SUMMARY
Surgical resection is one of the standard therapeutic choices for the treatment of hypopharyngeal cancer, whether or not combined with postoperative radiotherapy. The type of operation depends on the extension of the lesion and the subsites involved and often requires some form of reconstruction. Reconstructive strategies depend on whether the larynx, or part of it, has been preserved. We believe that the decisional flow-chart of the reconstructive methods after hypopharyngeal cancer resection should be based not only on the extent of resection, but also on the subsites involved. This report presents a literature review on the management of cancer of the hypopharynx and a proposal for a surgical decisional flow-chart.
KEY WORDS: Cancer, Hypopharynx, Flap reconstruction
RIASSUNTO
L'intervento chirurgico è il trattamento di scelta per il trattamento dei tumori ipofaringei, associato o meno a trattamento radioterapico post-operatorio. Il tipo di chirurgia dipende dall'estensione della lesione e dalle sottosedi interessate. Frequentemente si rende necessaria una ricostruzione e le strategie adottate dipendono dalla possibilità o meno di preservare la laringe. Riteniamo quindi che l'algoritmo decisionale per la ricostruzione o meno dopo chirurgia dell'ipofaringe debba essere basato non solo sull'estensione della resezione ma anche sulla sottosede coinvolta. Questo lavoro presenta una revisione della letteratura sulla gestione del cancro dell'ipofaringe ed una proposta di algoritmo decisionale chirurgico.
Introduction
Squamous cell carcinoma of the hypopharynx accounts for about 5% of all head and neck cancers and includes primary hypopharyngeal tumours and advanced tumours from other sites, most notably the larynx 1. The most frequently affected site is the pyriform sinus representing 70% of cases, followed by the retrocricoid region (15- 20%) and the posterior wall (10-15%) 2-4.
Carcinomas in this region are generally more common in males, aged around 55 years, the exception being tumours in the retrocricoid region, seen in about 30% of British women and unrelated to alcohol consumption or smoking, which are the two main risk factors for hypopharyngeal cancer 5.
Given the late presentation of symptoms and considerable submucosal spreading of the tumour, squamous cell carcinoma of the hypopharynx is usually detected in advanced stage (III and IV), often with locoregional and/or distant metastases and, consequently, has a poor prognosis 6.
Treatment options include radiotherapy, chemotherapy and surgery, alone or combined 7. Early cancers of the hypopharynx can be treated with radiotherapy alone. In terms of locoregional control and survival rates, results are comparable to those of partial surgery 8.
However, radiotherapy alone does not appear to provide a satisfactory outcome in advanced tumours compared to radical surgery and eventual adjuvant radiotherapy, in terms of locoregional control and survival. In fact, mean five-year survival in patients treated with radiotherapy alone is estimated to be between 12.7 and 13.9%. Survival rates among patients undergoing radical surgery followed by post-operative radiotherapy range from 25-60% 6. Moreover, salvage surgery performed after high dose radiotherapy gives poor results and has high comorbidity rates, especially considering the formation of salivary fistulas 7-12. Neoadjuvant chemotherapy for head and neck tumours has been studied to establish whether it improves the outcome of surgery and radiotherapy albeit with poor results, or as a palliative treatment 11 12. Several authors claim that chemotherapy, alone or combined with radiotherapy, appears to ensure control over locoregional recurrence and disease-free survival, with results comparable to those achieved by surgical procedures, preserving speech and swallowing, for a better outcome 9.
Another therapeutic approach involves concurrent chemoradiation, which appears to guarantee good results in terms of five-year survival (30.7%) 9. However, the highly toxic effects of this concurrent treatment should be taken into account. In the long term, patients often complain of persisting severe dysphagia that sometimes requires percutaneous endoscopic gastrostomy; a further possible complication is pharyngeal-oesophageal stenosis needing multiple dilation procedures. Some patients, instead, continue to rely on tracheal cannula 13.
Surgical resection, more or less radical, continues to be the standard therapeutic choice, whether or not combined with postoperative radiotherapy, even in advanced-stage patients or in those who are unfit to tolerate the other forms of treatment mentioned above. Surgical resection, followed by radiation therapy if necessary, has a higher disease- free survival rate compared to protocols aimed at organ preservation (chemo-radiotherapy) (five-year survival 52 vs. 42%), particularly in the event of large tumours and the presence of neck metastases 9. In addition, primary surgery ensures greater tumour extirpation resulting in better disease control.
Preoperative management
The most suitable therapeutic approach for the management of hypopharygeal carcinomas must be decided after a thorough preoperative assessment of the patient. Precise data regarding the patient's medical history is the first crucial diagnostic tool. Dysphonia and dysphagia are the first symptoms reported by patients; associated symptoms may also include reflex otalgia and dyspnoea if the tumour is large. Collection of data regarding medical history must be followed preferably by high definition endoscopic examination with narrow band imaging (NBI) and/or autofluorescence (AF). Although they use different principles, these methods allow a much clearer definition of the areas affected by the tumour compared to standard white light endoscopy, thus ensuring better clinical staging of the disease 14 15.
Some subsites, such as the pyriform sinus and the retrocricoid area, are difficult to explore with a fiberscope. However, evidence of indirect signs like oedema and hyperaemia of the mucosa and/or the presence of impaired salivary drainage should point to the need for further diagnostic investigation 7 16.
