Abstract
Purpose of review
The role of regulatory T cells (Treg) in peripheral tolerance has been studied extensively in transplantation research. Recently, mast cells have been shown to play an indispensable role in allograft tolerance. The purpose of this review is to inform the reader on the current standings of the role of mast cells in dominant tolerance with an emphasis on the interaction of mast cells with Treg.
Recent findings
Mast cells are required to sustain peripheral tolerance via Treg. Treg can stabilize mast cells degranulation by contact-dependent mechanisms through the interaction of OX40 and its ligand OX40L, and by production of soluble factors, such as interleukin-10 and transforming growth factor-β. Conversely, the activation and subsequent degranulation of mast cells break peripheral tolerance.
Summary
Both mast cells and Treg are needed to create a local immunosuppressive environment in the transplant. Treg are not only necessary to suppress effector T-cell responses but also to stabilize mast cells. Mast cells in return could contribute to the immunosuppressive state by release of transforming growth factor-β, interleukin-10 and specific proteases. However, the molecular basis for mast cells control of Treg suppression in organ transplantation is still unresolved.
Keywords: dominant tolerance, mast cells, regulatory T cells
Introduction
Peripheral tolerance, subdivided in linked suppression and infectious tolerance, is explained by the generation of regulatory T cells (Treg) in the periphery called adaptive Treg (aTreg) [1]. The importance of aTreg in organ transplantation has been reported in clinical and animal studies [2,3]. Additionally, mast cells are necessary in the establishment of peripheral tolerance [4,5], and multiple studies [6,7,8••,9,10,11•,12] have addressed the impact of Treg on the stabilization of mast cells by several mechanisms. Conversely, how mast cells influence Treg or contribute to suppression of alloreactive immune responses needs yet to be elucidated. On the basis of current knowledge of mast cells, we will discuss some of the possible modes of interaction.
Background
The establishment of an adaptive immune system is dependent on a complex series of events to avoid self-reactivity. With regard to T-cell development, progenitors migrate from bone marrow to the thymus in which rigorous selection steps take place known as ‘central tolerance’. During this process, a small proportion of thymocytes upregulates the forkhead transcription factor forkhead box P3 (FoxP3) [13–15]. These T cells, called natural Treg (nTreg), have profound suppressive capacity in the development and function of effector T cells (Teff) [16]. They are able to silence self-reactive Teff that have escaped thymics election as shown in autoimmunity studies [17,18]. Furthermore, during antigen presentation by dendritic cells in the absence of costimulation, nTreg are able to induce a suppressor phenotype in naive T cells (called adaptive Treg, aTreg). This active process is known as infectious tolerance.
In allogeneic organ transplantation, the introduction of foreign proteins leads to the rapid expansion of Teff and subsequent rejection. Both experimental and clinical studies [2,3] have shown the importance of Treg in organ transplantation. Many experimental approaches have been designed to induce a population of Treg with specificity for these alloantigens to allow the allogeneic tissue to be accepted. Indeed, when looking at cellular composition in accepted grafts large numbers of Treg are present. Curiously, another cell type is also abundantly present: the mast cell.
Mast cells are members of the innate immune system and can be found at locations that are in close contact with the outside world such as skin, lung and intestinal mucosa. They are characterized by staining of granules with basic dyes [19], such as toluidine blue, and can also be detected by antibody staining for the highly expressed stem cell factor receptor, c-kit, in combination with the high affinity immunoglobulin E (IgE) receptor, FcεRI [20]. Until recently, they have been considered proinflammatory in both protection against parasitic infections and allergies. This side of the mast cells is based on the activation and subsequent degranulation mediated by cross-linking of FcεRI by IgE [20,21]. The immediate response leads to the release of a wide array of proinflammatory mediators, chemotactic factors and proteolytic enzymes, inducing a rapid inflammation and tissue remodeling [21]. Further, mast cells aid in wound healing by releasing factors that promote fibrogenic activities, platelet activation and recruitment of leukocytes to fight off possible infection [22–26]. Thus, although detrimental in allergies, the role of mast cells in mounting an immune response to defend against parasites and in maintaining the physical barrier is indispensable.
