Abstract
Objective
To investigate the potential contribution of germline sequence alterations in the BAP1 gene in uveal melanoma (UM) patients with possible predisposition to hereditary cancer.
Design
A total of 53 unrelated UM patients with high risk for hereditary cancer and five additional family members of one proband were studied. Mutational screening was carried out by direct sequencing.
Results
Of the 53 UM patients studied, a single patient was identified with a germline BAP1 truncating mutation, c. 799 C→T (p.Q267X), which segregated in several family members and was associated with UM and other cancers. Biallelic inactivation of BAP1 and decreased BAP1 expression were identified in the UM, lung adenocarcinoma and meningioma tumours from three family members with this germline BAP1 mutation. Germline BAP1 variants of uncertain significance, likely non-pathogenic, were also identified in two additional UM patients.
Conclusion
This study reports a novel hereditary cancer syndrome caused by a germline BAP1 mutation that predisposes patients to UM, lung carcinoma, meningioma, and possibly other cancers. The results indicate that BAP1 is the candidate gene in only a small subset of hereditary UM, suggesting the contribution of other candidate genes.
INTRODUCTION
Uveal melanoma (UM), including choroidal and ciliary body tumours, is the most common primary intraocular tumour in adults. We previously reported that features suggestive of a hereditary cancer predisposition were present in nearly 12% of 121 unselected UM patients.1 The phenotype of cancer in these families is diverse and includes cancers other than UM. These findings are consistent with observations by other investigators suggesting predisposition of a subset of UM patients to other cancers.2 Several genes have been suggested as candidates in hereditary UM including CDKN2A, BRCA2 and p14/ARF.3–5 However, germline alteration in any of these candidates is extremely rare in UM.
BAP1 (BRCA1 associated protein-1) is a deubiquitinating enzyme with an ubiquitin carboxyterminal hydrolase function.6 BAP1 is located on chromosomal region 3p12. Monosomy of chromosome 3 is the most common somatic alteration in UM, reported in about 50% of primary tumours, and it is associated with aggressive tumours. A recent study identified a high frequency (27/57, 47.4%) of somatic mutations in BAP1 in primary UM.7 It also identified mutations in two out of the three metastatic lesions included in their study. The mutations were almost exclusively identified in tumours with a gene expression profile pattern strongly associated with early development of metastatic disease (class 2 tumours8) and were seen more commonly in UM with monosomy 3.7 A germline mutation in BAP1 was also detected in a single patient (1.7%) with no available family history.7
BAP1 has been suggested to be a tumour suppressor gene with a role in cell proliferation and growth inhibition.8,9 It has been suggested that the interaction of BAP1 with host cell factor-1 (HCF-1) is critical for its growth inhibition function.10,11 In addition to UM, somatic mutations in BAP1 have been identified in breast and lung cancers.6,9 However, germline pathogenic mutations have not been identified in patients with breast cancer.12,13
In the following study we investigated the frequency of germline sequence alterations in the BAP1 gene in 53 unrelated UM patients with a strong hereditary cancer risk.
PATIENTS AND METHODS
Patient selection
The study population represents patients with UM seen at the ophthalmology and/or the clinical cancer genetics programs at The Ohio State University, or referred to our program. The research was approved by The Ohio State University Cancer institutional review board.
A total of 53 unrelated patients were included in this study, including five patients with one or more relatives diagnosed with UM. Genomic DNA of five additional family members of one proband (FUM036) was available for sequencing. In addition, tumour tissues from three of the FUM036 family members were available for genotyping, sequencing, and immunohistochemistry.
Family histories of most of these patients were previously reported.1,5 Inclusion criteria included at least one of the following: (1) early age at diagnosis (<30 years); (2) personal history of UM plus an additional primary tumour(s) (excluding lung, non-melanoma skin cancers and cervical cancer due to their high environmental predisposition); or (3) a significant family history of other cancers as previously defined.1 Five of the 53 patients had a family history of UM. All patients tested negative for pathogenic mutations in the familial cutaneous melanoma predisposition genes CDKN2A, p14/ARF, and exon 2 of CDK4.5 Five patients with apparent breast cancer predisposition were negative for pathogenic BRCA1 and BRCA2 gene mutations based on clinical testing.