In these cases a direct microlaryngoscopy with rigid angled optics is mandatory, eventually associated with NBI and/or AF. This allows the surgeon not only to carry out a systematic evaluation of the upper aerodigestive tract and oesophagus (searching for signs of any synchronous tumours), but also to perform targeted biopsies of the lesions visualized 7 11 12.
Tumour staging should then be completed with computed tomopgraphy (CT)-scan, MRI or positron emission tomography (PET)-CT imaging. CT determines both the locoregional extent and depth of invasion of the tumour as well as lymph node involvement. If the tumour extends laterally, the thyroid cartilage, the first barrier to cancer spread, may present signs of sclerosis in the event of initial invasion, or be completely interrupted. Sagittal sections are able to determine whether the tumour has invaded the paraglottic and pre-epiglottic space, (if tumour volume extends inward and forward) 11.
Compared to CT, MRI offers a better resolution of soft tissue and a higher sensitivity in evaluating cartilage infiltration (albeit CT has better specificity); however, it is much more sensitive to the slightest movement of patients and therefore, is not routinely used in the clinical staging of hypopharyngeal tumours 11. Compared to other imaging techniques, PET is less frequently used to clinically stage tumours in the hypopharynx region. Despite its high sensitivity in identifying tumour presence, it has a much lower specificity due to false positive results caused, for instance, by inflammatory processes or foci of infection, which is not uncommon in this region 12.
Integrated PET-CT has been introduced to improve spatial specificity and resolution. Some studies report that PETCT seems to have a sensitivity equal to PET alone, but a considerably higher specificity compared to CT alone, both in initial staging (90.5 vs. 62.2%, p < 0.01) and during follow-up (97.2 vs. 74.4%, p < 0.01) 17. Furthermore, combined PET-CT appears to be very useful in the search for an unknown primary tumour presenting with laterocervical metastases 18. PET and/or PET-CT are therefore particularly indicated for a more thorough assessment of synchronous tumours, distant metastases and retropharyngeal lymph node involvement, which are decisive factors in the choice of treatment 19 20 as well as during follow-up. Thanks to their sensitivity in detecting the slightest disease persistence or recurrence, salvage treatment can be initiated earlier 21 22.
Ultrasound scanning of the neck is rapid, non-invasive and inexpensive, and can assess the status of laterocervical lymph nodes and provide a diagnosis through fine needle aspiration cytology. However, it achieves a high sensitivity and specificity only if performed by experts with a long-standing experience in this diagnostic field 23.
Decisional flow charts
The surgical management of the hypopharyngeal cancer depends on the lesion's extension and the subsites involved, and often requires some form of reconstruction. Reconstructive strategies for patients with defects of the upper aero-digestive tract are extremely versatile and depend on whether the larynx, or part of it, has been preserved. If the larynx is totally resected, in fact, a separate conduit for breathing and swallowing are established and restoration of the principal functions to this region are markedly different. Urken et al., in 1997, proposed a classification system based on the anatomic and functional regions of the laryngopharynx 24; in this scheme, the division of the hypopharynx into the lateral and posterior walls is useful to reflect if a defect needs to be resurfaced after partial or radical surgery.
In 2003, Disa et al. 25 proposed a classification based on the types of defect of the pharyngo-oesophageal segment after total laryngectomy so as to choose the most suitable reconstruction method. The following resection methods were described:
Type 0: minimal defects of the pharyngo-oesophageal segment that are amenable to primary closure
Type I: partial lesions affecting less than 50% of the pharyngo- oesophageal segment but are not amenable to primary closure
Type II: partial lesions affecting more than 50% of the pharyngo-oesophageal segment
Type III: extended longitudinal lesions involving other anatomical regions (nasopharynx, oropharynx, floor of the mouth or jaw)
Type IV: pharyngo-oesophageal defect with extension to cervical oesophagus.
These Authors claimed that type 0 lesions can be closed primarily. For a type I or II defect, the use of a pharyngeal strip of native mucosa is recommended to prevent a circumferential scar forming at the proximal or distal end of the repair 25 26; if the native tissues have been exposed to radiation and/or chemotherapy, the risk of development of a fistula or a stricture is high.
To prevent this complication, Urken et al. 27 proposed a new classification scheme:
Type 0: minimal defects of the pharyngo-oesophageal segment that are amenable to primary closure
Type I: non-circumferential defect with a presence of a viable strip of mucosa, measuring a minimum of 2 cm in width
Type II: circumferential defect extended no further cephalad than the level of the vallecula
Type III: circumferential or non-circumferential defect extended cephalad to the level of the vallecula
Type IV: any resection that extends caudal to the level of the clavicles.
Moreover, they include a superscript "i" to indicate the wound healing is impaired because of prior therapy and likely to be problematic and the superscript "s" to indicate the necessity of a flap of skin for wound closure.
We believe that the decisional flow-chart of the reconstructive methods after surgery of the hypopharynx should be based not only on the extent of resection, but also on the subsites involved.
Partial resections
Figure 1 shows our proposal of decisional flow-chart in case of partial resections of the hypopharynx, i.e. in T1, T2 and some T3 tumours involving only one of the subsites of the hypopharynx (lateral wall, pyriform sinus, posterior wall). Above all, the choice of surgical procedure depends on the close proximity of hypopharyngeal mucosa to the larynx: in fact, in most cases, this usually involves having to carry out partial or total resection of the larynx itself.
Fig. 1.
