueckert et al. 1999). The FA images were then affine registered and resampled to 1 × 1 × 1 mm MNI152 space. Transformations derived from the FA maps were then applied to the other diffusivity maps (DR and DA) for matched processing of all image volumes.
All MNI-transformed FA images were then averaged to create a mean FA image used to generate a common WM tract skeleton. An FA value of 0.2 was used to threshold the skeleton in order to minimize partial voluming effects after warping across subjects. Next, each participant’s spatially normalized FA image was projected onto the FA skeleton in order to account for residual misalignments between participants after the initial nonlinear registration. Finally, each subject’s DR and DA maps in MNI space were projected onto the common tract skeleton, using the pipeline for non-FA data provided by TBSS, which employs the projection vectors from each individual’s FA-to-skeleton transformation (Smith et al. 2006).
A main goal of the study was to determine if SES shows a neuroprotective effect in WM regions that undergo significant age-related declines. As a first step, a between-group comparison was performed to identify age-related changes in FA, with sex, IQ, and SES scores included as nuisance covariates. A permutation nonparametric test (using 5,000 permutations) was employed using a threshold-free cluster enhancement, and results were thresholded at P < 0.05 (corrected for multiple comparisons).
SES–FA correlations
As a second step, multiple regression analyses were performed to explore potential SES–FA relationships within regions showing age-related FA changes. Toward this end, an inclusion mask was first generated to include all voxels showing higher FA in the young group compared to the senior group. This inclusion mask was then resubmitted into the TBSS algorithm in order to search for SES–FA relationships within regions showing age-related FA declines. Age, sex, and IQ were included as covariates of no interest. Because the multiple regression analyses were restricted to (masked by) regions showing age-related FA declines, a statistical threshold of P < 0.001 (uncorrected) was employed with a cluster threshold of 20 voxels. For visualization purposes, all statistical maps were dilated using FSL’s tbss_fill.
Region of interest analyses
Results from the analyses described above demonstrated SES–FA correlations in the senior group in three frontal WM regions. Region of interest (ROI) analyses were conducted to further characterize the neurobiological bases and functional significance of these SES–FA correlations.
Component diffusivity analyses.
Multiple regression analyses were performed with SES as the dependent variable and age, sex, IQ, and mean DR and DA values from ROIs demonstrating SES–FA correlations as predictor variables. Standardized beta values were then assessed to determine the unique contribution of each independent variable. The largest standardized beta value indicates the independent variable that makes the strongest unique contribution to explaining the variance in SES in regions showing SES–WM integrity relationships in healthy seniors.
Correlation of FA and working memory.
The second ROI analysis was intended to explore relationships between working memory (i.e., digit backwards) and WM integrity in the three frontal ROIs that showed a correlation between SES and FA in the senior group. Partial correlations were used to determine the relationship between FA and working memory performance while controlling for sex and IQ.
Results
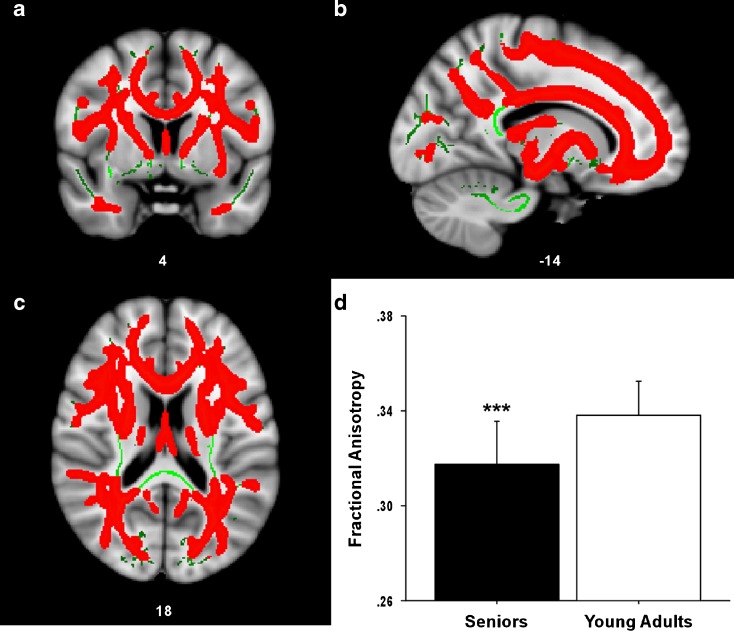
Demographic information for both groups is listed in Table 1. Results from the between-group comparison of FA are presented in Fig. 1. The senior group demonstrated significantly lower mean FA values (red) across a large portion, but not all, of the WM skeleton (green) after controlling for sex, IQ, and SES. The average age-related reduction in FA was significant across the entire WM skeleton F(1, 79) = 33.16, P < 0.001. There were no regions with significantly higher FA in the senior group compared to the young adult group.