DNA extraction, mutational screening, and genotyping
Germline DNA was extracted from peripheral blood mononuclear cells at the Human Cancer Genetics sample bank at The Ohio State University using a simple salting out procedure.14 Tumour DNA was extracted from archival material using Qiagen DNeasy kit (Qiagen, Valencia, California, USA). Primers and PCR conditions for sequencing of all exons of the BAP1 gene and the adjacent intronic sequences are listed in supplemental table 2. Mutational screening was carried out by direct sequencing of fragments obtained by PCR using an Applied Biosystems 3730 DNA sequencer (Applied Biosystems, Foster City, California, USA). Mutational screening for the hereditary melanoma candidate genes (CDKN2A, p14/ARF, and exon 2 of CDK4) were carried out, as previously described, in probands of families that were not included in the previous study.5 For BAP1 the sequence results were read by aligning with the reference sequence provided by Genebank accession number NM_004656.2, utilising the Sequencher software (Version 4.8, Gene Codes Corp, Ann Arbor, Michigan, USA). All identified sequence variations were confirmed at least once in an independent PCR experiment.
Genotyping was carried out on the tumour tissues of three individuals from family FUM036, one lung adenocarcinoma (individual III.1, figure 1), one meningioma (individual III.2, figure 1), and one UM (individual III.6, figure 1). The three patients had germline mutation in BAP1. A total of 15 micro-satellite markers on chromosome 3 were used for genotyping, including three markers (D3S3026, D3S3561, and D3S1578) flanking the BAP1 gene (figure 2).
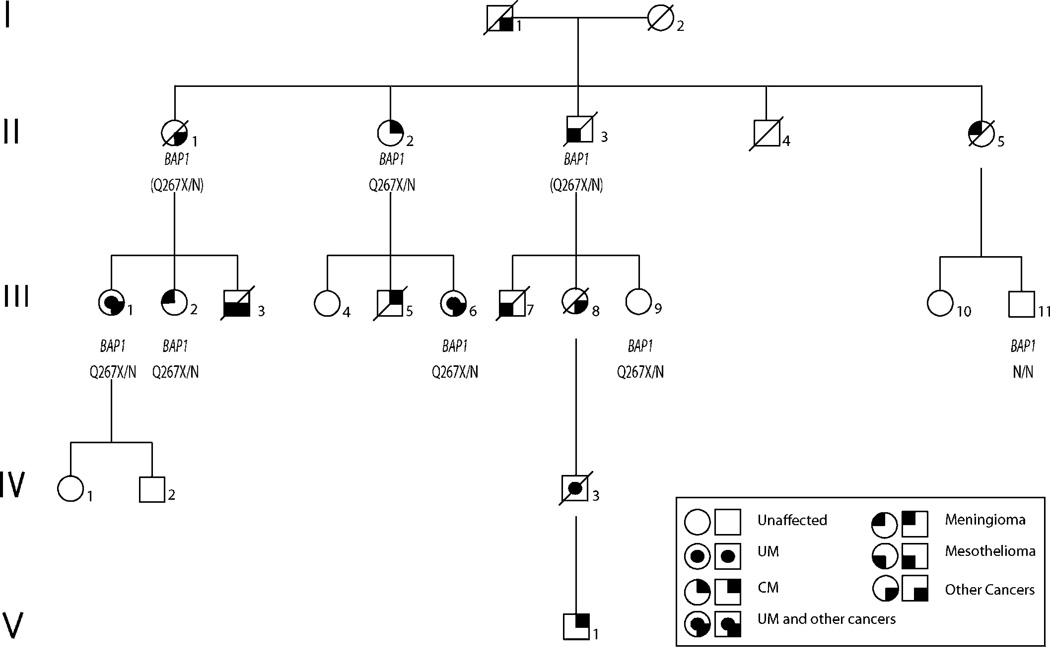
Figure 1.