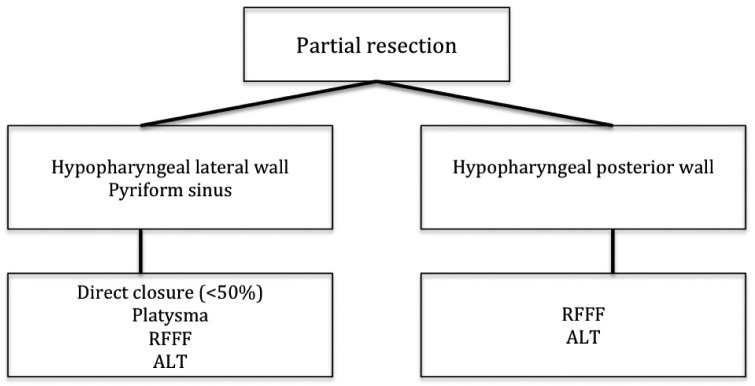
Principles of reconstruction after partial resection of the hypopharynx.
The resection defect of tumours affecting the lateral wall of the pyriform sinus can be closed directly if the extent of the lesion is less than 50%; otherwise, reconstruction is needed, preferably with a thin, pliable flap.
Reconstructive techniques may involve the choice of a pedicled myocutaneous platysma flap, a radial forearm free flap (RFFF) or anterolateral thigh flap (ALT) 28 29. If the tumour has invaded the entire pyriform sinus, conservative surgery for advanced lateral pharyngo-laryngeal tumours is feasible only in selected cases:
pharyngo-laryngeal tumours limited to one wall with or without extension into the pyriform sinus apex;
the tumour must not cross the midline of the retrocricoid region;
the tumour must not extend cranially infiltrating the inter-arytenoid mucous membrane (putting contralateral arytenoid cartilage at risk);
the integrity and movement of the healthy vocal cord should be preserved to re-establish functional speech;
tumour extension into the posterior hypopharyngeal wall need not be an absolute contraindication to surgery as long as the lateral wall of the contralateral pyriform sinus is not affected.
One surgical approach that can be used in these cases is that put forward by Urken 24. This procedure involves vertical hemilaryngectomy and removal of half of the hyoid bone, the epiglottis, thyroid cartilage and the cricoid, if the tumour involves the pyriform sinus apex. The following step is the reconstruction of the anatomical defect with a RFFF, together with the harvesting of a segment of rib cartilage so as to recreate the glottic plane. Hagen suggested modifying this technique, eliminating the use of cartilage to widen the remaining breathing space in the larynx 30.
Instead, when the tumour affects the posterior wall of the hypopharynx without extension below the arytenoid plane and into the cervical oesophagus, the choice of reconstruction method has to consider functional outcome in terms of swallowing. In the past, this tumour site required total laryngectomy due to the lack of reconstructive techniques. Nowadays, however, the larynx can be preserved as a result of the introduction of free flaps 31 32.
Reconstruction of the posterior wall can be carried out using a platysma flap, particularly if less than 50% of the mucosa has to be resected. This pedicled flap has the advantage of being thin and pliable with a reduced operating time compared to free flaps. However, traction on the vascular pedicle involves a high risk of flap necrosis. For this reason, reconstruction should use a RFFF or ALT if the clinical patient's condition is good. Some Authors 32 consider the RFFF an excellent reconstructive choice in these kinds of patients; however, they do report a high risk of chronic aspiration with the resulting need for long-term enteral feeding (71%) 32.
In accordance with this, Lydiatt et al. retain that partial laryngectomy and subsequent reconstruction with a RFFA is feasible with acceptable morbidity in patients with no underlying diseases (ASA 1 and 2); this procedure is unadvisable in patients for whom anaesthesia poses a higher risk (ASA ≥ 3) 33.
In our case-series of 165 hypopharyngeal reconstructions performed between November 1995 and April 2012, 41 patients (25%) underwent reconstruction following partial pharyngectomy with partial or total resection of the larynx. Hemipharyngo-total laryngectomy was performed in 15% of patients; partial pharyngectomy in 2% of cases and 8% of patients underwent vertical hemipharyngolaryngectomy according to Urken's technique 24.
Table I summarizes the type of ablative surgery and reconstruction performed. A pedicle flap was used in 46% of these cases (group A); while a free flap was conducted in the other 54% (group B).
Table I.
Type of reconstruction vs. type of partial pharyngectomy.
| Type of surgery | Group A | Group B | Total | |||
|---|---|---|---|---|---|---|
| PM | Platysma | SCM | RFFF | ALT | ||
| Urken | - | - | - | 13 (32%) | - | 13 (32%) |
| Hemipharyngo-TL | 16 (40%) | - | 1 (2%) | 7 (18%) | 1 (2%) | 25 (62%) |
| Partial pharyngectomy | 1 (2%) | 1 (2%) | - | 1 (2%) | - | 3 (6%) |
| Total | 19 (46%) | 22 (54%) | 41 (100%) | |||
TL: total laryngectomy, PM: pectoralis major, SCM: sternocleidomastoid muscle, RFFF: radial forearm free flap, ALT: anterolateral tight flap.
Table II reports the analysis of complications in the different types of partial pharyngectomies. Minor complications, i.e. those requiring only medical treatment, occurred in 27% of the cases; major complications, i.e. those requiring re-intervention, occurred in 12% of the cases; while flap necrosis occurred in 2 cases of Urken's technique.