Table 1.
Demographic data
| Subjects | Number | Sex (F/M) | Age | SES | IQ |
|---|---|---|---|---|---|
| Young Adults | 40 | 24/16 | 33.3 (4.3) | 29.4 (12.4) | 120.1 (19.76) |
| Older Adults | 44 | 25/19 | 66.2 (7.5) | 28.4 (11.0) | 123.6 (21.15) |
Values are means and values in parentheses are standard deviations
F female, M male
Fig. 1.
Age-related reductions in FA. Statistical map showing WM regions (a coronal, b sagittal, c transverse sections) where FA was significantly greater (red) in younger adults compared to seniors. The anatomic underlay used for illustration is the MNI152 T1-weighted 1-mm brain. The registered average FA skeleton is represented in green. d The average FA value across the entire WM skeleton is presented in the inset graph for both seniors and young adults. The average age-related reduction in FA was significant across the entire WM skeleton
SES–FA correlations
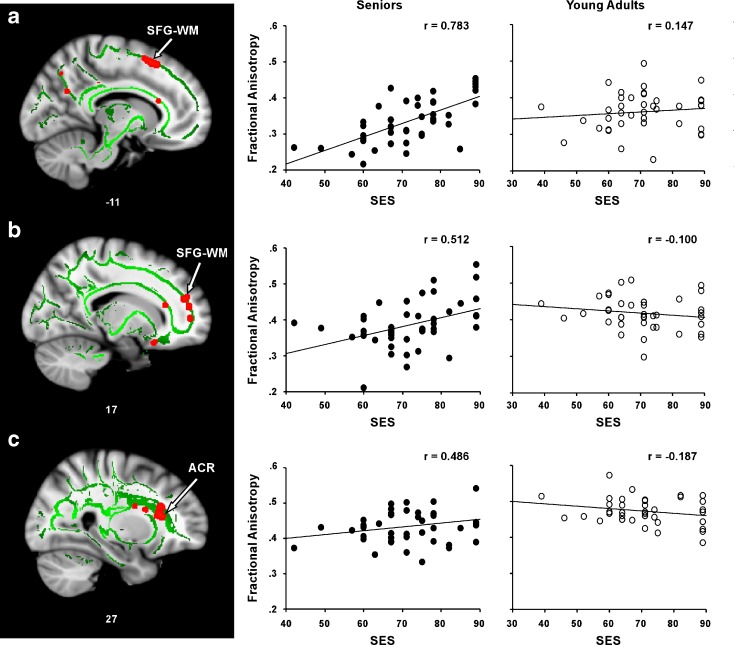
Figure 2 presents the results of the voxelwise multiple regression analysis between SES and FA in the senior group, and regression plots for the three regions consisting of ≥20 contiguous voxels (MNI coordinates are listed in Table 2). The correlation coefficients represent partial correlations after controlling for within-group variance in age, sex, and IQ. A positive correlation was observed between SES and FA in left superior frontal gyrus (SFG) WM, right SFG–WM, and the right anterior corona radiata (ACR) in the senior group. Specifically, the senior group demonstrated a positive correlation between SES and FA in left SFG–WM (r = 0.783, P < 0.001), right SFG–WM (r = 0.512, P < 0.001), and right ACR (r = 0.486, P < 0.001).
