Abstract
It has been well established that working memory abilities decrease with advancing age; however, the specific time point in the adult life span at which this deficit begins and the rate at which it advances are still controversial. There is no agreement on whether working memory declines equally for visuospatial and verbal information, and the literature disagrees on how task difficulty may influence this decay. We addressed these questions in a lifespan sample of 1,500 participants between 21 and 80 years old. The n-back task was used, with letters and circles presented at different positions around an imaginary circle, to evaluate working memory in the verbal and visuospatial domains, respectively. The participants’ task was to judge whether the current stimulus matched a stimulus that was shown n trials prior. Both domains were evaluated in two levels of difficulty: 1-back and 2-back. The comparison across decades showed that discrimination in the visuospatial and 1-back tasks started to decline earlier in women than in men; however, discrimination was equal between the sexes in the verbal and 2-back tasks. Performance on tasks in the visuospatial domain exhibited more pronounced decline than in those in the verbal domain. The rate of decline in working memory accuracy was superior in 2-back tasks than in 1-back tasks, independent of the domain. These results revealed that the effects of aging on working memory are less dependent on the type of information and more reliant on the resources demanded by the task.
Key words: Aging, Verbal working memory, Visuospatial working memory, Domain, Difficulty, Sex differences
Introduction
Working memory is defined as the ability to temporarily maintain information to actively process it in order to achieve specific goals. Complex cognitive tasks, such as language comprehension, reasoning or arithmetic calculations, depend on this memory system, and thus, its integrity is essential for everyday living. Numerous studies comparing the extremes of age groups have reported that working memory performance in older adults significantly differs from that in young adults (for a meta-analysis, see Bopp and Verhaeghen 2005). However, few studies have attempted to establish when working memory begins to decline during adulthood.
Dobbs and Rule (1989) addressed this question in a lifespan sample of 228 adults between 30 and 99 years of age and found that working memory significantly declined after 60 years of age. In this study, working memory was measured by a task that required the determination of the specific digit located in lag 0, 1 or 2 from a list of random digits read to the participant. Conversely, Park et al. (2002) observed that this type of memory declined uniformly after 20 years of age, utilizing a lifespan sample of 345 adults between 20 and 92 years of age. In this study, working memory was measured through four span tasks that simultaneously combined processing and storing demands. For example, one of the visuospatial span tasks required participants to draw in a sheet grid the position and orientation of lines that were presented during sets of increasing numbers of trials in which participants had to judge whether three irregular shapes were equal. Although age-related effects on working memory have been described (Borella et al. 2008; Hale et al. 2011; Salthouse 1992, 1995; Salthouse and Babcock 1991), the specific moment when this type of working memory began to decline has not been reported.
The markedly different ages at which working memory began to decline in these previous studies may be explained by the divergent methods used to measure this type of memory. In fact, the different procedures used to measure working memory have not been proven to examine the same components from this system. The span tasks used by Park et al. (2002) evaluated the two main functions outlined by Baddeley and Hitch (1974) to describe the working memory system, storing and processing; however, the lag task used by Dobbs and Rules (1989) included storage, binding each item to a specific temporal order, and retrieval, but processing was not required. The lag and span tasks may also be conceived as simple and complex span tasks, respectively. The simple span tasks measure mainly storage, whereas the complex span tasks evaluate cognitive control functions that coordinate storage and processing simultaneously (Hale et al 2011; Unsworth et al 2009). Although both tasks entail interference control, the span tasks most likely challenged this mechanism significantly more than the lag task, as the processing requirements were unrelated to the storage demands. The span tasks mimic the realistic everyday situations in which information must be kept in mind while undertaking other unrelated tasks. Therefore, the span tasks may not be conceived as pure working memory tasks but as double tasks that challenge our ability to successfully divide our attention. Indeed, for this reason, this task has been extensively used to investigate interference control mechanisms, such as inhibition (e.g., Robert et al. 2009).
The existing empirical evidence is still insufficient to determine when and how working memory declines across adulthood. Therefore, the aim of the present study was to determine the specific time point in the adult lifespan when visuospatial and verbal working memory begin to decline and to establish the rate at which this descent occurs. To accomplish these goals, we examined working memory by means of the widely used n-back task. This procedure, introduced by Kirchner (1958), requires participants to judge whether the current item is equal or not equal to the one presented n trials before. The task involves storage, binding each item to its temporal order, retrieval, updating the item and its temporal order, monitoring and interference control from items that do not correspond to the specific lag under evaluation. Thus, this task examines several of the characteristics that have been used to describe the working memory system. Moreover, updating tasks, such as the n-back procedure, are moderately (r = 0.55) (Shamosh et al. 2008) to highly correlated (r = 0.96) (Schmiedek et al. 2009) with span tasks, and both types of tasks successfully predict reasoning abilities (Schmiedek et al. 2009). Nevertheless, see Kane et al. (2007) for contradicting results. Additionally, the construct validity of updating working memory tasks demonstrated that this type of procedure is a reliable indicator of working memory (Oberauer et al. 2000). Furthermore, according to Schmiedek et al. (2009), the complex span and updating tasks measure working memory with equal efficiency and use the same working memory mechanisms of building, maintaining and updating bindings.
However, a shortcoming of the n-back task is that it is highly vulnerable to lures, i.e., items that are identical to the target item but that do not mach the lag under evaluation. Lures at a lag smaller than the one under evaluation produced higher intrusion costs in reaction times in older adults compared to young adults (Oberauer 2005). Because several studies have reported that interference control mechanisms are less efficient in older adults (e.g., Cansino et al. 2011; De Beni and Palladino 2004), we created sequences of stimuli for the n-back task without lures. Therefore, working memory was measured without the need for additional interference control mechanisms that could overestimate the working memory decline across adulthood, as these mechanisms are more challenging for older adults.
A second aim of the present study was to examine whether both verbal and visuospatial working memory decline equally across the adult lifespan. Although some authors (Park et al. 2002; Borella et al. 2008) observed that the working memory for both types of material decreased equally across the adult lifespan, results have been controversial in studies that have compared age groups at both extremes. Age-related effects were observed to be greater for visuospatial than for verbal working memory in some studies (e.g., Bopp and Verhaeghen 2007; Fiore et al. 2012; Myerson et al. 1999), but the opposite was found in another study (Vecchi et al. 2005). These contradictory results may be due to the difficulty in equating the tasks for both domains, especially when diverse processing demands are also required, such as in span tasks. According to the model by Baddeley and Hitch (1974), the visuospatial sketch and phonological loop slave systems are responsible for the storage and rehearsal of the information from their specific domain, but they are not responsible for the processing or manipulation of this information, which directly depends on the central executive. We attempted to measure visuospatial and verbal working memory under equivalent conditions to determine whether working memory declines equally for both slave systems. An equal decline in working memory for both systems may be evidence that the domain-general central executive functions are diminished across the adult lifespan, independent of the specific domain that is under evaluation. However, an alternative explanation could be that an equal decline in the two domain-specific stores occurs that is independent of the central executive decline or a decline in only one domain-general store with age. By contrast, if working memory deteriorates unevenly for visuospatial and verbal information, the effects of aging on working memory will prove to be domain-specific.
The third purpose of the study was to examine the effects of task difficulty on working memory performance across the adult lifespan. The only study (Dobbs and Rule 1989) that has evaluated this issue in a lifespan sample found that working memory declined as the lag of the reported digit increased from 0 to 2, but as previously mentioned, significant differences across age groups were found after 60 years of age. The comparison of age groups at both extremes have provided substantial evidence that supports the fact that older adults are less efficient in performing working memory tasks than young adults when memory load increases (e.g., Lustig et al. 2001; De Beni and Palladino 2004). The n-back task is particularly suitable for evaluating the effects of task difficulty on working memory because all of the processes involved in the task increase uniformly as the lag increases. In the present study, we examined verbal and visuospatial working memory at two levels of difficulty: 1-back and 2-back. By examining working memory at two levels of difficulty, we could investigate whether performance on more difficult tasks decreases as a function of age at a faster rate than performance on less difficult tasks.
These two levels were chosen because some authors (Daffner et al. 2011; Missonnier et al. 2004) have found an equivalent performance in young and older adults with the 1-back task but others have observed differences in accuracy but not d-prime values (Schulze et al. 2011); additionally, age differences in the 2-back task have been consistent (e.g., Daffner et al. 2011). Clarification of whether there is actually a decrease in working memory performance when the task demand is low is essential, as some authors, by means of functional magnetic resonance imaging (fMRI) (Mattay et al. 2006; Schulze et al. 2011), have reported a larger activation in the prefrontal cortex of older adults relative to young adults when performing the 1-back task, despite equivalent behavioral performance. These results have suggested that older adults make use of compensatory mechanisms to maintain their working memory performance at the same level as young adults. However, the comparison of relatively small extreme age groups may not provide data sensitive enough to clearly detect whether aging produces an effect in less-demanding working memory tasks. By testing a large lifespan sample, we were able to reliably determine the slope of working memory decline for both low- and high-demand tasks.
