Abstract
Although evidence suggests that loneliness may increase risk for health problems, the mechanisms are not well understood. Immune dysregulation is one potential pathway; elevated proinflammatory cytokines such as interleukin-6 (IL-6) increase risk for health problems. Among healthy adults exposed to acute stress (N=134), lonelier participants exhibited greater synthesis of tumor necrosis factor-alpha (TNF-α) and IL-6 by lipopolysaccharide (LPS) stimulated peripheral blood mononuclear cells (PBMCs) than less lonely participants. Similarly, among post-treatment breast cancer survivors exposed to acute stress (N=144), lonelier participants exhibited greater synthesis of IL-6 and interleukin-1 beta (IL-1β) by LPS stimulated PBMCs than less lonely participants. Although in the expected direction, loneliness was unrelated to Study 2 TNF-α. Thus, two different populations demonstrated that lonelier participants' PBMCs produced more cytokines in response to stress than less lonely participants, reflecting a proinflammatory phenotype. These data provide a glimpse into the pathways through which loneliness may impact health.
Keywords: loneliness, inflammation, psychoneuroimmunology
The desire for social connection is a strong impetus behind human behavior (Leary & Cox, 2008; Maslow, 1968). The importance of the need to belong is not surprising given the significance of group living for humans' survival throughout their evolutionary past; humans were most likely to thrive when they were part of a network of people who were invested in their welfare and who looked out for their well-being (Tooby & Cosmides, 1996). Over time, this ultimately led to a fundamental need to form close and caring bonds with other people (Baumeister & Leary, 1995).
Because the need for social connection is central to human nature, the failure to fulfill this need should be detrimental to mental and physical health. Indeed, loneliness is strongly linked to poor health (Hawkley & Cacioppo, 2010). For example, people who were lonelier reported worse physical health, experienced more chronic diseases, and were more likely to develop coronary heart disease than their socially connected counterparts (Sugisawa, Liang, & Liu, 1994; Thurston & Kubzansky, 2009). Furthermore, lonely people had 45% lower odds of survival compared to those who were not lonely, even after accounting for important sociodemographic and health-relevant risk factors (Holt-Lunstad, Smith, & Layton, 2010). This effect is on par with the negative health effects of obesity and inactivity (Holt-Lunstad, Smith, & Layton, 2010), providing compelling evidence for the importance of close relationships for well-being.
Immune dysregulation is one potential pathway through which loneliness may influence health. In fact, recent theoretical work argued that the links among loneliness, stress, and inflammation (a key immunological mechanism) were critical to understanding the health implications of loneliness (Hawkley, Bosch, Engeland, Marucha, & Cacioppo, 2007). Inflammation is modulated by stress and has strong ties to health; excessive and chronic inflammation is linked to age-related diseases such as cardiovascular disorders, neurodegenerative disorders, and frailty (Ershler & Keller, 2000; Hansson, 2005).
A growing body of evidence suggests that loneliness may be linked to dysregulated immune function, including elevated inflammation. For example, lonelier medical students had higher Epstein - Barr virus (EBV) antibody titers than less lonely students, indicating poorer cellular immune control over the latent virus (Glaser, Kiecolt-Glaser, Speicher, & Holliday, 1985). Similarly, lonelier HIV-infected men had higher human herpesvirus 6 (HHV-6) antibody titers than their more socially connected counterparts (Dixon et al., 2006). Lonelier medical students and lonelier psychiatric inpatients exhibited less natural killer cell activity, an important anti-tumor and anti-viral defense, than those with more social connections (Kiecolt-Glaser, Ricker, et al., 1984; Kiecolt-Glaser, Garner, et al., 1984). People who were lonelier had a poorer immune response to an influenza vaccine than those who were less lonely (Pressman et al., 2005). Compared with their socially connected counterparts, lonelier people had higher monocyte chemotactic protein-1 (MCP-1; Hackett, Hamer, Endrighi, Brydon, & Steptoe, in press), a cytokine implicated in inflammatory diseases such as rheumatoid arthritis and atherosclerosis (Deshmane, Kremlev, Amini, & Sawaya, 2009). In addition, compared with people more socially connected, lonelier individuals exhibited up-regulation of proinflammatory genes and down-regulation of anti-inflammatory genes (Cole et al., 2007).
