Abstract
The secretion of fluid and electrolytes by salivary gland acinar cells requires the coordinated regulation of multiple ion channel and transporter proteins, signaling components, and water transport. Importantly, neurotransmitter stimulated increase in the cytosolic free [Ca2+] ([Ca2+]i) is critical for the regulation of salivary gland secretion as it regulates several major ion fluxes that together establish the sustained osmotic gradient to drive fluid secretion. The mechanisms that act to modulate these increases in [Ca2+]i are therefore central to the process of salivary fluid secretion. Such modulation involves membrane receptors for neurotransmitters, as well as mechanisms that mediate intracellular Ca2+ release, and Ca2+ entry, as well as those that maintain cellular Ca2+ homeostasis. Together, these mechanisms determine the spatial and temporal aspects of the [Ca2+]i signals that regulate fluid secretion. Molecular cloning of these transporters and channels as well as development of mice lacking these proteins has established the physiological significance of key components that are involved in regulating [Ca2+]i in salivary glands. This review will discuss these important studies and the findings which have led to resolution of the Ca2+ signaling mechanisms that determine salivary gland fluid secretion.
Keywords: Calcium homeostasis, Calcium influx, Ion channels, Fluid secretion, Salivary gland cells, Physiology, Knockout-mouse models
Introduction
Ca2+ has a pivotal role in the physiological function of both excitable and non-excitable cells [1, 2]. It has been conclusively established that Ca2+ is the primary intracellular factor that regulates secretion in exocrine gland cells, including fluid secretion in salivary and lacrimal glands and protein secretion in pancreas [3–5]. Unlike in the pancreas, protein secretion in salivary glands is primarily controlled by β-adrenergic stimulation and mediated by cAMP-dependent activation of protein kinase-A. Moreover, the exact mechanism involved in protein secretion, i.e. granule fusion, in salivary gland cells is not yet understood. Although the contributions of various ion transporters to electrolyte and fluid secretion are also quite distinct in both gland types, there is remarkable similarity in the basic Ca2+ signaling mechanism(s) that regulate protein secretion in pancreas and fluid secretion in salivary glands. This review will primarily focus on our current understanding of the regulation of Ca2+ signaling in salivary gland cells and the role of Ca2+ in fluid secretion. We will briefly discuss aspects which have been noted to be distinct in the two types of glands.
Historically salivary glands have been widely used in studies to determine the mechanism(s) of fluid secretion and Ca2+ signaling. These early studies demonstrated that under normal physiological conditions, salivary glands maintain a continuous low level of saliva flow, often referred to as “resting” or “basal” secretion, which is dramatically increased upon demand, i.e. during a meal or other masticatory and gustatory stimuli. The regulation of secretion in the salivary glands is achieved by autonomic sympathetic and parasympathetic stimuli via a coordinated sequence of signal transduction and intracellular signaling events. These events include activation of membrane receptors and associated signal transduction proteins in the plasma membrane, generation of intracellular second messengers, calcium mobilization, and stimulation of ion transport pathways.
Salivary gland cells have receptors for a number of neurotransmitters which are localized in the plasma membrane which when stimulated lead to increases in fluid and protein secretion [3, 4]. Major receptors associated with salivary gland fluid secretion are muscarinic, α-adrenergic, and purinergic receptors. Receptor activation leads to activation of Gαq/11 family of G-proteins and phosphatidylinositol 4, 5, bisphosphate (PIP2)-specific phospholipase C (PLC), which results in hydrolysis of the membrane bound phospholipid, PIP2, and generation of inositol 1, 4, 5, trisphosphate (IP3) and diacylglycerol. IP3 diffuses into the cytosol and binds to the IP3 receptor (IP3R) localized on the endoplasmic reticulum (ER) membrane. This induces release of Ca2+ from the ER Ca2+ store(s) via the IP3R. Three subtypes of the IP3 receptor have been described (IP3R1, IP3R2 and IP3R3), of which IP3R2 and 3 are the major subtypes found in exocrine gland cells [4–10]. These are concentrated in the apical pole of the cells. The localization of IP3Rs in the acinar cells have important functional consequences. In pancreatic acinar cells, internal Ca2+ release originates in the luminal region of the cell and at lower levels of stimulation this signal is restricted to this region of the cell [6, 9–11]. At higher levels of stimuli the signal subsequently propagates to the basal regions of the cell. In salivary gland acinar cells the first signal is initiated in the apical region but unlike the pancreatic cells, the signal propagates to the basal region at either low or high levels of stimuli [12, 13]. The net result of the IP3-induced release of Ca2+ from the ER is an increase in the [Ca2+]i, which is the triggering event for the stimulation and sustained regulation of fluid secretion in salivary gland acini and protein secretion in pancreatic acini [4, 5]. While intracellular Ca2+ release is sufficient to trigger fluid secretion, sustained secretion is dependent on Ca2+ influx. In salivary gland acini, this influx is primarily mediated by store-operated calcium entry (SOCE) [4, 14]. Remarkably, this Ca2+ entry is activated by the intracellular Ca2+ release process [15, 16]. Thus, the coordinated activation and regulation of intracellular Ca2+ release and Ca2+ entry result in generating optimal [Ca2+]i signals that are required to drive sustained fluid secretion and also for regulation of other critical cellular functions.