Mast cells are not known for their immune suppressive capacities, and many correlative studies [27] show an increased number of mast cells in rejecting grafts, suggesting that mast cells play a role in preventing graft tolerance. However, the initial work by Zelenika et al. [28] showed a high expression of mast cells-related gene products in tolerant grafts, emphasizing the beneficial role for mast cells in maintaining peripheral tolerance. Additionally, our laboratory showed, in a skin graft model, the functional need for mast cells during the initiation phase of tolerance [5]. This finding was later confirmed in a heterotopic heart transplant model [4]. The duality of mast cells as positive and negative regulators of the immune response is only beginning to be resolved.
Dominant tolerance
As mentioned above, tolerance can be defined depending on the mechanism involved in its establishment. In this regard, recessive tolerance is accomplished by deletion of alloreactive T cells. On the contrary, dominant tolerance is explained by the generation of aTreg and manifested as linked suppression and infectious tolerance, which will be discussed below [1].
Regulatory T cells
Early observations suggested that a specific population of CD4+ T cells is responsible for the prevention of autoimmune diseases. Elimination of these cells through genetic mutation in the Foxp3 gene, in the mutant mouse strain scurfy [29] and the human X-linked recessive syndrome immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) [30], resulted in profound systemic autoimmunity. FoxP3 is expressed in regulatory CD25+CD4+ T cells, and retroviral transduction of FoxP3 in naive CD4+CD25− T cells engendered suppressive properties among these cells [14]. In addition, transfer of this subset of CD4 T cells was able to protect against the development of autoimmunity [31]. Therefore, FoxP3 is regarded as a key marker that defines Treg.
It was shown that Treg suppresses the proliferation of the effector population by inhibition of interleukin (IL)-2 secretion on the target cells. This can either be contact dependent by activation of its T-cell receptor [32] through the expression of granzyme A [33] or B [34] or in a contact-independent manner via IL-10 [35], IL-35 or transforming growth factor beta (TGF-β) [36]. Further research demonstrated that more than one population of Treg can be found, which can be divided in two main groups. As described above, nTreg and aTreg, the latter including several distinct populations: regulatory type 1 T cell (Tr1) (IL-10-producing T cells) [35], T helper cell type 3 (Th3) (TGF-β-producing T cells) [36] and a recently described subset of reversion-resistant Treg derived in the presence of the vitamin A metabolite retinoic acid [37].
Linked suppression and infectious tolerance
Davies et al. [38] showed that copresentation of tolerated self-antigen with nontolerated alloantigen on the same antigen-presenting cell led to tolerance to both self-antigens, now known as linked suppression. This process is independent of CD8+ T cells, showing that the newly induced Treg are sufficient for this effect [39].
Another mechanism was revealed when naive T cells from untreated mice were transferred into tolerated mice in which all T cells were deleted by thymectomy and CD4 antibody treatment. These naive T cells were able to break the established tolerance [40]. Surprisingly, when the infused naive lymphocytes are allowed to coexist for 2 weeks with the tolerated T-cell repertoire before deleting this endogenous pool of lymphocytes, tolerance to skin grafts was maintained [41]. These elegant experiments clearly demonstrate that naive T cells can become suppressive by the coexistence with tolerant cells and was named ‘infectious tolerance’. Years later, the same observations were confirmed using a cardiac allograft transplantation model [42].
The mast cells–regulatory T cell axis in dominant tolerance
Although the role of Treg in the maintenance of peripheral tolerance is quite established, other cells of the immune system contribute significantly. As mentioned previously, mast cells are known for their proinflammatory properties, and it was surprising that mast cells were absolutely required for the establishment of tolerance toward an allogeneic transplant [4,5]. Still unclear are the mechanisms of interaction between the Treg and the mast cells that help maintain a favorable mast cell–Treg axis in dominant tolerance.
Proinflammatory and immune suppressive mast cells
It is known that during the maintenance phase of dominant tolerance, mast cells have a detrimental effect on graft survival, especially in highly vascularized tissues such as kidney, heart and lung. This effect has been attributed to the slow non-IgE-mediated degranulation causing fibrosis and intima hyperplasia [4,43,44]. In favor of this notion, a retrospective study [45] among allergic rhinitis patients who received a kidney transplant showed more severe episodes of rejection compared with non-atopic transplanted patients, suggesting that the release of proinflammatory mediators caused by IgE-mediated degranulation of mast cells induces the overt inflammation. We confirmed this finding in a skin graft model with ovalbumin-sensitized mice. Subsequent local challenge led to acute rejection of the graft, showing that mast cell degranulation breaks established acquired tolerance (unpublished observation).