Much less is known about the links between loneliness and inflammation in the context of acute stress. However, related evidence suggests that lonely people are more psychologically reactive to stress than those who are not lonely. For example, although loneliness was not associated with the frequency of major life stressors or traumatic events, lonelier individuals felt more stressed and reported greater anxiety than those who were less lonely (Cacioppo et al., 2000). Similarly, lonelier people experienced everyday activities as more stressful and threatening, even though there were no loneliness-related differences in type or frequency of daily activities (Hawkley, Burleson, Berntson, & Cacioppo, 2003). Because lonely people are highly stress reactive and stress modulates inflammation (Glaser & Kiecolt-Glaser, 2005), loneliness may be linked to proinflammatory cytokine production in response to an acute stressor. Indeed, initial evidence demonstrated that the proinflammatory cytokine interleukin-6 (IL-6) and the interleukin-1 receptor antagonist (IL-1Ra) were elevated after acute stress among those experiencing greater loneliness compared with those who were less lonely (Hackett et al., in press).
Overview of Current Research
The goal of the current research was to fill an important gap in the literature by examining whether loneliness was linked to stress-related proinflammatory cytokine production. We selected tumor necrosis factor – alpha (TNF-α) and IL-6 as our primary inflammation measures because of their pervasive use in the psychoneuroimmunology (PNI) literature and their strong ties to age-related diseases (Ershler & Keller, 2000; Hansson, 2005).
The current research consisted of two samples: 1) healthy middle-aged adults and 2) breast cancer survivors who had completed cancer treatment (except for SERMs/aromatase inhibitors) between 2 months and 3 years prior to enrollment in the study. We hypothesized that, compared to their socially connected counterparts, lonelier people would exhibit greater TNF-α and IL-6 production capacity in response to an acute laboratory stressor.
Study 1
The first sample was purposefully selected on the basis of health; only healthy sedentary overweight people without major comorbidities were eligible to participate. This sample allowed us to examine our hypothesos in a relatively homogeneous sample free of health problems that could influence cytokine production.
Methods
Participants
Participants (N = 134) were from the baseline pre-randomization sample of a clinical trial assessing the potential health benefits of omega-3 who were recruited through advertisements and media announcements. Individuals were ineligible to participate if they had a convulsive, autoimmune, or inflammatory disease, or if they had diabetes, chronic obstructive pulmonary disease, symptomatic ischemic heart disease, liver/kidney failure, gastroesophageal reflux disease, a prior history of cancer (except basal or squamous cell), excessively high triglycerides or low-density lipoprotein (LDL) cholesterol, or a body mass index (BMI) under 22.5 or over 40. People were also excluded if they engaged in more than 3 hours of vigorous physical exercise per week, were taking medications for depression, anxiety, cholesterol, or cardiovascular problems, or were pregnant, nursing, vegetarians, alcoholics/drug abusers, or smokers. Additional sample characteristics are listed in Table 1. The Ohio State University Institutional Review Board approved the project; all subjects provided written informed consent prior to participation.
Table 1.