A Historical Perspective of Calcium Signaling in Salivary Acinar Cells
Studies reported almost three decades ago show that Ca2+ is involved in the regulation of fluid secretion in the salivary gland [17, 18]. Experiments with perfused salivary gland preparations demonstrated that sustained fluid secretion was achieved only when Ca2+ was present in the external medium; while in the absence of external Ca2+, fluid secretion was only stimulated transiently. Similar effect of external Ca2+ was obtained in measurements of agonist stimulated Rb+ efflux in salivary gland cells (Rb+ is used as a surrogate for K+, a critical cation in fluid secretion). Increase of Rb+ efflux from the cells was biphasic with an initial rapid transient increase which was independent of external Ca2+ and a lower sustained release which was completely dependent on the external [Ca2+]. More direct involvement of Ca2+ in salivary gland physiology was demonstrated by studies showing a flux of Ca2+ following stimulation of salivary gland cells with muscarinic and α1-adrenergic receptor agonists [18]. The rates of Ca2+ uptake as well as that of Ca2+ release were increased. Thus, it was suggested that intracellular Ca2+ homeostasis was altered and the Ca2+ permeability of the cell membrane was increased following agonist stimulation of cells. Furthermore, the release of Ca2+ from the cell was temporally correlated with the activation of K+ efflux and increase in fluid secretion, while Ca2+ uptake was associated with prolonged K+ channel activity and fluid secretion. Experiments examining uptake of isotopic Ca2+ into the cell suggested the involvement of an internal Ca2+ store into which Ca2+ was sequestered, or released from following stimulation. Further, use of a permeabilized cell system demonstrated the presence of an ATP-dependent Ca2+ uptake mechanism and a Ca2+ store in the ER of exocrine gland cells which was proposed to be the likely agonist-sensitive Ca2+ store. A landmark study in the field of Ca2+ signaling was the demonstration that IP3 induced the release of Ca2+ from ER Ca2+ store(s) [19]. This important finding linked the increase in inositol lipid turnover following receptor stimulation to intracellular Ca2+ mobilization and enzyme secretion in pancreatic acinar cells. This study has been critical to our present understanding of the receptor-stimulated Ca2+ signaling since a similar mechanism is present in all non-excitable cells.
The introduction of fluorescent probes for measuring [Ca2+]i in the early 1980s revolutionized the field of Ca2+ signaling. The use of these dyes not only confirmed previous suggestions which were made regarding Ca2+ mobilization events in the salivary glands but also clearly established the sequence and spatial aspects of the [Ca2+]i changes at a cellular level. Studies using fluorescent dyes to monitor [Ca2+]i rapidly evolved from studies in cell populations to those in single cells. A primary observation made was that cell stimulation induced biphasic increase in [Ca2+]i; an initial rapid increase in [Ca2+]i which was transient in nature and decreased to a lower more sustained elevation [4, 5, 9, 10, 17]. Further, the initial increase in Ca2+ was not altered by removing Ca2+ from the external medium, suggesting that it was primarily due to release from an internal Ca2+ store while the subsequent, sustained, increase in Ca2+ was completely dependent on the presence of external Ca2+ and could be inhibited by the addition of La3+ or Ca2+ chelators to the external medium. These results confirmed that the second phase of Ca2+ increase was due to Ca2+ influx into the cell from the external medium. These studies firmly established that while fluid secretion as well as Rb+ and Cl− efflux could be stimulated by intracellular Ca2+ release, prolonged secretion was dependent on the sustained elevation in [Ca2+]i which was achieved primarily by Ca2+ influx. It has now been demonstrated that sustained activation of the three ion flux systems that are critical in the regulation of fluid secretion, namely the KCa channels, the Na+/K+/Cl− cotransporter, and TMEM16A channel are all dependent on Ca2+ influx as is the membrane trafficking of the water channel, AQP5 [5]. Studies in single cells also demonstrated an important feature of Ca2+ signaling. It was shown that low concentrations of agonists produced oscillatory changes in [Ca2+]i, some of which were also spatially restricted within a certain domain in the cell. The pattern of these oscillations was highly cell-type and agonist specific [6, 9, 10]. These studies also provided interesting clues to the underlying mechanisms that contribute to cytosolic [Ca2+]i increase.
Mechanisms that Contribute to Cytosolic [Ca2+]i Increase
The major mechanisms that contribute to regulation of [Ca2+]i following agonist stimulation of cells are Ca2+ channels in the intracellular membranes, including the ER, and those in the plasma membrane, calcium pumps, and mitochondria. The role of mitochondria in the spreading of cytosolic calcium waves has been studied somewhat in detail in some exocrine gland cells and the function of the Ca2+ pumps have also been examined in isolated cells preparations [3, 4, 6, 8–11, 13]. Here we will focus the discussion on the intracellular Ca2+ release and Ca2+ entry mechanisms.