However, we and others [4,5] have shown that for the establishment of tolerance, mast cells are essential. Further, in the murine skin graft model, increased levels of IL-9 were found. IL-9 is a cytokine abundantly secreted by Treg and is known to enhance mast cells growth and chemotaxis. The observation that IL-9 neutralization leads to graft loss confirms that IL-9 is an important molecular link between Treg and mast cells [5].
Regulatory T cell dampens the proinflammatory properties of mast cells
Recently, it was reported that Treg can directly stabilize mast cells by desensitizing mast cells against FcεRI-mediated degranulation. It has been shown in an in-vitro system with bone marrow-cultured mast cells that Treg can downregulate FcεRI in a contact-dependent manner [11•]. Additionally, Gri et al. [8••] showed that Treg can increase cyclic AMP (cAMP) and inhibit intracellular calcium flux in mast cells degranulation by OX40–OX40 ligand (OX40L) interaction in vitro and in vivo. This mechanism of mast cells desensitization was independent of IL-10 and TGF-β [11•].
However, IL-10, TGF-β and also IL-4 have been shown to have an impact on mast cells development, survival and FcεRI expression by different mechanism [6,7,10,12,46]. It was observed in signal transducer and activator of transcription 6 (STAT6)−/− mice that STAT6 signaling was absolutely required for IL-4-mediated FcεRI down-regulation [46], and the synergistic effect of IL-10 was not impaired [6]. In the presence of IL-4, the expression of the FcεRI-β-subunit was decreased, with minimal impact on the expression of the α-subunit, and no effect was observed on the expression of the γ-subunit. This down-regulation of FcεRI resulted in reduced responsiveness during late phase response characterized by infiltration of leukocytes and measured by tumor necrosis factor alpha (TNF-α) release. However, the immediate responses (that is, the release of granular content) were not altered, as measured by β-hexoaminidase release [6]. In the case of IL-10, it was shown that FcεRI downregulation was STAT3 dependent and also induced reduction of STAT5, Akt, Syk and Fyn in mast cells [12]. The latter four molecules are part of different pathways known to be involved in IgE-mediated degranulation [47–49]. However, the effect of IL-10 treatment had a significant impact on the immediate response to IgE [12]. Lastly, TGF-β impacts the rate of protein synthesis of the IgE receptor and not RNA expression, suggesting that TGF-β may regulate mast cells functions via posttranslation mechanisms [7]. The effects of TGF-β on protein synthesis have recently been shown to be SMAD dependent [50].
These observations clearly show that, during dominant tolerance, Treg not only play an important role in suppressing effector T-cell development and function but are also needed to regulate the responsiveness of mast cells to IgE. That Treg have a diminished suppressive capacity in atopic patients [51–53] could, therefore, contribute to increased mast cells degranulation and thus aggravating allergic inflammation, whereas in graft tolerance, the Treg suppress this proinflammatory response by suppressing FcεRI expression.
Possible contributions of mast cells to dominant tolerance
Although it is clear that Treg can directly influence mast cells function, little is known about the effects of mast cells on Treg. Here, we discuss some of the possible mechanisms that mast cells employ to regulate acquired peripheral tolerance.