Fig. 2.
SES is correlated with FA in seniors. A positive correlation between SES and FA in the senior group was observed in a WM underlying the left superior frontal gyrus (SFG–WM), b WM underlying the right superior frontal gyrus SFG–WM, and c the right anterior coronal radiata (ACR). The numbers below each slice represent x coordinates in MNI space. The right panel shows scatter plots of the correlations between mean FA and SES in each WM region for each group (seniors = filled circles; young adults = open circles). Note: To render FA–SES regression plots more interpretable, participants’ SES values on the ISP were converted such that higher scores now reflected higher levels of SES (by subtracting each participant’s score from 100)
Table 2.
Location and size of white matter regions showing selective positive SES–FA correlations in the senior group
| Region | X | Y | Z | Cluster size | Correlation seniors | Correlation young adults |
|---|---|---|---|---|---|---|
| L superior frontal gyrus white matter | −11 | 21 | 51 | 47 | 0.783* | 0.147 |
| R superior frontal gyrus white matter | 18 | 48 | 21 | 20 | 0.512* | −0.100 |
| R anterior corona radiata | 27 | 25 | 13 | 27 | 0.486* | −0.187 |
Values represent partial correlations after controlling for within-group variance in age, sex, and IQ; r values are displayed
L left, R right
*P < 0.001
In contrast, in the younger adult group, no relationship was observed between SES and FA in the three frontal regions showing correlations in the senior group: left SFG–WM ROI (r = 0.147, P = 0.193), right SFG–WM ROI (r = −0.100, P = 0.279), or right ACR ROI (r = −0.187, P = 0.134). In addition, multiple regression analysis conducted across the brain’s WM (i.e., not restricted to regions showing age-related FA declines) revealed no regions showing a positive SES–FA relationship in the younger group.
Region of interest analyses
Component diffusivity analyses.
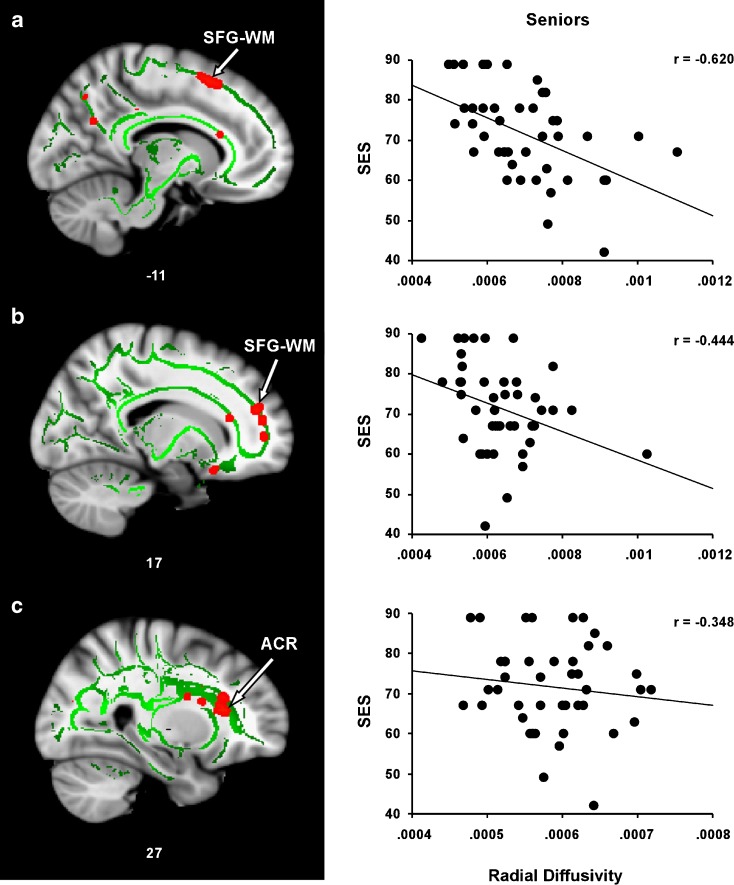
