Abstract
Fruit and vegetable (FV) intake, which is often low in older people, may be associated with improved muscle strength and physical function. However, there is a shortage of intervention trial evidence to support this. The current study examined the effect of increased FV consumption on measures of muscle strength and physical function among healthy, free-living older adults. A randomized controlled intervention study was undertaken. Eighty-three participants aged 65–85 years, habitually consuming ≤2 portions of FV/day, were randomised to continue their normal diet (≤2 portions/day), or to consume ≥5 portions of FV/day for 16 weeks. FV were delivered to all participants each week, free of charge. Compliance was monitored at baseline, 6, 12 and 16 weeks by diet history and by measuring biomarkers of micronutrient status. Grip strength was measured by a hand-held dynamometer, while lower-extremity physical function was assessed by performance-based measures. Eighty-two participants completed the intervention. The 5 portions/day group showed greater change in daily FV consumption compared to the 2 portions/day group (P < 0.001). This was reflected in significant increases in biomarkers of micronutrient status. No significant differences were evident in change in physical function between the two groups. However, there was a trend towards a greater change in grip strength in the 5 portions/day compared to the 2 portions/day group (mean change at 16 weeks ± SD, 2.04 ± 5.16 and 0.11 ± 3.26 kg, respectively, P = 0.06). Increased FV consumption may modestly increase grip strength but has no effect on physical function in healthy older adults.
Keywords: Physical function, Muscle strength, Fruit and vegetables, Ageing
Introduction
Ageing is associated with numerous anatomical and physiological changes which can adversely affect both physical function and muscle strength, thus contributing to an increased risk of falls, fractures and disability. Loss of muscle strength and flexibility, and impaired balance and reaction time are the most common factors associated with risk of falls (Myers et al. 1996). Lower-extremity physical function, in particular, is regarded as a strong predictor of disability, falls and hip fractures in older adults (Guralnik et al. 1994a, 1995; Dargent-Molina et al. 1996). Furthermore, lower-extremity physical function is thought to decline more rapidly than upper body function with age (Aoyagi and Shephard 1992).
Although many studies have examined the role of nutrient intake in skeletal health, nutritional factors seem to have been somewhat overlooked as potentially contributing to age-related decline in physical function and muscle strength. The physiological, social and economic changes that are associated with ageing can lead to a compromised nutritional status (Finch et al. 1998; Meydani 2002), and a substantial proportion of older populations may have low fruit and vegetable (FV) intakes (Wakimoto and Block 2001; Appleton et al. 2009). However, a growing body of observational evidence suggests that increased FV consumption and/or nutrients associated with a diet high in FV, such as carotenoids and vitamin C, may be associated with improved physical function and muscle strength (Cesari et al. 2004a; Houston et al. 2005; Myint et al. 2007; Lauretani et al. 2008; Robinson et al. 2008; Tomey et al. 2008). To our knowledge, this hypothesis has not yet been comprehensively tested in dietary intervention studies with muscle strength and physical function endpoints and involving free-living older adults.
A few intervention studies have been conducted; however, these have largely focused either on the effect of an overall healthy lifestyle on physical function (Demark-Wahnefried et al. 2006; Morey et al. 2009) or on the effects of antioxidant supplements on exercise performance and post-exercise recovery rather than physical function (Clarkson and Thompson 2000; Takanami et al. 2000). Furthermore, previous studies have relied on subjective measures of physical function rather than direct measures (Demark-Wahnefried et al. 2006; Morey et al. 2009). Although the exact mechanism through which FV may influence physical function and muscle strength is not entirely understood, it may be related to their high antioxidant and carotenoid content which may protect against oxidative stress and inflammation (Mecocci et al. 1999; Cesari et al. 2004b). More recent evidence also suggests that the alkaline salts which are abundant in FV may help to preserve muscle mass in older adults (Dawson-Hughes et al. 2008).
The aim of this study was to test the hypothesis that increased consumption of FV can have a positive effect on measures of physical function and muscle strength in healthy older adults aged 65 years and over. This study also examined the correlation between change in nutrient status and change in muscle strength and physical function over a 16-week period. This paper presents the secondary outcomes of a randomised controlled parallel-group trial which was primarily designed to compare the effect of increasing FV intake to the recommended 5 portions/day with a control low FV diet (≤2 portions/day) on clinically relevant measures of immune function in healthy older adults (Gibson et al. 2012).
Materials and methods
Study design and recruitment of participants
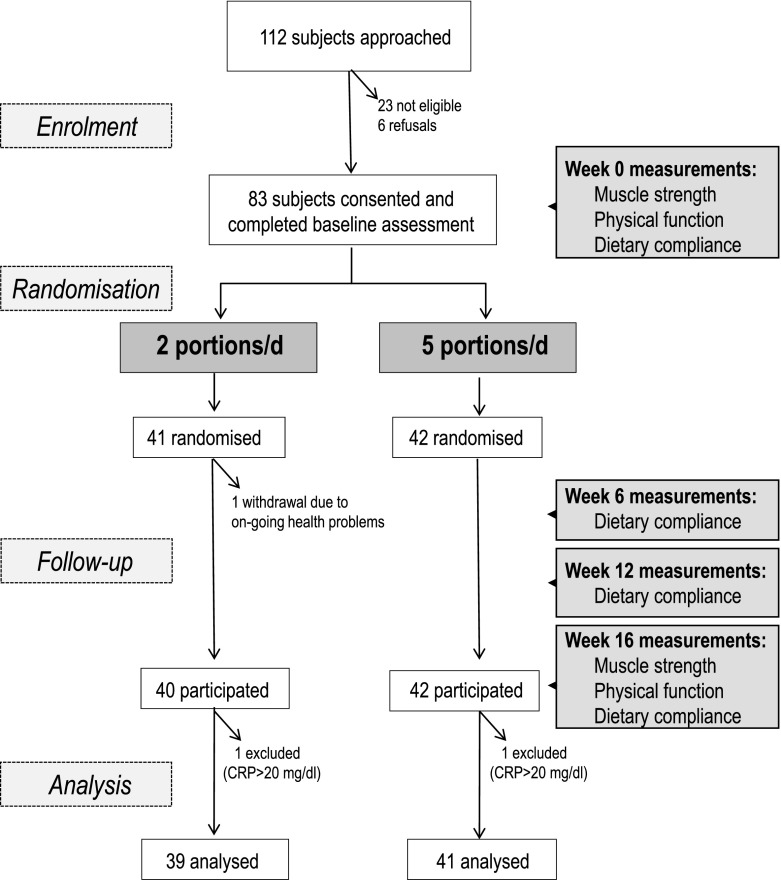
This study [named the Ageing and Dietary Intervention Trial (ADIT)] was a randomised controlled trial designed to examine the effect of the recommended 5 portions FV/day compared to ≤2 portions/day on measures of muscle strength and physical function. The study design is summarised in Fig. 1. Participants were recruited between October 2006 and June 2008 through press releases to local media, older people’s networks, newsletters and bulletins, presentations to older peoples’ community groups and from hospital outpatient clinics. All subsequent study visits (baseline, weeks 6, 12 and 16) were conducted in the participants’ own homes.