Mast cells produce IL-10 and TGF-β, two suppressive cytokines. As such, mast cells are able to suppress T-cell proliferation and could possibly generate aTreg via the production of these cytokines. Indeed, mast cells can downregulate antigen-specific T-cell proliferation after mosquito bites in an IL-10-mediated fashion, suggesting that IL-10 is one of the immune suppressive mediators released by mast cells [54]. That IL-10 plays an important role in suppressing immune responses was emphasized by a study [9] in contact dermatitis, in which IL-10 derived from mast cells significantly reduced the skin disease as measured by leukocyte infiltration and inflammation. Moreover, this group [9] and others [55,56] showed in both rodents and humans that low levels of either ultraviolet A (UVA) or UVB irradiation lead to activation of mast cells in the skin with the subsequent release of both IL-10 and histamine. Additionally, type I interferons (IFNs) induce IL-10 and TGF-β secretion by human mast cells; however, it also downregulates OX40L [57]. As has been shown in the murine bladder carcinoma (MB49) model, tumors can induce IL-10 production by the infiltrating leukocytes, thereby contributing to the immunosuppressive environment [58]. Although the role of mast cells in tumors is not clear yet, their presence has been linked to a bad prognosis. IL-10 derived from mast cells could contribute to the generation of tumor-specific aTreg. Therefore, next to the Treg, it is likely that one of the main sources of IL-10 during tumor development is the mast cell [59,60]. The possible positive effects of immune suppression in transplantation have mostly been attributed to the presence of Treg, whereas the negative effect of fibroses and intima hyperplasia mostly point at the mast cells [43,61–64]. However, the actual impact of the mast cells-derived IL-10 and TGF-β on dominant tolerance has yet to be established.
Mast cells express the serotonin-specific transporter (SERT) that enables them to take up and store serotonin. When mast cells get activated by IgE, the stored serotonin can be released. Serotonin is considered an accessory ‘third’ signal for T-cell proliferation and is involved in early T-cell activation of both naive CD4+ and CD8− T cells [65–67]. It is, therefore, plausible that under tolerant conditions mast cells actively deplete the local environment of serotonin needed for robust T-cell responses.
Moreover, the ability to present antigen in the context of major histocompatibility complex class II (MHC-II) could also imply that they are able to influence T-cells responses. More recently, it has been shown that mast cells express other costimulatory molecules of both the TNF superfamily [OX40L, CD30 ligand (CD30L), Fas and glucocorticoid-induced TNF receptor (GITR)] as well as the B7 family [CD80, CD86, PD ligand 1 (PD-L1) and PD-L2], making them bona fide antigen-presenting cells [68]. Recently, Nakano et al. [69••] showed that Notch signaling was required for upregulation of both MHC-II and OX40L on mast cells. However, these mast cells skewed naive T cells to a Th2 phenotype, inducing the production of IL-4, IL-10 and IL-13 and suppressing the production of IFN-γ. It is known that the environment in a graft is Th2 skewed [70], therefore suggesting that antigen presentation by mast cells, under tolerant conditions, could benefit graft acceptance.
Lastly, the release of a wide array of de-novo-generated (proteolytic) enzymes could modulate the direct cytokine environment. Although nothing is known about the role of mast cell proteases in organ transplantation, we observed upregulation of monocyte chemoattractant protein 1 (MCP1) and MCP5 in tolerated allogeneic grafts [5]. Recently, the study [71] of a patient who had received a kidney transplant also showed elevated levels of mast cells tryptases in the blood, although no correlation was found with graft function.
A barrier with addressing the role of mast-cell-derived mediators in tolerance and immunity is the ability to conditionally control the production of defined mediators by mast cells. However, with the development of mast cells-specific protease driven Cre knock-in mice [72•,73•], the technology to temporally, conditionally and spatially control mast cells synthesis of mediators in vivo is now possible.
Conclusion
There is little doubt that mast cells are regulators of adaptive immune responses. Figure 1 shows the described interactions between mast cells and T cells in a very simplified form. Many results come from observations without having defined the mediators that underlie the phenomena. However, it is recognized that there is a complex set and series of interactions between mast cells and Treg. The two faces of mast cells, either as inducers of inflammation or regulators of tolerance, are likely based on whether mediators are released by degranulation or secretion, the nature of the subsets of mast cells that are involved and other environmental cues that control mast cells phenotype. Defining the molecular basis for mast cells regulation of immunity and tolerance is the quest for the future.
Figure 1. Interaction of mast cells with T cells under inflammatory and tolerant conditions.