Table II.
Type of complications vs. type of partial pharyngectomy.
| Urken | Hemipharyngo-TL | Partial pharyngectomy | Total | |
|---|---|---|---|---|
| Minor | 2 (15%) | 7 (28%) | 2 (66%) | 11 (27%) |
| Major | 3 (23%) | 2 (8%) | - | 5 (12%) |
| Flap necrosis | 2 (15%) | - | - | 2 (5%) |
| Total | 7/13 (53%) | 9/25 (36%) | 2/3 (66%) | 18/41 (44%) |
TL: total laryngectomy.
Circular resections
Figure 2 shows the decisional flow chart to be used in case of circumferential resection of the hypopharynx. The surgical treatment of advanced stage tumours (T3- T4) necessitates a different reconstruction method depending on whether or not the tumour extends into nearby structures (cervical oesophagus, neck, oropharynx). In particular, if the tumour extends downward in the upper mediastinum, or in case of a synchronous tumour of the thoracic oesophagus, circumferential pharyngolaryngectomy needs to be associated with total oesophageal resection 8 34 35. In this case, gastric pull-up is the standard procedure due to its excellent blood supply, the relative ease of positioning and the single pharyngogastric anastomosis 35-37.
Fig. 2.
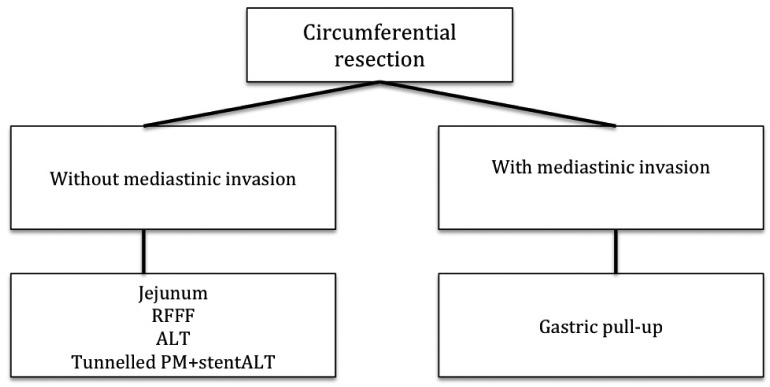
Principles of reconstruction after circumferential resection of the hypopharynx.
A study published by Dudhat et al. analyzed the incidence of complications that may occur after gastric pullup. These are divided into intraoperative complications (rupture of the trachea and pleura), post-operative complications (detachment from the anastomosis, hypocalcaemia, secondary haemorrage and abdominal wound dehiscence), and long-term complications, such as tracheal stoma stricture and gastric reflux. The authors conclude that gastric pull up involves minimal mortality, acceptable morbidity and short hospital stay 38.
If hypopharyngeal tumour extension is limited to the neck above sternum, the aim of reconstruction must bear in mind the two main functions of the hypopharynx, such as swallowing and speech. In the former case, contraction of the hypopharyngeal walls moves the food bolus into the oesophagus, while in the latter distension and vibration of the pharyngeal walls is responsible for phonation. Thus, reconstruction does not simply consist in replacing a 'tube', but remodelling a structure able to restore the functions of the hypopharynx as closely as possible 39.
The need to re-establish these functions gave rise to the idea of using a bowel flap tube due to its excellent intrinsic peristaltic properties. These characteristics are able to repair anatomical defects and peristalsis of the hypopharynx better than other flaps, and improves the quality of the patient's remaining life span 40 41.
Reconstruction of the hypopharynx using a jejunal flap requires two surgical teams. Disadvantages include a high risk of necrosis, fistulas and bowel complications 42.
Another problem is the discrepancy between the lumen of the flap and the pharyngeal defect when the resection extends to the oro- or nasopharynx.
Two reconstructions to overcome this problem have been proposed in our previous study. One solution is the creation of an end-to side anastomosis of the cranial end, easy and rather quick to perform, but with risk of kinking, stenosis, or creation of a blind loop; the second possibility is the creation of a jejunal reservoir. This technique is more difficult, longer, with a high risk of salivary fistulas, even though the recovery of the swallowing function is much more effective 43.
Other authors have reported the possibility to use other free flaps, such as RFFF and ALT, which are equally thin and pliable. In fact, these flaps have been shown to involve a much lower percentage of complications, such as fistulas and stenoses caused by scar tissue, compared to the jejunal flap, as well as offering comparable if not better speech production outcomes. 44 45.
The lower rate of complications at the donor site, the opportunity to close the donor site primarily and that of using a myocutaneous flap are advantages that lead some authors to prefer the ALT rather than the RFFF to reconstruct this anatomical site 1 46 47.
If a RFFF or ALT are chosen, these should be tunnelled and sutured to the prevertebral fascia instead of being tubed, to reduce the incidence of strictures and fistulas. A salivary stent has to be used during tunnel reconstruction to avoid salivary fistulas and to reduce retraction of scar tissue 48.
In case of poor clinical conditions or poor prognosis, it is better to adopt a quick and easy reconstruction. In this case, the first choice is represented by the pectoralis major flap (PM) 49 50.
This pedicled flap has an excellent blood supply which allows for single-stage reconstruction of the defect with minimal donor site morbidity; furthermore, its thickness can be used to fill large defects and helps to protect the carotid artery. However, the flap is often too bulky to allow tailoring into a tube for the reconstruction of circumferential defects of the pharyngo-oesophageal segment without the risk of stricture of the neopharyngeal lumen 6 51.
