Abstract
The action of taxifolin on the angiotensin-converting enzyme (ACE) and the formation of reactive oxygen and nitrogen species (ROS/RNS) in the aorta of aging rats and rats treated with nitric oxide synthase inhibitor (Nω-nitro-l-arginine methyl ester (L-NAME)) or dexamethasone have been studied. The ACE activity in aorta sections was determined by measuring the hydrolysis of hippuryl-l-histidyl-l-leucine, and the ROS/RNS production was measured by oxidation of dichlorodihydrofluorescein. It was shown that taxifolin at a dose of 30–100 μg/kg/day decreases the ACE activity in the aorta of aging rats and of rats treated with L-NAME or dexamethasone to the level of the ACE activity in young control rats. Taxifolin (100 μg/kg/day) was found to also reduce the amount of ROS/RNS in the aorta that increased as a result of L-NAME intake. L-NAME treatment increases the contribution of 5-lipoxygenase and NADPH oxidase to ROS/RNS production in the aorta, while taxifolin (100 μg/kg/day) decreases the contribution of these enzymes to the normal level.
Keywords: Aging, Angiotensin-converting enzyme, Aorta, Dexamethasone, L-NAME, Taxifolin, ROS/RNS
Introduction
Flavonoids reduce the risk of atherosclerosis and cardiovascular diseases (CVD) and decrease the death rate from CVD (Reed 2002; Maron 2004; Nandave et al. 2005; Grassi et al. 2010). In vitro and in vivo studies of flavonoids demonstrated that they favorably influence the cardiovascular system. In his review, Reed (2002) listed the following effects of flavonoids: suppression of oxidation of low-density lipids (LDL), suppression of thrombocyte aggregation, inhibition of enzymes mediating the response of immune cells to LDL and their absorption by epithelium macrophages, induction of endothelium-dependent vasodilation, and reduction of the total and LDL cholesterol. There is evidence indicating that the key effect of flavonoids in reducing the risk of atherosclerosis is a decrease of the oxidative stress in vessels (Duarte et al. 2001; Galisteo et al. 2004; Hishikawa et al. 2005; Grassi et al. 2010). For example, the flavonoid quercetin diminishes the concentration of isoprostane F2a and malondialdehyde (markers of oxidative stress and peroxidation, respectively) (Morrow and Roberts 1996; Kitts et al. 1998; Duarte et al. 2001) in rat urine and raises the concentration of the endogenous antioxidant glutathione (Galisteo et al. 2004). A cardinal regulator of the concentration of reactive oxygen species (ROS) and, consequently, of oxidative stress in vessels is the product of angiotensin-converting enzyme (ACE) angiotensin II (Dzau 2001; Munzel and Keaney 2001). Angiotensin II activates NADPH oxidase (Griendling et al. 1994; Rajagopalan et al. 1996; Landmesser et al. 2002), which results in enhanced ROS formation in vessels, provoking inflammation and fibrosis (Mehta and Griendling 2007; Choi et al. 2008), promotes cell division, and increases the expression of the monocyte chemoattractant protein in vessels (Heeneman et al. 2007). These effects of angiotensin II cause atherosclerosis of the vessels, their thickening, and cardiac insufficiency (Kim and Iwao 2000).
The previously mentioned data on the important role of angiotensin II in the pathogenesis of atherosclerosis demonstrate that the antiatherosclerotic effect of flavonoids may be due to the inhibition of ACE. Indeed, there are numerous reports showing that the ACE activity in vitro is inhibited by different flavonoids (Kameda et al. 1987; Hansen et al. 1996; Lacaille-Dubois et al. 2001; Actis-Gorettaa et al. 2003, 2006; Braga et al. 2007; Loizzo et al. 2007). There are some data on the influence of flavonoids on the ACE activity in endothelial cells and vessels. The results of Meunier et al. (1987) can be considered as indirect evidence that flavonoids inhibit the ACE activity in vessels. It was shown that the vasoconstrictive action of angiotensin I in rabbits is suppressed by the intravenous introduction of procyanidolic oligomers (5 μg/kg). The direct effect of another flavonoid genistein on the activity of ACE and its expression in endothelial cells in vitro, in serum, and in vessels was studied by Xu et al. (2006). Genistein suppressed the expression and activity of ACE in cells, vessels, and serum. In this work, the effect of genistein on the ACE activity was studied after the short-time treatment of normal rats, and the ACE activity was determined in aorta homogenate. The effect of flavonoids on the ACE activity in rats with increased ACE activity (aging rats and Nω-nitro-l-arginine methyl ester (L-NAME)-treated or dexamethasone-treated rats) was not studied. In addition, the ACE activity in homogenate can considerably differ from that (determined) in tissue (Korystova et al. 2012). We have previously developed methods for measuring the ACE activity (Emel’yanov et al. 2012; Korystova et al. 2012) and the amount of reactive oxygen species (ROS)/reactive nitrogen species (RNS) in rat aorta sections (Korystov et al. 2009). In the present study, we have examined the effect of taxifolin (dihydroquercetin) on the ACE activity in aortas of aging rats and rats treated with the nitric oxide (NO) synthase inhibitor or dexamethasone. Previously, we demonstrated that the ACE activity in the aorta of aging rats and rats treated with these agents increases (Korystova et al. 2012). We also determined the influence of taxifolin on the amount of ROS/RNS in the aorta and the contributions of different enzymes to their production after the treatment of rats with the NO synthase inhibitor.