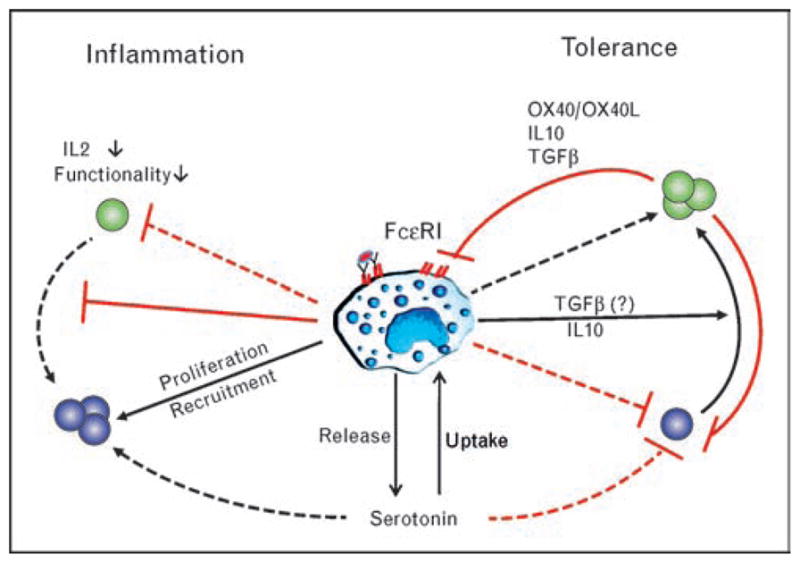
The binding of allergen IgE to the high affinity IgE receptor FcεRI arms the MC. Subsequent encounter with the allergen leads to the immediate release of granular content leading to a proinflammatory response as seen in allergies. This response leads to suppression of Treg functionality and recruitment and proliferation of Teff among other proinflammatory leukocytes. However, under tolerant condition, the MCs are needed to establish an immunosuppressive environment. The Treg present in the graft not only suppress Teff but also the proinflammatory properties of the MC mainly by influencing the expression of the FcεRI. IL, interleukin; IgE, immunoglobulin E; MC, mast cell; OX40L, OX40 ligand; Teff, effector T cell; Treg, regulatory T cell; TGF-β, transforming growth factor beta.
 , regulatory T cell;
, regulatory T cell;
 , effector T cell;
, effector T cell;
 , mast cell.
, mast cell.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 449).
- 1.Graca L, Chen TC, Le Moine A, et al. Dominant tolerance: activation thresholds for peripheral generation of regulatory T cells. Trends Immunol. 2005;26:130–135. doi: 10.1016/j.it.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 4.Boerma M, Fiser WP, Hoyt G, et al. Influence of mast cells on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20:256–265. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 5.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie SR, DeMartino RR, Zhu J, et al. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 7.Gomez G, Ramirez CD, Rivera J, et al. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Gri G, Piconese S, Frossi B, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. The first study to define the molecules involved in the contact-dependent interaction between Tregs and mast cell. The OX40–OX40L interaction resulted in increased cAMP levels and decreased calcium flux in the mast cells, thereby inhibiting degranulation. This interaction might be impaired in allergies, but on the contrary could play an important role in establishment of tolerance towards an allogeneic transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimbaldeston MA, Nakae S, Kalesnikoff J, et al. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 10.Kashyap M, Bailey DP, Gomez G, et al. TGF-beta1 inhibits late-stage mast cell maturation. Exp Hematol. 2005;33:1281–1291. doi: 10.1016/j.exphem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11•.Kashyap M, Thornton AM, Norton SK, et al. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–2043. doi: 10.4049/jimmunol.180.4.2039. The authors show a mutual interaction between mast cells and T cells. Mast cells actively recruit both Tregs and Teffs, whereas the Tregs lead to reduced FcεRI expression on the mast cells in a contact-dependent manner. Both T-cell subsets were able to induce IgE-mediated cytokine production in the mast cells, thereby showing that T cells can directly impact mast cell responses. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy Norton S, Barnstein B, Brenzovich J, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 15.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich P. Contributions to the theory and practice of histological staining. Leipzig: Leipzig University; 1878. [Google Scholar]
- 20.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 21.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Wong GW, Ghildyal N, et al. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J Biol Chem. 1997;272:31885–31893. doi: 10.1074/jbc.272.50.31885. [DOI] [PubMed] [Google Scholar]
- 23.Kanwar S, Kubes P. Ischemia/reperfusion-induced granulocyte influx is a multistep process mediated by mast cells. Microcirculation. 1994;1:175–182. doi: 10.3109/10739689409148272. [DOI] [PubMed] [Google Scholar]
- 24.Kauhanen P, Kovanen PT, Reunala T, Lassila R. Effects of skin mast cells on bleeding time and coagulation activation at the site of platelet plug formation. Thromb Haemost. 1998;79:843–847. [PubMed] [Google Scholar]
