Abstract
Telomere biology disorders are a complex set of illnesses defined by the presence of very short telomeres. Individuals with classic dyskeratosis congenita have the most severe phenotype, characterized by the triad of nail dystrophy, abnormal skin pigmentation, and oral leukoplakia. More significantly, these individuals are at very high risk of bone marrow failure, cancer, and pulmonary fibrosis. A mutation in one of six different telomere biology genes can be identified in 50–60% of these individuals. DKC1, TERC, TERT, NOP10 and NHP2 encode components of telomerase or a telomerase-associated factor, and TINF2, a telomeric protein. Progressively shorter telomeres are inherited from generation to generation in autosomal dominant dyskeratosis congenita, resulting in disease anticipation. Up to 10% of individuals with apparently acquired aplastic anemia or idiopathic pulmonary fibrosis also have short telomeres and mutations in TERC or TERT. Similar findings have been seen in individuals with liver fibrosis or acute myelogenous leukemia. This report reviews basic aspects of telomere biology and telomere length measurement, and the clinical and genetic features of those disorders that constitute our current understanding of the spectrum of illness caused by defects in telomere biology. We also suggest a grouping schema for the telomere disorders.
Keywords: telomere, telomere biology disorder, dyskeratosis congenita, bone marrow failure, idiopathic pulmonary fibrosis
INTRODUCTION
Over the past five to ten years, it has become increasingly clear that alterations in telomere integrity can directly impact human health. Telomeres are the specialized nucleoprotein structures present at the ends of chromosomes, and are comprised of long nucleotide repeats (TTAGGG)n bound by a host of telomere-specific and so-called ‘accessory’ proteins (1;2). The essential function of telomeres derives from the exquisite sensitivity cells have to double-stranded (ds) DNA ends and the robust mechanisms to detect and repair DNA ends created by DNA ds breaks. Telomeres protect the DNA ends present naturally at the termini of linear chromosomes from being acted upon as if they were broken ends, thereby inhibiting telomere fusions, illegitimate recombination and genomic instability.
Because the semiconservative DNA replication machinery is unable to replicate terminal DNA at chromosome ends, telomeric sequence is lost with each round of DNA replication (3). Consequently, telomeres shorten with aging. In peripheral blood leukocytes, the cells most extensively studied, the rate of attrition is greatest during the first year of life (4). Thereafter, telomeres shorten more gradually. When the extent of telomeric DNA loss exceeds a critical threshold, a robust antiproliferative signal is triggered, leading to cellular senescence or apoptosis. Thus, telomere attrition is thought to contribute to aging phenotypes (1).
With the discovery in 1985 of telomerase (5), the enzyme that extends telomeric nucleotide repeats, there has been rapid progress both in our understanding of basic telomere biology, and also in the connection of telomere biology to human disease. While the initial focus had been on the relevance of telomere biology to cancer, we now appreciate that there is a spectrum of human disease that originates from inherent defects in telomere length maintenance, making an understanding of telomere biology disorders of importance to a broader spectrum of clinicians. This review will describe the basis of human telomere length variation and inheritance, various methods available to measure telomere length, how a diagnosis of a telomere biology disorder can be established, and, lastly, the medical complications associated with short telomeres.
TELOMERASE AND TELOMERE ASSOCIATED PROTEINS
The elucidation of factors associated with telomerase through basic biology research has been crucial to the identification of genes mutated in telomere biology disorders. A diagram of these proteins is shown in Figure 1. Telomerase consists of two core components, TERT, a reverse transcriptase, and TERC, an RNA that contains a template for telomere repeat addition (3). The telomerase complex catalyzes the synthesis and extension of terminal telomeric DNA. Telomerase is developmentally regulated, with expression in most human tissues only during the first few weeks of embryogenesis (6). From the neonatal period onward, telomerase activity is largely repressed, except in certain highly-proliferative tissues such as skin, intestine, and bone marrow, which are thought to contain stem cell-like subpopulations, as well as in dividing lymphocytes, ovaries, and testes (6–9). Telomerase is also upregulated in most cancer cells, reflecting the need for telomere maintenance for proliferative potential (7;10).