Pedigree of family FUM036. Individuals III.1, II.2, III.2, III.6, III.9 were heterozygous for a truncating mutation (c. 799 C→T, p.Q267X) in BAP1 (designated Q267X/N in the figure). Individuals II.1 and II.3 are obligate carriers (inferred genotypes are shown in parentheses). Individual III.11 was negative for the mutation (designated N/N). No other individuals were tested. CM, cutaneous melanoma; UM, uveal melanoma.
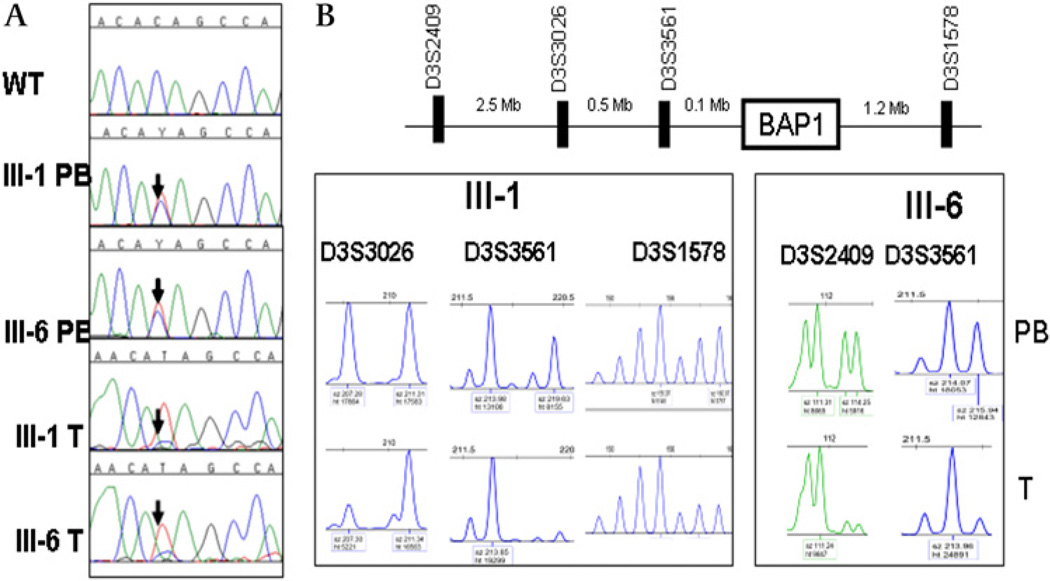
Figure 2.
Biallelic loss of BAP1 in tumours of the patients with germline mutation. (A) A germline truncating mutation (c. 799 C→T, p.Q267X) was observed in two patients with uveal melanoma (UM) (III.1 PB and III.6 PB) from the same family (FUM036). The mutation was also observed in the tumour tissues (III.1 T, a lung adenocarcinoma, and III.6 T, a UM) with loss of the wild type allele. (B) Genotyping of lung adenocarcinoma (from individual III.1) and UM (from individual III.6) tumours shows loss of heterozygosity of markers in close proximity to BAP1. PB, peripheral blood DNA; T, tumour DNA; WT, wild type.
Immunohistochemsitry
Immunohistochemistry was carried out on the same three tumour tissues noted above from family FUM036—that is, lung adenocarcinoma (individual, III.1), meningioma (individual, III.2), and UM (Individual, III.6). Two BAP1 antibodies were utilised, a mouse monoclonal (Clone C4) used at 1:500 dilution (SantaCruz Biotechnology, Santa Cruz, California, USA)8 and a rabbit polyclonal (N terminal clone) used at 1:150 dilution (ABGENT, San Diego, California, USA). The positive control was a tissue array of multiple normal tissues. Specificity of the antibodies was validated by immunohistochemistry on UM and non-tumour tissue microarrays (data not shown). Immunostaining without the primary antibody was used as negative control. Positive staining was assessed by a pathologist (MHA) using a Nikon Eclipse i50 bright-field microscope with Nikon digital sight DS-U1 5MP digital camera (Nikon, Japan).