The total SES variance explained by the model was 63.3 % in the left SFG–WM ROI (R = 0.796, F(5, 38) = 13.13, P < 0.001), 35.3 % in the right SFG–WM ROI (R = 0.549, F(5, 38) = 4.14, P < 0.004), and 30.2 % in the right ACR ROI (R = 0.549, F(5, 38) = 3.28, P < 0.015). DR made the strongest contribution to the variance in SES in each of these three regions [left SFG–WM ROI (beta = −1.30, P < 0.001), right SFG–WM ROI (beta = −0.57, P = 0.006), and right ACR ROI (beta = −0.62, P = 0.001)]. The unique contribution of both DR and DA are listed in Table 3. Scatter plots illustrating the relationship between DR and SES in each ROI are present in Fig. 3. The correlation coefficients represent partial correlations after controlling for within-group variance in age, sex, and IQ.
Table 3.
Unique contributions of radial and axial diffusivity measures in predicting SES
| Region | Standardized beta coefficients | |
|---|---|---|
| DR | DA | |
| L superior frontal gyrus white matter | −1.30 | 0.78 |
| R superior frontal gyrus white matter | −0.57 | 0.27 |
| R anterior corona radiata | −0.62 | 0.29 |
Fig. 3.
Relationship between SES and DR in regions showing a positive SES–FA correlation in seniors. Regression plots show the negative relationship between SES and DR in a WM underlying the left superior frontal gyrus (SFG–WM), b WM underlying the right superior frontal gyrus SFG–WM, and c the right anterior coronal radiata (ACR)
Correlation of FA and working memory.
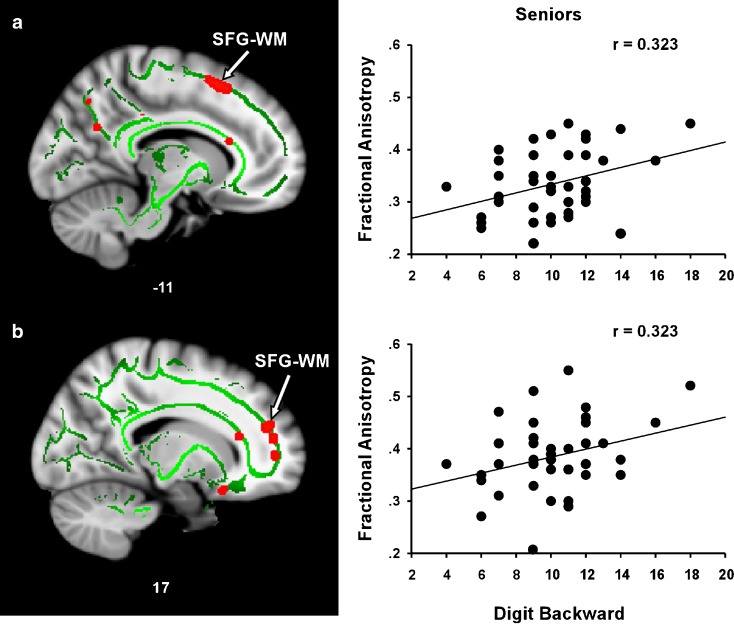
Figure 4 presents the correlations between FA and working memory in regions that showed a positive SES–FA relationship in healthy seniors. The correlation coefficients represent partial correlations after controlling for within-group variance in age, sex, and IQ. A positive correlation was observed between FA and DB in the left (r = 0.323, P = 0.019) and right (r = 0.323, P = 0.019) SFG–WM ROIs. Similarly, an FA–DB trend was observed in the right ACR ROI (r = 0.237, P = 0.066). For the young adults, no correlation was observed between FA and DB in the left SFG ROI (r = 0.060, P = 0.363), the right SFG ROI (r = −0.115, P = 0.249), or the right ACR ROI (r = −0.110, P = 0.259).