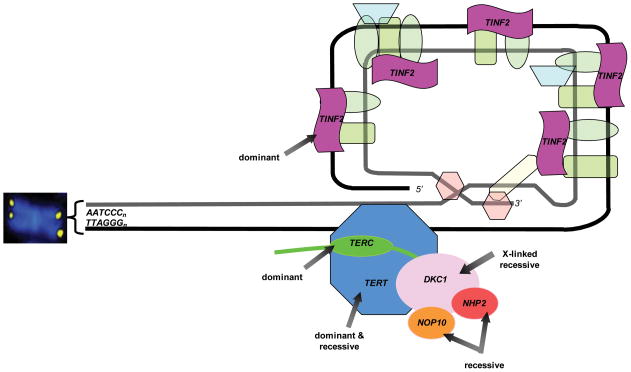
Figure 1. Schematic of genes mutated in telomere biology disorders.
The chromosome end consists of a (TTAGGG)n repeats that fold back to form a t-loop. This structure is capped by a six protein complex termed “shelterin.” The telomerase enzyme complex consists of TERT, TERC, DKC1, NOP10, NHP2, and GAR1 (not shown). The inheritance pattern of mutations in these genes is noted. Approximately 50–60% of patients with classic dyskeratosis congenita will have a mutation in one of these genes.
Abbreviations: TINF2, TRF1-interacting nuclear factor 2; TERT, telomerase; TERC, telomerase RNA component DKC1, dyskerin; NOP10, NOLA3, nucleolar protein family A, member 3; NHP2, NOLA2, nucleolar protein family A, member 2
TERT and TERC are sufficient for telomerase reconstitution in vitro, but additional factors are required for telomerase biogenesis, localization and activity in vivo. Importantly, dyskerin is associated with catalytically-active telomerase via TERC and is required for normal TERC levels and telomerase activity in vivo (11). Dyskerin, which is encoded by DKC1, associates with H/ACA box-containing small nucleolar RNAs (snoRNAs), including TERC (12). It is also involved in post-transcriptional pseudouridylation and forms a ribonucleoprotein complex with NOP10, NHP2, and GAR1(13;14). These factors are also found in a complex with TERC; they do not mediate its pseudouridylation, but instead appear to be involved in the proper stability, regulation or trafficking of telomerase (15). NAF1, which is exchanged for GAR1 in the dyskerin/NOP10/NHP2 complex, and the ATPases pontin and reptin, are also required for assembly of the catalytically competent telomerase complex, but are not associated with the mature enzyme (16;17). Lastly, there is the protein TCAB1, which associates with active telomerase by binding to the CAB box motif of TERC, and is responsible for the localization of telomerase to nuclear Cajal bodies, a critical step for telomere maintenance (18). Given the importance of these factors, we speculate that mutation in one or more may be identified in human telomere biology disorders in the future.
In addition to telomerase, proteins that bind to telomeric DNA are important for normal telomere maintenance and function. These include shelterin, a six protein telomere-specific complex which serves a number of functions including inhibiting the DNA damage response at telomeres, negatively regulating telomerase, and promoting telomere replication and sister telomere cohesion (2). The importance of this complex is underscored by the finding that mutations in one of the shelterin components, TIN2, which is encoded by TINF2, have been found to cause very short telomeres and dyskeratosis congenita (DC: see below) (19).
TELOMERE LENGTH AND INHERITANCE
Telomere length measurements in a variety of organisms have revealed tremendous variability between species. Individual species, however, maintain their germline telomeres broadly around a defined set point. In humans, telomeres are several kilobases (kb) in length. Telomere length is inversely proportional to age. Although telomere length varies from tissue to tissue, it is correlated within an individual (20–22) (Gadalla et al., under review). This likely reflects the proliferative history of the constituent cells, the level of telomerase activity, and other factors that may lead to telomere attrition. Thus, highly proliferative tissues may exhibit shorter telomere length.
Genetic factors have been shown to impact telomere length in healthy populations, with heritability estimates ranging from 36 – 82%(23–26). Telomere length correlated significantly with paternal, but not maternal telomere length in a number of studies, suggesting a possible role for genomic imprinting (27;28). Additionally, inheritance of telomere length follows a polygenic pattern, consistent with a quantitative trait. A number of quantitative trait linkage and genome-wide association studies (GWAS) have been performed to identify genetic factors that modulate telomere length. Several studies have identified single nucleotide polymorphisms (SNPs) that may contribute to telomere length regulation (29). For example, SNPs were identified in an intron in BICD1, which encodes a protein involved in vacuolar trafficking, and to a region between BRUNOL4 and VPS34, which encode a putative RNA binding protein and a lipid kinase involved in autophagy, respectively (30). The significance of these associations is unclear, although it is notable that vacuolar protein sorting (VPS) genes have been widely implicated in telomere maintenance in a yeast model system (31). Most recently, a SNP was identified in a GWAS of mean telomere length in a region 1.5 kb downstream from TERC (32). Whether the SNP affects TERC expression levels has yet to be evaluated, however, this is an attractive hypothesis as cells are very sensitive to reductions in TERC (see below).