Materials and methods
Animals and aorta preparation
Male Wistar rats weighing 330 and 530 g at an age of 11 weeks (N = 123) and 44 weeks (N = 12) (the animal collection of the Institute of Theoretical and Experimental Biophysics, Pushchino, Russia) were used. The rats were maintained in animal facilities with free access to water and standard rat chow (normal salt). The Local Ethics Committee Criteria for Care and Use of Laboratory Animals were carefully observed. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Eleven-week-old rats were used in all experiments with L-NAME and dexamethasone treatment. Forty-four-week-old rats were used in experiments with taxifolin treatment. The animals of L-NAME experimental groups were given the NO synthase inhibitor L-NAME (1 mg/ml) or L-NAME and taxifolin dissolved in drinking water for 5 or 12 days. When the rats consumed L-NAME and taxifolin, these substances were simultaneously added to drinking water. In another experimental group, dexamethasone with a daily dose of 30 μg/kg was injected into the peritoneal cavity for 8 days. When the effect of taxifolin on the dexamethasone-treated rats was studied, the rats were given taxifolin solution (1 μg/ml) to drink. Routine monitoring showed that the rats consumed approximately 100 ml of drinking water/1 kg of body weight each day irrespective of whether or not L-NAME or taxifolin were contained in water, and the drinking regime did not change throughout all treatment protocols. At this rate of water drinking, each rat received daily 100 mg of L-NAME and/or from 10 to 100 μg of taxifolin/kg of body weight. The aorta was prepared according to Korystov et al. (2009). At the end of the treatment protocols, animals were anesthetized by ether, and the thorax was dissected before the injection of heparin (500 U) into the heart to prevent blood clotting. The procedure from the beginning of operation to the removal of the aorta took <3 min. The rats were under anesthesia during this period and died shortly after the injection of heparin. A greater part of the adventitial fat adherent to the aorta was cleaned in situ. Then, the aorta was removed, rinsed with cold (4 °C) 10 mM Hank’s–HEPES solution, pH 7.4, and placed into the same solution. The residuary fat was carefully cleaned; care was taken not to damage the endothelium. The aorta was cut into eight 4- to 5-mm sections beginning with the point at which the aorta became parallel to the vertebral column. Sections 1–5 were in the thorax aorta and sections 7 and 8 were in the abdominal aorta irrespective of the age of rats. Section 6 was in the thorax aorta part in 11-week-old rats and in the abdominal aorta part in 44-week-old rats. The aortic sections were numbered from 1 to 8, starting with the section adjacent to the aortic arch. The aortic sections were cut lengthwise, turned inside out with the endothelium outside, and attached to the tip of a plastic pipette with a polyester thread. After the measurements of the ACE activity, the aortic sections were taken away from the pipette, flattened, and their linear dimensions were determined using a slide gauge to an accuracy of 0.1 mm.
Measurements of ACE activity in the aorta
The ACE activity was determined by measuring the hydrolysis of hippuryl-l-histidyl-l-leucine (Hip-His-Leu) using the method of Ackermann et al. (1998), with a modification from Miyamoto et al. (2002). Briefly, isolated rat aorta sections were placed in Hank’s–HEPES solution, pH 7.4 (450 μl), and incubated for 10 min at 37 °C with shaking (25 Hz, amplitude 1 mm) for adaptation before the addition of the ACE substrate. The reaction was started by the addition of 10 mM Hip-His-Leu (50 μl). After 30 min of incubation at 37 °C, the reaction was stopped by the addition of 1,000 μl of 0.1 N NaOH. After stirring the reaction mixture, aorta sections were taken out of the solution, and their dimensions and weight were determined. A 200-μl aliquot of the remaining solution was incubated with 50 μl of o-phthaldialdehyde (20 mg/ml in dimethyl sulfoxide) for 30 min at 37 °C, and the reaction was stopped by the addition of 2 ml of 0.8 N HCl. The samples were centrifuged at 3,000×g at 4 °C for 5 min, and fluorescence was measured using an MF44 Perkin-Elmer fluorimeter at excitation and emission wavelengths of 360 and 500 nm, respectively. For determining the ACE activity, a standard curve was generated using His-Leu. The ACE activity was expressed as picomoles of Hip-His-Leu hydrolyzed per minute per square millimeter of the inner aorta surface (the endothelium surface). The average ACE activity in the aorta was determined by averaging the ACE activities of all eight sections for each rat, and then these values were averaged for all rats used in the experiment.