Fig. 4.
Relationship between FA and working memory (digit backward) in regions showing a positive SES–FA correlation in seniors. The left (a) and right (b) SFG–WM regions showing a positive correlation between SES and FA also showed a positive FA–DB correlation. The right panel shows scatter plots of the correlations between mean FA and DB
Discussion
The present study represents the first systematic exploration of the relationship between socioeconomic status (SES) and WM microstructural integrity relevant to normal aging. Strengths of our study include the use of a TBSS method with rigorously validated registration methods, exploration of three main components of the diffusion tensor (FA/DR/DA), and the control of age, sex, and IQ variables in all analyses. Seniors demonstrated significantly lower FA across widespread portions of WM. However, high SES offset age-related FA declines in several frontal WM regions. In addition, the relative preservation of WM integrity in these regions was positively associated with working memory span. The details of these findings are discussed below.
We observed diffuse age-related reductions in FA in frontal, parietal, occipital, and temporal WM, consistent with previous results (Abe et al. 2002; Madden et al. 2004; O'Sullivan et al. 2001; Pfefferbaum et al. 2000). Within regions showing age-related WM integrity declines, we observed positive SES–FA relationships in several frontal regions in the senior group. Specifically, in the senior group, positive SES–FA correlations were observed in bilateral portions of WM underlying the superior frontal gyri (SFG–WM) and in the right anterior corona radiata (ACR). The location of the observed SES–FA relationship within frontal regions is consistent with a view that high levels of SES are associated with an increasing emphasis on high-level decision making, planning, and goal-directed behavior (Andersen et al. 2004; Kristensen et al. 2002). The positive SES–WM integrity relationship in the senior group could be the result of biological mechanisms (i.e., higher WM integrity leads to higher SES) or environmental mechanisms (i.e., SES is neuroprotective of WM integrity). These possibilities are not mutually exclusive and may both be at work in some interactive fashion.
To identify potential neuroprotective effects, we included a young group that was matched with the senior group for mean SES, SES variance, IQ, and gender. Despite this matching, the observed SES–FA correlations in frontal WM were selective for the senior group. The unique correlations in the senior group raise the possibility that high SES may neuroprotect some age-related frontal WM integrity declines. The anatomical specificity of the observed SES–frontal relationship may in part reflect that frontal WM tracts, because they are especially vulnerable to age-related declines (Head et al. 2004; O'Sullivan et al. 2001; Salat et al. 2005a, b), also carry greater chance of incurring protective benefits from high SES than other regions.
Although the neurobiological mechanisms contributing to a potential SES–WM integrity relationship are still unclear, cognitive stimulation, and the subsequent activation of cortical neurons, may play a significant role because axon myelination appears to be triggered by neural activity (Bradl and Lassmann 2010; Gyllensten and Malmfors 1963; Omlin 1997). Support for such a mechanism comes from studies exploring the effects of cognitive stimulation (cognitive training) on WM integrity (Engvig et al. 2011; Lovden et al. 2010). For example, Lovden et al. (2010) demonstrated that cognitive training in young adults and healthy seniors results in increased WM integrity in frontal commissural tracts, suggesting that regular cognitive stimulation may help contribute to dynamic changes in WM microstructural integrity at any age.