RESULTS
Of the 53 unrelated patients included in our study, we identified germline variants in BAP1 in three patients (probands of families FUM036, FUM060, and FUM147). The proband of FUM060 had an intronic c.123-48T→G variant while the proband of FUM147 had a synonymous mutation c.1026C→T. Splice site prediction, utilising both NetGene 2 version 2.4215 and NNSPLICE V.0.9 software,16 indicated that these variants are not potential splice sites, suggesting that they are likely not pathogenic. The proband of FUM060 had UM at the age of 47 years, meningioma at age 47, and a family history of breast and lung cancers. The proband of FUM147 had UM at age 29 and a family history of breast cancer, cutaneous melanoma, and colon cancer.
In family FUM036, we identified six germline polymorphisms in the proband (individual III.1, figure 1), who presented with UM, lung adenocarcinoma, and a strong family history of cancer. One of the BAP1 variants identified is a truncating mutation (c. 799 C→T, p.Q267X), another variant is a synonymous single nucleotide polymorphism (SNP)rs28997577(c1002 A→T), while the remaining four variants are intronic variants (c.650-26T→A, c.931+70A→G, c.931+117_118delCC, and c.1891-30G→C) of uncertain significance. The truncating mutation in BAP1 identified in this family is located at amino acid 267 proximal to the nuclear localisation region located at amino acids 717–722, and it was not reported in the 1000 genomes project (browser.1000genomes.org)
Germline DNA sequencing of BAP1 was performed on five relatives of the proband of family FUM036. The same BAP1 variants, including the p.Q267X mutation, were identified in four of those individuals and were inherited as a single linkage disequilibrium block (figure 1). The mutation and variants were detected in patients with cutaneous melanoma (individual II.2), meningioma (individual III.2), UM and neuroendocrine carcinoma (individual III.6), and in one individual who was cancer-free at the age of 55 years (individual III.9). In addition, two patients from family FUM036 are obligate carriers for the BAP1 mutation, one with abdominal adenocarcinoma, suspected to be ovarian per the patient’s clinical notes (individual II.1), and one with mesothelioma (individual II.3). One individual (individual III.11) tested negative for the truncating mutation and the other BAP1 variants and had no history of cancer.
As noted above, tumour tissues were available from three individuals from FUM036 (lung adenocarcinoma from individual III.1, meningioma from individual III.2, and UM from individual III.6). A gene expression based assay, conducted in an outside clinical laboratory, indicated that the UM is a class 2 tumour.17
Genotyping revealed loss of heterozygosity of all informative microsatellite markers, including markers in close proximity of BAP1, on the chromosome 3p arm in all three tumours (figure 2). Markers on the q arm showed retention of heterozygosity of all markers in the lung tumour and the meningioma, and loss of heterozygosity of some of the markers in the UM (data not shown). Sequencing of the tumour tissues showed loss of the normal allele, indicating biallelic inactivation of BAP1 in these tumours (figure 2). Immunohistochemistry of the tumour tissues showed a decrease in BAP1 expression in the three tumours and loss of nuclear localisation in the UM (from individual III.6) and lung adenocarcinoma (from individual III.1) tumours (supplemental figure 1). The two antibodies tested showed similar results. No other tumour tissue was available from the family for testing.
DISCUSSION
We report a novel cancer predisposition syndrome caused by a germline truncating mutation in the BAP1 gene. Cancers segregating with the mutation in this family included UM plus lung adenocarcinoma, UM plus neuroendocrine carcinoma, as well as meningioma, abdominal adenocarcinoma, and cutaneous melanoma (figure 1).