The final question we addressed is whether the efficiency of working memory differs between the sexes across the adult lifespan. To date, there is no agreement among studies. Exclusively considering data obtained with n-back tasks, Li et al. (2010) found no accuracy or speed differences between genders in a verbal version of the n-back task using letters as stimuli. However, a neurophysiological difference was measured by functional near infra-red spectroscopy (fNIRS): women displayed less diffuse activation at the prefrontal cortex than men. This finding was interpreted as evidence that women have a more efficient hemodynamic response. Also in the verbal domain (using letters and digits as stimuli), Speck et al. (2000) observed with fMRI that men had a bilateral or right-lateralized activation at the prefrontal and parietal cortices, whereas this activation was left-lateralized in women. Contrary to the results of the study by Li et al., women outperformed men in both tasks in this study, even in the more difficult version (2-back). In contrast, when testing with letters (Schmidt et al. 2009), words and faces (Haut and Barch 2006), neither behavioral nor functional activation (with fMRI) differences were observed between the sexes. In the visuospatial domain, one study (Lejbak et al. 2011) observed that men outperformed women in the n-back task. This finding agrees with several studies that have reported that men are superior to women in diverse visuospatial tasks (for meta-analytic studies see Masters and Sanders 1993; Voyer et al. 1995). Sex differences in these studies were only examined in young adults; thus, the possibility of a steadier pattern of working memory across adulthood remains unexplored with the n-back task.
The effects of aging, sex, domain and difficulty on working memory efficiency were separately described above because, to date, no study has examined all of these aspects together across the adult lifetime. However, in the present study, all of these factors were simultaneously examined to elucidate how they affect working memory performance across the healthy adult lifespan, both independently and in interaction. Moreover, we examined the effects of these factors on reaction time responses, a variable that has not been previously assessed for working memory across adulthood.
Methods
Participants
A sample of 1,500 individuals between 21 and 80 years of age participated in the study. From each decade included in the age range, 250 individuals participated (half of them were women). Participants were recruited over a period of 6 and a half years through appeals to community groups, advertisements, flyers and word of mouth. All participants signed an informed consent and received a financial payment as compensation for their participation in the study. The study was approved by the Bioethics Committee of the School of Medicine at the National Autonomous University of Mexico. For inclusion in the study, participants must not have been addicted to drugs or alcohol; not have taken any medication that alters the nervous system for the previous 6 months; not have neurological or psychiatric diseases; not have suffered head trauma; have at least 8 years of education; have normal or corrected-to-normal vision; and have obtained a score ≤20 on the Beck Depression Inventory (BDI) (Beck 1987), a score ≥24 on the Mini-Mental State Exam (MMSE) (Folstein et al. 1975), and a score ≥ 26 on the vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised (WAIS-R) (Wechsler 1981). These psychological tests were administered to ensure that participants were not suffering from depression, dementia, or intellectual difficulties, respectively. Table 1 shows participants’ characteristics and the scores that they obtained in the tests.
Table 1.
Subjects’ characteristics and performance on neuropsychological tests
| Decade | Age (years) | Education (years) | Vocabulary Scale (WAIS-R) | Mini-Mental State | Beck’s Depression Inventory | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| 21–30 | 23.68 (2.40) | 24.25 (2.40) | 15.85 (1.65) | 16.04 (1.96) | 12.89 (1.47) | 13.14 (1.38) | 29.16 (1.04) | 29.20 (1.06) | 6.17 (4.97) | 6.10 (5.05) |
| 31–40 | 35.74 (3.05) | 34.74 (2.81) | 15.44 (3.35) | 16.54 (3.55) | 12.50 (1.53) | 12.66 (1.77) | 28.98 (1.16) | 28.78 (1.13) | 6.86 (5.39) | 5.07 (4.49) |
| 41–50 | 46.33 (2.90) | 46.30 (3.09) | 14.13 (4.07) | 15.79 (4.34) | 12.62 (1.75) | 12.70 (1.77) | 28.70 (1.36) | 28.70 (1.16) | 7.14 (5.16) | 6.02 (4.82) |
| 51–60 | 55.52 (2.87) | 54.77 (2.90) | 13.72 (4.57) | 15.11 (4.58) | 12.69 (1.63) | 12.68 (1.62) | 28.38 (1.49) | 28.58 (1.23) | 6.50 (4.82) | 5.30 (4.74) |
| 61–70 | 65.39 (2.88) | 65.50 (2.77) | 12.57 (4.06) | 14.81 (5.04) | 12.50 (1.81) | 13.02 (1.95) | 28.34 (1.40) | 28.33 (1.34) | 7.82 (5.55) | 5.38 (4.45) |
| 71–80 | 74.72 (2.64) | 74.89 (2.58) | 12.37 (4.77) | 13.39 (4.40) | 13.02 (1.82) | 12.69 (1.63) | 28.27 (1.41) | 27.74 (1.60) | 7.95 (5.05) | 7.79 (4.96) |
Means and standard deviations are indicated in parenthesis. In each of the six decades, 125 women and 125 men participated
Table 2 displays the results of the analyses of variance (ANOVA) that were conducted to examine the participants’ education level and performance on the neuropsychological tests. The ANOVAs included the factors of decade (six total) and sex. The factors of decade and sex were significant for each participant’s years of education. Men (mean ± standard error [SE] = 15.28 ± 0.15) had slightly more years of education than women (14.01 ± 0.15). Additionally, individuals from the second decade (21–30) of life had more years of education (15.94 ± 0.25) than those from the fifth (51–60) (14.42 ± 0.25), sixth (61–70) (13.69 ± 0.25) and seventh (71–80) (12.88 ± 0.25) decades, according to Tukey post hoc tests. Individuals from the third decade (31–40) (15.99 ± 0.25) had more years of education than those from the fourth decade (41–50) (14.96 ± 0.25) and from the subsequent decades.
Table 2.
ANOVA analyses of the participants’ education level and their performance on the neuropsychological tests
| Factors | F | df | P | η p 2 | |
|---|---|---|---|---|---|
| Education | Decade | 24.08 | 5,1488 | <0.001 | 0.08 |
| Sex | 37.81 | 1,1488 | <0.001 | 0.03 | |
| Decade and sex | 1.85 | 5,1488 | 0.10 | 0.01 | |
| Vocabulary Scale (WAIS-R) | Decade | 2.17 | 5,1488 | 0.06 | 0.01 |
| Sex | 1.50 | 1,1488 | 0.22 | <0.01 | |
| Decade and sex | 1.72 | 5,1488 | 0.13 | 0.01 | |
| Mini-Mental State | Decade | 25.93 | 5,1488 | <0.001 | 0.08 |
| Sex | 1.55 | 1,1488 | 0.21 | <0.01 | |
| Decade and sex | 2.41 | 5,1488 | 0.04 | 0.01 | |
| Beck’s Depression Inventory | Decade | 5.43 | 5,1488 | <0.001 | 0.02 |
| Sex | 19.47 | 1,1488 | <0.001 | 0.01 | |
| Decade and sex | 2.15 | 5,1488 | 0.06 | 0.01 |
The analysis on the normalized scores of the vocabulary test (WAIS-R) was not significant. The factor of decade and the interaction between decade and sex turned out to be significant for the analysis conducted on the MMSE scores. According to pairwise comparisons, sex differences were observed only in the last decade, in which women performed higher than men (p = 0.001) (Table 1). In addition, women in their second decade performed better than those ≥41 years of age (p ≤ 0.005), women in their third decade outperformed those ≥51 years of age (p < 0.001), and women in their fourth decade outperformed those ≥61 years of age (p < 0.03). Men in their second decade had higher scores that those from all other decades (p ≤ 0.01), men in their third (p ≤ 0.005) and fourth (p < 0.02) decades outperformed those ≥61 years of age, and men in their fifth (p < 0.001) and sixth (p < 0.001) decades outperformed those in the last decade. The ANOVA conducted on BDI scores was significant for the factors of decade and sex. Women (7.08 ± 0.18) had higher scores than men (5.95 ± 0.18), and Tukey tests revealed that individuals in the last decade (7.87 ± 0.31) had higher scores than those in their second (6.14 ± 0.31), third (5.97 ± 0.31), fourth (6.58 ± 0.31), fifth (5.90 ± 0.31) and sixth (6.60 ± 0.31) decades.
Stimuli
For the verbal version of the n-back task, we used 12 uppercase letters (B, F, G, K, L, N, P, Q, R, S, T and X) with a vertical visual angle of 1.5° and a horizontal visual angle of approximately 1°. The letters were presented at the center of a white screen in a dark gray color to maintain a low contrast. Conversely, for the visuospatial n-back task, a dark gray circle with a diameter visual angle of 1.5° was used. The circle was displayed in one of 12 possible positions around the center of the screen, as in an imaginary clock. The distance between the circle and the center of the screen was 4°. A black cross (vertical and horizontal visual angles of 0.5°) was continuously displayed at the center of the screen, which was white in color. The letters and positions for the verbal and visuospatial tasks, respectively, were selected randomly and with the same probability.
Procedure
Each participant attended two sessions. Prior to being invited to attend the first session, prescreening questions were asked to the potential participants to elucidate whether they fulfilled the inclusion criteria. The first session was conducted in a silent room in which only the participant and experimenter were present. In this session, the participants were interviewed to further determine if they satisfied the inclusion criteria. Then, participants performed the psychological tests, and their vision was tested. Participants that were suitable for the study were requested to provide their informed consent. Afterward, the participants were further interviewed about their health and other aspects of their lifestyle.