Study 1 sample characteristics.
| Characteristic | Category | Number (%) (N = 134) |
Mean (SD) |
|---|---|---|---|
| Race | White | 105(77.8) | --- |
| Black | 22(16.3) | --- | |
| Other | 7(5.2) | --- | |
|
| |||
| Education | High school or below | 7(5.2) | --- |
| Some college or college graduate | 81(60) | --- | |
| Graduate or professional training | 46(34) | --- | |
|
| |||
| Marital Status | Single | 18(13.4) | --- |
| Married | 92(68.1) | --- | |
| Separated/divorced/widowed | 24(16.6) | --- | |
|
| |||
| Sex | Male | 42(31.1) | --- |
| Female | 92(68.1) | --- | |
|
| |||
| Age | N/A | --- | 51.01(7.75) |
|
| |||
| BMI | N/A | --- | 30.83(4.7) |
|
| |||
| SAD | N/A | --- | 22.96(3.14) |
|
| |||
| Loneliness | N/A | --- | 37.78(9.53) |
Procedure
Participants arrived at the Clinical Research Center (CRC; a hospital research unit) at 7:45 a.m. and a catheter was inserted in their arm. After eating a standardized breakfast and resting for 20 minutes, blood was drawn to assess baseline levels of stimulated cytokine production. Next, participants completed the Trier Social Stress Test, a well validated stressor consisting of an impromptu speech and a mental arithmetic task (Kirschbaum, Pirke, & Hellhammer, 1993). Participants spent 10 minutes preparing a speech about why they were the best candidate for a job. Then they delivered a 5 minute speech (without notes or aids) in front of a video camera and two panel members who were trained to remain neutral and unresponsive. Participants also completed a 5 minute mental arithmetic serial subtraction task in front of the same panel. Additional blood samples were collected 45 minutes and 2 hours post-stressor.
Questionnaires
Loneliness was measured with the UCLA loneliness scale, which assessed perceptions of social isolation and loneliness (Russell, 1996). The scale is highly reliable, demonstrates construct and convergent validity, and is one of the most commonly used loneliness measures.
The Pittsburgh Sleep Quality Index measured sleep quality over the past month via a combination of subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI can distinguish between people with and without sleep disturbances, indicating acceptable discriminant validity. The measure provided a way to assess the links between loneliness and stimulated cytokine production independent of sleep, which can influence cytokine levels (Faraut, Boudjeltia, Vanhamme, & Kerkhofs, 2012).
Exercise was measured with a combination of one-item about hours of vigorous exercise per week and a shortened version of the Community Healthy Activities Model Program for Seniors questionnaire, a well-validated measure of physical activity among middle-aged and older adults (CHAMPS: Stewart et al., 2001). High levels of exercise are associated with low levels of inflammation (Shephard, 2002). Accordingly, the exercise index allowed us to disentangle the relationships among loneliness, inflammation, and exercise.
Participants answered questions about their age, smoking status, weekly average alcohol consumption, current medication use, and highest level of education. Educational level was used as an index of SES because some women in our sample did not work outside of the home. In addition, education is less vulnerable to current economic conditions than income and job status. We also assessed participants' sagittal abdominal diameter (SAD), an index of abdominal fat measured via a person's abdominal height while laying flat. SAD was used as an adiposity measure because belly fat is one major source of proinflammatory cytokines (Mohamed-Ali et al., 1997). In addition, SAD is more precise than body mass index (BMI), another common adiposity measure, because BMI can be influenced by muscle mass.
Immune Assays
Lipopolysaccharide (LPS, a bacterial endotoxin) stimulated production levels of TNF-α and IL-6 were measured using an electrochemilluminescence method with Meso Scale Discovery kits, and read using the Meso Scale Discovery Sector Imager 2400. The stored culture supernatant samples for each subject were assayed for all the cytokine markers in one run, thus using the same controls for all nine time points for each person. To assess LPS-stimulated cytokine production, PBMC cultures, 1 × 106 cells/ml, were incubated for 24 hours in 3 ml RPMI 1640 media containing 10% human male serum either with or without 1.0 μg/ml LPS. After 24 h the cells were pelleted by centrifugation (2000 rpm for 5 min) and the supernatants removed and stored at -80°C. The dose and duration were based on evidence that the effects of dietary n-3 supplementation (which was part of the parent randomized controlled trial) are best demonstrated through the use of low LPS concentrations to stimulate peripheral blood mononuclear cells (PBMCs; Calder, 2004; Fritsche, 2006). Sensitivity for LPS-stimulated cytokine production is 2.4 pg/ml. The intra-assay coefficient of variation was 3.16% for TNF-α and 4.56% for IL-6. The inter-assay coefficient of variation was 15.35% for TNF-α and 12.13% for IL-6.
