Abstract
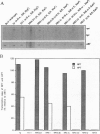
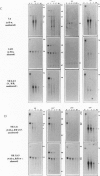
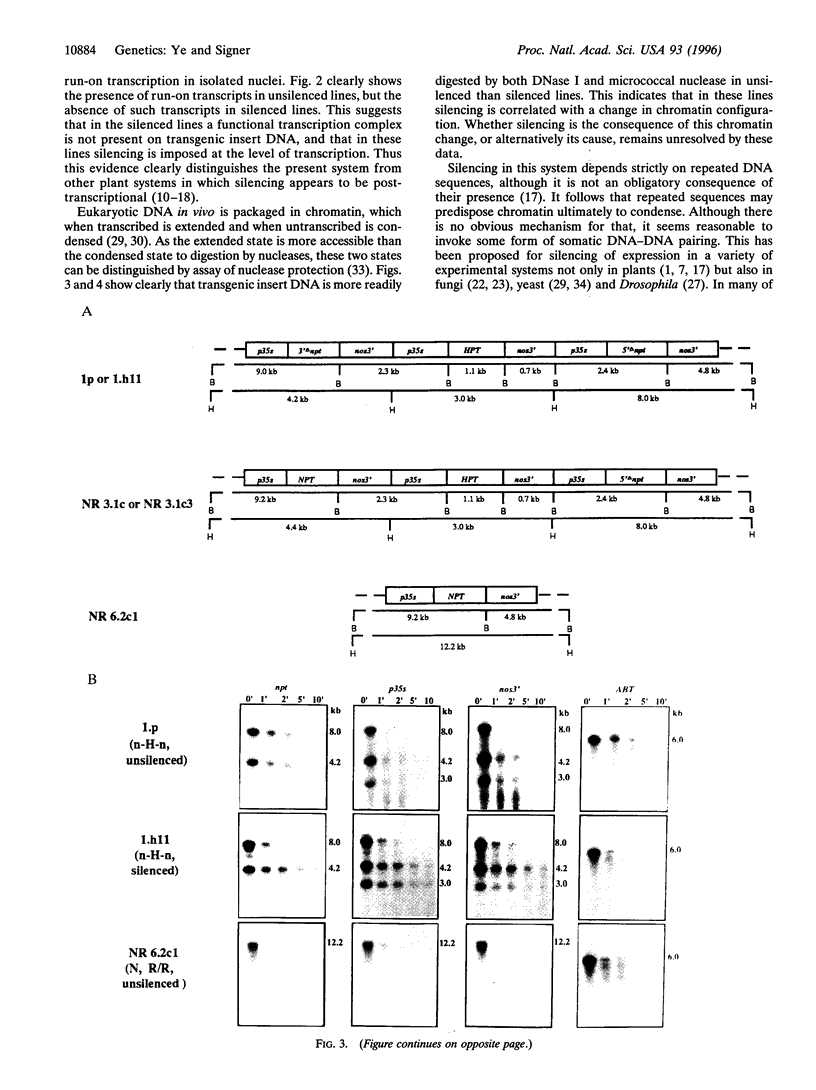
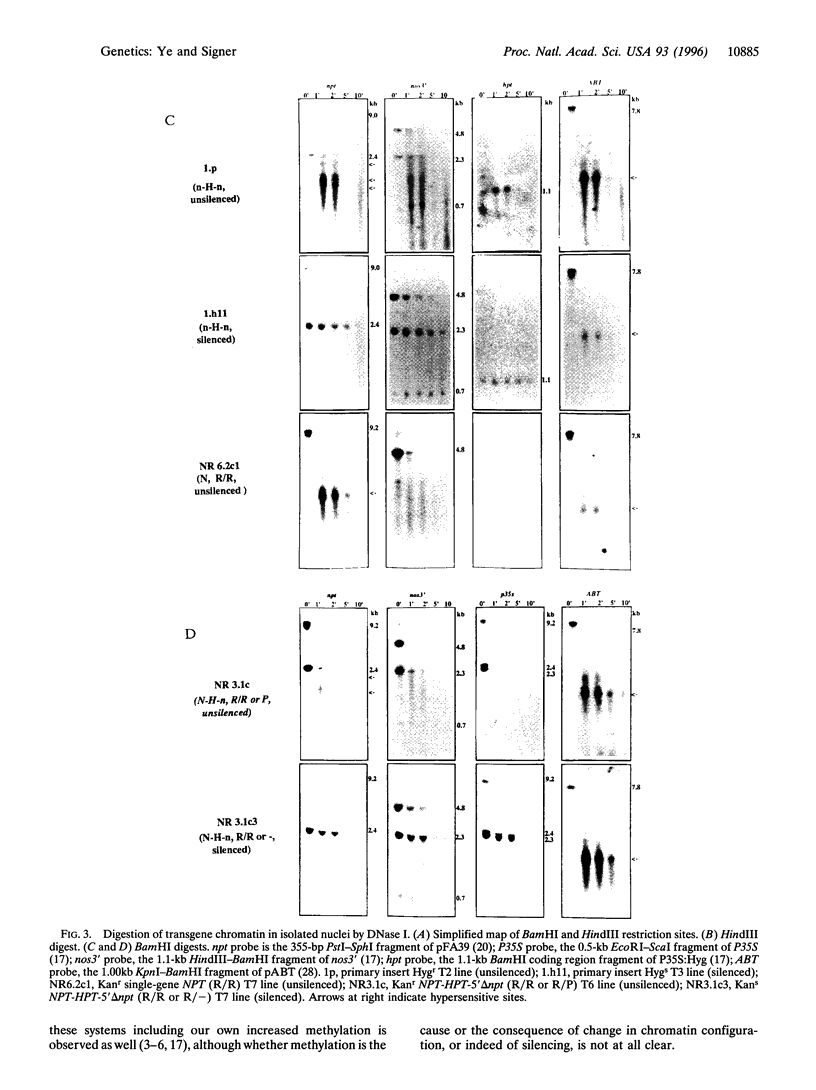
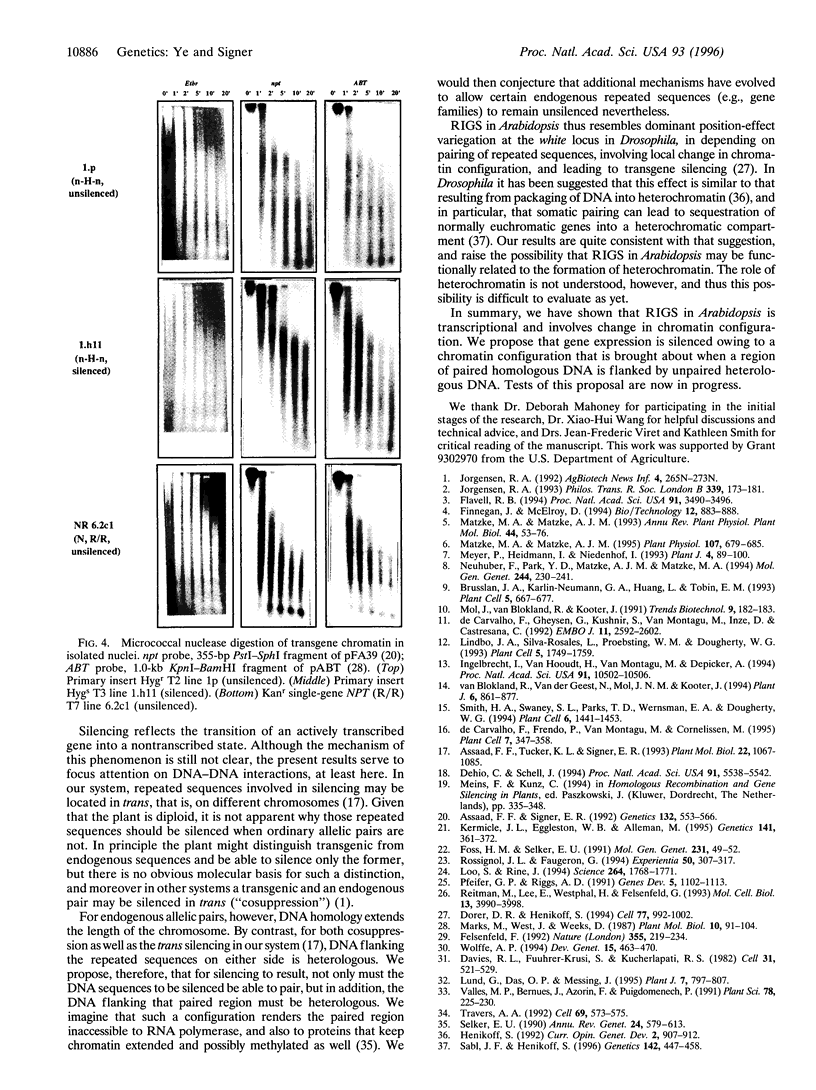
We have previously reported repeat-induced gene silencing (RIGS) in Arabidopsis, in which transgene expression may be silenced epigenetically when repeated sequences are present. Among an allelic series of lines comprising a primary transformant and various recombinant progeny carrying different numbers of drug resistance gene copies at the same locus, silencing was found to depend strictly on repeated sequences and to correlate with an absence of steady-state mRNA. We now report characterization, in nuclei isolated from the same transgenic lines, of gene expression by nuclear run-on assay and of chromatin structure by nuclease protection assay. We find that silencing is correlated with absence of run-on transcripts, indicating that expression is silenced at the level of transcription. We find further that silencing is also correlated with increased resistance to both DNase I and micrococcal nuclease, indicating that the silenced state reflects a change in chromatin configuration. We propose that silencing results when a locally paired region of homologous repeated nucleotide sequences is flanked by unpaired heterologous DNA, which leads chromatin to adopt a local configuration that is difficult to transcribe, and possibly akin to heterochromatin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaad F. F., Signer E. R. Somatic and germinal recombination of a direct repeat in Arabidopsis. Genetics. 1992 Oct;132(2):553–566. doi: 10.1093/genetics/132.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad F. F., Tucker K. L., Signer E. R. Epigenetic repeat-induced gene silencing (RIGS) in Arabidopsis. Plant Mol Biol. 1993 Sep;22(6):1067–1085. doi: 10.1007/BF00028978. [DOI] [PubMed] [Google Scholar]