The IP3-Sensitive Ca2+ Channel
One of the best characterized and most important Ca2+ channels in cells is the IP3-sensitive Ca2+ channel in the ER which allows Ca2+ to be rapidly released from the ER Ca2+ stores into the cytosol [19, 20]. The free [Ca2+] in the ER lumen is estimated to be between 70 and >300 μM, thus providing a large driving force for Ca2+ release from this pool. This channel is regulated in a complex way by a number of different factors and proteins and in turn IP3R regulates a number of key signaling events in the cell. As mentioned above, two major isoforms IP3R2 and IP3R3 are found in salivary gland acinar cells which are concentrated in the luminal end of the cell. This localization is consistent with the observation that agonist stimulated rise in [Ca2+]i initiated at the luminal pole of the cell. It has been well established that Ca2+ stimulates IP3-mediated Ca2+ release at lower concentrations and inhibits it at higher concentrations, >300 nM. The ability of Ca2+ to increase Ca2+ release is completely dependent on IP3. As the IP3 concentration increases, the receptor is more sensitive to lower [Ca2+]. This feed-forward and feedback regulation by Ca2+ ensures an open state of the channel when [Ca2+]i is low whereas when cytosolic Ca2+ levels are high, release from the ER Ca2+ store is restricted. Such regulation provides a process by which the ER Ca2+ stores can be protected and [Ca2+]i can be tightly regulated within the range required for the physiological function of the cell. Such regulation of IP3R can also account for [Ca2+]i oscillations, although not in all cell types.
Ca2+ Influx
Although evidence for the involvement of Ca2+ influx in salivary fluid secretion was provided almost 30 years ago [18] the molecular mechanism(s) involved in regulating this process proved to be a major challenge in the field of salivary gland physiology as well as calcium signaling in non-excitable cells. Interestingly, some of the earliest studies of Ca2+ influx were performed with salivary gland cells. These studies showed that Ca2+ influx into cells was increased several fold following stimulation of the cells with an agonist and inactivated when the stimulus was removed or an antagonist was added giving rise to the suggestion that Ca2+ influx was activated either directly as a result of agonist binding to the receptor, i.e. via receptor-operated Ca2+ entry (ROC) or by an intracellular second messenger via a second messenger operated entry (SMOC). An important study demonstrated that when cells were treated with the SERCA inhibitor, thapsigargin, there was slow depletion of internal Ca2+ stores and activation of Ca2+ influx which provided conclusive evidence that internal Ca2+ store depletion per se was the signal for activation of Ca2+ influx while refilling induced inactivation. This Ca2+ influx was termed capacitative Ca2+ entry (CCE) and currently is also referred to as store-operated Ca2+ entry (SOCE) [15]. SOCE is the primary mode of Ca2+ influx in salivary gland cells following stimulation with muscarinic and α1-adrenergic agonists [4, 5, 14].
The first model proposed by Putney for the regulation of SOCE suggested that the internal Ca2+ store is physically linked to the plasma membrane such that Ca2+ entering the cell directly enters the ER Ca2+ store from where it is released via the IP3-sensitive channel [15]. According to this model the resting level of Ca2+ in the ER exerted an inhibitory effect on the Ca2+ influx pathway and when the Ca2+ level decreased, following IP3-induced Ca2+ release, the inhibitory effect was removed and Ca2+ influx activated. However, using Mn2+ as a Ca2+ surrogate ion researchers clarified that the route of Ca2+ entry into ER was via the cytosol; i.e. Ca2+ entered the cell from where it was pumped into ER by the SERCA activity [15]. This Ca2+ entry pathway was shown to be involved in regulating key cellular functions; including exocrine secretion, platelet aggregation, endothelial cell permeability and migration, cell proliferation, T-lymphocyte activation, and mast cell degranulation. Thus identifying the mechanisms and components of this pathway has been a major focus of interest in the field of Ca2+ signaling.
A major problem in resolving this important mechanism was the lack of knowledge regarding the molecular components of the SOCE channel. Several models have been proposed to explain the mechanism(s) by which plasma membrane channels mediating SOCE are regulated; i.e. how the status of the ER-[Ca2+] is sensed and transmitted to the plasma membrane to regulate SOCE [15, 16, 21]. Two major hypotheses which have been proposed are the conformational coupling hypothesis and the diffusible messenger hypothesis. According to the former the depletion of Ca2+ in the ER lumen is detected by IP3R which undergoes a conformational change that is transmitted to the plasma membrane channels. However, later studies excluded a direct role for IP3R in gating the SOCE channel (further discussed below). The second model proposed the involvement of a diffusible metabolite that was released from the ER together with Ca2+. However, this model has not been supported with consistent and conclusive data and several studies have ruled out the possibility of such a diffusible factor. Other possible models include recruitment of channels to the plasma membrane by trafficking. While the exact mechanism involved in SOCE was not established until very recently, it was evident from early studies that the physical proximity of the ER and plasma membrane was critical in the activation of SOCE and that the activation was a relatively rapid process.