The second session took place in a sound-dampened chamber, approximately 1 week after the first session. In this session, participants performed the working memory tasks and a source memory task (data published by Cansino et al. 2012). Participants performed the tasks while seated in a high-back armchair located 100 cm away from the monitor screen. Participants responded by utilizing two keys that were set horizontally on a response panel, which was located on a left or right platform placed on the arm of the chair at a comfortable distance, according to the participants’ handedness. Participants performed the verbal and visuospatial n-back tasks in counterbalanced order, and within each domain, the two levels of difficulty (1-back and 2-back) were also counterbalanced across participants. Prior to performing each of the four n-back tasks, participants carried out brief versions of each task as training. The tasks were conducted with the software E-Prime v1.0 from Psychological Software Tools (Pittsburgh, PA, USA).
Working memory paradigm
Each trial from both the verbal and visuospatial n-back tasks started with the presentation of the stimulus (letter or circle) for 300 ms, followed by a period of 2,700 ms. After this time, the next stimulus was displayed. Participants were allowed to provide their response during the 3,000-ms period following the onset of the stimulus. In the verbal 1-back task, participants were requested to indicate whether the current letter was equal or not equal to the one presented in the last trial; in the 2-back task, participants were instructed to judge if the present letter was equal or not equal to the one displayed two trials prior. In the visuospatial version, participants were required to indicate whether the current circle was presented in the same position as the one displayed in the last trial (1-back) or two trials before (2-back). Participants performed 72 trials from each of the four tasks, from which 33 % of the trials were target (letters or positions equal to the one of the current trial, according to the level of difficulty).
Data analysis
Data from the first trial and from the two first trials of the 1-back and 2-back tasks, respectively, were excluded from the analysis because a target did not occur in these trials. The ability of each participant to separate a signal from noise was determined for each task using d-prime values. The d-prime values were selected for analyses because they provide an accurate estimation of the participants’ discrimination level, independent of the criterion for completing the task. In contrast, correct response analysis may overestimate working memory performance because the number of correct responses does not discriminate between misses and false alarms, and thus, a proportion of these responses may be attributable to lucky guesses. The mean reaction times for correct responses in each working memory task were logarithmically transformed to test for age differences under proportional measurements (Oberauer 2001). Reaction times and d-prime values were examined in two independent mixed-model analyses of covariance (ANCOVAs) with decade (six total) and sex as between factors, and domain (verbal and visuospatial) and difficulty (1-back and 2-back) as within factors, using years of education as a covariant. The data were stratified in decades for these analyses to establish the exact decade in adulthood in which each of the dependent variables begins to decline. Pairwise comparisons were adjusted for multiple comparisons with a Sidak test, and significant interactions were followed up by Tukey tests. Multiple regression analyses were also conducted on the d-prime values and reaction times from each n-back task to statistically control for the significant differences observed in the background variables across decades and between the sexes and to estimate their effects on each of the dependent variables. Because the MMSE and BDI scores correspond to ordinal measurements, they were first quantified to create interval scale scores using optimal scaling techniques (Meulman 2000). Hierarchical multiple regression analyses were computed for each variable: in the first step, years of education, MMSE-quantified scores and BDI-quantified scores were included, followed by a second step with sex and a third step with years of age. The scores from the vocabulary subtest were not included in these analyses because this background variable was not significantly different across decades or sexes. In addition, the data from each dependent variable were modeled as a function of age using linear, quadratic, and cubic regressions. The best model among the three was determined by Akaike's information criterion (AIC) (Akaike 1974). Although the analyses were conducted on transformed variables, the reaction times are also presented in milliseconds for the sake of clarity. The significance level was set at p < 0.05.
Results
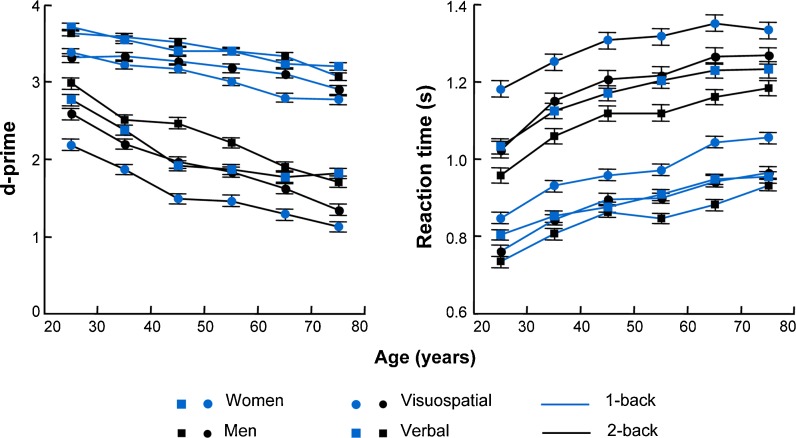
The results of the ANCOVA performed on d-prime values are presented in Table 3. The analysis on these values revealed that the two-way interactions between domain and sex, difficulty and decade, difficulty and sex, and domain and difficulty were significant. Additionally, the three interactions between domain, decade and sex, and between difficulty, decade, and sex turned out to be significant after controlling for years of education. Post hoc Tukey tests revealed that, for both sexes, discrimination was superior in the verbal (expressed as adjusted means, women: 2.75 ± 0.02; men: 2.86 ± 0.02) relative to the visuospatial (women: 2.31 ± 0.02; men: 2.55 ± 0.02) domain. In addition, in all decades, performance was superior in the 1-back tests compared to the 2-back tasks (Fig. 1). In the 1-back tasks, individuals in their second decade outperformed those ≥51 years of age, those in their third decade were superior to those ≥61 years of age, and those in their fourth decade were superior to those in their seventh decade. In the 2-back tasks, d-prime values differed significantly among the three first decades, between individuals from the fourth decade and those ≥61 years of age, and between those from the fifth and seventh decades. Post hoc analyses also revealed that men (2.11 ± 0.02) outperformed women (1.83 ± 0.02) in 2-back tasks and that both sexes were superior in 1-back tasks (women: 3.24 ± 0.02; men: 3.30 ± 0.02) relative to 2-back tasks. Furthermore, discrimination was superior in tasks from the verbal domain (1-back: 3.42 ± 0.01; 2-back: 2.19 ± 0.02) compared to that in tasks from the visuospatial domain (1-back: 3.12 ± 0.02; 2-back: 1.75 ± 0.02). Lastly, in both domains, d-prime values were superior in the 1-back tasks compared to the 2-back tasks.
Table 3.
ANCOVA results for discrimination and log-transformed reaction times, with years of education as a covariant
| F | df | P | η p 2 | |
|---|---|---|---|---|
| d-prime | ||||
| Decade | 93.91 | 5,1487 | <0.001 | 0.24 |
| Sex | 49.41 | 1,1487 | <0.001 | 0.03 |
| Domain | 42.00 | 1,1487 | <0.001 | 0.03 |
| Domain × decade | 0.69 | 5,1487 | 0.63 | <0.01 |
| Domain × sex | 18.81 | 1,1487 | <0.001 | 0.01 |
| Domain × decade × sex | 2.40 | 5,1487 | 0.04 | 0.01 |
| Difficulty | 615.13 | 1,1487 | <0.001 | 0.29 |
| Difficulty × decade | 32.63 | 5,1487 | <0.001 | 0.10 |
| Difficulty × sex | 42.80 | 1,1487 | <0.001 | 0.03 |
| Difficulty × decade × sex | 4.14 | 5,1487 | 0.001 | 0.01 |
| Domain × difficulty | 11.45 | 1,1487 | 0.001 | 0.01 |
| Domain × difficulty × decade | 1.25 | 5,1487 | 0.28 | <0.01 |
| Domain × difficulty × sex | 0.16 | 1,1487 | 0.69 | <0.01 |
| Domain × difficulty × decade × sex | 0.91 | 5,1487 | 0.48 | <0.01 |
| Reaction times | ||||
| Decade | 50.52 | 5,1487 | <0.001 | 0.15 |
| Sex | 80.42 | 1,1487 | <0.001 | 0.05 |
| Domain | 42.85 | 1,1487 | <0.001 | 0.03 |
| Domain × decade | 0.60 | 5,1487 | 0.70 | <0.01 |
| Domain × sex | 22.21 | 1,1487 | <0.001 | 0.02 |
| Domain × decade × sex | 0.77 | 5,1487 | 0.57 | <0.01 |
| Difficulty | 293.52 | 1,1487 | <0.001 | 0.17 |
| Difficulty × decade | 2.93 | 5,1487 | 0.01 | 0.01 |
| Difficulty × sex | 0.00 | 1,1487 | 0.99 | <0.01 |
| Difficulty × decade × sex | 0.78 | 5,1487 | 0.57 | <0.01 |
| Domain × difficulty | 0.18 | 1,1487 | 0.67 | <0.01 |
| Domain × difficulty × decade | 1.84 | 5,1487 | 0.10 | 0.01 |
| Domain × difficulty × sex | 0.68 | 1,1487 | 0.41 | <0.01 |
| Domain × difficulty × decade × sex | 1.80 | 5,1487 | 0.11 | 0.01 |
Fig. 1.
