Abstract
Regulatory T cells (Tregs) are potent immune modulators, but their precise role in HIV pathogenesis remains incompletely understood. Most studies to date have focused on frequencies or phenotypes of “bulk” Treg populations. However, although antigen-specific Tregs have been reported in other diseases, HIV-1-epitope specific Tregs have not been described to date. We here report the first identification of functional HIV-1-Gag-specific regulatory T cells using human leukocyte antigen class II tetramer staining in HIV-1-infected individuals.
Regulatory T cells (Tregs) are potent immune modulators and serve an important function in human immune homeostasis. Despite an increasing body of data on regulatory T cells in HIV-1 infection, their role in HIV-1 pathogenesis remains inadequately understood. Although some data argue in favor of a beneficial effect of Tregs through impact on HIV-1-associated immune activation [1] and more recently viral replication [2], other data support a deleterious effect by suppressing critical virus-specific immune responses [3, 4]. Controversy also remains about the fate of regulatory T cells during progressive HIV-1 infection, with some studies reporting declining Treg numbers and other studies demonstrating increased Treg frequencies [3, 5, 6]. Although “bulk” Treg populations have been studied extensively in recent years in the context of HIV-1 infection, no reliable data is available on HIV-1-specificity of regulatory T cells and whether these cells are induced in infected individuals.
Part of the challenges in detecting antigen-specific Treg populations relate to the limited availability of direct visualization tools such as human leukocyte antigen (HLA) class II tetramers. Another barrier to the study of HIV-1-specfic Tregs lies in the paucity of even “bulk” Treg populations in HIV-1-infected individuals. We and others recently reported median frequencies of CD4+CD25+CD127−FOXP3+ regulatory T cells of 4.5–7% (range 0.99%–13.1%) of the CD4+ T cell population in untreated infected individuals, with absolute Treg numbers declining over time during disease progression [4, 6, 7], further complicating the detection of small HIV-1-epitope-specific Treg subpopulations ex vivo.
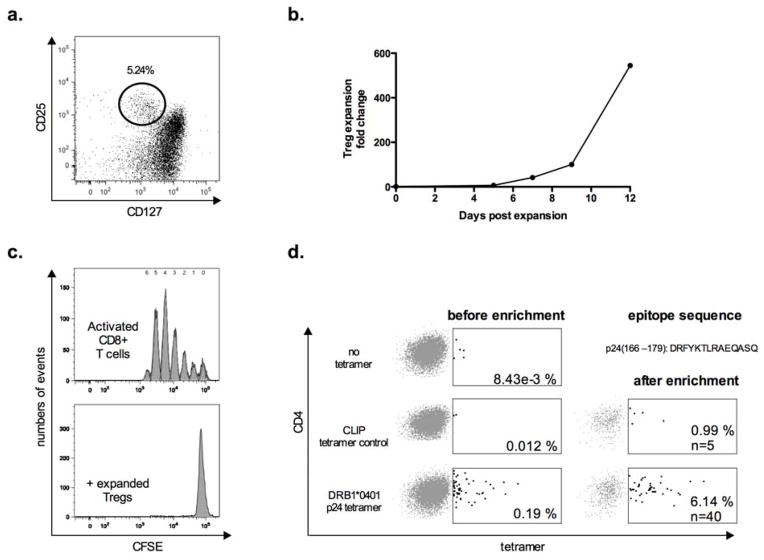
Antigen-specific Tregs have been reported and successfully visualized by HLA-class II tetramers in murine and human studies of transplantation [8], diabetes, influenza [9] and autoimmunity [10, 11]. In order to screen for HIV-1-specific regulatory T-cell populations, we first flow-sorted (gating scheme represented in Fig. 1a) and expanded Tregs ex vivo from eight HLA-DRB1*0401-expressing HIV-1-infected individuals (four individuals with chronic untreated progressive HIV-1 infection, three HIV elite controllers with undetectable HIV-1 viremia in the absence of therapy and one HAART-treated individual with undetectable HIV-1 viral load), using anti-CD3/anti-CD28-coated microbeads and IL-2 [12]. During the 12-day in vitro culture Tregs expanded by a median of 580 fold (IQR 186 and 871) (Fig. 1b), and were tested for their suppressive capacity by standard CFSE T cell proliferation assays on day 7 using autologous bead stimulated cryopreserved peripheral blood mononuclear cells (PBMCs) as responder cells. Expanded Tregs were highly suppressive (Fig. 1c), displayed the phenotype of ‘activated’ Tregs (CD4+CD45RA−FOXP3hi)[13], and expressed high levels of classical Treg markers (HELIOS, CTLA4, FOXP3, CD39, CD25)(data not shown). Expanded Tregs were demethylated at the Treg-specific demethylation region locus of the FOXP3 gene as evidenced by epigenetic analysis, suggesting true origin from the regulatory T cell lineage, as opposed to activation-induced transient FOXP3 upregulation [14]. We next stained the Treg lines with phycoerythrin (PE)-conjugated HLA class II tetramers specific for the HIV-p24-Gag