Resolving Receptor-Mediated Ca2+ Signaling Mechanisms in Salivary Gland Acini
Receptors
Identification of the specific muscarinic acetylcholine receptor (mAChR) subtypes mediating stimulation of salivary secretion was important not only for clarifying the components of salivary gland Ca2+ signaling but also of considerable clinical interest. It was reported earlier that the M(1) and M(3) subtypes are the major muscarinic acetylcholine receptors although their physiological relevance was not resolved until later by studies from Mikoshiba and Wess laboratories [22, 23]. These studies confirmed the earlier findings by demonstrating that carbachol-induced [Ca2+]i increase was markedly impaired in submandibular gland cells from mice lacking M(3) receptors and completely absent in those lacking both M(1) and M(3) receptors. This demonstrated that M(3) and M(1) play major and minor roles, respectively, in the cholinergically induced [Ca2+]i increase. Two-dimensional Ca2+-imaging analysis revealed a patchy distribution of M(1) in submandibular gland acini while there was ubiquitous distribution of M(3). In vivo administration of a high dose of pilocarpine (10 mg kg−1, s.c.) to M(3) knockout mice (M(3)−/− mice) caused salivation comparable to that in wild-type mice, while no salivation was induced in M(1)/M(3)−/− mice, indicating that salivation in M(3)−/− mice is caused by an M(1)-mediated [Ca2+]i increase. In contrast, a lower dose of pilocarpine (1 mg kg−1, s.c.) failed to induce salivation in M(3)−/− mice, but induced abundant salivation in wild-type mice, indicating that M(3)-mediated salivation has a lower threshold than M(1)-mediated salivation. In addition, M(3)−/−, but not M(1)−/−, mice, had difficulty in eating dry food, as shown by frequent drinking during feeding, suggesting that salivation during eating is mediated by M(3) and that M(1) plays no practical role in it. These results provide conclusive evidence the M(3) subtype is essential for parasympathetic control of salivation.
Protease-activated receptor-2 (PAR-2) is expressed in the salivary glands and when stimulated with an agonist, SLIGRL-OH, induced salivary flow in mice lacking M(3) and M(1) muscarinic receptors. In PAR-2−/− mice SLIGRL-OH-stimulated secretion was abolished [24]. Furthermore, compared with the secretion in WT mice, PAR-2-mediated salivary secretion and [Ca2+]i response were enhanced in mice lacking M(3) or both M(1) and M(3) mAChRs, in which mAChR-stimulated secretion and [Ca2+]i response in acinar cells were severely impaired. Although the mechanism underlying the enhanced PAR-2-mediated salivary secretion in M(3)-deficient mice is not clear, the result suggests the presence of some compensatory mechanism involving PAR-2 in the salivary glands deficient in cholinergic activation.
GPCR-Signal Transduction Mechanisms
While functional studies of either salivary or pancreatic secretion have not been carried out with mice lacking Gαq/11 or PLCβ, these proteins are associated with muscarinic receptor-mediated Ca2+ signaling. They are co-localized with receptors and key Ca2+ signaling proteins and this association has also been biochemically verified by co-immunoprecipitation. Spinophilin (SPL) and neurabin (NRB) are structurally similar scaffolding proteins. SPL binds regulators of G protein signaling (RGS) proteins and regulate the intensity of Ca2+ signaling by GPCRs. Studies with SPL(−/−) and NRB(−/−) mice show that SPL and NRB reciprocally regulate Ca2+ signaling by GPCRs. Deletion of SPL in mice enhanced binding of RGS2 to NRB and Ca2+ signaling by αAR, whereas deletion of NRB enhanced binding of RGS2 to SPL and reduced Ca2+ signaling by αAR. This was due to reciprocal modulation by SPL and NRB of the potency of RGS2 to inhibit Ca2+ signaling by αAR. These findings suggest a novel mechanism of regulation of GPCR-mediated Ca2+ signaling in which SPL/NRB form a functional pair of opposing regulators that modulate Ca2+ signaling intensity by GPCRs [25, 26].
RGS proteins accelerate the GTPase activity of Gα subunits to determine the duration of the stimulated state and control G protein-coupled receptor-mediated cell signaling. RGS2 is an RGS protein that shows preference toward Gα(q). In cells derived from RGS2(−/−) mice the kinetics of IP3 production was modified without an effect on the peak level of IP(3) [27]. The cells were also adapted to deletion of RGS2 by reducing Ca2+ signaling excitability. Reduced excitability was achieved by adaptation of all transporters to reduce Ca2+ influx into the cytosol. These findings highlight the central role of RGS proteins in Ca2+ signaling and reveal a prominent plasticity and adaptability of the Ca2+ signaling apparatus. It is highly likely that such modulation of Ca2+ signaling will significantly impact fluid secretion.