- 25.Mekori YA, Galli SJ. [125I]fibrin deposition occurs at both early and late intervals of IgE-dependent or contact sensitivity reactions elicited in mouse skin. Mast cell-dependent augmentation of fibrin deposition at early intervals in combined IgE-dependent and contact sensitivity reactions. J Immunol. 1990;145:3719–3727. [PubMed] [Google Scholar]
- 26.Thomas VA, Wheeless CJ, Stack MS, Johnson DA. Human mast cell tryptase fibrinogenolysis: kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry. 1998;37:2291–2298. doi: 10.1021/bi972119z. [DOI] [PubMed] [Google Scholar]
- 27.Jahanyar J, Koerner MM, Loebe M, et al. The role of mast cells after solid organ transplantation. Transplantation. 2008;85:1365–1371. doi: 10.1097/TP.0b013e31816fc0a3. [DOI] [PubMed] [Google Scholar]
- 28.Zelenika D, Adams E, Humm S, et al. The role of CD4+ T-cell subsets in determining transplantation rejection or tolerance. Immunol Rev. 2001;182:164–179. doi: 10.1034/j.1600-065x.2001.1820113.x. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 30.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 32.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman WJ, Verbsky JW, Tollefsen BL, et al. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 34.Gondek DC, Lu LF, Quezada SA, et al. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 35.Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 36.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies JD, Leong LY, Mellor A, et al. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607. [PubMed] [Google Scholar]
- 39.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–5816. [PubMed] [Google Scholar]
- 40.Qin SX, Wise M, Cobbold SP, et al. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990;20:2737–2745. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 41.Qin S, Cobbold SP, Pope H, et al. ‘Infectious’ transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 42.Onodera K, Lehmann M, Akalin E, et al. Induction of ‘infectious’ tolerance to MHC-incompatible cardiac allografts in CD4 monoclonal antibody-treated sensitized rat recipients. J Immunol. 1996;157:1944–1950. [PubMed] [Google Scholar]
- 43.Colvin RB, Dvorak HF. Letter: basophils and mast cells in renal allograft rejection. Lancet. 1974;1:212–214. doi: 10.1016/s0140-6736(74)92512-4. [DOI] [PubMed] [Google Scholar]
- 44.Yousem SA. The potential role of mast cells in lung allograft rejection. Hum Pathol. 1997;28:179–182. doi: 10.1016/s0046-8177(97)90103-9. [DOI] [PubMed] [Google Scholar]
- 45.Seung LM, Lorincz AL. Incidence of acute renal transplant rejection in atopic individuals. Arch Dermatol. 1994;130:584–588. [PubMed] [Google Scholar]
- 46.Ryan JJ, DeSimone S, Klisch G, et al. IL-4 inhibits mouse mast cell Fc epsilonRI expression through a STAT6-dependent mechanism. J Immunol. 1998;161:6915–6923. [PubMed] [Google Scholar]
- 47.Barnstein BO, Li G, Wang Z, et al. Stat5 expression is required for IgE-mediated mast cell function. J Immunol. 2006;177:3421–3426. doi: 10.4049/jimmunol.177.5.3421. [DOI] [PubMed] [Google Scholar]
- 48.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 49.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Gomez G, Yu SH, et al. TGF-beta1 attenuates mediator release and de novo kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–7272. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25 high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy. 2008;63:67–74. doi: 10.1111/j.1398-9995.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 52.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 53.Thunberg S, Akdis M, Akdis CA, et al. Immune regulation by CD4+CD25+ T cells and interleukin-10 in birch pollen-allergic patients and nonallergic controls. Clin Exp Allergy. 2007;37:1127–1136. doi: 10.1111/j.1365-2222.2007.02739.x. [DOI] [PubMed] [Google Scholar]