Lifespan measures of d-prime values and reaction times in the working memory tasks of both domains (visuospatial and verbal) and levels of difficulty (1-back and 2-back) for each sex, adjusted for years of education. Error bars represent standard errors
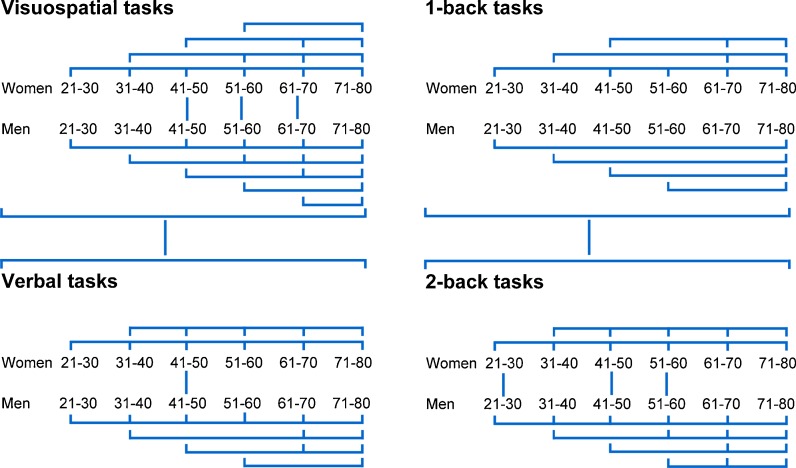
The results of the Tukey tests performed to identify significant three-way interactions among the d-prime values are graphically depicted in Fig. 2. Tukey tests showed that the interaction between domain, decade and sex was because, relative to women, discrimination was superior only for men in their fourth decade in tasks from the verbal domain (Fig. 1); however, in tasks from the visuospatial domain, this superiority was observed in the fourth, fifth and sixth decades. In all decades, d-prime values in the verbal tasks were higher than in the visuospatial tasks for both sexes. Additionally, discrimination in the visuospatial domain diminished unevenly between sexes. The first significant decline in d-prime values occurred in the third decade in women and in the fourth decade in men. For tasks in the verbal domain, d-prime values diminished significantly for both sexes between the second decade of live and after 31 years of age.
Fig. 2.
Representation of the significant interactions between domain, decade and sex effects (left) and between difficulty, decade and sex (right) effects on d-prime values, determined by the Tukey test. The numbers correspond to the age range of each decade. The lines indicate differences between sexes, domains, difficulties and decades. For all significant differences between the sexes, men had higher levels of discrimination than women. Additionally, performance was superior in all verbal and 1-back tasks relative to visuospatial and 2-back tasks, respectively
The Tukey tests that were conducted to elucidate the interaction between difficulty, decade and sex showed that, relative to women, the d-prime values for the 2-back tasks were higher in men from the second, fourth and fifth decades (Fig. 1). Additionally, d-prime values were superior in all 1-back tasks compared to 2-back tasks for both sexes from all decades. In the 1-back tasks, d-prime values diminished differently with age for each sex. In women, the first significant decline was between the second decade of life and after 41 years of age. In men, these values decreased significantly between the second decade of life and after 71 years of age. In the 2-back tasks, men and women in their second decade exhibited higher d-prime values than individuals ≥31 years of age.
Results of the ANCOVAs that were conducted on log-transformed reaction times during correct responses are presented in Table 3. The two-way interactions between domain and sex and between difficulty and decade were significant after controlling for years of education. Post hoc Tukey tests showed that women (visuospatial: 1,130 ± 6.5; verbal: 1,028 ± 6.4) had longer reaction times than men (visuospatial: 1,037 ± 6.5; verbal: 972 ± 6.4) in tasks from both domains. Likewise, both sexes were faster in tasks from the verbal domain than in those from the visuospatial domain. In all decades, reaction times were faster in the 1-back tasks than in the 2-back tasks (Fig. 2). Additionally, in the 1-back tasks, reaction times significantly increased between individuals in their second decade and those ≥31 years of age, between participants in their third decade and those ≥61 years of age, and between individuals in their fourth and fifth decades and those ≥71 years of age. However, in the 2-back tasks, reaction times were faster for individuals in their second decade than those ≥31 years of age and for participants in their third decade than those ≥51 years of age.
The results from the multiple regression analyses that were conducted on d-prime values are presented in Table 4. The performance in all four tasks was explained by background variables that included years of education, MMSE and BDI scores between R2 = 0.04 and R2 = 0.11, according to the results from the first step of the model. The outcomes of the second step of the model revealed that sex exerts a significant effect in both visuospatial tasks (1-back and 2-back), but in the verbal domain, sex only influenced performance in the high-difficulty task. The proportion of variance explained by sex was between R2 = 0.01 and R2 = 0.03 in these three tasks. The results from the third step of the model showed that, for the visuospatial and verbal tasks, age influenced performance in the low-difficulty tasks by R2 = 0.06 and R2 = 0.07, respectively, and in the high-difficulty tasks by R2 = 0.14 in both tasks. The rate of working memory decline per year, indicated by the slope (b-value) of the fitted line that was predicted by age, i.e., the d-prime values, was similar in both domains, regardless of whether the task was difficult or easy; the decreases were −0.02 and −0.01 per year for the 2-back and 1-back tasks, respectively. The slopes of the regression lines were not significantly different between domains when the difficulty was low (F(1,1,498) = 0.01, p = 0.91, ηp2 < 0.01) or when it was high (F(1,1,498) = 0.18, p = 0.67, ηp2 < 0.01). In both the visuospatial (F(1,1,498) = 99.85, p < 0.001, ηp2 = 0.06) and the verbal (F(1,1,498) = 103.13, p < 0.001, ηp2 = 0.06) domains, the slopes of the regression lines were significantly different for the 1-back and 2-back tasks.
Table 4.
Results of the multiple regression analyses performed on d-prime values
| Step 1 | Step 2 | Step 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE b | Β | b | SE b | Β | b | SE b | Β | |
| Visuospatial 1-back task | |||||||||
| Education (years) | 0.018 | 0.004 | 0.112*** | 0.016 | 0.004 | 0.099*** | 0.007 | 0.004 | 0.041 |
| MMSEa | 0.085 | 0.017 | 0.127*** | 0.089 | 0.017 | 0.133*** | 0.048 | 0.017 | 0.071** |
| BDIa | −0.048 | 0.017 | −0.071** | −0.044 | 0.017 | −0.065* | −0.040 | 0.017 | −0.059* |
| Sexb | 0.118 | 0.034 | 0.088*** | 0.125 | 0.033 | 0.093*** | |||
| Age (years) | −0.010 | 0.001 | −0.256*** | ||||||
| R 2 | 0.042 | 0.049 | 0.106 | ||||||
| ΔR 2 | 0.007 | 0.057 | |||||||
| Visuospatial 2-back task | |||||||||
| Education (years) | 0.052 | 0.005 | 0.240*** | 0.046 | 0.005 | 0.212*** | 0.026 | 0.005 | 0.121*** |
| MMSEa | 0.143 | 0.022 | 0.158*** | 0.154 | 0.022 | 0.170*** | 0.066 | 0.021 | 0.073*** |
| BDIa | −0.070 | 0.022 | −0.077** | −0.059 | 0.022 | −0.065** | −0.050 | 0.020 | −0.056* |
| Sexb | 0.336 | 0.044 | 0.185*** | 0.352 | 0.040 | 0.194*** | |||
| Age (years) | −0.021 | 0.001 | −0.402*** | ||||||
| R 2 | 0.108 | 0.141 | 0.282 | ||||||
| ΔR 2 | 0.033 | 0.141 | |||||||
| Verbal 1-back task | |||||||||
| Education (years) | 0.021 | 0.004 | 0.150*** | 0.021 | 0.004 | 0.151*** | 0.012 | 0.004 | 0.086*** |
| MMSEa | 0.085 | 0.015 | 0.146*** | 0.084 | 0.015 | 0.146*** | 0.044 | 0.014 | 0.077** |
| BDIa | −0.072 | 0.015 | −0.125*** | −0.072 | 0.015 | −0.125*** | −0.068 | 0.014 | −0.118*** |
| Sexb | −0.007 | 0.029 | −0.006 | 0.000 | 0.028 | 0.000 | |||
| Age (years) | −0.010 | 0.001 | −0.289*** | ||||||
| R 2 | 0.074 | 0.074 | 0.147 | ||||||
| ΔR 2 | 0.000 | 0.073 | |||||||
| Verbal 2-back task | |||||||||
| Education (years) | 0.041 | 0.005 | 0.195*** | 0.038 | 0.005 | 0.179*** | 0.018 | 0.005 | 0.087*** |
| MMSEa | 0.166 | 0.022 | 0.188*** | 0.173 | 0.022 | 0.195*** | 0.087 | 0.021 | 0.098*** |
| BDIa | −0.073 | 0.022 | −0.083*** | −0.067 | 0.022 | −0.076** | −0.059 | 0.020 | −0.067** |
| Sexb | 0.195 | 0.044 | 0.110*** | 0.211 | 0.040 | 0.119*** | |||
| Age (years) | −0.020 | 0.001 | −0.405*** | ||||||
| R 2 | 0.099 | 0.111 | 0.253 | ||||||
| ΔR 2 | 0.012 | 0.143 | |||||||
aMMSE (Mini-Mental State Exam) and BDI (Beck’s Depression Inventory) scores were transformed with optimal scale techniques
bThe positive values of sex indicate that men performed better than women
*p < 0.05, **p < 0.01, ***p < 0.001
The proportion of variance explained by the background variables (years of education, MMSE and BDI scores) for log-transformed reaction times in all four working memory tasks was between R2 = 0.01 and R2 = 0.05 (Table 5). According to the results from the second step of the model, the proportion of variance explained by sex was less (~R2 = 0.02) for reaction times in the verbal domain than for those in the visuospatial domain (between R2 = 0.04 and R2 = 0.05). When age was included in the third step of the model, the effects of all background variables were not found to be any more significant, except for reaction times in the verbal 1-back task, in which MMSE and BDI were still slightly significant. The proportion of variance explained by age for reaction times in all four tasks was between R2 = 0.06 and R2 = 0.12. The slope of the fitted line that was predicted by age corresponds to an increase in reaction times of 0.3 ms per year in the verbal 1-back task and 0.4 ms per year in the rest of the tasks. The slopes of the regression lines were significantly different between the domains in the 1-back tasks (F(1,1,498) = 7.30, p = 0.007, ηp2 = 0.01) but not in the 2-back tasks (F(1,1,498) = 1.91, p = 0.17, ηp2 < 0.01). The slopes of the regression lines were significantly different between the low-difficulty and high-difficulty tasks in the visuospatial domain (F(1,1,498) = 20.56, p < 0.001, ηp2 = 0.01) but not in the verbal domain (F(1,1,498) = 0.91, p < 0.34, ηp2 < 0.01).