Measurements of ROS/RNS in the aorta
ROS/RNS were determined according to Korystov et al. (2009). The aorta from the aortic arch to the point of branching of kidney arteries was cut into seven 5-mm sections. The aortic sections were numbered from 1 to 7, starting with the section near the aortic arch. Sections 1–6 were used for ROS/RNS determination and section 7 was not incubated with dichlorodihydrofluorescein diacetate (DCFH2-DA). This section was used to determine endogenous fluorescent substances leaving the aorta after treatment with digitonin. The fluorescence of endogenous substances was subtracted from the fluorescence of extracts obtained from the sections incubated with DCFH2-DA. The aortic sections were cut lengthwise, turned inside out with the endothelium outside, and attached to the tip of the plastic pipette. Then, the aorta segments were placed in glass flasks in 2.5 ml Hank’s–HEPES solution, pH 7.4, and incubated for 30 min at 37 °C with shaking (25 Hz, 1 mm amplitude) without inhibitors for adaptation or with inhibitors for the determination of their effects on ROS/RNS. The inhibitors used were as follows: nordihydroguaiaretic acid (NDGA), an inhibitor of all lipoxygenases; baicalein, an inhibitor of 12- and 15-lipoxygenases; caffeic acid, an inhibitor of 5-lipoxygenase; diphenyleneiodonium (DPI), an inhibitor of NADPH oxidase; indomethacin and N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulfonamide (NS-398), inhibitors of cyclooxygenases; and L-NAME, an inhibitor of NO synthase. Then, 20 μm of DCFH2-DA was added, and the aorta segments were incubated for 20 min at 37 °C with shaking. The DCFH2-containing solutions were removed after the completion of incubation, and aorta sections were rinsed twice with cold Hank’s–HEPES solution. Then, the aorta segments were placed in citrate buffer (2.5 ml), pH 4, containing 0.02 % digitonin and incubated for 20 min at 37 °C with shaking. The aorta extracts were cooled to room temperature and kept at this temperature until fluorescence measurements. Fluorescence was measured at room temperature under stirring on an MF44 Perkin-Elmer fluorimeter at excitation and emission wavelengths of 475 and 525 nm, respectively. Dichlorofluorescein (DCF) fluorescence was measured at pH 7.
Drugs
Baicalein, caffeic acid, digitonin, DCFH2-DA, DPI, Hank’s solution, HEPES, Hip-His-Leu acetate salt, His-Leu, indomethacin, NDGA, L-NAME, NS-398, o-phthaldialdehyde, and Trypan blue were obtained from Sigma (USA), and heparin was a pharmaceutical preparation. A 10-mM DCFH2-DA stock solution was prepared in ethanol, stored at −20 °C, and diluted in Hank’s solution before use.
Statistical analysis
The results are expressed as the means ± standard error of the mean. The numbers of rats (N) used in the experiments are given in the figure legends. The significance of differences in multiple comparisons was determined using the analysis of variance and Tukey’s post hoc tests. P values <0.05 were considered significant.
Results
The effect of taxifolin on the activity of ACE
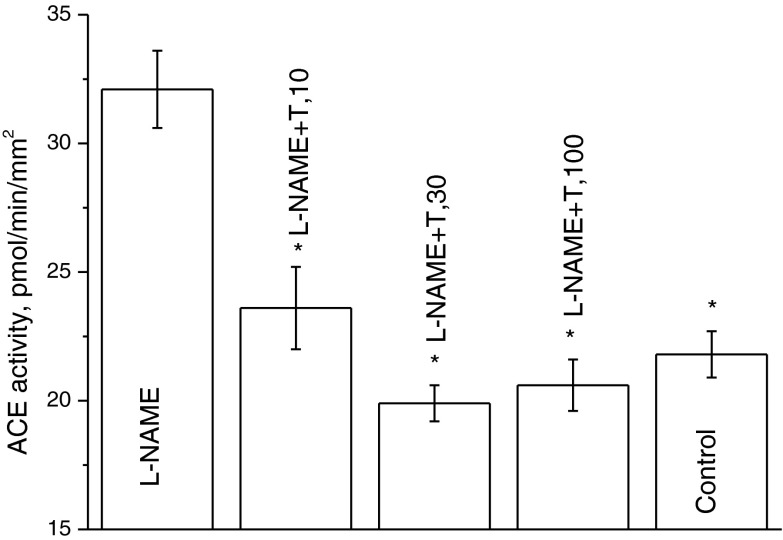
Figure 1 shows the effect of taxifolin on the ACE activity in the rat aorta enhanced by a 12-day introduction of L-NAME. As seen, the increase in the taxifolin dose causes a decrease in the ACE activity and, at a dose of 30 μg/kg/day, it already drops to 19.9 ± 0.7 pmol/min/mm2, which is lower than the value typical of 11-week-old rats that were not treated with L-NAME (21.8 ± 0.9 pmol/min/mm2; see Fig. 1, the last bar).
Fig. 1.