Data Analytic Strategy
The distributions of the immune data were checked for normality and the presence of outliers. Participants whose immune values were more than 4 standard deviations from the mean were dropped from the corresponding analyses. Specifically, we dropped 3 TNF-α and 3 IL-6 values, which were less than 1% of all samples. The results did not differ whether the outliers were included or excluded. The data for TNF-α and IL-6 were highly skewed. Accordingly, each measure was log10 transformed prior to analyses.
Mixed models were utilized to account for correlations within subjects because several observations were obtained for each participant. An unstructured variance-covariance matrix was fitted to estimate the error variance. We hypothesized that loneliness would be linked to stimulated cytokine production in response to the stressor such that high levels of loneliness would be associated with greater increases in stimulated cytokine production. To test reactivity to the stressor, we investigated whether loneliness predicted post-stress levels of stimulated cytokine production controlling for baseline levels of the corresponding cytokine. Adjusting for baseline created scores reflecting change in the outcome from pre to post stress.
Potential confounds were selected based on their theoretical and empirical relationships to cytokine levels and kept the the same within and across studies when possible. Every model adjusted for SAD, age, and gender. Models also included a random effect for the plate on which the assay was conducted when results suggested that plate number produced additional variability not explained by other predictors. All models were analyzed with SPSS 19.0 (IBM, New York) using a repeated and a random statement (when plate was included as a random effect). In ancillary analyses we examined health behaviors (e.g., sleep and exercise) as additional confounds.
We initially included the 3-way loneliness Χ time Χ gender interaction and the corresponding 2-way interactions in each model. None of the 2-way or 3-way interactions involving gender were significant and thus the gender interaction terms were omitted from all analyses.
Results
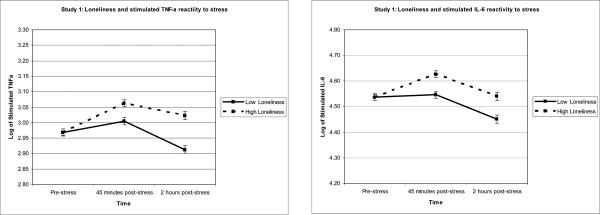
Reported results are unstandardized beta coefficients and are graphically represented in Figures 1a-b. Loneliness was unrelated to baseline synthesis of TNF-α or IL-6 by PBMCs stimulated with LPS (both p values > .623).
Figure 1.
Figure 1a and b. To illustrate the relationship between loneliness and inflammation, Study 1 averages of stimulated TNF-α and IL-6 production were graphed for participants 1 standard deviation above and below the mean of loneliness. Sample means were estimated using a model that included loneliness as a continuous measure and are adjusted for SAD, age, and gender.
To analyze the stimulated TNF-α production stress-reactivity data, we used a mixed model that included the covariates listed in the data analytic strategy, baseline stimulated TNF-α production, the main effects of loneliness and time (45 minutes vs. 2 hours post-stress), and the interaction between loneliness and time. In response to acute stress, participants who were lonelier exhibited significantly greater increases in the synthesis of TNF-α production by LPS stimulated PBMCs than participants who were less lonely, b = .004, F(1,108) = 7.70, p = .007. The non-significant interaction between loneliness and time indicated that the strength of the relationship between loneliness and stimulated TNF-α production was the same at both post-stress assessments, p = .124.