Fig. 1.
Flow chart showing overview of study design and progression of participants through the study
Healthy, free-living males and females, aged 65–85 years with low habitual FV consumption (≤2 portions/day), were recruited. Exclusion criteria were those on special diets, taking nutritional supplements (i.e. dietary or nutritional supplements including multivitamins) or medications known to affect immune function or absorption of nutrients; excessive alcohol consumption (>28 units/week for men, >21 units/week for women); BMI > 35 kg/m2; history of diabetes or dementia; Pneumovax II vaccination within previous 2 years; inability to provide informed consent; any other problem which would prevent adherence to a high FV diet; or recent infection (<3 weeks since completion of any antibiotic course or symptoms of viral illness).
Written informed consent was obtained from all individuals prior to participation. Ethical approval was obtained from the Office for Research Ethics Committees Northern Ireland (ORECNI). Research governance was approved by the Research Office, Belfast Health and Social Care Trust, who acted as sponsor for the study.
Randomisation and intervention
Participants were randomised using a block randomisation approach (block size, n = 8) with computer-generated random numbers to one of two arms—either to increase FV consumption to at least 5 portions/day (intervention group) or to follow their normal diet (therefore consuming ≤2 portions/day), for 16 weeks. Each participant was instructed as to what defined a portion and was provided with portion size guides. For the purposes of this study, a portion was defined using the UK Food Standards Agency guidelines, i.e. an 80-g serving (one apple, orange or banana, or three heaped tablespoons of vegetables, or 150 ml fruit juice; http://www.eatwell.gov.uk). All participants were supplied with a tablespoon and glass as an aid to portion size estimation. At the start of the study, participants were given a list of FV that were available in the local supermarket. Participants used this list to indicate their likes and dislikes of specific FV. This was subsequently used to guide the researcher, who was responsible for purchasing the FVs. Participants were allowed a free choice of FV for consumption over the study. No prescriptive list was offered to participants at any point. Participants were encouraged to consume as wide a variety of FV as possible as botanical diversity is thought to play an important role in determining the bioactivity of FV-rich diets (Thompson et al. 2006). At the time of FV selection, the researchers also discussed feasibility issues such as storage, cooking methods and preparation of composite dishes.
Over the course of the intervention, participants were asked to make a note of any recent infections or illness, and any newly prescribed medications and to minimise other changes to health and lifestyle behaviours. Participants were also asked not to commence any new dietary supplements or vitamin supplements during the course of the intervention.
Monitoring and maximising compliance
Compliance was monitored in all participants by diet history interview and laboratory assessment of micronutrient status at baseline, 6, 12 and 16 weeks. All participants received extensive personal dietetic advice and nutritional counselling to encourage incorporation of FV into their diet without, for example, compromising energy intake, and in line with their physical capabilities. Compliance was encouraged by provision of menu suggestions and recipes. In order to support dietary compliance while minimising personal expense and maximising food freshness during the intervention phase, each participant (in both intervention and control arms) received weekly home deliveries of FV from a local supermarket. The FV which were delivered by the supermarket were selected by the researcher each week and ordered using an online shopping system. The quantity of FV ordered depended on the group allocation, i.e. 5 or 2 portions/day. All participants were contacted at weekly intervals by telephone to monitor any difficulties, provide positive reinforcement and encourage compliance.
Dietary intake assessment
At baseline, weeks 6, 12 and 16, dietary data were collected using the 7-day diet history method (van Staveren et al. 1985). This consisted of a detailed, open-ended, one-to-one interview, the purpose being to ascertain the habitual food and nutrient intake of each participant and more specifically to assess their FV intake. Participants were asked to recall their usual weekly meal and snack food intake by reporting the frequency, amounts and methods of preparation of foods consumed. In the case of FV intake, participants were asked whether the fruit or vegetables were fresh, frozen, tinned, dried or pureed. Amounts of foods were reported in household measures (for example, 1 tablespoon) or natural measures (for example, one slice). Each dietary interview lasted approximately 45 min per person, depending on the complexity and stability of the eating patterns. Following completion of the diet history, all food quantities were converted into grams. Food Portion Sizes (Food Standards Agency 2006) was also used to quantify intakes. The mean number of daily FV portions consumed by each participant at baseline, weeks 6, 12 and 16 were assessed from the diet histories by two independent researchers who were blinded to the participants’ group allocation.
Blood sampling and laboratory analysis
At baseline, weeks 6, 12 and 16, fasting blood samples were collected from participants between 8:00 and 10:00 a.m. EDTA samples were stored on ice, while serum samples were stored at room temperature in the dark. All samples were processed and frozen at −80 °C within 2 h of collection.
Biochemical markers of nutritional status
Biochemical markers of nutritional status were assessed at baseline and at weeks 6, 12 and 16.
Serum concentrations of a panel of carotenoids (lutein, zeaxanthin, beta (β)-cryptoxanthin, alpha (α)-carotene, β-carotene and lycopene) were determined by reverse-phase high-performance liquid chromatography (HPLC) with diode array detection (Craft 1992). The intra-assay and inter-assay CV was <7 % and <10 %, respectively. Plasma ascorbate was measured by automated fluorimetric assay (Vuilleumier and Keck 1989). At 50.6 μmol/l, the inter-assay and intra-assay CV was 14.5 % and 11.6 %, respectively, while at 151.0 μmol/l, the inter-assay and intra-assay CV was 6.7 % and 1.4 %, respectively. Plasma vitamin B12 and folate concentrations were measured by competitive protein binding utilized by the SimulTRAC-SNB radioassay kit (ICN Pharmaceuticals, Costa Mesa, CA, USA). The inter-assay and intra-assay CV for vitamin B12 was 7.4 % and 6.8 %, respectively, and 5.6 % and 3.7 % for folate, respectively. 25-Hydroxy vitamin D (25(OH)D) was measured in serum samples (at baseline and week 16 only) by an enzyme-linked immunosorbent assay (ELISA) (Immunodiagnostic Systems Limited, Tyne and Wear, UK). The inter-assay and intra-assay CV for the 25(OH)D ELISA method was 9.3 % and 5.2 %, respectively. All laboratory assays were standardised against available international standards (e.g. National Institute of Standards and Technology materials for lipid-soluble vitamins and ascorbate), and quality control samples were included in every run. Laboratory analyses were carried out by investigators blinded to participants’ group allocation.
Measurement of other biochemical secondary endpoints
High-sensitivity C-reactive protein (CRP) concentrations were determined in serum using an immunoturbidimetric assay (Randox, Crumlin, Northern Ireland) on the ILab-600 biochemical analyser (Instrumentation Laboratories, Warrington, UK). The inter-assay and intra-assay CV for CRP was 0.7 % and 0.5 %, respectively.