The biallelic inactivation of BAP1 in the UM, meningioma and lung adenocarcinoma confirms that these tumours are part of the cancer phenotype in the family. A recent report identified two families with germline BAP1 mutations that presented with UM, cutaneous melanoma, and multiple naevi18 indicating that cutaneous melanoma and naevi may be part of the cancer phenotype in patients with germline BAP1 mutations. Whether other cancers observed in our family, such as mesothelioma, testicular cancer, and adrenocortical carcinoma, are part of the cancer phenotype caused by germline BAP1 alteration remains to be investigated. We cannot rule out the possibility of co-segregation of other cancer predisposition gene(s) in the family or co-occurrence of sporadic cancers. Only one out of seven individuals with the mutation was cancer-free (individual III.9). She was 55 years old at the time of testing and further monitoring is highly warranted due to the late onset of cancers (69, 72, and 75 years) in three individuals from the family. Further studies in additional families are needed to properly determine the full cancer phenotype and identify the degree of penetrance.
Germline BAP1 mutations have been analysed in a series of 47 French and 96 French Canadian families with high risk for breast and/or ovarian cancers that did not have detectable mutations in the BRCA1 and BRCA2 genes.12,13 No deleterious BAP1 mutation was detected in any of these families leading to the suggestion that BAP1 is not a breast cancer predisposing gene. However, further studies in other populations are recommended.
BAP1 is located in the tumour suppressor cluster at the 3p21 chromosomal region which shows deletion in many cancers including lung, breast, ovarian, pancreatic, and head and neck cancers.19 BAP1 has been reported as a candidate tumour suppressor in this region.19 Somatic mutations in BAP1 have been detected infrequently in lung and breast cancers,6,20 and more frequently in UM.7 In UM, somatic mutations in BAP1 were highly correlated with monosomy of chromosome 3 in tumours,7 suggesting that either mutation in BAP1 is a primary hit in tumours with monosomy 3 or that monosomy 3 is the primary hit in these tumours and BAP1 mutation is a secondary hit.
A previous study has identified a germline mutation of BAP1 in a female patient with UM who was diagnosed at the age of 53 years. The family history was not available for that patient.7 In that study, germline and somatic DNA from a total of 60 UM patients were evaluated. Although no family histories were provided for these patients, seven of them were younger than 45 years of age at the time of diagnosis, which is an earlier onset than the median age of 55 years generally reported for the diagnosis of UM. Taken together with the results of our study, it appears that germline mutations in BAP1 are the cause of hereditary cancer predisposition in a small subset of UM patients. Our data also suggest that other genes, in addition to BAP1, are important for hereditary cancer predisposition in UM. The frequency of germline mutations in other known candidate genes in UM patients, including BRCA2, CDKN2A, p14/ARF, and CDK4, is extremely low, suggesting the existence of one or more additional genes.3,5
In conclusion, we report a novel hereditary cancer syndrome caused by a germline BAP1 mutation that predisposes patients to UM, lung carcinoma, meningioma, and possibly other cancers. Our data suggest that BAP1 is the causative gene in a small subset of patients with hereditary UM and other cancers.
Acknowledgments
Funding This work is funded by the Patti Blow Research fund in Ophthalmology and by grant # IRG-67-003-47 from the American Cancer Society.
Footnotes
Competing interests None.
Ethics approval The Ohio State University Cancer Institutional Review Board.