To avoid this complication, several surgeons 52 have adapted a surgical technique put forward by Fabian in 1984 53 consisting of tunnelling the pectoralis major flap to reconstruct the lateral and anterior walls of the hypopharynx and covering the prevertebral fascia with a skin graft 53. In this way, the bulkiness caused by the flap itself is reduced, re-establishing a sufficiently wide lumen which is kept open thanks to the positioning of a salivary stent (which is removed after 4-6 weeks). The modification suggested by Spriano avoids using a skin graft and involves suturing the posterior walls of the oropharynx and cervical oesophagus directly to the prevertebral fascia, which will form the posterior wall of the neopharynx 52. This technique has been supported by Soussez et al., who also did not consider it necessary to place a salivary stent 54.
Several Authors believe that pedicled flaps should be preferred over free flaps due to the relative ease of their placement, reduced operating times and shorter hospital stay. Moreover, compared to jejunal flaps, they do not give rise to complications that may be linked to abdominal surgery 44.
There is no doubt that pedicled flaps must represent the standard choice for salvage surgery after primary chemoradiation protocols, due to the patient's poor general condition, advanced stage of the disease and low life expectancy 6.
Moreover, other authors state that the functional outcome is not linked to the intrinsic characteristics of the flap, but to the surgical expertise used during reconstruction 52 55-57. In our experience, 121 (73%) of the 165 patients underwent reconstruction following total laryngectomy and circumferential resection of the pharynx for squamous cell carcinoma of the hypopharynx. Thirty-five percent of the patients were reconstructed with a pedicle flap (group A); while in the remaining 65% of cases reconstruction was performed with a free flap (group B) (Table III).
Table III.
Type of reconstruction for circumferential resection.
| Type of surgery | Group A | Group B | Total | |||
|---|---|---|---|---|---|---|
| PM | LD | Jejunum | RFFF | ALT | ||
| CPL | 40 (33%) | 2 (2%) | 64 (53%) | 10 (8%) | 5 (4%) | 121 (100%) |
| Total | 42 (35%) | 79 (65%) | 121 (100%) | |||
CPL: circumferential pharyngolaryngectomy, PM: pectoralis major, SCM: sternocleidomastoid muscle, RFFF: radial forearm free flap, ALT: anterolateral tight flap, LD: latissimus dorsi.
Flap necrosis occurred in 7% of patients of the group A and in 13% of patients of the group B. Major and minor complications were similar in the two groups (Table IV).
Table IV.
Type of complications vs. type of partial pharyngectomy.
| Group A | Group B | Total | |
|---|---|---|---|
| Minor | 17 (40%) | 14 (18%) | 31 (26%) |
| Major | 4 (10%) | 23 (29%) | 27 (22%) |
| Flap necrosis | 3 (7%) | 13 (16%) | 16 (13%) |
| Total | 24/42 (57%) | 50/79 (63%) | 74/121 (61%) |
The results in terms of recovery of swallowing after reconstruction with PM flap are controversial. Some authors found no significant differences in terms of recovery of free diet in patients undergoing reconstruction with a PM flap compared with patients reconstructed with free flaps 58 59; others have observed longer periods of NG-tube feeding and more dietary restrictions in reconstructions with pedicled flaps 59 60. Our experience has shown a higher possibility of restoration of normal feeding with free flaps instead of PM flap 59 61.
If the recovery of swallowing function after circumferential resection is obviously required, the recovery of vocal function is, wrongly, regarded as secondary, probably because of the low life expectancy of these patients.
We have already demonstrated that oesophageal voice rehabilitation is often impossible for both free and pedicled flaps 59 61. Pedicled flaps are usually too thick and stiff to vibrate during the passage of air from the stomach to the mouth, the jejunum flap does not allow the passage of air for its intrinsic peristalsis and the RFFF or ALT flaps, although thin and pliable, require a high air pressure to vibrate 59 61. The shunt between the trachea and the flap, thanks to the high expiratory pressure provided by the lungs, allows the vibration of the walls of free flaps, but it is insufficient to overcome the resistance offered by the walls of the pedicled flap 59 61. A voice-prosthesis represents the only opportunity to restore the capacity of communication in these kind of patients 25 37 40 59 62 63.
Conclusions
Poor diagnosis generally affects the severity of hypopharyngeal carcinomas. This occurs because they are asymptomatic for a long time and have thus reached an advanced stage by the time they are diagnosed. Cervical metastases are often the first signs of disease. Organ-preservation protocols have low survival rates and can, however, give rise to serious complications. Surgery often involves extensive resection, but reconstructive techniques using free flaps can minimize functional complications using tissues that are able to re-establish contractions and vibration of the walls of the pharynx.
If free flap reconstruction is not possible, the surgical approach must resort to using pedicled flaps. Even in this event, however, a thorough surgical procedure can guarantee acceptable functional outcomes. The oncological surgeon should be aware of and able to perform all the radical and reconstructive procedures so as to guarantee the patient not only adequate disease management, but also the best possible quality of residual life.