Assessment of physical performance
Physical performance was assessed at baseline and at the end of week 16. All participants were asked to refrain from consuming high-caffeine drinks such as coffee or cola for 24 h prior to the physical performance assessment to minimise any potential effect on performance in the tests. The physical performance assessment included the following tests:
Assessment of muscle strength
Hand grip strength was assessed using a hand-held dynamometer. This method has recently been shown to be a reliable and valid instrument for assessing muscle strength and function (Abizanda et al. 2012; Stark et al. 2011; Arnold et al. 2010). The participant was asked to sit with their elbow flexed at 90°, with the upper arm tight against the body and the dynamometer positioned so that the dial faced outwards. Participants were asked to squeeze the handle of the dynamometer as hard as possible, in order to exert the best possible force. Measurements (in kilograms) were repeated three times with each hand. For data analysis, the mean grip strength was calculated using the best attempt from each hand.
Assessment of lower-extremity physical function
Lower-extremity physical function was determined by the Short Physical Performance Battery (SPPB; Guralnik et al. 2000). This battery assesses performance on three objective tests of physical function: (1) 8-ft. walking speed, (2) repeated chair stand (time taken to rise from a chair five times) and (3) standing balance.
Eight-foot walking speed test. An 8-ft. walk course was measured using a metal rule, with start and finish lines clearly marked. Each participant was asked to walk a distance of 8 ft. at their usual speed. The time taken in seconds to complete the walk was recorded. The test was then repeated. Walk speed was defined as the fastest time in seconds of the two walks. If required, participants were permitted to use any walking aids such as a walking stick.
Repeated chair stand test. Participants were asked to fold their arms against their chest and sit upright on a firm, padded armless stool which was positioned against a wall. Initially, participants were asked to rise from the stool once with arms folded across their chest. If they were able to perform this task successfully, they were then instructed to stand up and sit down five times as quickly as possible, again with arms folded across their chest. Participants were reminded to straighten their legs fully when rising off the chair. The total time taken in seconds to complete the five chair stands was recorded. The same stool was used for testing all participants.
-
Standing balance test. Balance was assessed by three tests, each one being progressively more challenging:
- Side-by-side: participants were instructed to stand with their feet side-by-side and hold the position for 10 s.
- Semi-tandem: participants were instructed to stand with one foot next to and halfway in front of the other for 10 s.
- Full-tandem: participants were instructed to stand with the heel of one foot directly in front of and touching the toes of the other foot for 10 s.
The semi-tandem and subsequent full-tandem positions were only attempted if the participant was able to perform the side-by-side position. Participants carried out the tests unaided. Participants were scored according to performance as follows: score 0 = unable to complete the test; 1 = able to hold a side-by-side stand for 10 s but unable to hold a semi-tandem stand for 10 s; 2 = able to hold a semi-tandem stand for 10 s but unable to hold a full tandem stand for more than 2 s; 3 = able to hold the full tandem stand for 3–9 s; and 4 = able to hold the full tandem stand for 10 s (Table 1).
Summary score of lower-extremity physical function. A composite score (SPPB score) for overall lower-extremity physical function was derived from performance on each of the three physical function tests, i.e. SPPB score = sum of scores from standing balance (as described above), 8-ft. walk test and repeated chair stand test (Table 1). The scoring system for the 8-ft. walk test and repeated chair stand test was based on quartiles of performance. In the 8-ft. walk test, the following scores were applied: 0 = unable to complete the test, 1 = ≥5 s to complete, 2 = 4 s to complete, 3 = 3 s to complete and 4 = ≤2 s to complete. In the repeated chair stand test, the following scores were applied: 0 = unable to complete the test, 1 = >12.25 s to complete, 2 = 12.25–10.01 s to complete, 3 = 10.0–9.01 s to complete and 4 = ≤9 s to complete. Summary scores could range from 0 (worst) to 12 (best) depending on performance, with higher scores indicative of a better performance. The internal consistency of the summary scale used in the current study was 0.61, as assessed by Cronbach’s alpha.
Table 1.
Scoring system used to calculate SPPB score
| Score | 8-ft. walk test | Repeated chair stand test | Standing balance test |
|---|---|---|---|
| 0 | Unable to complete test | Unable to complete test | Unable to complete test |
| 1 | ≥5 s | >12.25 s | Able to hold a side-by-side stand for 10 s but unable to hold a semi-tandem stand for 10 s |
| 2 | 4 s | 12.25–10.01 s | Able to hold a semi-tandem stand for 10 s but unable to hold a full tandem stand for more than 2 s |
| 3 | 3 s | 10.00–9.01 s | Able to hold the full tandem stand for 3 to 9 s |
| 4 | ≤2 s | ≤9.00 s | Able to hold the full tandem stand for 10 s |
SPPB short physical performance battery
Anthropometry and other lifestyle measurements
At baseline, height was measured to the nearest 0.1 cm using the Leicester portable height measure, and body weight was measured to the nearest 0.1 kg using calibrated digital weighing scales (Tanita HS-301, Tanita, Yiewsley, UK). Measurement of body weight was repeated at week 16. For both measurements, participants wore no shoes. Body mass index (BMI) was computed as weight (kilograms)/square of the height (metres). At baseline, physical activity was assessed using a validated self-report physical activity questionnaire from the British Women’s Heart and Health Study (Lawlor et al. 2002). This questionnaire was based on a physical activity questionnaire previously used in the British Regional Heart Study (Shaper et al. 1991), with the addition of questions on housework (Lawlor et al. 2002). A confidential questionnaire was used to obtain baseline demographic information including medical history, previous occupation, years in full-time education, smoking and drinking habits, and any previous occurrence of bone fractures. A similar but shorter questionnaire was used to document changes in lifestyle behaviours and physical activity at weeks 6, 12 and 16.
Statistical analyses
Statistical analyses were performed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were obtained for each variable of interest according to FV allocation group. Normally distributed continuous variables were summarised using mean ± SD. Skewed variables were log transformed for parametric analysis and were summarised using the geometric mean and interquartile range. Between-group comparisons of baseline values were made using independent samples t tests and chi-square analysis for continuous and categorical variables, respectively. Between-group comparisons of change in each outcome variable were made using independent samples t tests, and data presented as change (95 % CI). The association between change in self-reported FV intake and change in the primary endpoints were assessed using linear regression analysis. ANCOVA analyses were also performed to examine interactions between FV group and gender and between FV group and initial grip strength. The association between change in nutrient status and change in measures of lower-extremity physical performance and grip strength were tested using bivariate correlation analyses. A P value of <0.05 was considered statistically significant.
Results
A total of 83 individuals were recruited (Fig. 1). One participant failed to complete the 16-week intervention due to personal reasons unrelated to the study; therefore, a total of 82 participants completed the study.