Table 5.
Results of the multiple regression analyses conducted on log-transformed reaction times
| Step 1 | Step 2 | Step 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE b | Β | b | SE b | Β | b | SE b | Β | |
| Visuospatial 1-back task | |||||||||
| Education (years) | −0.003 | 0.001 | −0.151*** | −0.002 | 0.001 | −0.116*** | −0.001 | 0.000 | −0.032 |
| MMSEa | −0.010 | 0.002 | −0.119*** | −0.011 | 0.002 | −0.135*** | −0.004 | 0.002 | −0.046 |
| BDIa | 0.005 | 0.002 | 0.061* | 0.004 | 0.002 | 0.046 | 0.003 | 0.002 | 0.037 |
| Sexb | −0.039 | 0.004 | −0.233*** | −0.040 | 0.004 | −0.241*** | |||
| Age (years) | 0.002 | 0.000 | 0.372*** | ||||||
| R 2 | 0.050 | 0.103 | 0.223 | ||||||
| ΔR 2 | 0.052 | 0.121 | |||||||
| Visuospatial 2-back task | |||||||||
| Education (years) | −0.001 | 0.001 | −0.059* | −0.001 | 0.001 | −0.030 | 0.001 | 0.001 | 0.028 |
| MMSEa | −0.007 | 0.002 | −0.072** | −0.008 | 0.002 | −0.086*** | −0.002 | 0.002 | −0.025 |
| BDIa | 0.000 | 0.002 | −0.001 | −0.001 | 0.002 | −0.014 | −0.002 | 0.002 | −0.020 |
| Sexb | −0.038 | 0.005 | −0.198*** | −0.039 | 0.005 | −0.203*** | |||
| Age (years) | 0.001 | 0.000 | 0.253*** | ||||||
| R 2 | 0.010 | 0.048 | 0.104 | ||||||
| ΔR 2 | 0.038 | 0.056 | |||||||
| Verbal 1-back task | |||||||||
| Education (years) | −0.002 | 0.001 | −0.102*** | −0.002 | 0.001 | −0.081** | 0.000 | 0.001 | −0.009 |
| MMSEa | −0.010 | 0.002 | −0.117*** | −0.011 | 0.002 | −0.126*** | −0.004 | 0.002 | −0.050* |
| BDIa | 0.006 | 0.002 | 0.076** | 0.006 | 0.002 | 0.067** | 0.005 | 0.002 | 0.060* |
| Sexb | −0.023 | 0.004 | −0.135*** | −0.024 | 0.004 | −0.142*** | |||
| Age (years) | 0.002 | 0.000 | 0.319*** | ||||||
| R 2 | 0.037 | 0.054 | 0.143 | ||||||
| ΔR 2 | 0.018 | 0.089 | |||||||
| Verbal 2-back task | |||||||||
| Education (years) | −0.001 | 0.001 | −0.055* | −0.001 | 0.001 | −0.036 | 0.001 | 0.001 | 0.028 |
| MMSEa | −0.008 | 0.002 | −0.083*** | −0.009 | 0.002 | −0.092*** | −0.002 | 0.002 | −0.024 |
| BDIa | 0.003 | 0.002 | 0.034 | 0.002 | 0.002 | 0.026 | 0.002 | 0.002 | 0.019 |
| Sexb | −0.025 | 0.005 | −0.131*** | −0.026 | 0.005 | −0.137*** | |||
| Age (years) | 0.002 | 0.000 | 0.282*** | ||||||
| R 2 | 0.013 | 0.030 | 0.099 | ||||||
| ΔR 2 | 0.017 | 0.069 | |||||||
aMMSE (Mini-Mental State Exam) and BDI (Beck’s Depression Inventory) scores were transformed with optimal scale techniques
bThe negative values of sex indicate that men were faster than women
*p < 0.05, **p < 0.01, ***p < 0.001
Curve estimation
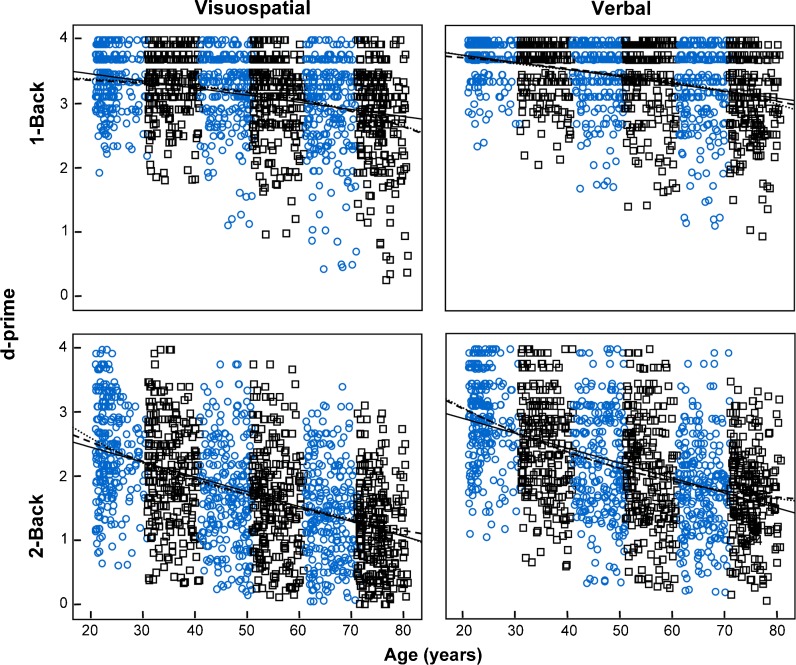
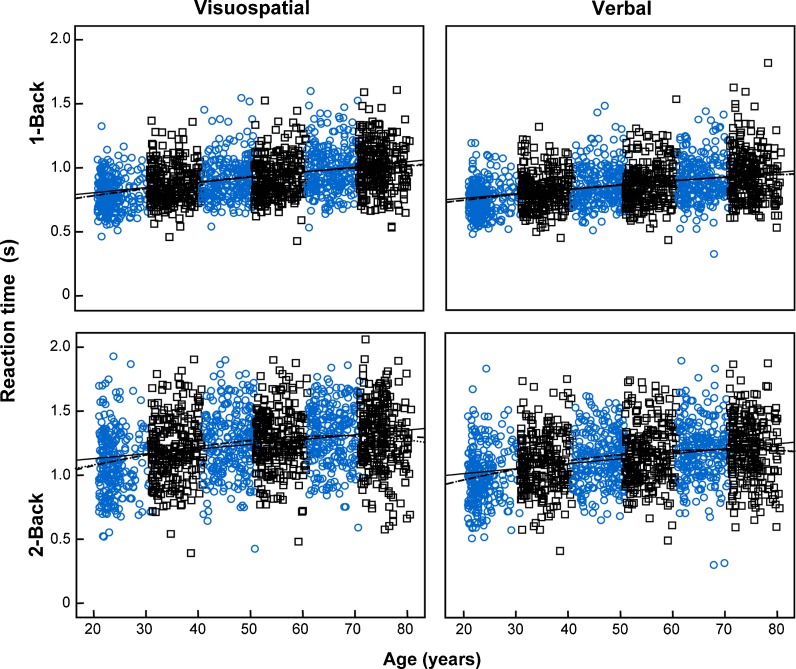
The results of the linear, quadratic, and cubic regressions are shown in Table 6. The fit curves are presented in Fig. 3 for d-prime values and in Fig. 4 for reaction times. The best-fit model was determined by the AIC model selection procedure. All variables were best modeled with quadratic regression, except for the d-prime values in the verbal 1-back task, which were best modeled by a linear fitting. Note that these analyses are based only on age effects, without the control of the possible influence of the background variables.