Functional Significance of InsP3R Family
InsP3R2 and InsP3-3 are the main receptors found in acinar cells leading to the question as to whether this is reflective of redundancy or do particular sub-types make specific contribution to the function of the gland. Genetic knockout of individual or a combination of InsP3R genes provided significant insight into these issues [28]. Futatsugi and colleagues reported that targeted ablation of either the type-2 or type-3 InsP3R individually, had no significant effects on muscarinic-receptor stimulated secretion and the animals had no overt phenotype. Consistent with these observations, the peak secretagogue-stimulated increases in [Ca2+]i were not altered by the deletion of InsP3R3 and only modestly impacted by the loss of the InsP3R2. In InsP3R2−/− acini, marked changes were only seen at low secretagogue or InsP3 concentrations. In both cases the spatial aspects of the signals were largely unaffected. Taken together these data indicate that the complement of InsP3R2 or InsP3R3 in isolation, or perhaps in combination with relatively low levels of InsP3R1, is sufficient to maintain signaling and preserve stimulated exocytosis. However, analysis of the compound InsP3R2/InsP3R3−/− animal revealed a much more striking phenotype. Although animals were born normally, they failed to survive past weaning, largely due to a failure to ingest and subsequently assimilate food. Even when fed wet mashed food to overcome the salivary deficit, the animals failed to thrive as a result of diminished pancreatic secretory function. The immediate cause of this was an almost complete absence of any measurable secretagogue-induced Ca2+ signal-even at supramaximal concentrations of agonist. The conclusion from these data is that while the residual expression of InsP3R1 is not sufficient to mount a Ca2+ signal, either the InsP3R2 or InsP3R3 in isolation. In addition, Ca2+ release via InsP3R was markedly influenced by the levels of cellular ATP, potentially linking the extent of Ca2+ release to the metabolic status of the cell. In acini, ATP (~Kd 40 μM) enhanced Ca2+ release at low levels of stimulation but did not influence release at high InsP3 levels. In contrast, in InsP3R2−/− mice presumably dominated by InsP3R3, ATP modulated release at all InsP3 levels although the Kd for this effect was 10 fold higher (~450 μM). Interestingly, the properties of the wild-type animal were essentially identical to those shown for the InsP3R2 in isolation, while the InsP3R2−/− mice mirrored those of the InsP3R3. These data indicate that while InsP3R2 and InsP3R3 are interchangeable for Ca2+ release, the individual InsP3Rs are not redundant. Further, when expressed together the properties of InsP3R2 dominate over InsP3R3. These results reveal IP3R2 and IP3R3 as key molecules in exocrine physiology underlying energy metabolism and animal growth. More importantly, the data show that there is minimal Ca2+ signaling including Ca2+ entry that occurs in the absence of IP3Rs, i.e. supported by PIP2 hydrolysis alone.
Ca2+ Entry
As discussed above, agonist-induced Ca2+ entry is required for sustained fluid secretion. Members of the transient receptor potential (TRP) channels have been proposed as components of the SOCE-channel [29–31]. Studies reported by Ambudkar and co-workers have provided evidence for the involvement of TRPC1 in SOCE in the human salivary gland cell line, HSG [4, 32–35]. These investigators provided further confirmation by carrying out studies using mice lacking TRPC1. Neurotransmitter-regulated salivary gland fluid secretion in TRPC1−/− mice was severely decreased (by 70%) [14]. Further, SOCE stimulated either by agonist- or thapsigargin (Tg) was significantly reduced in salivary gland acinar cells isolated from TRPC1−/− mice. Deletion of TRPC1 also eliminated sustained Ca2+-dependent potassium channel activity, which depends on Ca2+ entry and is required for fluid secretion. Expression of key proteins involved in fluid secretion and Ca2+ signaling was not altered. In addition, inhibitors of SOCE, 1 μM Gd3+ as well as 20 μM 2APB completely blocked SOCE in agonist and Tg-stimulated submandibular gland acini which demonstrated that SOCE is the primary Ca2+ entry pathway into these cells (this agrees with the lack of Ca2+ influx in acini from IP3R2 + IP3R3−/− mice discussed above). Together, these data demonstrate that reduced SOCE accounts for the severe loss of salivary gland fluid secretion in TRPC1−/− mice, thus providing the first evidence that SOCE supports fluid secretion. This important study finally established TRPC1 as a critical channel component of SOCE in salivary gland acinar cells which is essential for neurotransmitter-regulation of fluid secretion.
Another TRP channel protein, TRPC3, was shown to function as SOCE channel in vivo to mediate a significant portion of the receptor-stimulated Ca2+ influx in exocrine pancreatic cells. TRPC3-mediated Ca2+ influx in these cells affected the frequency of Ca2+ oscillations and moreover excessive Ca2+ influx by TRPC3 during supramaximal receptor stimulation was toxic to acinar cells and responsible in part to the cell stress and damage in pancreatitis. Such pancreatic damage was abrogated in TRPC3−/− mice. Therefore, it was suggested that inhibition of acinar cell TRPC3 and/or other Ca2+ influx channels may be considered as a strategy to control and reduce the severity of pancreatitis [36].