epitope DRFYKTLRAEQASQ (p24166–179). HLA-DR molecules with bound peptides from a self-protein, the invariant chain-derived CLIP peptide, were used as controls, as previously described [15]. Labeling with HIV-p24-Gag tetramers was considered positive when the T-cell population was more than threefold larger compared to control tetramers, as defined in our previous studies using the same tetramer constructs for HIV-1 specific CD4 effector T-cell populations [16]. Two out of the eight HIV-1 positive study subjects with chronic untreated progressive HIV-1 infection had detectable responses to the p24-Gag class II tetramer at a frequency of 0.19 and 0.05% of CD4 in the nonenriched Treg culture, respectively. After tetramer-positive T-cell enrichment over a magnetic column, using anti-PE-conjugated magnetic beads [16], this frequency was enriched to 6.14 and 0.23% of Tregs, respectively (representative example shown in Fig. 1d). No tetramer-specific cells were demonstrated in HIV-1 negative control subjects or individuals lacking HLA-DRB1*0401 expression. These data demonstrate that HIV-1-epitope-specific Tregs can be detected in HIV-1 infected individuals using HLA-class II tetramer technology.
Figure 1.
a) Representative example of CD4+ regulatory T-cell (Treg) staining by flow cytometry with gating strategy before flow-based sorting and Treg expansion. Tregs are defined as CD25+CD127low CD4+ T cells.
b) Mean expansion fold change of the expanded regulatory T-cells lines that were stained with HIV-1-p24-gag-specific HLA class II tetramer.
c) Representative histogram plots showing T-cell proliferation by CFSE dilution of CD8+ T cells after 4 days of culture following stimulation with anti-CD3/CD2/CD28 coated beads, cocultured with (lower histogram) or without expanded Tregs (upper histogram) at a ratio of 1:1 Treg per PBMC.
d) Example of PE-conjugated-HLA class II tetramer staining on expanded Tregs isolated from an individual with untreated chronic progressive HIV-1 infection before and after PE-enrichment over a magnetic column. The cells were incubated alone (upper dot plot), in presence of an HLA-DR0401 restricted HLA-class II tetramer loaded with a control CLIP peptide (middle dot plots) or the p24-Gag peptide DRFYKTLRAEQASQ (lower dot plots). Percentages refer to tetramer positive cells per total CD4+ T-cells. Equal numbers of input cells were used for all staining and enrichment procedures.
To our knowledge this visualization by HIV-1-Gag-specific HLA class II tetramers represents the first identification of HIV-1-specific regulatory T cells reported to date. While the epitope-specific Treg population was not readily detectable ex vivo from PBMCs, the magnitude of the tetramer-response after expansion was robust and comparable to frequencies for HLA-class II-restricted responses in previous reports from other disease settings. Furthermore, higher frequencies of HIV-1-specific Treg may be detectable using similar methods in lymphoid tissues such as the gut-associated lymphoid tissue, in which increased frequencies of bulk Tregs have recently been described [4]. The specificity of the p24-Gag-epitope tested overlaps with a previously described HIV-1-specific CD4+ effector T-cell response [16]. This finding is consistent with published data indicating that naïve and memory CD4+ Tregs can share the same TCR clonotypes as CD4+ non-Treg in humans [17] and observations in murine models that regulatory and effector CD4+ T cells may be driven by the same antigens [18]. Identification and further functional characterization of HIV-1 specific Tregs will also be important in HIV vaccine studies, in which vaccine strategies should be evaluated for their potential to induce not only HIV-1-specific effector populations, but also vaccine-induced HIV-1-specific regulatory T cells, which may negatively interfere with vaccine immunogenicity [19].