In the next model, we examined stimulated IL-6 production using a similar analytic strategy. In response to acute stress, lonelier participants exhibited significantly greater increases in IL-6 production by LPS stimulated PBMCs than those who were less lonely, b = .004, F(1,113) = 5.00, p = .027. The non-significant interaction between loneliness and time indicated that the strength of the relationship between loneliness and stimulated IL-6 production was the same at both post-stress assessments, p = .807.
Ancillary Analyses
We used a series of mixed models to examine whether the relationships among loneliness and stimulated cytokine production held after controlling for sleep quality over the past month, numbers of hours slept the night before the visit, smoking status, exercise levels, alcohol consumption, education level, and medication use. Non-steroidal anti-inflammatory drugs (NSAIDs) were the most common type of medication used in this sample (N = 24). The results were unchanged after adjusting for the above variables.
Study 2
The second sample was chosen with the goal of generalizing the Study 1 findings to a more diverse sample. In particular, the Study 2 sample was more heterogeneous in terms overall health, health behaviors, and medication use. The breast cancer survivor sample also allowed us to assess the links between loneliness and stress-related proinflammatory cytokine production among a group who recently experienced a major life stressor (i.e., cancer diagnosis and treatment). We made one addition to the Study 2 inflammation measures, interleukin-1 beta (IL-1β). We added IL-1β to expand our cytokine repertoire; stimulated IL-1β production is reliably modulated by acute stress (Steptoe, Hamer, & Chida, 2007). We hypothesized that, compared to their socially connected counterparts, lonelier people would exhibit greater stimulated TNF-α, IL-6, and IL-1β production in response to an acute laboratory stressor, reflecting a proinflammatory phenotype among lonely individuals.
Methods
Participants
Participants were stage 0-IIIA breast cancer survivors (N = 144) from the baseline pre- randomization sample of an ongoing clinical trial about cancer-related fatigue. Survivors were recruited through cancer clinics and media announcements if they had completed cancer treatment (except for SERMs/aromatase inhibitors) between 2 months and 3 years prior to enrollment in the study. Individuals were ineligible if they engaged in over 5 hours of vigorous physical activity per week, or if they had a BMI over 44, chronic obstructive pulmonary disease, symptomatic ischemic heart disease, uncontrolled hypertension, liver or kidney failure, or a prior history of cancer (except basal or squamous cell). The average age of participants was 51.44 (SD = 9.17, range 28-76) and the majority of participants were White (86%). Additional sample characteristics are listed in Table 2. The project was approved by The Ohio State University Institutional Review Board; all participants provided written informed consent before participating.
Table 2.
Study 2 sample characteristics.
| Characteristic | Category | Number (%)* (N = 144) |
Mean (SD) |
|---|---|---|---|
| Race | White | 124(86) | --- |
| Black | 15(10) | --- | |
| Other | 5(4) | --- | |
|
| |||
| Education | High school or below | 8(5.5) | --- |
| Some college or college graduate | 80(55.5) | --- | |
| Graduate or professional training | 56(39) | --- | |
|
| |||
| Marital Status | Single | 20(14) | --- |
| Married | 103(71.5) | --- | |
| Separated/divorced/widowed | 21(14.5) | --- | |
|
| |||
| Stage | 0 | 11(7.5) | --- |
| I | 63(44) | --- | |
| II | 56(39) | --- | |
| III | 14(9.5) | --- | |
|
| |||
| Age | N/A | --- | 51.44(9.17) |
|
| |||
| Months since Tx | N/A | --- | 11.35(7.84) |
|
| |||
| BMI | N/A | --- | 27.82(5.64) |
|
| |||
| SAD | N/A | --- | 20.74(3.23) |
|
| |||
| Loneliness | N/A | --- | 38.69(8.45) |
Procedure
Participants arrived at the CRC at 8:30 a.m. and a catheter was inserted in their arm. After eating a standardized breakfast and relaxing for 20 minutes, a blood sample was collected to assess baseline stimulated cytokine production. Next, subjects participated in the same Trier Social Stress Test as described in Study 1. Two additional blood samples were collected 45 minutes and 2 hours post-stressor.