Other Components of SOCE
Recently two novel proteins, STIM1 and the Orai proteins, were identified as critical components of SOCE [16, 37]. STIM1 is a multidomain, Ca2+ binding protein that functions as the ER Ca2+ sensor. At the resting state STIM1 is diffusely localized in the ER with its luminal EF hand domain bound to Ca2+. Depletion of ER Ca2+ results in dissociation of Ca2+ from the STIM1-EF hand domain as well as aggregation, translocation, and clustering of STIM1 in close proximity to the plasma membrane where it interacts with the channels involved in SOCE. Orai1 is a plasma membrane protein, with four transmembrane domains, that has now been established as the main poreforming component of store-operated CRAC (calcium release activated Ca2+) channels that are found in T lymphocytes and other hematopoietic cells. Orai1 is gated by STIM1 in response to store depletion and co-expression of Orai1 and STIM1 generated CRAC channel function [38–40]. As predicted by the contribution of TRPC1 to SOCE, STIM1 was shown to bind and activate TRPC channels, including TRPC1 and TRPC3 [34, 35, 41–43]. Co-expression of STIM1 and TRPC1 resulted in generation of non-selective cation channels that are distinct from CRAC channels in their characteristics. These channels have been referred to as store-operated Ca2+ (SOC) channels. STIM1 gates Orai1 and TRPC1 by different mechanisms [43, 44]. While the STIM1-SOAR domain in the C-terminus of STIM1 interacts with the C terminus of Orai1 to gate the CRAC channel, the C-terminal polybasic (KK) motif of STIM1 activates TRPC1 by an electrostatic gating mechanism which results in SOC channel activation [39, 45, 46]. In aggregate, these data finally validated the conformational coupling model for the regulation of SOCE and ruled out other previously proposed models.
More intriguingly, it was shown that TRPC1 function is completely dependent on Orai1 [31, 46, 47]. When endogenous Orai1 expression is suppressed, TRPC1 + STIM1 function is eliminated. How exactly Orai1 regulates TRPC1 is a critical and as yet unresolved question. While Orai1 knock-out mice appear to have severe growth problems, STIM1 knockout mice survive [37]. Salivary gland function in either mouse model has not yet been tested. However in a recent study, TRPC1, STIM1 or Orai1 were knocked down in isolated exocrine cell preparations and this lead to reduced SOCE and frequency of Ca2+ oscillations [48]. Thus, although the exact physiological role of Orai1 and STIM1 in exocrine gland function needs to be confirmed by using relevant mouse models, these recent data provide further demonstration for the requirement of all three proteins in Ca2+ signaling in exocrine acinar cells.
Spatial Aspects of Ca2+ Signaling Mechanisms
The highly polarized nature of epithelial secretory cells in exocrine glands necessitates targeting of functionally relevant proteins to specific locations in the cells. Further, key signaling proteins are assembled into complexes which confines the Ca2+ signaling apparatus, and [Ca2+]i signals, to specific cellular microdomains. Such high degree of compartmentalization in the cellular localization has been shown for several Ca2+ signaling molecules, including G protein coupled receptors and their associated proteins, Ca2+ influx channels at the plasma membrane and Ca2+ release channels in the endoplasmic reticulum [8, 49, 50]. Although the physiological significance of polarized Ca2+ signaling can be predicted and has been experimentally validated to some extent, little is known about the mechanism of targeting, assembly, and retention of Ca2+ signaling complexes. For example, the consequence of polarized enrichment of Ca2+ signaling complexes at the apical pole facilitates the generation of an apical Ca2+ signal at low physiological agonist concentrations and limits propagation of the signal to the basal pole [6, 7, 9, 10, 12]. Close examination of the initiation and propagation pattern of repetitive Ca2+ waves evoked by the same agonist reveal that consecutive Ca2+ waves originate from the exact same initiation site and propagate along the same pattern. This indicates that the initiation site of the Ca2+ signal is likely defined by stably localized Ca2+ signaling proteins at the initiation sites. Consistent with this prediction, immunolocalization revealed concentration of Ca2+ signaling proteins at the apical pole of polarized cells. The Ca2+ signaling complexes at the apical pole appear to be much more sensitive to agonist stimulation than the complexes at the basal pole. The polarized localization of proteins and organelles results in generation of Ca2+ microdomains, primarily by limiting the diffusion of Ca2+ in the cell and segregating locally generated Ca2+ signals. An example of this is found in pancreatic acinar cells where mitochondria cause a fire wall effect and restrict [Ca2+]i elevations to the apical region [9, 10, 13]. A particularly interesting microdomain was discovered recently by measurement of [Ca2+]i using TIRF microscopy [51]. These measurements revealed a very high and sustained Ca2+ microdomain underneath the plasma membrane (PM) when SOCE is activated. The exact components required to sustain this microdomain as well as the functional relevance of this Ca2+ signal is not yet known. It is suggested that such localized sustained [Ca2+]i elevation is likely utilized for regulation of specific functions such as ion channel activation, activation of Ca2+ -dependent signaling; e.g. calcineurin, NFAT and NFκB. The frequency and amplitude of the [Ca2+]i signal determine the type of pathway activated as each is regulated by a specific type of Ca2+ sensor.