Dependence of the ACE activity in aorta of rats receiving L-NAME for 12 days on the taxifolin dose. The age of rats at the end of the experiment was 11 weeks. N = 3–6 for each experimental point. *P < 0.05 vs. the ACE activity in aortas of rats treated with L-NAME only
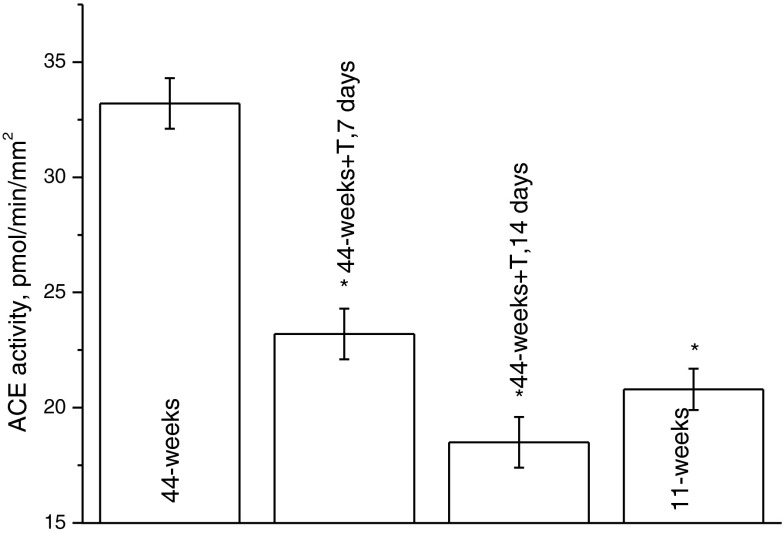
Figure 2 demonstrates the effect of the intake of taxifolin (100 μg/kg/day) on the ACE activity in 44-week-old rats. The ACE activity in 44-week-old rats increased to 33.2 ± 1.1 pmol/min/mm2. As seen from the figure, after 2 weeks of taxifolin intake, the ACE activity dropped to 18.5 ± 1.1 pmol/min/mm2, which is lower than the ACE activity in 11-week-old rats (see Fig. 2, the last bar) and typical for young (4-week-old) rats (see Korystova et al. 2012).
Fig. 2.
Dependence of the ACE activity in aortas of 44-week-old rats on the duration of taxifolin treatment (100 μg/kg/day). N = 3–6 for each time point. *P < 0.05 vs. the ACE activity in aortas of control 44-week-old rats
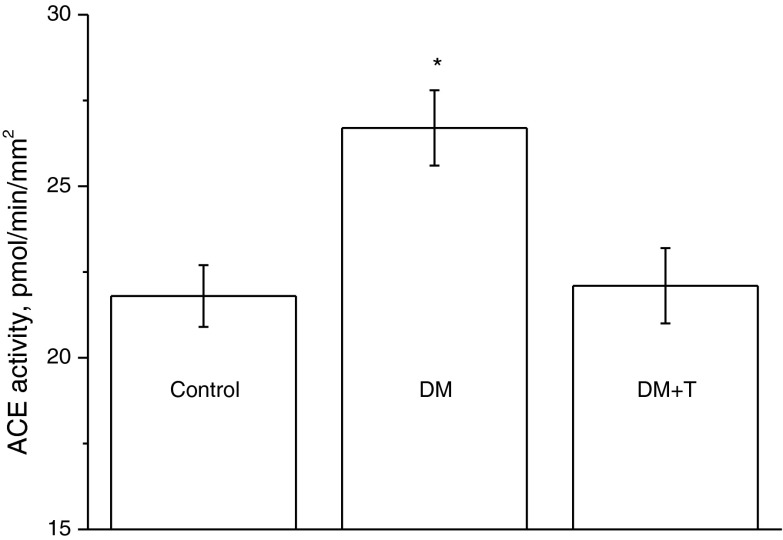
Figure 3 shows the data on the influence of taxifolin (100 μg/kg) on the ACE activity of rats that received dexamethasone at a dose of 30 μg/kg/day for 8 days. Dexamethasone enhanced the ACE activity in the aorta, and taxifolin reduced the ACE activity to the normal level.
Fig. 3.
The ACE activity in the aorta of control rats and rats treated with dexamethasone (30 μg/kg/day, 8 days—DM) or dexamethasone with taxifolin (100 μg/kg/day, 8 days—DM + T). The age of rats at the end of the experiment was 11 weeks. N = 3–6 for each experimental point. *P < 0.05 vs. the ACE activity in aortas of control rats
The effect of taxifolin on the formation of ROS/RNS
The data on the amount of ROS/RNS in the aorta segments of control rats and rats treated with L-NAME and L-NAME combined with taxifolin without inhibitors and in the presence of inhibitors of different ROS/RNS-forming enzymes are given in Table 1. In the aortas of rats that received L-NAME for 5 days, the amount of ROS/RNS increased by 25 % as compared with control rats (line 1). Taxifolin (100 μg/kg/day) diminished the amount of ROS/RNS to the level by 33 % lower than in the aortas of rats that received only L-NAME and by 16 % lower than in the aortas of control rats (line 1).
Table 1.