- Brusslan J. A., Karlin-Neumann G. A., Huang L., Tobin E. M. An Arabidopsis mutant with a reduced level of cab140 RNA is a result of cosuppression. Plant Cell. 1993 Jun;5(6):667–677. doi: 10.1105/tpc.5.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Dehio C., Schell J. Identification of plant genetic loci involved in a posttranscriptional mechanism for meiotically reversible transgene silencing. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5538–5542. doi: 10.1073/pnas.91.12.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer D. R., Henikoff S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell. 1994 Jul 1;77(7):993–1002. doi: 10.1016/0092-8674(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Flavell R. B. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss H. M., Selker E. U. Efficient DNA pairing in a Neurospora mutant defective in chromosome pairing. Mol Gen Genet. 1991 Dec;231(1):49–52. doi: 10.1007/BF00293820. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Position effect and related phenomena. Curr Opin Genet Dev. 1992 Dec;2(6):907–912. doi: 10.1016/s0959-437x(05)80114-5. [DOI] [PubMed] [Google Scholar]
- Ingelbrecht I., Van Houdt H., Van Montagu M., Depicker A. Posttranscriptional silencing of reporter transgenes in tobacco correlates with DNA methylation. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10502–10506. doi: 10.1073/pnas.91.22.10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermicle J. L., Eggleston W. B., Alleman M. Organization of paramutagenicity in R-stippled maize. Genetics. 1995 Sep;141(1):361–372. doi: 10.1093/genetics/141.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbo J. A., Silva-Rosales L., Proebsting W. M., Dougherty W. G. Induction of a Highly Specific Antiviral State in Transgenic Plants: Implications for Regulation of Gene Expression and Virus Resistance. Plant Cell. 1993 Dec;5(12):1749–1759. doi: 10.1105/tpc.5.12.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S., Rine J. Silencers and domains of generalized repression. Science. 1994 Jun 17;264(5166):1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Matzke AJM. How and Why Do Plants Inactivate Homologous (Trans)genes? Plant Physiol. 1995 Mar;107(3):679–685. doi: 10.1104/pp.107.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P., Heidmann I., Niedenhof I. Differences in DNA-methylation are associated with a paramutation phenomenon in transgenic petunia. Plant J. 1993 Jul;4(1):89–100. doi: 10.1046/j.1365-313x.1993.04010089.x. [DOI] [PubMed] [Google Scholar]