TELOMERE LENGTH MEASUREMENT
Several methods are available in the research setting to determine telomere length in whole cells and DNA preparations (reviewed in (33;34)). The current gold standard of telomere length determination is terminal restriction fragment (TRF) measurement on Southern blots. This method requires several hundred nanograms of high-quality DNA and uses restriction enzymes to digest telomeric DNA. The resultant fragments are run on a Southern blot and the median length determined. Telomere length can be determined on metaphase chromosomes and on fixed or imbedded tissues through the use of telomere-specific probes and fluorescence in situ hybridization (FISH) (35). The intensity of the telomeric fluorescent signal is measured relative to another DNA-specific probe. Single telomere length analysis (STELA), a PCR-based approach, is useful in understanding telomere length differences between chromosomes (36;37).
Quantitative-PCR (QPCR) has emerged as a method that is useful in larger epidemiologic studies of telomere length because it is a high-throughput platform (38;39). It requires small amounts of high-quality DNA and determines a ratio of telomere signal to single copy gene signal, which serves as a telomere length surrogate.
Telomere length measurement by automated multicolor flow cytometry combined with FISH (flow-FISH) on white blood cell (WBC) subsets is very useful in understanding telomere length differences in the immune system (35). Unlike other methods, it requires either fresh or cryopreserved blood samples but gives specific measurements on the WBC subset telomere length. Testing of telomere length in WBC subsets using the flow-FISH procedure is currently the only method of determining telomere length that is clinically available. Examples of results from flow-FISH telomere length testing are shown in Figure 2.
Figure 2.
Example of lymphocyte telomere length measured by flow cytometry with fluorescence in situ hybridization. Black circle, patient with classic dyskeratosis congenita; blue circle, patient with aplastic anemia; red circle, patient with isolated pulmonary fibrosis.
Abbreviations: kb, kilobases; %ile, percentile.
Graph courtesy of Drs. Blanche Alter and Peter Lansdorp
The method of telomere length measurement, cell type studied, as well as study design and statistical power, must be considered in the interpretation of data in studies evaluating telomere lengths in individuals with various conditions. For example, there can be considerable inter-individual variability in telomere length, even amongst age-matched subjects. Therefore, a large number of controls are essential to properly interpret data. Many earlier studies reported either the mean telomere length in patients compared with age-matched controls, or the difference between the average telomere lengths of patients and controls (deltaTEL). The measurements are useful in understanding the characteristics of a group of individuals, but not always applicable to the individual patient. In more recent studies, individual telomere length is plotted against the distribution of age-dependent telomere lengths observed in a large number of ‘normal’ individuals to determine an individual’s percentile telomere length (e.g., see Figure 2) (35;40).
THE TELOMERE BIOLOGY DISORDERS
Abnormalities in telomere biology resulting in clinically significant disease were first recognized in patients with dyskeratosis congenita (DC)(12). Studies of individuals with severe aplastic anemia (SAA), a frequent complication of DC, found that a subset of individuals with SAA had shorter telomeres and mutations in telomere biology genes that overlapped with those that cause DC (41–47). Short telomeres and mutations in the same genes have also been identified in a small percentage of individuals with pulmonary fibrosis (48;49), as well as non-alcoholic liver disease (50) and acute myelogenous leukemia (AML)(51;52). These disorders represent a broad spectrum of related diseases caused by defective telomere maintenance. This section will describe the clinical presentation, diagnosis, and overlapping features of these related disorders (Box 1).
Box 1. Telomere biology disorder groupings.
Individuals with short telomeres and/or a germline mutation in a telomere biology gene should be considered to have a telomere biology disorder. One schema for how they can be further grouped is presented below. The groupings are based on our current clinical knowledge. It is important to recognize that an individual’s placement in a particular group may change over time if additional disease manifestations become apparent.
Classical Dyskeratosis Congenita (DC) – patients with the mucocutaneous triad of dysplastic nails, skin pigmentation abnormalities, and oral leukoplakia, or those who have one component of the triad in combination with a hypoplastic bone marrow and two or more of the other somatic manifestations of DC as suggested by Vulliamy, et al. (74). These individuals have very short telomeres (<1st percentile for age) in leukocytes measured by flow-FISH (40).