Number of ROS/RNS without inhibitors in the aortas of control rats (without treatment), rats treated with L-NAME for 5 days (L-NAME), and rats treated with L-NAME and taxifolin (100 μg/kg/day) for 5 days (L-NAME + T) and in the presence of inhibitors
| Treatment of rats: | Without treatment | L-NAME | L-NAME + T | |
|---|---|---|---|---|
| ROS/RNS, DCF, pmol/h/cm2 (% to 1) | 1. Without inhibitors (100) | 18.4 ± 0.5 (100) | 23 ± 0.6*** (100) | 15.4 ± 0.6*** |
| 2. Baicalein, 0.1 μM | 6.5 ± 0.7** (35) | 10.1 ± 0.9** (44) | – | |
| 3. NDGA, 3 μM | 11.4 ± 0.6** (62) | 17.7 ± 0.7* (77) | – | |
| 4. Caffeic acid, 10 μM | 17.4 ± 0.7 (95) | 15.9 ± 1.1* (69) | 15.1 ± 1 (98) | |
| 5. DPI, 10 μM | 17.3 ± 1 (94) | 17.5 ± 1* (76) | 15.2 ± 1 (99) | |
| 6. Indomethacin, 30 μM | 20 ± 1.5 (109) | 26.3 ± 2 (114) | – | |
| 7. NS-398, 5 μM | 26.5 ± 1.4* (144) | 23.2 ± 2 (101) | – | |
| 8. L-NAME, 200 μM | 15.5 ± 1.3* (84) | 23.5 ± 0.9 (102) | – |
The age of rats at the end of the experiment was 11 weeks. N = 6–9 for each experimental point
*P < 0.05 vs. the number of ROS/RNS in aortas without inhibitors in each experimental group of rats; **P < 0.01 vs. the number of ROS/RNS in aortas without inhibitors in each experimental group of rats; ***P < 0.05 vs. the number of ROS/RNS in aortas of control rats without inhibitors
As seen in Table 1, under the action of the inhibitors, the amount of ROS/RNS in the aorta can both decrease and increase to different extents, the effects of the inhibitors differing in the aortas of control rats and rats treated with L-NAME. NDGA (line 3), a nonspecific inhibitor of lipoxygenases (Hamberg 1976), causes the greatest decrease in the amount of ROS/RNS in the aortas of control rats and rats treated with L-NAME, with the inhibiting effect of NDGA being more pronounced in the aortas of control rats than animals treated with L-NAME. The decrease in the amount of ROS/RNS in the presence of 3 μM NDGA can be partially explained by its antioxidant properties, which should have the same effect in the aortas of the two experimental groups of rats. Baicalein, as an inhibitor of 12- and 15-lipogenases (Deschamps et al. 2006), reduces essentially the amount of ROS/RNS in the aortas of control rats (line 2), whereas the effect of caffeic acid, a specific inhibitor of 5-lipoxygenase (Koshihara et al. 1984), is insignificant (line 4). In the aortas of rats treated with L-NAME, the effect of these inhibitors is the opposite: NDGA and baicalein have a lesser effect than in the aortas of control rats, and caffeic acid has a far greater effect. These data show that L-NAME diminishes the activity of 12- and 15-lipoxygenases and increases the activity of 5-lipoxygenase. The action of DPI (an inhibitor of NADPH oxidase) in the aortas of control rats and rats treated with L-NAME is also diverse. In the aortas of control rats, DPI slightly affects the amount of ROS/RNS (a decrease by 6 %, line 5), and in the aortas of rats treated with L-NAME, it decreases the amount of ROS/RNS by 24 % (line 5). The inhibitors of cyclooxygenases indomethacin and NS-398 do not change the amount of ROS/RNS in the aortas of rats treated with L-NAME but enhance it in the aortas of control rats by 10–40 % (lines 6 and 7). L-NAME, an inhibitor of NO synthase, does not affect the amount of ROS/RNS in the aortas of rats treated with L-NAME (the activity of this enzyme is already suppressed by the inhibitor that the rats received during drinking) and reliably (by 16 %) decreases it in the aortas of control rats (line 8). Consequently, the treatment of rats with L-NAME changes both the amount of ROS/RNS in the aorta and the contribution of different enzymes to their production. To study the action of taxifolin on the L-NAME-induced change of the contribution of enzymes to the production of ROS/RNS in the aorta, we have chosen caffeic acid and DPI as inhibitors whose effects reliably differ in control rats and rats treated with L-NAME. As seen from the data presented in Table 1 (lines 4 and 5), the intake of L-NAME combined with taxifolin (100 μg/kg) normalizes the effect of both caffeic acid and DPI on the formation of ROS/RNS in the rat aorta.