Contributors MHAR: concept and design, overall supervision of the study, review of family histories and hereditary risk classification, pathological assessment, analysis and interpretation of data, drafting the manuscript and final approval of the version to be published. RP: patient accrual, review family histories and hereditary risk classification, analysis and interpretation of data, drafting the manuscript and final approval of the version to be published. CMC: concept and design, patient accrual, analysis and interpretation of data, drafting the manuscript and final approval of the version to be published. JBM: analysis and interpretation of the data, revising the manuscript and final approval of the published version. BC: analysis and interpretation of the data, revising the manuscript and final approval of the published version. GB: analysis and interpretation of the data and revising the manuscript and final approval of the published version. PH: patients’ accrual, revising the manuscript and final approval of the published version. FHD: concept and design, patients’ accrual, funding of the study and review final manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Abdel-Rahman MH, Pilarski R, Ezzat S, Sexton J, Davidorf FH. Cancer family history characterization in an unselected cohort of 121 patients with uveal melanoma. Fam Cancer. 2010;9:431–438. doi: 10.1007/s10689-010-9328-7. [DOI] [PubMed] [Google Scholar]
- 2.Bergman L, Nilsson B, Ragnarsson-Olding B, Seregard S. Uveal melanoma: a study on incidence of additional cancers in the Swedish population. Invest Ophthalmol Vis Sci. 2006;47:72–77. doi: 10.1167/iovs.05-0884. [DOI] [PubMed] [Google Scholar]
- 3.Buecher B, Gauthier-Villars M, Desjardins L, Lumbroso-Le Rouic L, Levy C, De Pauw A, Bombled J, Tirapo C, Houdayer C, Bressac-de Paillerets B, Stoppa-Lyonnet D. Contribution of CDKN2A/P16 (INK4A), P14 (ARF), CDK4 and BRCA1/2 germline mutations in individuals with suspected genetic predisposition to uveal melanoma. Fam Cancer. 2010;9:663–667. doi: 10.1007/s10689-010-9379-9. [DOI] [PubMed] [Google Scholar]
- 4.Kannengiesser C, Avril MF, Spatz A, Laud K, Lenoir GM, Bressac-de-Paillerets B. CDKN2A as a uveal and cutaneous melanoma susceptibility gene. Genes Chromosomes Cancer. 2003;38:265–268. doi: 10.1002/gcc.10286. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Rahman MH, Pilarski R, Massengill JB, Christopher BN, Noss R, Davidorf FH. Melanoma candidate genes CDKN2A/p16/INK4A, p14ARF, and CDK4 sequencing in patients with uveal melanoma with relative high-risk for hereditary cancer predisposition. Melanoma Res. 2011;21:175–179. doi: 10.1097/CMR.0b013e328343eca2. [DOI] [PubMed] [Google Scholar]
- 6.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 7.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen DE, Rauscher FJ., 3rd BAP1, a candidate tumor suppressor protein that interacts with BRCA1. Ann N Y Acad Sci. 1999;886:191–194. doi: 10.1111/j.1749-6632.1999.tb09414.x. [DOI] [PubMed] [Google Scholar]
- 10.Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, Liu J, O’Rourke K, Dixit VM, Wilson AC. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem. 2009;284:34179–34188. doi: 10.1074/jbc.M109.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guénard F, Labrie Y, Ouellette G, Beauparlant CJ, Durocher F. INHERIT BRCAs. Genetic sequence variations of BRCA1-interacting genes AURKA, BAP1, BARD1 and DHX9 in French Canadian families with high risk of breast cancer. J Hum Genet. 2009;54:152–161. doi: 10.1038/jhg.2009.6. [DOI] [PubMed] [Google Scholar]
- 13.Coupier I, Cousin PY, Hughes D, Legoix-Né P, Trehin A, Sinilnikova OM, Stoppa-Lyonnet D. BAP1 and breast cancer risk. Fam Cancer. 2005;4:273–277. doi: 10.1007/s10689-005-2833-4. [DOI] [PubMed] [Google Scholar]
- 14.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouzé P, Brunak S. Splice site prediction in Arabidopsis thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res. 1996;24:3439–3452. doi: 10.1093/nar/24.17.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J Comput Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 17.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesner T, Obenauf A, Murali R, Fried I, Griewank K, Ulz P, Windpassinger C, Loy S, Wackernagel W, Wolf I, Becker J, Viale A, Lash A, Pirun M, Socci N, Ruetten A, Palmedo G, Ott A, Abramson D, Offit K, Cerroni L, Kutzner H, Bastian BC. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. Philadelphia (PA): Orlando, Florida; 2011. Apr 2–6, Germline mutations in BAP1 predispose to melanocytic nevi and melanoma. AACR; 2011. Abstract nr {LB-125} [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesson LB, Cooper WN, Latif F. Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene. 2007;26:7283–7301. doi: 10.1038/sj.onc.1210547. [DOI] [PubMed] [Google Scholar]
- 20.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]