Hoyeraal Hreidarsson Syndrome – individuals with documented cerebellar hypoplasia and additional manifestations of classical DC.
Revesz Syndrome – individuals with bilateral exudative retinopathy and additional manifestations of classical DC.
Mutation Carrier – asymptomatic or pre-symptomatic individuals with a mutation in a gene known to cause DC. These individuals are typically identified by screening of family members of affected individuals. In families in which affected individuals do not carry a mutation in one of the known six genes, an asymptomatic individual may be presumed to be a mutation carrier based on the presence of telomeres less than the 1st percentile for age.
DC-like – individuals who have several features suggestive of DC but do not yet meet diagnostic criteria. Examples could be a patient with bone marrow failure, developmental delay, and a family history of pulmonary fibrosis, in the absence of another diagnosis. Telomeres are typically short but may not be as short as in classical DC. A mutation in a telomere biology gene may or may not be identified. These patients may fulfill the criteria for classical DC over time and should be monitored appropriately.
Isolated Subtypes – individuals who carry a known mutation in a telomere biology gene and/or have telomeres less than the 1st percentile for age who have the isolated presentation of a condition known to be associated with a telomere biology disorder and dyskeratosis congenita. These conditions include isolated bone marrow failure, pulmonary fibrosis, unexplained liver disease, myelodysplastic syndrome, acute myelogenous leukemia, or early age of onset cancers within the DC spectrum. For example, a patient with isolated bone marrow failure and very short telomeres (<1st percentile for age), or one with isolated pulmonary fibrosis and a TERT mutation would be considered to have an isolated subtype. These patients, too, may fulfill the criteria for classical DC or the DC-like grouping over time.
Additional Considerations – The extent to which Mutation Carriers, DC-like, or Isolated Subtype individuals need monitoring for DC-related complications is unclear and should be considered on a case-by-case basis. For example, a three-year old with isolated bone marrow failure and a TINF2 mutation is at very high risk of complications during hematopoietic stem cell transplantation (HSCT) and should receive a modified HSCT regimen. On the other hand, a 70-year old with isolated pulmonary fibrosis and a TERT mutation may not have additional risks but their family members should be counseled for potential disease risk and the existence of genetic anticipation.
DYSKERATOSIS CONGENITA
Clinical features
The disorder now known as dyskeratosis congenita (DC) was first described between 1906 and 1910 (53). Case reports initially described the diagnostic triad of nail dystrophy, lacy reticular pigmentation of the neck and upper chest, and oral leukoplakia in males. As additional cases were identified, including in females, the presence of bone marrow failure (BMF), cancer, and numerous other medical problems were noted (54;55). Figure 3 shows features of the diagnostic triad. The clinical characteristics may also include abnormal pigmentation changes not restricted to the upper chest and neck, eye abnormalities (epiphora, blepharitis, sparse eyelashes, ectropion, entropion, trichiasis) (56), dental abnormalities (caries, periodontal disease) (57), esophageal stenosis, urethral stenosis, avascular necrosis of the femur and/or humerus, osteopenia, pulmonary fibrosis, and liver disease. Patients with DC are at risk of progressive BMF, myelodysplastic syndrome (MDS) or AML, and solid tumors (usually squamous cell carcinoma of the head/neck or anogenital cancer) (58).
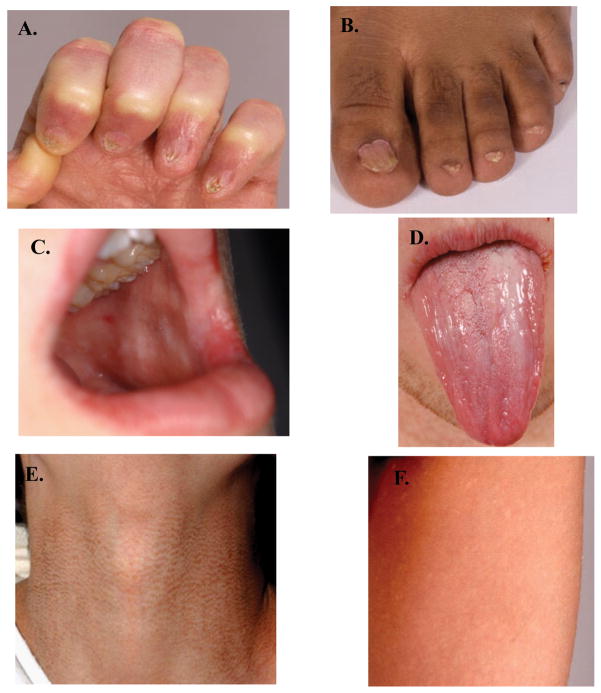
Figure 3. The diagnostic triad of dyskeratosis congenita.