Discussion
We studied the influence of taxifolin on the ACE activity in the aortas of aging rats and rats treated with L-NAME and dexamethasone, as well as on the contribution of various enzymes to the formation of ROS/RNS in the aortas of rats that received L-NAME. A low dose of taxifolin (30 μg/kg/day) reduces the L-NAME-enhanced ACE activity in the rat aorta to the control level (Fig. 1). In 44-week-old rats, the 2-week intake of taxifolin (100 μg/kg/day) decreases the ACE activity in the aortas to the level typical of young rats (Fig. 2). In addition, taxifolin (100 μg/kg/day) reduces the ACE activity in the aortas of dexamethasone-treated rats to the normal level (Fig. 3). These data on the ability of taxifolin to bring the ACE activity to the normal level have been obtained for the first time; however, it is possible to compare them with the influence of flavonoids on other manifestations of age-related vessel pathology or pathology caused by the NO synthase inhibitor or glucocorticoid hormones. As known, vessel remodeling in rats enhances with aging (Basso et al. 2007) and as a result of the effect of the NO synthase inhibitor (Takemoto et al. 1997; Katoh et al. 2001). In these cases, pathological changes in vessels are caused by enhanced ACE activity because ACE inhibitors prevent vessel remodeling (Takemoto et al. 1997; Katoh et al. 2001; Basso et al. 2007). Glucocorticoid hormones also increase the ACE activity in the aorta (Emel’yanov et al. 2012), blood pressure (Saruta 1996), and the risk of CVD (Nashel 1986; Souverein et al. 2004). As for the influence on vessel remodeling and blood pressure, the most studied flavonoid is quercetin, which makes 60–75 % of all flavonoids humans receive with the Western diet (Hertog et al. 1993; Sampson et al. 2002). Thus, it was shown that quercetin at a dose of 2.5 mg/kg/day suppresses vessel remodeling in mice deficient in apolipoprotein E (Hayek et al. 1997). At a dose of 10 mg/kg, it decreases blood pressure in spontaneously hypertensive rats (Duarte et al. 2001), as well as suppresses hypertension and removes all pathological changes in vessels and the heart of rats treated with L-NAME; at a dose of 5 mg/kg, some effects of quercetin were less pronounced than at a dose of 10 mg/kg (Duarte et al. 2001). Thus, in the experiments with rats, quercetin at doses of 3–10 mg/kg/day reduces vessel remodeling. Genistein in the same dose range inhibits the ACE activity in rat aorta (Hu et al. 2006). In his review, Maron (2004) summarized studies on the effects of flavonoids on the risk of atherosclerosis in humans. Most of the epidemiological data indicate that increasing the dose of flavonoids up to 30–40 mg/day (about 500 μg/kg/day) decreases the risk of CVD. This dose is an order of magnitude lower than quercetin doses used in experiments with animals, but from five to ten times higher than the taxifolin doses (30–100 μg/kg/day) that in our experiments completely normalized the ACE activity that was increased with age and by the treatment with the NO synthase inhibitor or the glucocorticoid hormone.
The mechanism of the action of flavonoids on the ACE activity in vivo was partly studied by an example of genistein (Xu et al. 2006). It was shown that genistein dose-dependently decreased ACE levels in rats both in vivo and in vitro. The effect was mediated by the estrogen receptor followed by the activation of the ERK1/2 pathway. Genistein also stimulated NO synthesis in vascular endothelial cells by the cyclic adenosine 3′,5′-monophosphate-dependent mechanism (Liu et al. 2004). This effect can be another cause of ACE suppression because there is a “cross talk” between eNOS expression/activity and tissue ACE expression/activity by feedback regulation (Linz et al. 1999).
At a dose of 100 μg/kg/day, taxifolin reduces the ROS/RNS formation that increased in the aorta of rats treated with the NO synthase inhibitor (Table 1, line 1). Though the antioxidant properties of flavonoids are well-known (Grassi et al. 2010), it is questionable that taxifolin at such a low dose can compete for ROS/RNS with endogenous antioxidant systems. It is more probable that this effect of taxifolin is due to the suppression of ROS/RNS production. Table 1 demonstrates that both the enhanced ROS/RNS production in rats induced by L-NAME and the effect of taxifolin are caused by a change in the contribution and, consequently, in the activities of enzymes forming ROS/RNS. In the aortas of control rats, the greatest contribution to the formation of ROS/RNS is made by 12- and 15-lipoxygenases because, of all inhibitors studied, their specific inhibitor baicalein at low concentrations produced the maximal effect. NDGA, another inhibitor of lipoxygenases, has a far greater effect, but it may be partly explained by the fact that, as an antioxidant, NDGA at the concentration used (3 μM) catches a portion of ROS/RNS. The inhibitors of cyclooxygenases somewhat increase the ROS/RNS production in the aortas of control rats. This effect may be caused by the fact that, upon oxidation of arachidonic acid with cyclooxygenases, a lesser amount of ROS is formed as compared to that formed upon oxidation with lipoxygenases. When the activity of cyclooxygenases is suppressed, a great portion of the substrate is oxidized with lipoxygenases, and the production of ROS is enhanced. The essential decrease in ROS/RNS formation caused by L-NAME suggests that part of ROS/RNS in the aortas of control rats is also formed by the NO synthase. The treatment of rats with L-NAME changes the contribution of these enzymes to ROS/RNS production in the aorta. The effect of the inhibitor of 12- and 15-lipoxygenases decreases, though insignificantly, and the contributions of 5-lipoxygenase and NADPH oxidase increase reliably as compared to that in the aortas of control rats. As should be expected, the NO synthase inhibitor does not produce any effect in L-NAME-treated rats because NO synthase is already suppressed by the inhibitor received during drinking. The increase in the contribution of 5-lipoxygenase to ROS/RNS formation may be explained by the activation of monocytes attached to the endothelium, followed by their transformation into macrophages when rats take L-NAME (Kato et al. 1996; Koyanagi et al. 2000). Both types of cells contain a significant amount of active (intranuclear) 5-lipoxygenase (Mehrabian and Allayee 2003). The increase in the contribution of NADPH oxidase is caused by its activation by angiotensin II (Griendling et al. 1994; Rajagopalan et al. 1996; Landmesser et al. 2002), the concentration of which rises upon treatment of rats with L-NAME because of the increase in the ACE activity (Takemoto et al. 1997; Korystova et al. 2012). There are data showing that the activation of NADPH oxidase with angiotensin II depends on 5-lipoxygenase: angiotensin II activates 5-lipoxygenase and LTB4, a product of 5-lipoxygenase, activates NADPH oxidase (Luchtefeld et al. 2003). The reduction in the activity of 15-lipoxygenase and the enhancement in the activity of 5-lipoxygenase may be vital for the induction of atherosclerosis with L-NAME because the products of 15-lipoxygenase have an anti-inflammatory action, and the products of 5-lipoxygenase are reagents that provoke inflammation in different tissues (Serhan 1997). In rats, the intake of taxifolin combined with the NO synthase inhibitor neutralizes the effect of L-NAME and the contribution of 5-lipoxygenase and NADPH oxidase becomes insignificant, the same as in control rats: the inhibitors of these enzymes do not affect ROS/RNS (Table 1, lines 4 and 5).