The primary endpoint of this intervention study was to examine between group differences in mean changes in the markers of immune function (Gibson et al. 2012). The current study focused on secondary endpoints which were to examine between-group differences in mean change in objective measures of physical function and muscle strength. Two participants who had serum C-reactive protein (CRP) values >20 mg/l at at least one timepoint during the study were excluded from all analyses, as acute inflammation may have affected the immune function endpoints.
Baseline physical characteristics of participants
The baseline physical characteristics of participants are described according to their allocated FV group (Table 2). Any significant imbalances between the groups at baseline are indicated (Table 2), and further statistical analysis was carried out using change in each variable of interest to minimise the effect of any imbalances on the comparison between intervention groups. There was a significantly higher proportion of men randomised to the 5 portions/day group (n = 21) than the 2 portions/day group (n = 7), and therefore, participants in the 5 portions/day group were significantly taller and heavier than those in the 2 portions/day group, although mean BMI was similar in both groups. Significantly more women in the 2 portions/day group had previously used HRT compared to those in the 5 portions/day group. There were no other significant differences evident between the two FV groups (P > 0.05) at baseline with respect to BMI, blood pressure, smoking status, alcohol status, current use of medication, bone fracture history, menopausal status (women only) and current use of drug treatment for osteoporosis. Participants in both groups reported similar mean baseline levels of total physical activity as assessed by self-report questionnaire.
Table 2.
Baseline characteristics of participants according to FV allocation
| Characteristic | 2 portions/day (n = 39) | 5 portions/day (n = 41) |
|---|---|---|
| Age, years | 71.1 ± 5.0 | 70.9 ± 5.0 |
| Men, % | 18 | 51* |
| Height, m | 1.6 ± 7.4 | 1.7 ± 7.4** |
| Weight, kg | 71.2 ± 12.4 | 78.9 ± 13.9*** |
| BMI, kg/m2 | 28.1 ± 4.5 | 28.5 ± 4.0 |
| Systolic blood pressure, mmHg | 150.5 ± 24.4 | 152.9 ± 20.9 |
| Diastolic blood pressure, mmHg | 84.1 ± 10.9 | 87.0 ± 10.9 |
| Current smokers, % | 8 | 2 |
| Former smokers, % | 35 | 44 |
| Alcohol consumers, % | 40 | 44 |
| Currently using medication, % | 93 | 93 |
| Currently on drug treatment for osteoporosis, % | 10 | 2 |
| Previous fracture or broken bone, % | 35 | 39 |
| Previous use of HRT, % | 42 | 17* |
| Age at menopause, approximate years, n = 54 | 47.1 ± 5.9 | 47.0 ± 5.5 |
| Years since menopause, n = 54 | 23.8 ± 8.3 | 21.7 ± 6.3 |
| CRPa, mg/L | 2.4 (1.3, 4.4) | 1.7 (1.0, 2.7) |
| Total physical activityb, h/week | 7.3 ± 7.1 | 8.9 ± 9.0 |
Values are mean ± SD or per cent unless noted otherwise
FV fruit and vegetable, HRT hormone replacement therapy, CRP C-reactive protein
*P < 0.01; **P < 0.001; ***P < 0.05 (significantly different from 2 portions/day group)
aVariable is logarithmically transformed and summarised as geometric mean (IQ range)
bTotal physical activity assessed by self-report questionnaire
Baseline and changes in fruit and vegetable consumption and micronutrient status during the intervention
There were no significant differences in FV intake between the two groups at baseline (Table 3). The usual FV intake of the participants at baseline was 1.4 ± 0.6 portions/day. The change in self-reported daily FV consumption was significantly different (P < 0.001) between the two groups at all timepoints with those in the 5 portions/day group consistently showing a larger increase in FV intake compared to those in the 2 portions/day group.
Table 3.
Self-reported FV intake (number of portions) at baseline and weeks 6, 12 and 16 according to group allocation
| Baseline | Week 6 | Week 12 | Week 16 | Change at 6 weeksa | Change at 12 weeksa | Change at 16 weeksa | ||
|---|---|---|---|---|---|---|---|---|
| 2 portions/day, n = 39 | F | 0.6 ± 0.5 | 0.9 ± 0.6 | 0.8 ± 0.5 | 1.0 ± 0.6 | 0.3 (0.1, 0.5) | 0.2 (0.1, 0.4) | 0.4 (0.2, 0.6) |
| V | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.2) | 0.0 (−0.1, 0.1) | |
| FV | 1.4 ± 0.7 | 1.7 ± 0.5 | 1.7 ± 0.5 | 1.8 ± 0.6 | 0.3 (0.1, 0.6) | 0.3 (0.1, 0.5) | 0.4 (0.1, 0.7) | |
| 5 portions/day, n = 41 | F | 0.7 ± 0.5 | 3.9 ± 1.2 | 3.7 ± 1.1 | 4.0 ± 1.2 | 3.2 (2.9, 3.6)* | 3.0 (2.7, 3.4)* | 3.3 (3.0, 3.7)* |
| V | 0.8 ± 0.3 | 2.1 ± 0.8 | 2.0 ± 0.6 | 2.0 ± 0.9 | 1.4 (1.2, 1.6)* | 1.2 (1.0, 1.4)* | 1.2 (0.9, 1.5)* | |
| FV | 1.4 ± 0.5 | 6.0 ± 1.3 | 5.6 ± 1.2 | 6.0 ± 1.3 | 4.6 (4.2, 5.0)* | 4.2 (3.8, 4.6)* | 4.6 (4.1, 5.0)* |
Values are mean ± SD or mean change (95 % CI)
F fruit, V vegetables, FV fruit and vegetables
*P < 0.001 (change at timepoint different from the 2 portions/day group)
aChange calculated as week 6 − baseline, week 12 − baseline and week 16 − baseline
In relation to micronutrient concentrations, there were no significant differences between the two groups at baseline, with the exception of zeaxanthin and lycopene concentrations which were significantly higher in the 2 portions/day group compared to the 5 portions/day group (P < 0.01 and P < 0.05, respectively; Table 4). The change in vitamin C (all timepoints), total carotenoids (week 16), zeaxanthin (all timepoints), β-cryptoxanthin (all timepoints), lycopene (all timepoints) and folate (week 6) differed significantly between the two intervention groups, being higher in the 5 portions/day group than in the 2 portions/day group. The change in vitamin B12 at week 6 was also significantly different between the two intervention groups; however, this change was higher in the 2 portions/day group compared to the 5 portions/day group. There was no significant difference between the two groups in change in 25(OH)D over the 16-week period.
Table 4.