Questionnaires
Loneliness and sleep quality were measured with the same questionnaires as Study 1. Exercise was assessed with one item about hours of vigorous activity in a typical week. Participants answered questions about their age, smoking status, weekly average alcohol consumption, current medication use, and highest level of education.
The Charlson index is a widely utilized comorbidity measure originally developed for breast cancer patients and later extended to cancer and non-cancer populations (Charlson, Szatrowski, Peterson, & Gold, 1994). The measure uses participants' self-reported health information to assign weights to 19 medical conditions based on their ability to influence 1-year mortality. The Charlson was included to account for potential links between comorbidities and immune function.
Immune Assays
Synthesis of TNF-α, IL-6, and IL-1β by LPS stimulated PBMCs were assayed using the same method as Study 1.
Data Analytic Strategy
The distributions of the immune data were checked for normality and the presence of outliers. Similar to Study 1, participants whose immune values were more than 4 standard deviations from the mean were dropped from the corresponding analyses. Specifically, we dropped 3 TNF-α values and 1 IL1-β value, which were less than 1% of all samples. The results did not differ whether the outliers were included or excluded. The distributions of TNF-α, IL-6, and IL1-β were moderately skewed and were thus square root transformed prior to analyses.
To test reactivity to the stressor, we employed the same mixed model analytic strategy as in Study 1. The control variables were the same as Study 1 except for the following changes: gender was omitted because all participants were women, and time since completion of cancer treatment, type of cancer treatment, and major medical comorbidities were added to tailor the covariates to a cancer population. Models also included a random effect for the plate on which the assay was conducted when results suggested that plate number produced additional variability not explained by other predictors. In ancillary analyses we examined health behaviors as additional confounds.
Results
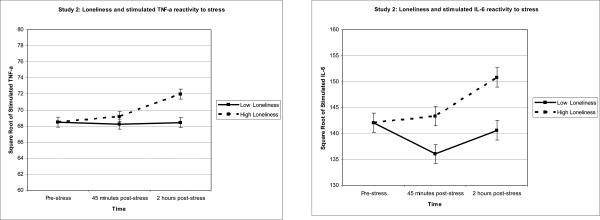
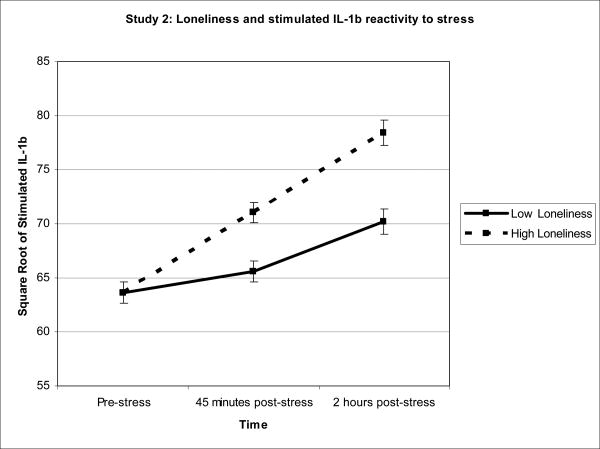
Reported results are unstandardized beta coefficients and are graphically represented in Figures 2a-c. There were no loneliness-related baseline differences in the synthesis of TNF-α, IL-6, or IL-1β by PBMCs stimulated with LPS (all ps > .768).
Figure 2.
Figure 2a-c. To illustrate the relationship between loneliness and inflammation, Study 2 averages of stimulated TNF-α, IL-6, and IL-1β production were graphed for participants 1 standard deviation above and below the mean of loneliness. Sample means were estimated using a model that included loneliness as a continuous measure and are adjusted for SAD, age, time since treatment, and comorbidities.