- 54.Depinay N, Hacini F, Beghdadi W, et al. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 55.Vocks E, Stander K, Rakoski J, Ring J. Suppression of immediate-type hypersensitivity elicitation in the skin prick test by ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 1999;15:236–240. doi: 10.1111/j.1600-0781.1999.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 56.Hart PH, Grimbaldeston MA, Swift GJ, et al. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita T, Kambe N, Uchiyama T, Hori T. Type I interferons attenuate T cell activating functions of human mast cells by decreasing TNF-alpha production and OX40 ligand expression while increasing IL-10 production. J Clin Immunol. 2006;26:512–518. doi: 10.1007/s10875-006-9043-1. [DOI] [PubMed] [Google Scholar]
- 58.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a nontumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- 59.Wasiuk A, de Vries VC, Hartmann K, et al. Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galinsky DS, Nechushtan H. Mast cells and cancer: no longer just basic science. Crit Rev Oncol Hematol. 2008;68:115–130. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Barr ML, Carey JN, Nishanian GP, et al. Addition of a mast cell stabilizing compound to organ preservation solutions decreases lung reperfusion injury. J Thorac Cardiovasc Surg. 1998;115:631–636. doi: 10.1016/S0022-5223(98)70328-9. discussion 636–637. [DOI] [PubMed] [Google Scholar]
- 62.Facoetti A, Fallarini S, Miserere S, et al. Histochemical study of cardiac mast cells degranulation and collagen deposition: interaction with the catecholaminergic system in the rat. Eur J Histochem. 2006;50:133–140. [PubMed] [Google Scholar]
- 63.Li QY, Raza-Ahmad A, MacAulay MA, et al. The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation. 1992;53:1047–1051. doi: 10.1097/00007890-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Zweifel M, Hirsiger H, Matozan K, et al. Mast cells in ongoing acute rejection: increase in number and expression of a different phenotype in rat heart transplants. Transplantation. 2002;73:1707–1716. doi: 10.1097/00007890-200206150-00004. [DOI] [PubMed] [Google Scholar]
- 65.Aune TM, Golden HW, McGrath KM. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J Immunol. 1994;153:489–498. [PubMed] [Google Scholar]
- 66.Laberge S, Cruikshank WW, Beer DJ, Center DM. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310–315. [PubMed] [Google Scholar]
- 67.Leon-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 69••.Nakano N, Nishiyama C, Yagita H, et al. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81. doi: 10.1016/j.jaci.2008.10.040. The authors defined a pathway that makes mast cells bona fide antigen-presenting cells. Notch signaling induced upregulation of both MHC-II and OX40L on the mast cells. Coculture of T cells with these mast cells resulted in proliferation and skewing to a Th2 phenotype. [DOI] [PubMed] [Google Scholar]
- 70.Singh N. Novel immune regulatory pathways and their role in immune reconstitution syndrome in organ transplant recipients with invasive mycoses. Eur J Clin Microbiol Infect Dis. 2008;27:403–408. doi: 10.1007/s10096-008-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barczyk M, Mysliwiec M, Kalinowski M, et al. Mast cells tryptase in patients after renal transplantation. Transplant Proc. 2008;40:3437–3439. doi: 10.1016/j.transproceed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 72•.Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163–166. doi: 10.1002/dvg.20378. Both Musch et al. [72•] and Scholten et al. [73•] developed mast cell-specific cre-expressing mice. Mast cell-deficient mice, such as the Wv/Wv and Wsh/Wsh, which are based on mutation in the cKit gene have many known and unknown variables that could alter the results, Therefore, these mice will prove to be invaluable to specifically address the role of mast cells and their mediators in inflammation and dominant tolerance. [DOI] [PubMed] [Google Scholar]
- 73•.Scholten J, Hartmann K, Gerbaulet A, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–315. doi: 10.1007/s11248-007-9153-4. Both Musch et al. [72•] and Scholten et al. [73•] developed mast cell-specific Cre-expressing mice. As reconstitution of mast cell deficient mice has many known and unknown variables that could alter the results, these mice will prove to be invaluable to specifically address the role of mast cells in inflammation and dominant tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]