Micronutrient status at baseline and during intervention according to FV allocation
| 2 portions/day, n max = 39 | 5 portions/day, n max = 41 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Week 12 | Week 16 | Change at 6 weeks | Change at 12 weeks | Change at 16 weeks | Baseline | Week 6 | Week 12 | Week 16 | Change at 6 weeks | Change at 12 weeks | Change at 16 weeks | |
| Vitamin C, μmol/l | 43.0 (39.8, 60.8) | 44.1 (38.8, 61.4) | 43.1 (40.1, 65.3) | 42.3 (37.0, 61.6) | 1.03 (0.93, 1.13) | 1.00 (0.91, 1.10) | 0.98 (0.87, 1.12) | 42.7 (35.1, 58.1) | 60.6 (52.2, 71.3) | 54.0 (41.1, 71.3) | 59.3 (52.5, 73.0) | 1.42* (1.27, 1.59) | 1.27** (1.13, 1.42) | 1.39* (1.23, 1.57) |
| Lutein, μmol/l | 0.15 (0.12, 0.21) | 0.17 (0.13, 0.23) | 0.16 (0.13, 0.23) | 0.16 (0.12, 0.22) | 1.07 (0.98, 1.18) | 1.07 (0.96, 1.18) | 1.03 (0.92, 1.16) | 0.14 (0.10, 0.20) | 0.17 (0.14, 0.22) | 0.17 (0.13, 0.24) | 0.17 (0.13, 0.24) | 1.18 (1.07, 1.31) | 1.16 (1.05, 1.29) | 1.18 (1.04, 1.34) |
| Zeaxanthin, μmol/l | 0.03**** (0.03, 0.04) | 0.03 (0.02, 0.05) | 0.03 (0.03, 0.05) | 0.03 (0.03, 0.05) | 1.00 (0.88, 1.12) | 1.01 (0.90, 1.14) | 1.02 (0.89, 1.17) | 0.02 (0.01, 0.04) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.05) | 0.03 (0.03, 0.04) | 1.41* (1.21, 1.65) | 1.39** (1.19, 1.63) | 1.42** (1.22, 1.65) |
| β-Cryptoxanthin, μmol/l | 0.06 (0.04, 0.10) | 0.06 (0.04, 0.10) | 0.06 (0.03, 0.09) | 0.06 (0.04, 0.09) | 1.03 (0.90, 1.18) | 0.99 (0.78, 1.25) | 1.12 (0.95, 1.32) | 0.06 (0.04, 0.09) | 0.08 (0.07, 0.13) | 0.08 (0.05, 0.12) | 0.08 (0.06, 0.14) | 1.50* (1.29, 1.75) | 1.37*** (1.15, 1.63) | 1.53*** (1.26, 1.86) |
| α-Carotene, μmol/l | 0.10 (0.07, 0.15) | 0.12 (0.08, 0.19) | 0.12 (0.09, 0.18) | 0.12 (0.08, 0.18) | 1.19 (0.98, 1.45) | 1.18 (0.99, 1.40) | 1.17 (0.96, 1.44) | 0.09 (0.05, 0.18) | 0.13 (0.09, 0.22) | 0.12 (0.06, 0.22) | 0.13 (0.08, 0.28) | 1.37 (1.09, 1.73) | 1.34 (1.05, 1.69) | 1.43 (1.04, 1.96) |
| β-Carotene, μmol/l | 0.25 (0.18, 0.38) | 0.28 (0.17, 0.44) | 0.28 (0.19, 0.43) | 0.31 (0.21, 0.49) | 1.13 (0.98, 1.32) | 1.13 (1.01, 1.27) | 1.26 (1.08, 1.47) | 0.25 (0.14, 0.45) | 0.29 (0.21, 0.48) | 0.30 (0.19, 0.47) | 0.31 (0.17, 0.51) | 1.19 (0.96, 1.45) | 1.20 (0.94, 1.53) | 1.23 (0.92, 1.64) |
| Lycopene, μmol/l | 0.51 (0.31, 0.99) | 0.46 (0.28, 0.75) | 0.48 (0.31, 0.78) | 0.43 (0.25, 0.75) | 0.91 (0.72, 1.17) | 0.94 (0.77, 1.14) | 0.85 (0.65, 1.12) | 0.31 (0.16, 0.74) | 0.43 (0.24, 0.81) | 0.45 (0.27, 0.70) | 0.42 (0.27, 0.75) | 1.39*** (0.99, 1.96) | 1.44*** (1.04, 1.99) | 1.34*** (0.95, 1.86) |
| Total carotenoidsa, μmol/l | 1.20 (0.81, 1.82) | 1.21 (0.90, 1.62) | 1.22 (0.98, 1.58) | 1.22 (0.83, 1.71) | 1.01 (0.87, 1.17) | 1.02 (0.91, 1.14) | 1.02 (0.89, 1.17) | 1.01 (0.56, 1.64) | 1.24 (0.92, 1.74) | 1.23 (0.94, 1.70) | 1.25 (0.92, 1.75) | 1.23 (1.04, 1.44) | 1.21 (1.04, 1.41) | 1.24*** (1.05, 1.46) |
| Folate, nmol/l | 20.0 (15.4, 30.6) | 19.7 (14.1, 31.9) | 19.7 (13.2, 29.7) | 20.3 (14.6, 30.6) | 0.98 (0.88, 1.10) | 0.98 (0.88, 1.10) | 1.01 (0.90, 1.14) | 22.4 (15.1, 31.3) | 25.7 (18.9, 30.8) | 24.4 (18.3, 31.5) | 23.6 (17.6, 30.3) | 1.15*** (1.04, 1.27) | 1.09 (0.98, 1.21) | 1.05 (0.94, 1.18) |
| Vitamin B12, pmol/l | 320 (237, 403) | 301 (213, 386) | 307 (217, 376) | 306 (212, 423) | 0.94 (0.89, 0.99) | 0.96 (0.89, 1.04) | 0.96 (0.89, 1.03) | 259 (208, 327) | 266 (197, 332) | 250 (191, 332) | 252 (192, 328) | 1.03*** (0.98, 1.07) | 0.97 (0.92, 1.01) | 0.97 (0.92, 1.02) |
| 25(OH)D, nmol/l | 50.7 (36.6, 71.7) | nm | nm | 47.4 (32.7, 67.1) | nm | nm | 0.93 (0.85, 1.02) | 57.6 (45.2, 73.5) | nm | nm | 56.2 (40.1, 79.9) | nm | nm | 0.97 (0.86, 1.10) |
Variables are logarithmically transformed and summarised as geometric mean (IQ range). Changes are presented as the geometric mean (95 % CI) of the 6-, 12- or 16-week-to-baseline ratios
FV fruit and vegetable, nm not measured
*P < 0.001; **P < 0.01; ***P < 0.05 (for comparison of change in micronutrient at timepoint between 2 and 5 portions/day); ****P < 0.05 (significantly different from 5 portions/day group at baseline)
aTotal carotenoids = sum of α-carotene + β-carotene + β-cryptoxanthin + lutein + zeaxanthin + lycopene
Baseline and changes in muscle strength, physical function and physical activity during the intervention
At baseline, hand grip strength was significantly higher in those allocated to the 5 portions/day group compared to the 2 portions/day group (P < 0.001; Table 5). As previously mentioned, further statistical analysis was carried out using change in grip strength to minimise the effect of any imbalances on the comparison between the intervention groups. In relation to individual measures of lower-extremity physical function, as assessed by standing balance, walk speed and the chair stand test, there were no significant differences evident between the two groups at baseline. Likewise, the SPPB score determined for lower-extremity physical function did not differ significantly between the two groups at baseline (Table 5).