References
- 1.Patel R, Goldstein D, Brown D, et al. Circumferential pharyngeal reconstruction: history, critical analysis of techniques, and current therapeutic recommendations. Head Neck. 2010;32:109–120. doi: 10.1002/hed.21169. [DOI] [PubMed] [Google Scholar]
- 2.Bahadur S, Thakar A, Mohanti BK, et al. Results of radiotherapy with, or without, salvage surgery versus combined surgery and radiotherapy in advanced carcinoma of the hypopharynx. J laryngol Otol. 2002;116:29–32. doi: 10.1258/0022215021910302. [DOI] [PubMed] [Google Scholar]
- 3.Godballe C, Jorgensen K, Hansen O, et al. Hypopharyngeal cancer: results of treatment based on radiation therapy and salvage surgery. Laryngoscope. 2002;112:834–838. doi: 10.1097/00005537-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Ho C, Ng W, Lam K, et al. Radial clearance in resection of hypopharyngeal cancer: an indipendent prognostic factor. Head Neck. 2002;24:181–190. doi: 10.1002/hed.10002. [DOI] [PubMed] [Google Scholar]
- 5.Archibald S, Young JEM. A pharyngo-cervical esophageal reconstruction. Clin Plast Surg. 2005;32:339–346. doi: 10.1016/j.cps.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Chu P, Chang S. Reconstruction of the hypopharynx after surgical treatment of squamous cell carcinoma. J Chin Med Assoc. 2009;72:351–355. doi: 10.1016/S1726-4901(09)70386-7. [DOI] [PubMed] [Google Scholar]
- 7.Bradley P. Cancer of the hypopharynx Operative techniques in otolaryngology. Head Neck Surg. 2005;16:55–66. [Google Scholar]
- 8.Jones AS. The management of early hypopharyngeal cancer: primary radiotherapy and salvage surgery. Clin Otolaryngol. 1992;17:545–549. doi: 10.1111/j.1365-2273.1992.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Wu H, Heo D, et al. Advanced hypopharyngeal carcinoma treatment results according to treatment modalities. Head Neck. 2001;23:713–717. doi: 10.1002/hed.1101. [DOI] [PubMed] [Google Scholar]
- 10.Alcock C, Fowler J, Haybittle J, et al. Salvage surgery following irradiation with different fractionation regimes in the treatment of carcinoma of the laryngo pharynx: experience gained from a British Institute of Radiology Study. J Laryngol Otol. 1992;106:147–153. doi: 10.1017/s0022215100118924. [DOI] [PubMed] [Google Scholar]
- 11.Campora E. Edition Scientifiques et Médicales Elsevier. 2010. Encyclopédie medico-chirurgicale, otorinolaringoiatria. [Google Scholar]
- 12.Harrison LB, Hong WK, Roy B. Head and Neck Cancer: A Multidisciplinary Approach. Third edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 13.Lambert L, Fortin B, Souulières D, et al. Organ preservation with chemoradiation for advanced laryngeal cancer: are we succeeding? Int J Radiat Oncol Biol Phys. 2010;76:398–402. doi: 10.1016/j.ijrobp.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe A, Taniguchi M, Tsujie H, et al. The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol Head Neck Surg. 2008;138:446–451. doi: 10.1016/j.otohns.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka S, Saito Y. Endoscopic diagnosis of pharyngeal carcinoma by NBI. Endoscopy. 2008;40:347–351. doi: 10.1055/s-2007-995433. [DOI] [PubMed] [Google Scholar]
- 16.Gil Z, Fliss D. Contemporary management of head and neck cancer. Isr Med Assoc J. 2009;11:296–300. [PubMed] [Google Scholar]
- 17.Ishikita T, Oriuchi N, Higuchi T. Additional value of integrated PET/TC over PET alone in the initial staging and follow-up of head and neck malignancy. Ann Nucl Med. 2010;24:77–82. doi: 10.1007/s12149-009-0326-5. [DOI] [PubMed] [Google Scholar]
- 18.Rudmik L, Lau HY, Matthews TW, et al. Clinical utility of PET/TC in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011;33:935–940. doi: 10.1002/hed.21566. [DOI] [PubMed] [Google Scholar]
- 19.Sigg MB, Steinert H, Gratz K, et al. Staging of head and neck tumors: [18F]fluorodeoxyglucose positron emission tomography compared with physical examination ans conventional imaging modalities. J Oral Maxillofac Surg. 2003;61:1022–1029. doi: 10.1016/s0278-2391(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 20.Chan SC, Lin CY, Ng SH, et al. 18F-FDG PET for retrpharyngeal lymph node metastsis in oropharyngel and hypopharyngeal cancers: impact of diagnosis and prediction analysis. Nucl Med Commun. 2010;31:260–265. doi: 10.1097/MNM.0b013e3283360133. [DOI] [PubMed] [Google Scholar]
- 21.Conessa C, Hervè S, Foehrenbach H, et al. FDG-PET scan in local follow-up of irradiated head and neck squamous cell carcinoma. Ann Otol Rhinol Laryngol. 2004;113:628–635. doi: 10.1177/000348940411300806. [DOI] [PubMed] [Google Scholar]
- 22.Yao M, Graham MM, Smuyh RB, et al. Value of FDG PET in assessment of treatment response and surveillance in headneck cancer patients after intensity modulated radiation treatment: a preliminary report. Int J Radiat Oncol Biol Phys. 2004;60:1410–1418. doi: 10.1016/j.ijrobp.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 23.Brekel MW, Castelijns JA, Snow GB. The role of modern imaging studies in staging and therapy of head and neck neoplasms. Semin Oncol. 1994;21:340–348. [PubMed] [Google Scholar]