- Neuhuber F., Park Y. D., Matzke A. J., Matzke M. A. Susceptibility of transgene loci to homology-dependent gene silencing. Mol Gen Genet. 1994 Aug 2;244(3):230–241. doi: 10.1007/BF00285450. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Riggs A. D. Chromatin differences between active and inactive X chromosomes revealed by genomic footprinting of permeabilized cells using DNase I and ligation-mediated PCR. Genes Dev. 1991 Jun;5(6):1102–1113. doi: 10.1101/gad.5.6.1102. [DOI] [PubMed] [Google Scholar]
- Reitman M., Lee E., Westphal H., Felsenfeld G. An enhancer/locus control region is not sufficient to open chromatin. Mol Cell Biol. 1993 Jul;13(7):3990–3998. doi: 10.1128/mcb.13.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J. L., Faugeron G. Gene inactivation triggered by recognition between DNA repeats. Experientia. 1994 Mar 15;50(3):307–317. doi: 10.1007/BF01924014. [DOI] [PubMed] [Google Scholar]
- Sabl J. F., Henikoff S. Copy number and orientation determine the susceptibility of a gene to silencing by nearby heterochromatin in Drosophila. Genetics. 1996 Feb;142(2):447–458. doi: 10.1093/genetics/142.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Smith H. A., Swaney S. L., Parks T. D., Wernsman E. A., Dougherty W. G. Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of nonessential RNAs. Plant Cell. 1994 Oct;6(10):1441–1453. doi: 10.1105/tpc.6.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. The reprogramming of transcriptional competence. Cell. 1992 May 15;69(4):573–575. doi: 10.1016/0092-8674(92)90218-2. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. Inheritance of chromatin states. Dev Genet. 1994;15(6):463–470. doi: 10.1002/dvg.1020150604. [DOI] [PubMed] [Google Scholar]
- de Carvalho Niebel F., Frendo P., Van Montagu M., Cornelissen M. Post-transcriptional cosuppression of beta-1,3-glucanase genes does not affect accumulation of transgene nuclear mRNA. Plant Cell. 1995 Mar;7(3):347–358. doi: 10.1105/tpc.7.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho F., Gheysen G., Kushnir S., Van Montagu M., Inzé D., Castresana C. Suppression of beta-1,3-glucanase transgene expression in homozygous plants. EMBO J. 1992 Jul;11(7):2595–2602. doi: 10.1002/j.1460-2075.1992.tb05324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]