Table 6.
Linear, quadratic, and cubic regression analyses results
| R 2 | Adj R 2a | F | df | P | AIC | Model | |
|---|---|---|---|---|---|---|---|
| d-prime | |||||||
| Visuospatial 1-back task | |||||||
| Linear | 0.085 | 0.084 | 138.62 | 1,1498 | <0.001 | −1323.2 | |
| Quadratic | 0.090 | 0.089 | 74.29 | 2,1497 | <0.001 | −1330.4 |
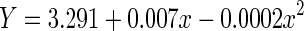
|
| Cubic | 0.091 | 0.089 | 49.68 | 3,1496 | <0.001 | −1328.9 | |
| Visuospatial 2-back task | |||||||
| Linear | 0.211 | 0.211 | 400.69 | 1,1498 | <0.001 | −647.0 | |
| Quadratic | 0.213 | 0.212 | 202.18 | 2,1497 | <0.001 | −648.1 |
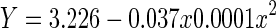
|
| Cubic | 0.214 | 0.212 | 135.52 | 3,1496 | <0.001 | −648.0 | |
| Verbal 1-back task | |||||||
| Linear | 0.117 | 0.116 | 197.99 | 1,1498 | <0.001 | −1826.5 | Y = 3.984 − 0.011 |
| Quadratic | 0.118 | 0.117 | 99.94 | 2,1497 | <0.001 | −1826.2 | |
| Cubic | 0.118 | 0.117 | 66.98 | 3,1496 | <0.001 | −1825.3 | |
| Verbal 2-back task | |||||||
| Linear | 0.212 | 0.211 | 402.60 | 1,1498 | <0.001 | −723.7 | |
| Quadratic | 0.219 | 0.218 | 209.83 | 2,1497 | <0.001 | −735.3 |
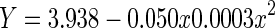
|
| Cubic | 0.219 | 0.217 | 139.82 | 3,1496 | <0.001 | −733.4 | |
| Reaction times | |||||||
| Visuospatial 1-back task | |||||||
| Linear | 0.158 | 0.157 | 280.16 | 1,1498 | <0.001 | −7705.5 | |
| Quadratic | 0.163 | 0.162 | 145.50 | 2,1497 | <0.001 | −7712.9 |
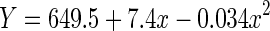
|
| Cubic | 0.163 | 0.161 | 96.94 | 3,1496 | <0.001 | −77100.9 | |
| Visuospatial 2-back task | |||||||
| Linear | 0.063 | 0.063 | 101.57 | 1,1498 | <0.001 | −7144.7 | |
| Quadratic | 0.079 | 0.078 | 64.09 | 2,1497 | <0.001 | −7167.6 |
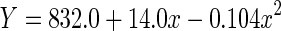
|
| Cubic | 0.079 | 0.077 | 42.84 | 3,1496 | <0.001 | −7166.0 | |
| Verbal 1-back task | |||||||
| Linear | 0.116 | 0.115 | 195.65 | 1,1498 | <0.001 | −7578.7 | |
| Quadratic | 0.119 | 0.117 | 100.69 | 2,1497 | <0.001 | −7581.9 |
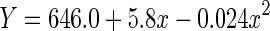
|
| Cubic | 0.119 | 0.117 | 67.08 | 3,1496 | <0.001 | −7579.9 | |
| Verbal 2-back task | |||||||
| Linear | 0.080 | 0.080 | 130.66 | 1,1498 | <0.001 | −7171.2 | |
| Quadratic | 0.095 | 0.094 | 78.36 | 2,1497 | <0.001 | −7193.0 |
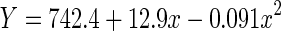
|
| Cubic | 0.095 | 0.093 | 52.25 | 3,1496 | <0.001 | −7191.1 | |
Akaike’s Information Criterion (AIC) was used to select the best model for each variable. The models are presented in milliseconds for reaction times
aAdj R 2 (adjusted R 2)
Fig. 3.
Discrimination (d-prime values) in the working memory tasks of both domains (visuospatial and verbal) and levels of difficulty (1-back and 2-back) by a continuous lifespan sample of 1,500 adults (250 from each decade, between 21 and 80 years of age) as a function of participant age. The shape and color of the markers distinguish the members of each decade. The solid, dashed, and dotted lines represent linear, quadratic, and cubic regression fits, respectively
Fig. 4.
Reaction times in the working memory tasks of both domains (visuospatial and verbal) and levels of difficulty (1-back and 2-back) by a continuous lifespan sample of 1,500 adults (250 from each decade, between 21 and 80 years of age) as a function of participant age. The shape and color of the markers distinguish the members of each decade. The solid, dashed, and dotted lines represent linear, quadratic, and cubic regression fits, respectively
Discussion
Difficulty and domain independently influenced working memory decline across the adult lifespan, as these two factors did not interact together with decade. Notably, sex separately interacted with each of these factors and with decade, indicating that it has an effect on working memory decline. This effect was shown to be completely absent in other types of memory, such as episodic memory (Cansino et al. 2012). Although the comparison across decades revealed that the age at which significant declines in working memory occurred was dependent on the domain, the difficulty of the task, and the sex of the individual, the regression analyses demonstrated that discrimination declined across all of the adult life span, indicating that there is not a specific time point in adulthood when working memory starts to decline. However, the rate at which this continuous working memory decay occurred was twice as fast in the high-difficultly tasks than in the low-difficultly tasks, an observation that is independent of the task domain. Below, we discuss the effects of domain and difficulty on discrimination across the adult life span, and then we discuss the effects of age on discrimination and reaction times for each domain and level of difficulty.
Domain
The decrease in working memory across the adult lifespan was influenced by the specific domain that was under evaluation. In the visuospatial domain, the first significant decline in d-prime values occurred in women after 31 years of age and in men after 41 years of age. However, in the verbal domain, the d-prime values decreased significantly in both sexes after 31 years of age. Thus, men’s discrimination in tasks in the visuospatial domain started to decay at a more advanced age than in tasks in the verbal domain. After this initial working memory decline, discrimination in tasks from the visuospatial domain displayed a more pronounced decay with advancing age tan those from the verbal domain. Some previous studies have observed performance differences between domains in both sexes (e.g., Bopp and Verhaeghen 2007; Fiore et al. 2012; Myerson et al. 1999), while others have not (Park et al. 2002; Borella et al. 2008). Importantly, this uneven decline between domains was observed only when the number of years of education was introduced as a covariant to control for its possible effects on the dependent variables. However, when the effects of the background variables and sex were controlled through multiple regression models, the proportion of variance for discrimination that was explained by age was identical in both domains (see below).
The dissimilar decline observed in the discrimination pattern for each domain indicates that the slave systems are unevenly affected by age, particularly domain-specific storage and rehearsal processes. Moreover, d-prime values in the visuospatial domain were generally lower than those in the verbal domain for both sexes across all decades. The fact that processing visuospatial information was more difficult, even for young adults, may explain why the visuospatial sketch was more greatly affected by age. Although the processing demands required from the central executive were equivalent for both domains, the results revealed a remarkable disadvantage for manipulating visuospatial information. Moreover, this drawback could not be attributed to the use of inefficient strategies, such as using a verbal code to remember the positions, because the difference in reaction times between verbal and visuospatial tasks was too short to perform this extra coding process (102 ms in women and 65 ms in men). Additionally, the stimuli were applied in both domains for a short period of time (300 ms) to prevent participants from verbally encoding the stimuli in the visuospatial tasks. One possible explanation could be that individuals are continuously exposed to verbal information, such as letters, and therefore, rehearsal abilities are more developed for this type of information. Conversely, retaining positions may have been an extremely novel mental activity, in which strategies were not fully developed enough to be implemented accurately.
Discrimination in the two domains was more greatly affected by age in women than in men. However, this vulnerability should not be misinterpreted as a tendency for women to perform less efficiently than men in working memory tasks, as differences between sexes in d-prime values are not generalized but specific. Between 41 and 70 years of age, men outperformed women in visuospatial tasks, and between 41 and 50 years of age, they outperformed women in verbal tasks. The absence of observed differences between the sexes in both domains in the two first decades is in agreement with the outcome reported by Robert and Savoie (2006). When those authors examined whether performance in the verbal and visuospatial domains was different between the sexes, in a sample of one hundred young adults using a wide variety of working memory tasks, they found that accuracy was equivalent. In the present study, the clear disadvantages in both domains observed in women in their fourth decade coincide with the mean age of menopause, which has been identified to be between 43.8 and 53 years of age in Latin American women (Palacios et al. 2010). Of the several symptoms that perimenopausal women experience, working memory difficulties are particularly significant (Weber et al. 2012). The change of steroid hormone levels during menopause (Korenman et al. 1978; Longcope et al. 1986), particularly the decline of estrogens, has been linked to the cognitive difficulties that women experience during this transitional period (e.g., Weber and Mapstone 2009).