Accordingly, low doses of taxifolin (30–100 μg/kg/day) normalize the ACE activity, the amount of ROS/RNS, and the contribution of enzymes to the formation of ROS/RNS that were changed by the agent initiating the vascular remodeling. A comparison of effective doses of quercetin and taxifolin demonstrates that taxifolin is a more likely candidate as a drug decreasing the risk of CVD than quercetin.
References
- Ackermann A, Fernandez-Alfonso MS, Sanchez-de-Rojas R, Ortega T, Paul M, Gonzales C. Modulation of angiotensin-converting enzyme by nitric oxide. Br J Pharmacol. 1998;124:291–298. doi: 10.1038/sj.bjp.0701836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis-Gorettaa L, Ottaviania JI, Keenb CL, Fragaa CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003;555:597–600. doi: 10.1016/S0014-5793(03)01355-3. [DOI] [PubMed] [Google Scholar]
- Actis-Gorettaa L, Ottaviania JI, Fragaa CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agricult Food Chem. 2006;54:229–234. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Braga FC, Serra CP, Viana Junior NS, Oliveira AB, Cortes SF, Lombardi JA. Angiotensin-converting enzyme inhibition by Brazilian plants. Fitoterapia. 2007;78:353–358. doi: 10.1016/j.fitote.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14:4295–4301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol. 2001;133:117–124. doi: 10.1038/sj.bjp.0704064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau VJ. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.HYP.37.4.1047. [DOI] [PubMed] [Google Scholar]
- Emel’yanov MO, Korystova AF, Kublik LN, Levitman MK, Shaposhnikova VV, Korystov YN. Low doses of ethanol decrease the activity of the angiotensin-converting enzyme in the aorta of aging rats and rats treated with a nitric oxide synthase inhibitor and dexamethasone. Clin Sci. 2012;122:75–81. doi: 10.1042/CS20110181. [DOI] [PubMed] [Google Scholar]
- Galisteo M, Garcia-Saura MF, Jimenez R, Villar IC, Zarzuelo A, Vargas F, Duarte J. Effects of chronic quercetin treatment on antioxidant defence system and oxidative status of deoxycorticosterone acetate-salt-hypertensive rats. Mol Cell Biochem. 2004;259:91–99. doi: 10.1023/B:MCBI.0000021360.89867.64. [DOI] [PubMed] [Google Scholar]
- Grassi D, Desideri G, Ferri C. Flavonoids: antioxidants against atherosclerosis. Nutrients. 2010;2:889–902. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Hamberg M. On the formation of thromboxane B2 and 12L-hydroxy-5, 8,10,14-eicosatetraenoic acid (12 ho-20:4) in tissues from the guinea pig. Biochim Biophys Acta. 1976;431:651–654. doi: 10.1016/0005-2760(76)90232-0. [DOI] [PubMed] [Google Scholar]
- Hansen K, Adsersen A, Smitt UW, Nyman U, Christensen SB, Schwartner C, Wagner H. Angiotensin converting enzyme (ACE) inhibitory flavonoids from Erythroxylum laurifolium. Phytomedicine. 1996;2:313–317. doi: 10.1016/S0944-7113(96)80075-4. [DOI] [PubMed] [Google Scholar]
- Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, Aviram M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice after consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.ATV.17.11.2744. [DOI] [PubMed] [Google Scholar]
- Heeneman S, Sluimer JC, Daemen M. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1111. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Fujita T. Oral flavonoid supplementation attenuates atherosclerosis development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:442–446. doi: 10.1161/01.ATV.0000148404.24271.fc. [DOI] [PubMed] [Google Scholar]
- Kameda K, Takaku T, Okuda H, Rimura Y. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J Nat Prod. 1987;50:680–683. doi: 10.1021/np50052a017. [DOI] [PubMed] [Google Scholar]
- Kato H, Hou J, Chobanian AV, Brecher P. Effects of angiotensin II infusion and inhibition of nitric oxide synthase on the rat aorta. Hypertension. 1996;28:153–158. doi: 10.1161/01.HYP.28.2.153. [DOI] [PubMed] [Google Scholar]
- Katoh M, Egashira K, Kataoka C, Usui M, Koyanagi M, Kitamoto S, Ohmachi Y, Takeshita A, Narita H. Regression by ACE inhibition of arteriosclerotic changes induced by chronic blockade of NO synthesis in rats. Am J Physiol Heart Circ Physiol. 2001;280:H2306–H2312. doi: 10.1152/ajpheart.2001.280.5.H2306. [DOI] [PubMed] [Google Scholar]
- Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- Kitts DD, Yuan YV, Godin DV. Plasma and lipoprotein lipid composition and hepatic antioxidant status in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Can J Physiol Pharmacol. 1998;76:202–209. doi: 10.1139/y98-010. [DOI] [PubMed] [Google Scholar]