Table 5.
Physical performance and muscle strength at baseline and during intervention according to FV allocation
| 2 portions/day (n max = 39) | 5 portions/day (n max = 41) | P valueb | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 16 | Change at 16 weeksa | Baseline | Week 16 | Change at 16 weeksa | ||
| Grip strength, kg | 24.2 ± 8.11* | 24.3 ± 8.55 | 0.11 ± 3.26 | 33.2 ± 10.3 | 35.2 ± 10.6 | 2.04 ± 5.16 | 0.06 |
| Balance, n | |||||||
| Score 1 | 0 | 0 | 2 | 0 | 0.38 | ||
| Score 2 | 1 | 3 | 1 | 1 | |||
| Score 3 | 3 | 3 | 2 | 4 | |||
| Score 4 | 35 | 33 | 36 | 36 | |||
| Walk speed, s | 3.62 ± 1.50 | 3.77 ± 1.61 | 0.15 ± 1.46 | 3.68 ± 2.10 | 3.63 ± 1.37 | −0.05 ± 1.48 | 0.54 |
| Chair stand, s | 11.5 ± 4.27 | 11.5 ± 4.69 | 0.00 ± 3.56 | 10.8 ± 4.57 | 10.2 ± 4.47 | −0.56 ± 4.01 | 0.51 |
| SPPB score | 9.05 ± 2.09 | 8.97 ± 1.87 | −0.08 ± 2.04 | 9.43 ± 2.30 | 9.49 ± 1.90 | 0.05 ± 1.63 | 0.76 |
Values are mean ± SD unless noted otherwise
SPPB score short physical performance battery score (= sum of standing balance score + walk speed score + chair stand score. SPPB score ranges from 3 to 12)
*P < 0.001 (significantly different from 5 portions/day group at baseline)
aChange calculated as week 16 − baseline
b P value represents difference in change from baseline to week 16 between groups, independent samples t test for continuous variables, chi-square test for categorical variables
The change in lower-extremity physical function (from both individual measures and SPPB score) over the course of the 16-week intervention did not differ significantly between the two groups. However, the difference in change in grip strength between the two intervention groups approached significance (P = 0.06) with those in the 5 portions/day group tending to show a larger change in grip strength over the course of the intervention compared to those in the 2 portions/day group (Table 5). Since we observed that grip strength differed between the intervention groups at baseline, reanalysis using a general linear model, with final grip strength as the dependent variable and initial grip strength and portion as predictors, confirmed the effect of FV on grip strength (P = 0.02). A further ANCOVA analysis also revealed no interaction between FV group and gender or between FV group and initial grip strength.
Change in physical activity was not directly measured over the course of the intervention; however, in the self-report physical activity questionnaire, eight participants (three participants in the 5 portions/day group and five participants in the 2 portions/day group) did report an increase in physical activity, while two participants (one in the 5 portions/day group and one in the 2 portions/day group) reported a decrease in physical activity over the 16-week intervention period. When the general linear model analysis was repeated with participants who reported a change in their physical activity level being excluded, the significant effect of FV on grip strength remained (P = 0.03).
Statistical analysis was initially carried out between the intervention groups to which participants were randomised, but was also repeated according to self-reported change in FV consumption. The results of this analysis showed that change in FV consumption was positively associated with the change in grip strength at 16 weeks (P = 0.02). An extra daily portion of FV was predicted to increase grip strength by 0.49 kg (95 % CI, 0.07, 0.90). The changes in measures of lower-extremity function (both individual measures and summary measure) were not associated with change in FV consumption (data not shown).
Association between change in muscle strength and physical function and change in nutrient status
A correlation analysis was carried out to examine the relationship between change in hand grip strength and lower-extremity physical function and change in micronutrient status. Correlations between change in micronutrient status and change in grip strength tended to be weak, with the exception of vitamin C whereby change in plasma concentration at week 16 was positively correlated with change in grip strength (r = 0.24, P = 0.04). A significant positive correlation was also evident between change in concentrations of zeaxanthin and β-cryptoxanthin at week 16 and change in standing balance (r = 0.35, P < 0.01 and r = 0.26, P = 0.02, respectively).
Discussion
This study showed that increased FV consumption improves grip strength in healthy older people. No other intervention studies have specifically examined the effect of FV consumption on grip strength. The findings therefore suggest that FV may enhance muscle strength in older adults. This finding may potentially have important implications, particularly since muscle strength is regarded as a key denominator in the cycle of frailty (Fried et al. 2001). There is limited scientific evidence regarding the effect of FV on muscle strength. A few observational studies have noted associations between vegetable intake and grip strength; however, these studies were cross-sectional (Robinson et al. 2008) or longitudinal in design (Lauretani et al. 2008). Another study also reported an association between urinary potassium excretion and lean body mass in older adults which led the authors to conclude that increased consumption of potassium-rich foods such as FV may help to conserve muscle mass (Dawson-Hughes et al. 2008).
We found no significant effect of increased FV consumption on measures of lower-extremity physical function. Other intervention studies (Demark-Wahnefried et al. 2006; Morey et al. 2009) have, however, suggested that a healthy diet, including increased FV intake, may enhance physical function, although these have largely focused on overall diet or lifestyle quality rather than intakes of specific food groups such as FV. Extrapolating results is also difficult since participants in these studies were overweight, long-term survivors of cancer (Morey et al. 2009) and older adults with a recent diagnosis of cancer (Demark-Wahnefried et al. 2006) hence results are not directly applicable to healthy, older adults. These studies also used self-report questionnaires as a means of assessing general physical function and ability to carry out activities of daily living, rather than using direct measures of physical function. Some observational studies have found that individuals with “healthier” diets or higher FV-containing diets were more likely to have better functional health (Duffy and MacDonald 1990; Stafford et al. 1998; Houston et al. 2005; Myint et al. 2007; Tomey et al. 2008). Again, these studies relied on self-report questionnaires to assess general functional limitations (Duffy and MacDonald 1990; Stafford et al. 1998; Houston et al. 2005; Myint et al. 2007; Tomey et al. 2008) rather than direct measures. In addition, many studies relied on a food frequency questionnaire to assess dietary intake (Houston et al. 2005; Myint et al. 2007; Tomey et al. 2008), which may have inadequately captured habitual FV intake.
In the current study, the increase in FV consumption as a result of the intervention resulted in concomitant changes in overall nutrient status. The change in vitamin C status was positively correlated with change in grip strength, thus supporting the positive effect of FV on grip strength. Cross-sectional results from the InCHIANTI study similarly showed positive correlations between plasma antioxidant concentrations and muscle strength (Cesari et al. 2004a). Evidence from other intervention studies regarding the effect of FV-related nutrients on physical function is limited, and the primary outcomes and participants of other studies were considerably different from the current study, with most focusing on the effect of antioxidants on exercise performance rather than physical function (Clarkson and Thompson 2000; Takanami et al. 2000).