Taken together these results show for the first time that HIV-1-specific regulatory T cells can be successfully visualized and isolated from HIV-1-infected individuals. The ability to identify and track HIV-1-specific regulatory T cells opens new opportunities to gain insight into the role of Tregs in HIV pathogenesis.
Acknowledgments
We thank all individuals who participated in this study as well as the Ragon Institute Clinical Platform for critical support with cohort coordination and specimen acquisition.
We thank Nilufer Seth for providing the protocol for class II tetramer staining and enrichment.
This work was supported in part by research funding from the Elisabeth Glaser Pediatric AIDS Foundation (Pediatric HIV Vaccine Program Award MV-00-9-900-1429-0-00 to MMA), MGH/ECOR (Physician Scientist Development Award to MMA), NIH NIAID (KO8 AI074405 and AI074405-03S1 to MMA). These studies were furthermore supported by the Bill & Melinda Gates Foundation and the Terry and Susan Ragon Foundation. This publication resulted in part from research supported by the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NCCAM, FIC, and OAR.
Footnotes
M.M.A, and M. Angin were responsible for study concept and design. M.K., M. Angin, K.W.W. participated in data generation and experiments. K.W.W. also kindly generated and provided tetramer reagents and protocols. M. Angin and M.M.A analyzed, interpreted all data and drafted the manuscript. M. Altfeld and B.D.W were involved in the establishment of the patient cohorts used and revised the manuscript. All authors have read and approved the text as submitted to AIDS.
Conflict of interest:
The authors declare that they have no competing interests.
References
- 1.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;82:8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Fernandez ME, Rueda CM, Rusie LK, Chougnet CA. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood. 2011 May 19;117(20):5372–80. doi: 10.1182/blood-2010-12-323162. Epub 2011 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazekas de St Groth B, Landay AL. Regulatory T cells in HIV infection: pathogenic or protective participants in the immune response? AIDS. 2008;22:671–683. doi: 10.1097/QAD.0b013e3282f466da. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Increased Frequency of Regulatory T-Cells Accompanies Increased T-Cell Immune Activation in Rectal Mucosa of HIV+ Non-Controllers. 2011 Nov;85(21):11422–34. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 6.Schulze Zur Wiesch J, Thomssen A, Hartjen P, Toth I, Lehmann C, Meyer-Olson D, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2010;85:1287–1297. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angin M, Kwon DS, Streeck H, Wen F, King M, Rezai A, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis. 2012 May 15;205(10):1495–500. doi: 10.1093/infdis/jis236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc Natl Acad Sci U S A. 2008;105:3479–3484. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long SA, Walker MR, Rieck M, James E, Kwok WW, Sanda S, et al. Functional islet-specific Treg can be generated from CD4+CD25- T cells of healthy and type 1 diabetic subjects. Eur J Immunol. 2009;39:612–620. doi: 10.1002/eji.200838819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 12.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 15.Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seth N, Kaufmann D, Lahey T, Rosenberg ES, Wucherpfennig KW. Expansion and contraction of HIV-specific CD4 T cells with short bursts of viremia, but physical loss of the majority of these cells with sustained viral replication. J Immunol. 2005;175:6948–6958. doi: 10.4049/jimmunol.175.10.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheinberg P, Melenhorst JJ, Hill BJ, Keyvanfar K, Barrett AJ, Price DA, Douek DC. The clonal composition of human CD4+CD25+Foxp3+ cells determined by a comprehensive DNA-based multiplex PCR for TCRB gene rearrangements. J Immunol Methods. 2007;321:107–120. doi: 10.1016/j.jim.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litjens NH, Boer K, Betjes MG. Identification of circulating human antigen-reactive CD4+ FOXP3+ natural regulatory T cells. J Immunol. 2012;188:1083–1090. doi: 10.4049/jimmunol.1101974. [DOI] [PubMed] [Google Scholar]
- 19.Macatangay BJ, Szajnik ME, Whiteside TL, Riddler SA, Rinaldo CR. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS One. 2010;5:e9852. doi: 10.1371/journal.pone.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]