- Korystov YN, Emel’yanov MO, Korystova AF, Levitman MK, Shaposhnikova VV. Determination of reactive oxygen and nitrogen species in rat aorta using the dichlorofluorescein assay. Free Radic Res. 2009;43:149–155. doi: 10.1080/10715760802644686. [DOI] [PubMed] [Google Scholar]
- Korystova AF, Emel’yanov MO, Kublik LN, Levitman MK, Shaposhnikova VV, Kim YA, Korystov YN. Distribution of the activity of the angiotensin-converting enzyme in the rat aorta and changes in the activity with aging and by the action of L-NAME. Age. 2012;34:821–830. doi: 10.1007/s11357-011-9282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984;792:92–97. doi: 10.1016/0005-2760(84)90287-X. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Egashira K, Kubo-Inoue M, Usui M, Kitamoto S, Tomita H, Shimokawa H, Takeshita A. Role of transforming growth factor-1 in cardiovascular inflammatory changes induced by chronic inhibition of nitric oxide synthesis. Hypertension. 2000;35:86–90. doi: 10.1161/01.HYP.35.1.86. [DOI] [PubMed] [Google Scholar]
- Lacaille-Dubois MA, Franck U, Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8:47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.HYP.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz W, Wohlfarta P, Scholkensa BA, Malinskib T, Wiemer G. Interactions among ACE, kinins and NO. Cardiovasc Res. 1999;43:549–561. doi: 10.1016/S0008-6363(99)00091-7. [DOI] [PubMed] [Google Scholar]
- Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 3′,5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–5539. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, Hufner A, Menichini F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae) Phytother Res. 2007;21:32–36. doi: 10.1002/ptr.2008. [DOI] [PubMed] [Google Scholar]
- Luchtefeld M, Drexler H, Schieffer B. 5-Lipoxygenase is involved in the angiotensin II-induced NAD(P)H oxidase activation. Biochem Biophys Res Commun. 2003;308:668–672. doi: 10.1016/S0006-291X(03)01456-6. [DOI] [PubMed] [Google Scholar]
- Maron DJ. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004;6:73–78. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- Mehrabian M, Allayee H. 5-Lipoxygenase and atherosclerosis. Cur Opin Lipidol. 2003;14:447–457. doi: 10.1097/00041433-200310000-00005. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Meunier M-T, Villié F, Jonadet M, Bastide J, Bastide P. Inhibition of angiotensin I converting enzyme by flavanolic compounds: in vitro and in vivo studies. Planta Med. 1987;53:12–15. doi: 10.1055/s-2006-962606. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Murata S, Nishio A. Role of ACE and NEP in bradykinin-induced relaxation and contraction response of isolated porcine basilar artery. Naunyn-Schmiedeberg’s Arch Pharmacol. 2002;365:365–370. doi: 10.1007/s00210-002-0543-0. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Munzel T, Keaney JF. Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation. 2001;104:1571–1574. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- Nandave M, Ojha SK, Arya DS. Protective role of flavonoids in cardiovascular diseases. Nat Prod Radiance. 2005;4:166–176. [Google Scholar]
- Nashel DJ. Is atherosclerosis a complication of long-term corticosteroid treatment. Am J Med. 1986;80:925–929. doi: 10.1016/0002-9343(86)90639-X. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42:301–316. doi: 10.1080/10408390209351919. [DOI] [PubMed] [Google Scholar]
- Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–1420. doi: 10.1016/S0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertens Res. 1996;19:1–8. doi: 10.1291/hypres.19.1. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity. Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts ACG, Leufkens HGM, Walker BR. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case–control study. Heart. 2004;90:859–865. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, Egashira K, Usui M, Numaguchi K, Tomita H, Tsutsui H, Shimokawa H, Sueishi K, Takeshita A. Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J Clin Invest. 1997;99:278–287. doi: 10.1172/JCI119156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-Y, Yang C, Li S-N. Effects of genistein on angiotensin-converting enzyme in rats. Life Sci. 2006;24:828–837. doi: 10.1016/j.lfs.2006.02.035. [DOI] [PubMed] [Google Scholar]