The superior performance of men between 41 and 70 years of age relative to women in tasks from the visuospatial domain was not compared in previous studies because this particular age range has not been previously examined. Moreover, the late onset of these sex differences that was observed in the current study cannot be attributed to a different visuospatial memory decline across these decades because both sexes showed exactly the same pattern. In young adults, diverse tasks have been used to investigate differences in visuospatial working memory between genders (e.g., Vecchi and Girelli 1998; Kaufman 2007); however, the n-back task is rarely used (Lejbak et al. 2011). In agreement with the findings of the present study, Lejbak et al. (2011) found that men were superior to women in a spatial 2-back task, although a different age range (17–28 years) was used in that study.
One possible explanation might be the fact that each sex follows a different decline of steroid hormones across the adult lifespan. Testosterone is particularly important in visuospatial working memory, as it has been observed that in women, high levels of androgens are associated with better performance in spatial tasks (Kimura and Hampson 1994), while moderate (Hampson 1995) or high (e.g., Hooven et al. 2004; Christiansen and Knussmann 1987) levels benefit men in these type of tasks. Women experience a sudden decrease in hormone levels during menopause, whereas in men, hormones decline gradually across adulthood. Specifically, testosterone decreases approximately 1 % per year after the age of 19 (Mooradian and Korenman 2006). Therefore, if testosterone plays an important role in the performance of visuospatial tasks, it is possible that the sex differences that we observed in these types of tasks were significant after 41 years due to the dramatic changes in steroid hormone levels in women. This finding has not been confirmed in humans, but it has been reported in rats. A similar late-onset sex difference on spatial memory was observed at an age that coincided with the beginning of estrous cycle deficiencies in female rats (Markowska 1999). Additionally, it has been well documented that steroid hormones act on brain regions that are relevant to memory, such as the basal forebrain and hippocampus (for reviews, see McEwen et al. 1997; Veiga et al. 2004).
Difficulty
The effects of aging on discrimination were also modulated by difficulty. In low-difficulty tasks (1-back) from both domains, working memory started to decline in the fourth decade for women and in the last decade (71 years old) for men, according to d-prime values. In contrast, when the task was highly demanding (2-back), working memory showed a significant decline in d-prime values at an age as early as 31 years, independent of the participants’ sex. This early decline agrees with the results observed when working memory was examined via span tasks (Park et al. 2002), indicating that span tasks and 2-back tasks have similar levels of difficulty. Because task difficulty is directly related to resource demands (Wickens 1991), both tasks appear to require an equivalent intensity of resource allocation to achieve an appropriate performance. These resources start to decrease early in the adult lifespan, according to the present study and the results reported by Park et al. (2002).
Moreover, it was observed that men outperformed women from the second, fourth and fifth decades in 2-back tasks. Thus, young women from 21 to 30 years of age experience a disadvantage relative to men in high-difficulty tasks; this disadvantage reappears in the decades that correspond to the menopausal period. The significant differences in the high-difficulty tasks were observed even though women’s discrimination level unexpectedly remained steady between 41 and 80 years of age, whereas men showed a decline. Although the effect size (ηp2 = 0.01) of these sex differences was extremely low, it should be considered genuine evidence that women showed a disadvantage in high-difficulty tasks. This outcome further confirms the proposal that sex differences in working memory arise when the task requires active processing (Cattaneo et al. 2006; Vecchi and Girelli 1998). Although these authors used different tasks than the ones used here, they also found that the demands of the task affect sex differences and not the specific domain. Therefore, this particular sex difference seems to be the consequence of the fact that women implement less efficient central executive processes or strategies when the task difficulty increases and not the result of ineffective processing mechanisms at the level of the slave systems.
As expected, d-prime values were superior in the 1-back task than in the 2-back task in both sexes across all decades. Moreover, the proportion of variance explained by age for discrimination in 2-back tasks (mean for both domains: R2 = 0.14) was twice that for 1-back tasks (mean for both domains: R2 = 0.07). This outcome could be interpreted as evidence that both the proportion of variance explained by age and the task difficulty increased monotonically in an equivalent way. However, it is also possible that this result could indicate that the 1- and 2-back tasks encompass different processes. According to Oberauer (2005), the 1-back task can be accomplished through familiarity, while the 2-back task requires recollection. This proposal is based on the fact that in 1-back tasks, the last item encountered is the most active, and if the following item is identical, a familiarity criterion can be used to answer the task. Familiarity processes in working memory can be activated without the need to retrieve any additional information, such as the identity of the item or its temporal order. Conversely, the success in 2-back tasks necessarily entails processes such as the binding between the item and its temporal order, the control of the interference provoked by previous items, retrieval and monitoring. It is likely that only storage, the time information is maintained, and updating processes, which are more demanding as a function of task difficulty, are indubitably common to both the 1- and 2-back tasks.
The use of primary memory (i.e., the ability to consciously maintain information for a brief period of time) could also be sufficient to solve the 1-back tasks. Unsworth and Engle (2007) proposed that simple span tasks rely on primary memory, whereas complex span tasks depend mainly on secondary memory. Discrimination decreased as a function of age at a faster rate in the 2-back tasks than in the 1-back tasks. However, this differing effect of age on working memory performance at the two levels of difficulty is not sufficient to establish whether the memory processes underling the two levels of difficulty in the n-back are different, as has been shown for the scan tasks. Hale et al. (2011) reported a similar decrease in rate for simple and complex span tasks within the verbal and spatial domains, finding suggesting that even if the 1-back and simple span tasks rely on primary memory, the 2-back and complex span do not seem to depend on the same memory processes.
The observation of different anatomical networks that underlie the solution of the 1- and 2-back tasks could partially account for the proposal that the 1-back task can be solved by familiarity or primary memory instead of properly working memory. However, few fMRI studies (Carlson et al. 1998; Honey et al. 2002) have observed different anatomical networks for these two tasks. The most consistent finding has been that the activity from the dorsolateral prefrontal cortex increases as a function of memory load (Braver et al. 1997; Callicott et al. 1999; Veltman et al. 2003), denoting that both tasks depend on the same functional mechanisms. Although automatic processes, such as familiarity or primary memory, might be sufficient to answer the 1-back task, the decision to employ them depends on each individual strategy, making them impossible to verify.
Discrimination
The effects of age on discrimination for each domain and level of difficulty were examined after separating the effects of the background variables and sex. The proportion of variance explained by age for d-prime values depends on the task difficulty rather than the domain because this proportion was almost identical in both domains for the same level of difficulty. Moreover, the slopes of the regression lines were identical in both domains in the high- and low-difficulty tasks. Furthermore, the rate of decline of the d-prime values per year was equivalent for both domains, either in the low- (−0.01) and high- (−0.02) difficulty tasks, according to the multiple regression models.
The effects of age on discrimination in the verbal 1-back task were best predicted by a linear model; however, in the rest of the tasks, the effects of age were best predicted by quadratic models. In the low-difficulty task in the visuospatial domain, the trajectory showed that working memory performance increased up to 18 years of age (an age not tested in the present data) and began to decrease thereafter. Quadratic models produced the best fits for discrimination in the visuospatial and verbal 2-back tasks. However, these quadratic models predict that verbal task performance will improve at 83 years of age and visuospatial task performance will improve at an age that is well beyond normal life expectancy. Therefore, for both the visuospatial and verbal 2-back tasks, there was a linear discrimination decline between 21 and 80 years of age, because in the age range tested in the present study, a continuous decline was observed.
Reaction times
The current study is the first lifespan study in which reaction times during working memory tasks have been measured. Thus, to date, only reaction time data from extreme age groups are available in the scientific literature. The longer reaction times observed for high-difficulty and visuospatial tasks across all decades mirrored the results obtained for d-prime values, indicating that reaction times directly reflect the processing time required for each type of task. Moreover, men were faster than women in all the tasks, denoting that even when discrimination levels are equivalent between sexes, men achieve this performance in shorter time. The majority of the studies (e.g., Schmidt et al. 2009; Goldstein et al. 2005; Speck et al. 2000) failed to find sex differences in working memory reaction times. However, in tasks from the visuospatial domain, there is some evidence that men are faster than women (Loring-Meier and Halpern 1999). Although the women were less rapid than men in performing the tasks, the fact that women were not less efficient in all tasks indicates that slower reaction times did not negatively impact their working memory efficiency. Instead, this outcome reflects that women likely utilized different processing strategies, and/or they also spent time monitoring their response to ensure their accuracy.
Considering the effects of age on reaction times, both sexes showed the same pattern of decline across decades, characterized by a significantly rapid decline between the second and third decades in all tasks, and by a second significant decline in the fifth and sixth decades for 2-back and 1-back tasks, respectively. The effects of age on the reaction times measured in the four tasks were best predicted by quadratic models, characterized by an increase in reaction times as a function of age, followed by their decrease. For low-difficulty tasks, the moment at which the reaction times began to diminish was well beyond the years of life expectancy; however, for high-difficulty tasks, this moment occurred at 67 and 71 years of age for tasks from the visuospatial and verbal domain, respectively. Although there was an apparent reduction in reaction times after these ages, this reduction should be negligible, as the ANCOVA results indicated that reaction times remained stable in the last two examined decades.