Interestingly, TRPC1 is primarily localized in the basal and lateral regions of acinar cells while TRPC3 is also seen in the apical region of the cell [14, 48]. Orai1 appears to be localized in the lateral membrane towards the luminal region and possibly at very low levels in the basal membrane [48, 49]. Following cell stimulation, STIM1 moves to the lateral and basal region of the cells where it co-localizes with Orai1 and TRPC channels which are localized there. The localization of these key components of SOCE suggests that they can contribute to the apical and basal [Ca2+]i signals. It is important to note that ion flux components as well as AQP water channels display polarized localization in acinar cells, consistent with the vectorial transport of ions and water from the basolateral region of the cell to the apical pole. Ca2+ regulated KCa channels as well as NKCC1 are localized in the basolateral region of acinar cells while TMEM16A and AQP5, which are also dependent on [Ca2+]i increase, are localized in the luminal membrane [4, 5]. Thus, it can be speculated that these different ion channels could be regulated by local changes in [Ca2+]i which are generated by the activity of Ca2+ channels residing in those specific cellular domains. The exact local and global [Ca2+]i increases contributed by Orai1 channels, localized in the lateral and luminal region of the cells, as well as TRPC1 which are localized in the basolateral regions in response to stimulation of cells by agonists needs to be determined. However, it is reasonable to hypothesize that amplitude and spatiotemporal signature of the [Ca2+]i increase will determine which ion channels involved in fluid secretion are regulated. Future studies should be directed to addressing some of these very physiologically relevant issues.
Conclusions and Questions
In summary, in the past four decades since the identification of Ca2+ as a critical factor in salivary gland function, several key components that are involved in generating and regulating cellular [Ca2+] signals have now been identified (Fig. 1). If we consider the muscarinic receptor signaling system, one of the main G-protein couples receptor signaling pathways that regulates salivary gland function, we now know that M(1) and M(3) receptors are functionally relevant. Downstream from these receptors, Gαq/11, PLCβ, and RGS proteins are involved in signal transduction and tuning the level of stimulation. Activation of the receptor and downstream signal transduction components leads to PIP2 hydrolysis and generation of IP3. While the role of DAG, other than in activation of PKC, is not yet known, IP3 binds to IP3R initiating intracellular Ca2+ release. IP3R2 and IP3R3 have been shown by Mikoshiba and colleagues to have critical and non-redundant functions in determining the magnitude and pattern of [Ca2+]i signals. This important study also showed that non-IP3R-dependent mechanisms do not have any significant contribution to agonist-stimulated [Ca2+]i signals. Interestingly, localization of these receptors in the cell is consistent with the spatial aspects of receptor-mediated [Ca2+]i increases and modulation of local cellular functions. Importantly, key aspects of the elusive Ca2+ influx pathway have now been elucidated. Studies from the Ambudkar lab have shown that TRPC1 is a major contributor to SOCE in salivary gland acinar cells and thus in the regulation of salivary fluid secretion. Muallem and co-workers have reported the relevance of TRPC1 in pancreatic acini and also demonstrated that TRPC3 contributes to SOCE in pancreatic acinar cells. Further, TRPC channels are regulated by STIM1 and Orai1. Important insights into the organization of the Ca2+ signaling mechanisms have now been provided. Studies from several leading researchers demonstrate that Ca2+ signaling proteins as well as Ca2+ signals and cellular functions are compartmentalized in cells. Concentrated localization of IP3Rs in the luminal region of the cells is consistent with the [Ca2+]i signals detected at that location as well as the main secretory functions that are triggered at the luminal membrane by Ca2+ elevations. Furthermore, consistent with the predicted site of Ca2+ influx, key components of SOCE; TRPC1, Orai1, and STIM1, are localized in the lateral and basal membrane regions in stimulated cells. Although the physiological relevance of Orai1 and STIM1 in the exocrine glands have yet to be described, the occurrence of these two proteins in areas where TRPC1 is located points out to the close functional interactions between these proteins. Together, these studies elucidate the key components of Ca2+ signaling in exocrine gland cells and provide insight into the spatiotemporal aspects of their contribution to salivary gland function. Further studies should address how exactly the Ca2+ signals are sensed and detected. What Ca2+ sensors are involved and how the Ca2+ signal is translated to regulate specific cellular functions? The mechanisms involved in the targeting and assembly of signaling complexes to various cellular locations is another area that needs to be examined in great detail. Of course, finally the effect of pathology or disease on Ca2+ -signaling or alternatively the contribution of Ca2+ signaling, or its deregulation, to exocrine gland dysfunction is a crucial area that will need to be addressed. Such studies will not only provide us with a comprehensive knowledge of the physiology of these important glands but also allow greater understanding of pathology and disease and lead to identification of new clinical targets and development of new therapeutic strategies.