In partial support of this view, joint consideration of the other major components of the diffusion tensor showed that the positive SES–FA correlations in the left SFG–WM, right SFG–WM, and right ACR were primarily explained by negative SES–DR correlations. Interestingly, similar findings have been reported in frontal tracts in association with a different kind of reserve variable, cardiorespiratory fitness (Johnson et al. 2012). The convergence of these findings raises the possibility that maintenance of myelin integrity within frontal WM tracts may serve as one common mechanism of brain reserve. However, future longitudinal research will be required to determine if the adoption of positive lifestyle variables can attenuate the rate of myelin loss in cognitively normal seniors.
Finally, to determine the potential functional significance of maintained frontal WM integrity in aging, we explored the relationship between working memory (i.e., DB) and WM integrity in frontal regions showing positive SES–FA correlations in the senior group. We observed a positive relationship between microstructural integrity and DB in WM underlying the left and right SFG. The observed association has high face validity because WM underlying bilateral portions of the SFG include tract connections with the left and right dorsolateral prefrontal cortex, brain regions that play central roles in working memory function (D'Esposito et al. 1995; Salmon et al. 1996).
The present study has several caveats that highlight the need for future work in this field. First, the cross-sectional design used in this study is unable to provide definitive information on cause-and-effect relationships between SES and WM integrity. Furthermore, the cross-sectional design did not allow us to account for potential cohort effects (Deary et al. 2003). Second, SES is considered to be a proxy indicator for other lifestyle variables known to contribute to reserve (such as physical activity, access to health care, and dietary habits). These variables could contribute to the present SES correlations and should be explored in future work. Additionally, our younger and older group samples demonstrated a relatively high mean IQ. Future work will be required to determine if our findings generalize to groups with average IQs. Third, the underlying biophysical properties derived from the diffusion tensor still need to be elucidated in humans. Although animal model studies have established a link between demyelination and DR (Song et al. 2002, 2005), this link is not as well established in humans (Wheeler-Kingshott and Cercignani 2009). Lastly, future studies should explore the contribution of genetic factors, such as the presence of apolipoprotein-E4 allele, as genetics can interact with environmental variables to influence cortical structure (Anttila et al. 2004; den Heijer et al. 2004; Dufouil et al. 2000).
In conclusion, our results suggest a potential neuroprotective effect of SES on frontal WM integrity. The positive SES–FA correlations observed were primarily driven by negative DR–SES correlations, suggesting that SES may buffer age-related changes in myelin. The functional significance of high WM integrity in these frontal regions was demonstrated through positive correlations with working memory span. These findings suggest that SES may help maintain frontal WM integrity and working memory function in aging. On the flip side, our results draw further attention to the fact that disparities in SES affect not only one’s status in society but also the health of the aging brain.
Acknowledgments
This study was supported by NIH grant AG033036 and NSF grant BCS 0814302. We thank Sara E. Cilles for her assistance in recruiting, scanning, and testing participants. In addition, we gratefully acknowledge our collaborators at the Sanders-Brown Center on Aging at the University of Kentucky.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23(3):433–441. doi: 10.1016/S0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Andersen I, Burr H, Kristensen TS, Gamborg M, Osler M, Prescott E, Diderichsen F. Do factors in the psychosocial work environment mediate the effect of socioeconomic position on the risk of myocardial infarction? Study from the Copenhagen Centre for Prospective Population Studies. Occup Environ Med. 2004;61(11):886–892. doi: 10.1136/oem.2004.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329(7465):539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Sole-Padulles C, Junque C, Fernandez-Espejo D, Bargallo N, Rami L, Molinuevo JL, Bartres-Faz D. Specific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. Am J Geriatr Psychiatr Off J Am Assoc Geriatri Psychiatr. 2011;19(1):33–42. doi: 10.1097/JGP.0b013e3181e448e1. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5(3):421–428. doi: 10.1037/0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. doi: 10.1016/S1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D, Sole-Padulles C, Junque C, Rami L, Bosch B, Bargallo N, Falcon C, Sanchez-Valle R, Molinuevo JL. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol. 2009;80(2):256–259. doi: 10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::AID-MRM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Polvikoski T, Sulkava R. Education, the brain and dementia: neuroprotection or compensation? Brain J Neurol. 2010;133(Pt 8):2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. NeuroImage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS (1960) Handbook for the individual or group culture fair intelligence test. In: U.S.A. IfPaAT, editor
- Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, Wright MJ, Thompson PM. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage. 2011;54(3):2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Leaper SA, Murray AD, Staff RT, Whalley LJ. Cerebral white matter abnormalities and lifetime cognitive change: a 67-year follow-up of the Scottish Mental Survey of 1932. Psychol Aging. 2003;18(1):140–148. doi: 10.1037/0882-7974.18.1.140. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, van Duijn CM, Hofman A, Breteler MM. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. Am J Clin Nutr. 2004;80(4):992–997. doi: 10.1093/ajcn/80.4.992. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378(6554):279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4(4):500–503. doi: 10.1037/0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P, Alperovitch A. Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology. 2000;11(3):280–284. doi: 10.1097/00001648-200005000-00009. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2011;33(10):2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer's disease. NeuroImage. 2010;52(4):1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822(3):416–422. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Field D, Hammond SM. Measuring memory span. Int J Psychol. 1999;34(5–6):359–363. doi: 10.1080/002075999399693. [DOI] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function—a quantitative investigation in mice. J Embryol Exp Morpholog. 1963;11:255–266. [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Social class and mental illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen TS, Borg V, Hannerz H. Socioeconomic status and psychosocial work environment: results from a Danish national study. Scand J Public Health. 2002;59:41–8. [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lovden M, Bodammer NC, Kuhn S, Kaufmann J, Schutze H, Tempelmann C, Heinze HJ, Duzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48(13):3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. NeuroImage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Fratiglioni L, Bandinelli S, Ferrucci L. Socioeconomic status during lifetime and cognitive impairment no-dementia in late life: the population-based aging in the Chianti Area (InCHIANTI) Study. J Alzheimers Dis JAD. 2011;24(3):559–568. doi: 10.3233/JAD-2011-101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin FX. Optic disc and optic nerve of the blind cape mole-rat (Georychus capensis): a proposed model for naturally occurring reactive gliosis. Brain Res Bull. 1997;44(5):627–632. doi: 10.1016/S0361-9230(97)00283-9. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical "disconnection" as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/WNL.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB. Effect of age on event-based and time-based prospective memory. Psychol Aging. 1997;12(2):314–327. doi: 10.1037/0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::AID-MRM13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain J Neurol. 1996;119(Pt 5):1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. doi: 10.1037/0894-4105.7.3.273. [DOI] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J Int Neuropsychol Soc: JINS. 2000;6(1):52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89(5):2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70(2):296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28(7):1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer's disease. Neurobiol Aging. 2010;31(7):1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargallo N, Jurado MA, Barrios M, Molinuevo JL. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2009;30(7):1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA: J Am Med Assoc. 1994;271(13):1004–1010. doi: 10.1001/jama.1994.03510370056032. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45(1):55–60. doi: 10.1212/WNL.45.1.55. [DOI] [PubMed] [Google Scholar]
- Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37(5):590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Burger K, Reiser MF, Herpertz S, Moller HJ, Hampel H. White matter microstructure in relation to education in aging and Alzheimer's disease. J Alzheimers Dis: JAD. 2009;17(3):571–583. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36(4):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Wechsler DS. Wechsler adult intelligence scale, 3rd edn. (WAIS-3®) San Antonio: Harcourt Assessment; 1997. [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Mag Reson Med: Off J Soc Mag Reson Med / Soc Mag Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wiegersma S, Meertse K. Subjective ordering, working memory, and aging. Exp Aging Res. 1990;16(1–2):73–77. doi: 10.1080/07340669008251530. [DOI] [PubMed] [Google Scholar]