In summary, the difficulty of the task and the domain independently influenced discrimination across the adult lifespan. However, considering the effects of aging on working memory performance, the difficulty of the task exerts a greater influence than its domain. Women in their third decade experienced their first significant decline in discrimination in both domains and in the high-difficulty tasks, whereas in the low-difficulty tasks, this decline occurred in their fourth decade. For men, discrimination decreased significantly in the third decade for the verbal and 2-back tasks, in the forth decade for the visuospatial tasks, and in the seventh decade for the 1-back tasks. Remarkably, the proportion of variance of working memory decline explained by age across the adult life span does not exceed 14 %; this proportion is half of that explained by age for other types of memory, such as episodic memory (Cansino et al. 2012). This indicates that several other factors must be examined to further explain the changes that occur in working memory with advancing age.
Acknowledgements
The study was supported by grants from the National Council of Science and Technology (CONACYT 98801) and the National Autonomous University of Mexico (DGAPA PRIDE IN304202 IN300206 IN300309 ID300312). We thank the National Institute of Older Persons (INAPAM) for allowing us to invite community groups to participate in the study.
References
- Akaike H. A new look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GA, editor. Recent advances in learning and motivation. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Beck AT. Beck Depression Inventory. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. J Gerontol B- Psychol. 2005;60B:P223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Age-related differences in control processes in verbal and visuo-spatial working memory: storage, transformation, supervision, and coordination. J Gerontol B- Psychol. 2007;62B:P239–P246. doi: 10.1093/geronb/62.5.p239. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, De Beni R. Working memory and inhibition across the adult life-span. Acta Psychol. 2008;128:33–44. doi: 10.1016/j.actpsy.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Jones K, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cansino S, Guzzon D, Martinelli M, Barollo M, Casco C. Effects of aging on interference control in selective attention and working memory. Mem Cognition. 2011;39:1409–1422. doi: 10.3758/s13421-011-0109-9. [DOI] [PubMed] [Google Scholar]
- Cansino S, Estrada-Manilla C, Hernández-Ramos E, Martínez-Galindo JG, Torres-Trejo F, Gómez-Fernández T, et al. The rate of source memory decline across the adult lifespan. Dev Psychol Online first publication. 2012 doi: 10.1037/a0028894. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rämä P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Postma A, Vecchi T. Gender differences in memory for object and word locations. Q J Exp Psychol. 2006;59:904–919. doi: 10.1080/02724980543000079. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Sun X, Tarbi EC, Riis JL, McGinnis SM, et al. Mechanisms underlying age- and performance-related differences in working memory. J Cognitive Neurosci. 2011;23:1298–1314. doi: 10.1162/jocn.2010.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beni R, Palladino P. Decline in working memory updating through ageing: intrusion error analyses. Memory. 2004;12:75–89. doi: 10.1080/09658210244000568. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Fiore F, Borella E, Mammarella IC, De Beni R. Age differences in verbal and visuo-spatial working memory updating: evidence from analysis of serial position curves. Memory. 2012;20:14–27. doi: 10.1080/09658211.2011.628320. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram MW, Anagnoson R, Breiter H, Makris N, Goodman JM, et al. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Hale S, Rose NS, Myerson J, Strube MJ, Sommers M, Tye-Murray N, et al. The structure of working memory abilities across the adult life span. Psychol Aging. 2011;26:92–110. doi: 10.1037/a0021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Spatial cognition in humans: possible modulation by androgens and estrogens. J Psyquiatr Neurosci. 1995;20:397–404. [PMC free article] [PubMed] [Google Scholar]
- Haut KM, Barch DM. Sex influences on material-sensitive functional lateralization in working and episodic memory: men and women are not all that different. Neuroimage. 2006;32:411–422. doi: 10.1016/j.neuroimage.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Honey GD, Fu CH, Kim J, Brammer MJ, Croudace TJ, Suckling J, et al. Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. Neuroimage. 2002;17:573–82. [PubMed] [Google Scholar]
- Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42:782–790. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, Colflesh GJH. Working memory, attention control, and the n-back task: a question of construct validity. J Exp Psychol Learn. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Kimura D, Hampson E. Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Curr Dir Psychol Sci. 1994;3:57–61. [Google Scholar]
- Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- Korenman SG, Sherman BM, Korenman JC. Reproductive hormone function: the perimenopausal period and beyond. Clin Endocrinol Meta. 1978;7:625–43. doi: 10.1016/s0300-595x(78)80012-7. [DOI] [PubMed] [Google Scholar]
- Lejbak L, Crossley M, Vrbancic M. A male advantage for spatial and object but not verbal working memory using the n-back task. Brain Cogn. 2011;76:191–196. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Li T, Luo Q, Gong H. Gender-specific hemodynamics in prefrontal cortex during a verbal working memory task by near-infrared spectroscopy. Behav Brain Res. 2010;209:148–53. doi: 10.1016/j.bbr.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Longcope C, Franz C, Morello C, Baker R, Johnston CC. Steroid and gonadotropin levels in women during the peri-menopausual years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- Loring-Meier S, Halpern DF. Sex differences in visuopatial working memory: components of cognitive processing. Psychon B Rev. 1999;6:464–471. doi: 10.3758/bf03210836. [DOI] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. J Exp Psychol Gen. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters MS, Sanders B. Is the gender difference in mental rotation disappearing? Behav Genet. 1993;23:337–341. doi: 10.1007/BF01067434. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- Meulman JJ. Optimal scaling methods for multivariate categorical data analysis. SPSS, Chicago: SPSS White Papers; 2000. [Google Scholar]
- Missonnier P, Gold G, Leonards U, Costa-Fazio L, Michel JP, Ibáñez V, et al. Aging and working memory: early deficits in EEG activation of posterior cortical areas. J Neural Transm. 2004;111:1141–1154. doi: 10.1007/s00702-004-0159-2. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Korenman SG. Management of the cardinal features of andropause. Am J Ther. 2006;13:145–160. doi: 10.1097/01.mjt.0000132252.80403.c9. [DOI] [PubMed] [Google Scholar]
- Myerson J, Hale S, Rhee SH, Jenkins L. Selective interference with verbal and spatial working memory in young and older adults. J Gerontol B-Psychol. 1999;54B:P161–P164. doi: 10.1093/geronb/54b.3.p161. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Removing irrelevant information from working memory: a cognitive aging study with the modified Sternberg task. J Exp Psychol Learn. 2001;27:948–957. [PubMed] [Google Scholar]
- Oberauer K. Binding and inhibition in working memory: individual and age differences in short-term recognition. J Exp Psychol Gen. 2005;134:368–387. doi: 10.1037/0096-3445.134.3.368. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Süβ HM, Schulze R, Wilhelm O, Wittmann WW. Working memory capacity — facets of a cognitive ability construct. Pers Indiv Differ. 2000;29:1017–1045. [Google Scholar]
- Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13:419–428. doi: 10.3109/13697137.2010.507886. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Robert M, Savoie N. Are there gender differences in verbal and visuospatial working-memory resources? Eur J Cogn Psychol. 2006;18:378–397. [Google Scholar]
- Robert C, Borella E, Fagot D, Lecerf T, Ribaupierre A. Working memory and inhibitory control across the life span: intrusion errors in the reading span test. Mem Cognition. 2009;37:336–345. doi: 10.3758/MC.37.3.336. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influences of processing speed on adult age differences in working memory. Acta Psychol. 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in learning. Swiss J Psychol. 1995;51:102–l12. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock R. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- Schmidt H, Jogia J, Fast K, Christodoulou T, Haldane M, Kumari V, et al. No gender differences in brain activation during the n-back task: an fMRI study in healthy individuals. Hum Brain Mapp. 2009;30:3609–3615. doi: 10.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F, Hildebrandt A, Lövdén M, Wilhelm O, Lindenberger U. Complex span versus updating tasks of working memory: the gap is not that deep J Exp Psychol Learn. 2009;35:1089–1096. doi: 10.1037/a0015730. [DOI] [PubMed] [Google Scholar]
- Schulze ET, Geary EK, Susmaras TM, Paliga JT, Maki PM, Little DM. Anatomical correlates of age-related working memory declines. J Aging Res. 2011;2011:606871. doi: 10.4061/2011/606871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11:2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007;114:104–32. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway JM, Engle RW. Complex working memory span tasks and higher-order cognition: a latent-variable analysis of the relationship between processing and storage. Memory. 2009;17:635–54. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Vecchi T, Girelli L. Gender differences in visuo-spatial processing: the importance of distinguishing between passive storage and active manipulation. Acta Psychol. 1998;99:1–16. doi: 10.1016/s0001-6918(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Vecchi T, Richardson JTE, Cavallini E. Passive storage versus active processing in working memory: evidence from age-related variations in performance. Eur J Cogn Psychol. 2005;17:521–539. [Google Scholar]
- Veiga S, Melcangi RC, DonCarlos LL, Garcia-Segura LM, Azcoitia I. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–70. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Weber MT, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16:694–700. doi: 10.1097/gme.0b013e318196a0c9. [DOI] [PubMed] [Google Scholar]
- Weber MT, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19:735–41. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wickens CD. Processing resources and attention. In: Damos DL, editor. Multiple task performance. Basingstoke, England: Taylor & Francis; 1991. pp. 3–34. [Google Scholar]