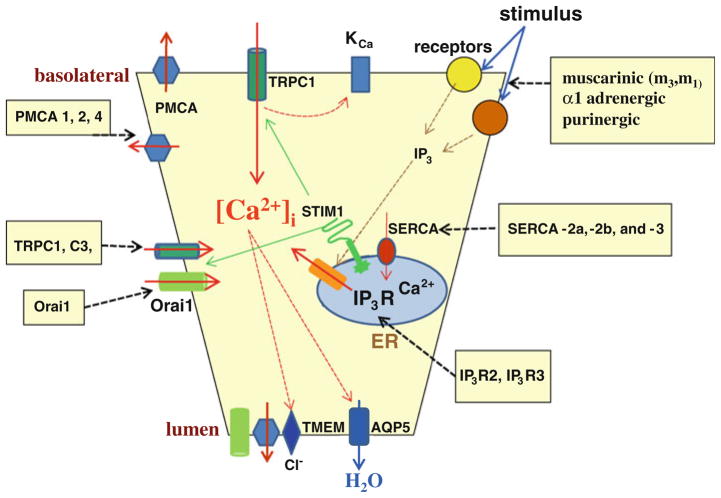
Fig. 1.
Ca2+ signaling mechanisms regulating salivary gland fluid secretion. The figure shows Ca2+ mobilizing events in acinar cells that are initiated by a stimulus and lead to fluid secretion. All molecular components, and the cellular domains, are labeled. Dashed arrows indicate mechanisms regulated by [Ca2+]i increase and those inducing [Ca2+]i increase. Boxes list the signaling proteins identified in these cells
References
- 1.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40(5–6):405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 4.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid, electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 5.Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–S8. doi: 10.1097/ICO.0b013e31817f246e. [DOI] [PubMed] [Google Scholar]
- 6.Yule DI. Subtype-specific regulation of inositol1, 4,5-trisphosphate receptors: controlling calcium signals in time and space. J Gen Physiol. 2001;117(5):431–434. doi: 10.1085/jgp.117.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 8.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 10.Petersen OH. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium. 2003;33:337–344. doi: 10.1016/s0143-4160(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 11.Sneyd J, Tsaneva-Atanasova K, Bruce JI, Straub SV, Giovannucci DR, Yule DI. A model of calcium waves in pancreatic and parotid acinar cells. Biophys J. 2003;85(3):1392–1405. doi: 10.1016/S0006-3495(03)74572-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce JI, Giovannucci DR, Blinder G, Shuttleworth TJ, Yule DI. Modulation of [Ca2+]i signaling dynamics and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J Biol Chem. 2004;279(13):12909–12917. doi: 10.1074/jbc.M309070200. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA. 2007;104(44):17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 15.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231(1):10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 16.Parekh AB, Putney JW., Jr Store-operated calcium channels. Phys Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 17.Putney JW., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- 18.Putney JW, Jr, Weiss SJ. Relationship between receptors, calcium channels, and responses in exocrine gland cells. Methods Cell Biol. 1981;23:503–511. doi: 10.1016/s0091-679x(08)61516-2. [DOI] [PubMed] [Google Scholar]
- 19.Streb H, Heslop JP, Irvine RF, Schulz I, Berridge MJ. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985;260(12):7309–7315. [PubMed] [Google Scholar]
- 20.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007;74:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 21.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114(Pt 12):2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558(Pt 2):561–575. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66(2):260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama T, Nakamura T, Obara K, Inoue H, Mishima K, Matsumoto N, Matsui M, Manabe T, Mikoshiba K, Saito I. Up-regulated PAR-2-mediated salivary secretion in mice deficient in muscarinic acetylcholine receptor subtypes. J Pharmacol Exp Ther. 2007;320(2):516–524. doi: 10.1124/jpet.106.113092. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Zeng W, Kim MS, Allen PB, Greengard P, Muallem S. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 2007;26(11):2768–2776. doi: 10.1038/sj.emboj.7601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7(4):405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 27.Luo X, Ahn W, Muallem S, Zeng W. Analyses of RGS protein control of agonist-evoked Ca2+ signaling. Methods Enzymol. 2004;389:119–130. doi: 10.1016/S0076-6879(04)89008-6. [DOI] [PubMed] [Google Scholar]
- 28.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309(5744):2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 29.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci USA. 1996;93(26):15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 31.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. J Biol Chem. 2000;275(5):3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 33.Ambudkar IS. Ca2+ signaling microdomains: platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27(1):25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans. 2007;35(Pt 1):96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- 35.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42(2):213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137(4):1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 39.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136(5):876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 41.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42(2):205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282(12):9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9(6):636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32(3):439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11(3):337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/ Orai1/TRPCs. FEBS Lett. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store operated TRPC1-STIM1 channels. J Biol Chem. 2008;283(19):12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12(2):232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lur G, Haynes LP, Prior IA, Gerasimenko OV, Feske S, Petersen OH, Burgoyne RD, Tepikin AV. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr Biol. 2009;19(19):1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem. 2005;280(13):12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- 51.Won JH, Yule DI. Measurement of Ca2+ signaling dynamics in exocrine cells with total internal reflection microscopy. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G146–G155. doi: 10.1152/ajpgi.00003.2006. [DOI] [PubMed] [Google Scholar]