This study is, to our knowledge, the first intervention study to specifically examine the effect of increased FV consumption on physical function and muscle strength in older adults. Previous studies examining dietary intake in relation to physical function and muscle strength have mostly been observational in design, and therefore, only associations have been reported, rather than causal effects. In addition, by selecting low FV consumers in the current study, the possibility of demonstrating an effect of increased FV consumption was maximised. A further strength of the current study is that physical function was assessed by direct measures rather than relying on self-report questionnaires. Hand grip strength, measured as an indicator of general muscle strength (Rantanen et al. 2003), has previously been associated with decline in functional capacity (Lauretani et al. 2003), disability (Rantanen et al. 1999) and mortality (Rantanen et al. 2003). Results from a study by Avlund (1994) also showed a strong positive correlation between grip strength and both total body strength and lower-extremity strength in adults aged 75 years and older. The SPPB, which was used to assess physical function, is regarded as a reliable and accurate indicator of physical function and muscle strength (Guralnik et al. 1995). It has also been shown to be associated with subsequent disability, nursing home admission and mortality (Guralnik et al. 1994a, 1995, 2000) and has been used as a means of ranking the physical function of community-dwelling individuals who had no evident disability (Guralnik et al. 1994b). Moreover, the SPPB was chosen since it is easy to administer in an individual’s home, especially if space is limited, and it generally has low respondent burden (Guralnik et al. 2000). It provides a summary measure of physical function by combining performance scores from three assessments of lower-extremity function, thus increasing the reliability and sensitivity of the measure (Ostir et al. 2002). Measuring both lower-extremity physical function and hand grip strength, as a measure of upper-extremity muscle strength, enabled us to evaluate overall physical function. Furthermore, unlike other studies which were conducted in middle-aged adults (Stafford et al. 1998; Tomey et al. 2008), older homebound adults (Sharkey et al. 2003) or those with degrees of disability (Semba et al. 2007; Alipanah et al. 2009), the current study recruited healthy, free-living older men and women, thus making our findings more applicable to the older general population.
As stated previously (Gibson et al 2012), overall compliance with the intervention was good, as reflected by the increase in self-reported FV consumption in the intervention group, as well as the increase in biomarkers of nutrient status associated with FV consumption. This confirms that the differing dietary instructions provided to all participants according to their FV allocation were effective. In terms of the FV commonly selected by participants, we observed that bananas, apples, oranges, grapes and pears were the most commonly selected fruits, while carrots, cabbage, broccoli, peas, tomatoes and Brussels sprouts were the most commonly chosen vegetables.
The study limitations also warrant comment. Firstly, hand grip strength differed between the two groups at baseline, with measurements being significantly higher in those allocated to the 5 portions/day group. This most likely reflects the fact that more men were allocated to the 5 portions/day group (51 %) compared to the 2 portions/day group (18 %). However, none of the findings in the study were altered by this gender imbalance or difference in initial grip strength. The short duration of the intervention may have also limited our findings. This was an adequate length of time for observing changes in the primary outcome measures, i.e. markers of immune function and nutrient status, but it may have been too short a duration to detect an appreciable change in lower extremity physical function. It also remains plausible that the 5 portions/day may not have been adequate to have a significant impact on physical function. However, a key aim of the current study was to keep the intervention in line with current government guidelines which recommend the consumption of 5 portions of FV/day. A further limitation relates to the physical performance measurements. The majority of participants performed well on the physical function tests which resulted in SPPB scores falling within a narrow range. This would suggest that the SPPB may have not been sensitive enough to detect differences in physical function among healthy individuals. Finally, the assessment of dietary intake using the diet history method may have not accurately captured habitual dietary intake, particularly as it relies on memory recall, which for some older adults can pose a challenge. However, a recent systematic review which examined dietary assessment methods for micronutrient intake in elderly people reported greater correlations with most micronutrients when a diet history was used to assess dietary intake, compared to a food frequency questionnaire (Ortiz-Andrellucchi et al. 2009).
In conclusion, the current findings suggest a positive effect of FV consumption on grip strength. Although increasing FV consumption to 5 portions/day did not significantly modulate lower-extremity physical function in this cohort of healthy older adults, it is possible that FV may play an important role which was simply not detectable in the current study due to the short duration of the intervention, or due to the sensitivity of the tool used to measure physical function. The limitations presented thus highlight the need for further randomised controlled intervention studies in older adults which are appropriately powered and robustly designed, with an adequate duration.
Acknowledgments
The authors wish to acknowledge Prof. Chris Patterson and Dr. Chris Cardwell, Queen’s University Belfast, for providing statistical advice, Mr. Cyril McMaster for carrying out the ELISA measurements and Dr. Caroline Mercer for undertaking the serum lipid and CRP analysis. J.V.W. was the principal investigator, and I.S.Y. and D.E. were co-investigators and were responsible for the project conception, development of overall research plan and study oversight. C.E.N. was primarily responsible for writing the manuscript and for managing the day-to-day running of the study including participant recruitment and study execution (including dietary assessment, data collection, dietary analysis, blood and urine sample collection and processing, endpoint assessment of physical function and muscle strength, data input and statistical analysis); S.E.C.M. and A.G. assisted with participant recruitment and study execution; S.E.C.M. provided technical assistance with laboratory extractions and analysis and was responsible for vitamin C analysis. C.E.N., the corresponding author, had the primary responsibility for the final content. All authors contributed to the drafts, revisions and proof reading of the final manuscript. The work was undertaken in the Nutrition & Metabolism Research Group, Centre for Public Health, Queen’s University Belfast (QUB) and also in collaboration with Ria Banerjee, Unilever R&D, UK who provided training in physical performance methods and provided the final physical function and accelerometer data. The study was funded by the UK Food Standards Agency and Unilever, UK and was registered at www.clinicaltrials.gov, registration number NCT00858728.