- 24.Urken M, Blackwell K, Biller H. Reconstruction of the laryngopharynx after hemicricoid/hemithyroid cartilage resection. Arch Otolaryngol Head Neck Surg. 1997;123:1213–1222. doi: 10.1001/archotol.1997.01900110067009. [DOI] [PubMed] [Google Scholar]
- 25.Disa J, Pusic A, Hidalgo D, et al. Microvascular reconstruction of the hypopharynx: defect classification, treatment algorithm, and functional outcome based on 165 consecutive cases. Plast Reconstr Surg. 2003;111:652–660. doi: 10.1097/01.PRS.0000041987.53831.23. [DOI] [PubMed] [Google Scholar]
- 26.Anthoi N, Tibirna G, Suharski, et al. Free flaps for type III pharyngoesophageal defects after enlarged ablative surgery for advanced cancer of larynx and hypopharynx. Microsurgery. 2003;23:189–193. doi: 10.1002/micr.10133. [DOI] [PubMed] [Google Scholar]
- 27.Urken M, editor. Atlas of Regional and Free Flaps for Head and Neck Reconstruction. VII edition. Philadelphia, PA: Lippincott, Williams & Wilkins; 2011. [Google Scholar]
- 28.Ho MW, Houghton L, Gillmartin E, et al. Outcomes following pharyngolaryngectomy reconstruction with the anterolateral thigh (ALT) free flap. Br J Oral Maxillofac Surg. 2012;50:19–24. doi: 10.1016/j.bjoms.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Yu P, Hanasono MM, Skoracki RJ, et al. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer. 2010;116:1718–1724. doi: 10.1002/cncr.24947. [DOI] [PubMed] [Google Scholar]
- 30.Hagen R. Functional long-term results following hemipharyngo- hemilaryngectomy and microvascular reconstruction using radial farearm flap. Laryngorhinootologie. 2002;81:233–242. doi: 10.1055/s-2002-25036. [DOI] [PubMed] [Google Scholar]
- 31.Putten L, Spasiano R, Bree R, et al. Flap reconstruction of the hypopharynx: a defect orientated approach. Acta Otorhinolaryngol Ital. 2012;32:288–296. [PMC free article] [PubMed] [Google Scholar]
- 32.Jol J, Quak J, Bree R, et al. Larynx preservation surgery for advanced posterior pharyngeal wall carcinoma with free flap reconstruction: a critical appraisal. Oral Oncol. 2003;39:552–558. doi: 10.1016/s1368-8375(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 33.Lydiatt WM, Kraus DH, Cordeiro PG, et al. Posterior pharyngeal carcinoma resection with larynx preservation and radial forearm flap reconstruction: a preliminary report. Head Neck. 1996;18:501–505. doi: 10.1002/(SICI)1097-0347(199611/12)18:6<501::AID-HED3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Pesko P, Sablijak P, Bjelovic M, et al. Surgical treatment and clinical course of patents with hypopharyngeal carcinoma. Dis esophagus. 2006;19:248–253. doi: 10.1111/j.1442-2050.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferahkose Z, Bedirli A, Kerem M, et al. Comparison of free jejunal graft with gastric pull-up reconstruction after resection of hypopharyngeal and cervical esophageal carcinoma. Dis esophagus. 2008;21:340–345. doi: 10.1111/j.1442-2050.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 36.Schustermann MA, Shestak K, Vries EJ, et al. Reconstruction of the cervical esophagus: free jejunal transfer versus gastric pull-up. Plast Reconstr Surg. 1990;85:16–21. doi: 10.1097/00006534-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Puttawibul P, Pompatanarak C, Sangthong B, et al. Results of gastric pull-up reconstruction for pharyngolaryngo-oesophagectomy in advanced head and neck cancer and cervical oesophageal squamous cell carcinoma. Asian J Surg. 2004;27:180–185. doi: 10.1016/S1015-9584(09)60029-4. [DOI] [PubMed] [Google Scholar]
- 38.Dudhat SB, Mistry RC, Fakih AR. Complications following gastric transposition after total laryngo-pharyngectomy. Eur J Surg Oncol. 1999;25:82–85. doi: 10.1053/ejso.1998.0605. [DOI] [PubMed] [Google Scholar]
- 39.Benazzo M, Bertino G, Occhini A, et al. Functional outcomes in patients reconstructed with flaps following surgery for hypopharyngeal cancer. Acta Otolaryngol. 2006;26:127–132. [PMC free article] [PubMed] [Google Scholar]
- 40.Bertino G, Benazzo M, Occhini A, et al. Reconstruction of the hypopharynx after free jejunum flap failure is a second free jejunum transfer feasible? Oral Oncol. 2008;44:61–64. doi: 10.1016/j.oraloncology.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Benazzo M, Occhini A, Rossi V, et al. Jejunum free flap in hypopharynx reconstruction: cases series. BMC Cancer. 2002;2:13–25. doi: 10.1186/1471-2407-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azizzadeh B, Yafai S, Rawnsley JD, et al. Radial forearm free flap pharyngoesophageal reconstruction. Laryngoscope. 2001;111:807–810. doi: 10.1097/00005537-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Benazzo M, Bertino G, Gatti P, et al. Atypical reconstructions with free jejunum flap after circumferential pharyngolaryngectomy. Microsurgery. 2007;27:17–20. doi: 10.1002/micr.20300. [DOI] [PubMed] [Google Scholar]