To analyze the stimulated TNF-α production stress-reactivity data, we used a mixed model that included the covariates listed in the data analytic strategy, baseline stimulated TNF-α production, the main effects of loneliness and time (45 minutes vs. 2 hours post-stress), and the interaction between loneliness and time. Loneliness was unrelated to changes in stimulated TNF-α production from pre to post stress, b = .14, F(1,129) = 2.33, p = .130. However, the pattern was in the expected direction with lonelier participants exhibiting greater stimulated TNF-α production in response to stress than less lonely participants. The non-significant interaction between loneliness and time indicated that the strength of the relationship between loneliness and stimulated TNF-α production was the same at both post-stress assessments, p = .137.
In the next set of models, we analyzed the stimulated IL-6 and IL-1β production data using a similar mixed model strategy. In response to acute stress, lonelier participants exhibited greater synthesis of IL-6 and IL-1β by PBMCs stimulated with LPS than those who were less lonely, b = .53, F(1,129) = 4.48, p = .036 and b = .42, F(1,131) = 7.21, p = .008 respectively. The non-significant interactions between loneliness and time indicated that the strength of the relationships between loneliness and stimulated IL-6/IL-1β production were the same at both post-stress assessments, p = .602 and p = .359 respectively.
Ancillary Analyses
Similar to Study 1, we used a series of mixed models to examine whether the relationships between loneliness and stimulated cytokine production held after controlling for sleep quality over the past month, numbers of hours slept the night before the visit, smoking status, exercise levels, alcohol consumption, education level, and medication use. Non-steroidal anti-inflammatory drugs (NSAIDs, N = 65), cancer-related medications (SERMs/aromitase inhibitors; N = 133), and depression medications (N = 64) were the most common type of medications used in this sample. The patterns were unchanged after adjusting for the above variables.
Discussion
In accord with psychological data demonstrating that lonely people are highly stress-reactive, people who were lonelier exhibited more stimulated TNF-α, IL-6, and IL-1β production in response to an acute stressor than those who were less lonely. Furthermore, the results were highly consistent across two different populations, healthy sedentary overweight adults and breast cancer survivors1, suggesting that lonely individuals exhibit a proinflammatory phenotype.
The current results are consistent with theoretical speculation and empirical evidence demonstrating that close and caring relationships are essential to mental and physical well-being (Baumeister & Leary, 1995); lonelier participants had larger stimulated cytokine production responses to acute stress than less lonely participants, and inflammation is linked to a variety of age-related diseases (Ershler & Keller, 2000; Hansson, 2005). Indeed, compared to their socially connected counterparts, lonelier people experience a wide array of health problems ranging from increased coronary heart disease incidence to premature mortality (Holt-Lunstad et al., 2010; Thurston & Kubzansky, 2009). Accordingly, inflammation driven by excessive cytokine production may be one key mechanism linking loneliness to poor health.
Other factors may work independently or in tandem with changes in inflammation to influence health. For example, compared to people more socially connected, lonelier people reported poorer sleep quality, a strong predictor of negative health outcomes (Cacioppo et al., 2002; Strine & Chapman, 2005). In the current study, ancillary analyses demonstrated that lonelier people exhibited greater stimulated proinflammatory cytokine production in response to stress than less lonely people regardless of sleep quality. These data suggest that sleep quality does not explain loneliness-related immune alterations. Accordingly, loneliness may independently influence health via both sleep and immune dysregulation.
Mechanistically, both the autonomic and neuroendocrine systems influence stress-related inflammation. Norepinephrine stimulates the release of proinflammatory cytokines by inducing nuclear factor-kappaB (NF-κB) transcription, an intracellular signaling molecule that regulates proinflammatory cytokine gene expression (Bierhaus et al., 2003; Kohm & Sanders, 2000). Furthermore, parasympathetic activity can reduce inflammation via the cholinergic anti-inflammatory pathway that induces acetylcholine release (Tracey, 2009). Because stress reduces parasympathetic activity, stress ultimately results in elevated cytokine production. Accordingly, research incorporating the autonomic, neuroendocrine, and immune consequences of loneliness would help provide a complete picture about the ways that these physiological systems influence each other to affect health.