References
- Abizanda P, Navarro JL, Garcia-Tomas MI, Lopez-Jimenez E, Martinez-Sanchez E, Paterna G. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community dwelling older persons. Arch Gerontol Geriatr. 2012;54:21–27. doi: 10.1016/j.archger.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Alipanah N, Varadhan R, Sun K, Ferrucci L, Fried LP, Semba RD. Low serum carotenoids are associated with a decline in walking speed in older women. J Nutr Health Aging. 2009;13:170–175. doi: 10.1007/s12603-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y, Shephard RJ. Aging and muscle function. Sports Med. 1992;14:376–396. doi: 10.2165/00007256-199214060-00005. [DOI] [PubMed] [Google Scholar]
- Appleton KM, McGill R, Woodside JV. Fruit and vegetable consumption in older individuals in Northern Ireland: levels and patterns. Br J Nutr. 2009;102:949–953. doi: 10.1017/S0007114509332122. [DOI] [PubMed] [Google Scholar]
- Arnold CM, Warkentin KD, Chilibeck PD, Magnus CR. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J Strength Cond Res. 2010;24:815–824. doi: 10.1519/JSC.0b013e3181aa36b8. [DOI] [PubMed] [Google Scholar]
- Avlund K. Maximal isometric muscle strength and functional ability in daily activities among 75-year-old men and women. Scand J Med Sci Sports. 1994;4:32–40. doi: 10.1111/j.1600-0838.1994.tb00403.x. [DOI] [Google Scholar]
- Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, Atkinson H, Martin A, Guralnik JM, Ferrucci L. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;59:242–248. [Google Scholar]
- Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72(Suppl):637S–646S. doi: 10.1093/ajcn/72.2.637S. [DOI] [PubMed] [Google Scholar]
- Craft NE. Carotenoid reversed-phase high-performance liquid chromatography methods: reference compendium. Methods Enzymol. 1992;213:185–205. doi: 10.1016/0076-6879(92)13121-D. [DOI] [PubMed] [Google Scholar]
- Dargent-Molina P, Favier F, Grandjean H, Baudoin C, Schott AM, Hausherr E, Meunier PJ, Breart G. Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/S0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Ceglia L. Alkaline diets favour lean tissue mass in older adults. Am J Clin Nutr. 2008;87:662–665. doi: 10.1093/ajcn/87.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, Cohen HJ. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy ME, MacDonald E. Determinants of functional health of older persons. Gerontologist. 1990;30:503–509. doi: 10.1093/geront/30.4.503. [DOI] [PubMed] [Google Scholar]
- Finch S, Doyle W, Lowe C, Bates CJ, Prentice A, Smithers G, Clarke PC. National diet and nutrition survey: people aged 65 years and over. London: TSO; 1998. [Google Scholar]
- Food portion sizes. 3. London: TSO; 2006. [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gibson A, Edgar JD, Neville CE, Gilchrist SECM, McKinley MC, Patterson CC, Young IS, Woodside JV. Effect of fruit and vegetable consumption on immune function in older people: a randomised controlled trial. Am J Clin Nutr. 2012;96:1429–1436. doi: 10.3945/ajcn.112.039057. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging. 1994;6:410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- Houston DK, Stevens J, Cai J, Haines PS. Dairy, fruit, and vegetable intakes and functional limitations and disability in a biracial cohort: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2005;81:515–522. doi: 10.1093/ajcn.81.2.515. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartalli B, Cavazzini C, Di lorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63:376–383. doi: 10.1093/gerona/63.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Taylor M, Bedford C, Ebrahim S. Is housework good for health? Levels of physical activity and factors associated with activity in elderly women. Results from the British Women’s Heart and Health Study. J Epidemiol Community Health. 2002;56:473–478. doi: 10.1136/jech.56.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. doi: 10.1016/S0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Meydani M. The Boyd Orr lecture. Nutrition interventions in aging and age-associated disease. Proc Nutr Soc. 2002;61:165–171. doi: 10.1079/PNS2002144. [DOI] [PubMed] [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AH, Young Y, Langlois JA. Prevention of falls in the elderly. Bone. 1996;18(Suppl):87S–101S. doi: 10.1016/8756-3282(95)00384-3. [DOI] [PubMed] [Google Scholar]
- Myint PK, Welch AA, Bingham SA, Surtees PG, Wainwright NW, Luben RN, Wareham NJ, Smith RD, Harvey IM, Day NE, et al. Fruit and vegetable consumption and self-reported functional health in men and women in the European Prospective Investigation into Cancer-Norfolk (EPIC-Norfolk): a population-based cross-sectional study. Public Health Nutr. 2007;10:34–41. doi: 10.1017/S1368980007222608. [DOI] [PubMed] [Google Scholar]
- Ortiz-Andrellucchi A, Sanchez-Villegas A, Doreste-Alonson J, de Vries J, de Groot L, Serra-Majem L. Dietary assessment methods for micronutrient intake in elderly people: a systematic review. Br J Nutr. 2009;102(Suppl 1):S118–49. doi: 10.1017/S0007114509993175. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM, Women’s Health and Aging Study Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/S0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/S0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Varadhan R, Bartali B, Ferrucci L, Ricks MO, Blaum C, Fried LP. Low serum carotenoids and development of severe walking disability among older women living in the community: the Women’s Health and Aging Study I. Age Ageing. 2007;36:62–67. doi: 10.1093/ageing/afl122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee G, Weatherall R. Physical activity and ischaemic heart disease in middle-aged British men. Br Heart J. 1991;66:384–394. doi: 10.1136/hrt.66.5.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey JR, Giuliani C, Haines PS, Branch LG, Busby-Whitehead J, Zohoori N. Summary measures of dietary musculoskeletal nutrient (calcium, vitamin D, magnesium, and phosphorus) intakes is associated with lower-extremity physical performance in homebound elderly men and women. Am J Clin Nutr. 2003;77:847–856. doi: 10.1093/ajcn/77.4.847. [DOI] [PubMed] [Google Scholar]
- Stafford M, Hemingway H, Stansfeld SA, Brunner E, Marmot M. Behavioural and biological correlates of physical functioning in middle aged office workers: the UK Whitehall II study. J Epidemiol Community Health. 1998;52:353–358. doi: 10.1136/jech.52.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark T, Walker B, Phillips JK, Fejer R, Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3:472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Takanami Y, Iwane H, Kawai Y, Shimomitsu T. Vitamin E supplementation and endurance exercise: are there benefits? Sports Med. 2000;29:73–83. doi: 10.2165/00007256-200029020-00001. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Heimendinger J, Diker A, O’Neill C, Haegele A, Meinecke B, Wolfe P, Sedlacek S, Zhu Z, Jiang W. Dietary botanical diversity affects the reduction of oxidative biomarkers in women due to high vegetable and fruit intake. J Nutr. 2006;136:2207–2212. doi: 10.1093/jn/136.8.2207. [DOI] [PubMed] [Google Scholar]
- Tomey KM, Sowers MR, Crandall C, Johnston J, Jannausch M, Yosef M. Dietary intake related to prevalent functional limitations in midlife women. Am J Epidemiol. 2008;167:935–943. doi: 10.1093/aje/kwm397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staveren WA, de Boer JO, Burema J. Validity and reproducibility of a dietary history method estimating the usual food intake during one month. Am J Clin Nutr. 1985;42:554–559. doi: 10.1093/ajcn/42.3.554. [DOI] [PubMed] [Google Scholar]
- Vuilleumier JP, Keck E. Fluorometric assay of vitamin C in biological materials using a centrifugal analyser with fluorescence attachment. J Micronutr Anal. 1989;5:25–34. [Google Scholar]
- Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56(Suppl):S65–S80. doi: 10.1093/gerona/56.suppl_2.65. [DOI] [PubMed] [Google Scholar]