- 44.Yu P, Jan L, Reece G, et al. Comparison of clinical and functional outcomes and hospital costs following pharyngoesophageal reconstruction with the anterolateral thight free flap versus the jejunal flap. Plast Reconstr Surg. 2006;117:968–974. doi: 10.1097/01.prs.0000200622.13312.d3. [DOI] [PubMed] [Google Scholar]
- 45.Lewin J, Barringer D, May A, et al. Functional outcome after circumferential pharyngoesophageal reconstruction. Laryngoscope. 2005;115:1266–1271. doi: 10.1097/01.MLG.0000165456.01648.B8. [DOI] [PubMed] [Google Scholar]
- 46.Amin A, Bassiouny M, Elsebai H, et al. Fasciocutaneous free flaps for hypopharyngeal reconstruction. J Reconstr Surg. 2002;18:1–5. doi: 10.1055/s-2002-19702. [DOI] [PubMed] [Google Scholar]
- 47.Giordano L, Bondi S, Ferrario F, et al. Radial forearm free flap surgery: a modified skin-closure technique improving donor-site aesthetic appearance. Acta Otorhinolaryngol Ital. 2012;32:158–163. [PMC free article] [PubMed] [Google Scholar]
- 48.Varvares M, Cheney M, Gliklich R, et al. Use of the radial forearm fasciocutaneous free flap and Montgomery salivary bypass tube for pharingoesphageal reconstruction. Head Neck. 2000;22:463–468. doi: 10.1002/1097-0347(200008)22:5<463::aid-hed4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 49.Montemari G, Rocco A, Galla S, et al. Hypopharynx reconstruction with pectoralis major myofascial flap: our experience in 45 cases. Acta Otorhinolaryngol Ital. 2012;32:93–97. [PMC free article] [PubMed] [Google Scholar]
- 50.Castelli ML, Pecorari G, Succo G, et al. Pectoralis major myocutaneous flap: analysis of complications in difficult patients. Eur Arch Otorhinolaryngol. 2001;258:542–545. doi: 10.1007/s004050100389. [DOI] [PubMed] [Google Scholar]
- 51.Theogaraj DS, Merrit WH, Acharya G, et al. The pectoralis major musculocutaneous flap in single-stage reconstruction of the pharyngoesphageal region. Plast Reconstr Surg. 1980;65:267–276. doi: 10.1097/00006534-198003000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Spriano G, Piantanida R, Pellini R. Hypopharyngeal reconstruction using pectoralis major myocutaneous flap and prevertebral fascia. Laryngoscope. 2001;111:544–547. doi: 10.1097/00005537-200103000-00030. [DOI] [PubMed] [Google Scholar]
- 53.Fabian R. Reconstruction of the laryngopharynx and cervical esophagus. Laryngoscope. 1984;138:537–543. doi: 10.1288/00005537-198410000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Saussez S, Cuno A, Urbain F, et al. Reconstruction of circumferential oro- and hypopharyngeal defects with U-shaped pectoralis major myocutaneous flap. Otolaryngol Head Neck Surg. 2006;134:823–829. doi: 10.1016/j.otohns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Mc Crory AL, Magnuson S. Free tissue transfer versus pedicled flap in head and neck reconstruction. Laryngoscope. 2002;112:2161–2165. doi: 10.1097/00005537-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Shektman A, Silver C, Strauch B. A re-evaluation of hypopharyngeal reconstruction: pedicled flaps versus microvascular free flaps. Plast Reconstr Surg. 1997;100:1691–1696. doi: 10.1097/00006534-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Làszlò I, Paczona R, Czigner J, et al. Pharyngeal and hypopharyngeal reconstruction after mutilating surgery for malignant hypopharyngeal cancers. Eur Arch Otorhinolaryngol. 2001;258:292–295. doi: 10.1007/s004050100358. [DOI] [PubMed] [Google Scholar]
- 58.Guillamondegui O, Larson D, Goepfert H. Reconstruction of the hyphopharynx and cervical esophagus. In: Bull TR, Myers E, editors. Plastic reconstruction in the head and neck. London: Butterworths; 1986. pp. 31–31. [Google Scholar]
- 59.Mura F, Bertino G, Occhini A, et al. Advanced carcinoma of the hypopharynx: functional results after circumferential pharyngolaryngectomy with flap reconstruction. Acta Otorhinolaryngol Ital. 2012;32:154–157. [PMC free article] [PubMed] [Google Scholar]
- 60.Serafin D, DeLand M, Lesesne CB, et al. Reconstruction with vascularized composite tissue in patients with excessive injury following surgery and irradiation. Ann Plast Surg. 1982;8:35–54. doi: 10.1097/00000637-198201000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Julieron M, Germain M, Schwaab G, et al. Reconstruction par transplant libre de jejunum après pharyngolaryngectomie totale circulaire-Soixante-treize case. Ann Otolaryngol Chir Cervicofac. 1996;113:269–275. [PubMed] [Google Scholar]
- 62.Harii K, Eblhars S, Ono I, et al. Pharyngoesophageal reconstruction using a prefabricated forearm free flap. Plast Reconstr Surg. 1985;75:463–473. doi: 10.1097/00006534-198504000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Wookey H. The surgical treatment of carcinoma of the pharynx and upper esophagus. Surg Gynecol Obstet. 1942;75:449–506. [Google Scholar]