In sum, loneliness was linked to exaggerated proinflammatory cytokine production following an acute stressor, reflecting a proinflammatory phenotype among lonely individuals. The current study demonstrates that loneliness has immune consequences and provides a glimpse into the pathways through which social relationships can impact health and well-being.
Acknowledgments
Work on this project was supported in part by NIH grants CA126857, CA131029, AG029562, UL1RR025755, CA016058, and DE014320 as well as American Cancer Society Postdoctoral Fellowship Grants 121911-PF-12-040-01-CPPB and PF-11-007-01-CPPB, and a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center.
Footnotes
The one exception is that, although in the expected direction, the stimulated TNF-α production results were non-significant in Study 2.
All authors declare that there are no financial conflicts of interest.
References
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, Kowalewski RB, et al. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. International Journal of Psychophysiology. 2000;35(2–3):143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science. 2002;13(4):384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 fatty acids, inflammation, and immunity--relevance to postsurgical and critically ill patients. Lipids. 2004;39(12):1147–1161. doi: 10.1007/s11745-004-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. Journal of Interferon & Cytokine Research. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Cruess S, Kilbourn K, Klimas N, Fletcher MA, Ironson G, Baum A, et al. Social support mediates loneliness and human herpesvirus type 6 (HHV-6) antibody titers. Journal of applied social psychology. 2006;31(6):1111–1132. doi: 10.1111/j.1559-1816.2001.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased Interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51(1):245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Medicine Reviews. 2012;16(2):137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Fritsche K. Fatty acids as modulators of the immune response. Annual Review of Nutrition. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2012.03.016. (in press) [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress, and immunity: A role for cytokines. In: Plotnikoff NP, editor. Cytokines: Stress And Immunity. CRC Press; 2007. pp. 67–85. [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. Journal of Personality and Social Psychology. 2003;85(1):105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine. 2010;40(2):218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Garner W, Speicher C, Penn GM, Holliday J, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosomatic Medicine. 1984;46(1):7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Ricker D, George J, Messick G, Speicher C, Garner W, Glaser R. Urinary cortisol levels, cellular immunocompetency, and loneliness in psychiatric inpatients. Psychosomatic Medicine. 1984;46(1):15–23. doi: 10.1097/00006842-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunology Today. 2000;21(11):539–542. doi: 10.1016/S0167-5699(00)017473. [DOI] [PubMed] [Google Scholar]
- Leary MR, Cox CB. Belongingness motivation: A mainspring of social action. In: Gardner WL, Shah JY, editors. Handbook of Motivation Science. New York, NY: Guilford Press; 2008. pp. 27–40. [Google Scholar]
- Maslow AH. Toward a Psychology of Being. 2. Oxford, England: D Van Nostrand; 1968. [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. The Journal of Clinical Endocrinology and Metabolism. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24(3):297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Shephard RJ. Cytokine responses to physical activity, with particular reference to IL-6: sources, actions, and clinical implications. Critical Reviews in Immunology. 2002;22(3):165–182. [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Strine TW, Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Medicine. 2005;6(1):23–27. doi: 10.1016/j.sleep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. Journal of Gerontology. 1994;49(1):S3–S13. doi: 10.1093/geronj/49.1.S3. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosomatic medicine. 2009;71(8):836–842. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. Friendship and the banker's paradox: Other pathways to the evolution of adaptations for altruism. In: Runciman WG, Smith JM, Dunbar RIM, editors. Evolution of Social Behaviour Patterns in Primates and Man. New York, NY: Oxford University Press; 1996. pp. 119–143. [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. Understanding the links between social support and physical health: A life-span perspective with emphasis on the separability of perceived and received support. Perspectives on Psychological Science. 2009;4(3):236–255. doi: 10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]