A. Fingernail dysplasia, B. Toenail dysplasia, C. Leukoplakia of the buccal mucosa, D. Leukoplakia of the tongue, E, and F. Skin pigmentation abnormalities can be diverse and occur anywhere on the body. E. Hyperpigmentation of the neck, F. Hypopigmented areas on the upper thigh.
Two disorders, Hoyeraal-Hreidarsson syndrome and Revesz syndrome, represent severe variants of DC. Hoyeraal-Hreidarsson syndrome is characterized by many of the DC features listed above plus the presence of cerebellar hypoplasia (59;60). These patients also have significant developmental delay, and immunologic abnormalities. Individuals with Revesz syndrome have bilateral exudative retinopathy and intracranial calcifications, in addition to many features of DC (61;62). The determination of very short telomeres and mutations in telomere biology genes in both Hoyeraal-Hreidarsson (DKC1 and TINF2) and Revesz (TINF2) syndromes firmly establishes that these disorders are indeed within the DC spectrum (19;60;63).
Inheritance
DC is inherited in X-linked recessive, autosomal dominant, and autosomal recessive forms. Approximately one-half of patients with DC have a mutation identified in one of six known genes, DKC1, TERC, TERT, TINF2, NHP2, and NOP10 (Table 1) (55;64). The mutations in the X-linked recessive gene, dyskerin (DKC1), were the first link to the role of telomere biology in DC pathogenesis (12;65). Fibroblasts from patients with DKC1 mutations were shown to have very short telomeres (12). This is thought to be due to a decrease in TERC in DKC1 mutant cells which results in a deficiency in cellular telomerase activity (12).
Table 1. Genes that cause dyskeratosis congenita and other telomere biology disorders.
The approximate percent of patients with causative mutations in telomere biology genes in the disorders is shown.
| Approximate percent of patients with mutations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene, Protein name(s) | Chromosomal Locus | MIM # | Inheritance | # reported mutations | DCa | Isolated SAA | Isolated IPF | Isolated fibrotic liver disease | Isolated AML |
|
DKC1 DKC1, dyskerin |
Xq28 | 300126 | XLR | >40 | 17–36 | NR | NR | NR | NR |
|
TINF2 TIN2, TERF1 (TRF1)-interacting nuclear factor 2 |
14q11.2 | 604319 | AD | ~17 | 11–24 | 5b | NR | NR | NR |
|
TERC TERC, telomerase RNA component |
3q26.3 | 602322 | AD | ~40 | 6–10 | 1–5c | 1–3e | 3 familiesf | NR |
|
TERT TERT, telomerase reverse transcriptase |
5p15.53 | 187270 | AD, AR | ~40 | 1–7 | 3–4d | 7–12e | 2 familiesf | 2g |
|
NOP10 NOP10, NOLA3, nucleolar protein family A, member 3 |
15q14-q15 | 606471 | AR | 1 | <1 | NR | NR | NR | NR |
|
NHP2 NHP2, NOLA2 nucleolar protein family A, member 2 |
5q35.5 | 606470 | AR | 3 | <1 | NR | NR | NR | NR |
Based on reports from Dyskeratosis Congenita Registry (64) and National Cancer Institute’s Dyskeratosis Congenita cohort.
Derived from Du et al, based on 6 of 109 children with SAA who had a TINF2 mutation (98).
Derived from Yamaguchi et al with 3 of 210 patients with SAA and from Vulliamy et al with 2 of 17 patients with SAA reported to have a TERC mutation (44;45).
Derived from Yamaguchi et al with 7 of 200 patients with moderate or SAA reported to have a TERT mutation (46).
Based on reports of Tsakiri et al and Armanios et al who identified 5 individuals with TERT and 1 with TERC mutations from 46 families (49) and 5 individuals with TERT and 1 with TERC mutations from 72 probands (48) with IPF and no features of DC, respectively.
Number of families reported by Calado et al (50). Incidence in unrelated individuals is not known.
Calado et al reported that 20 of 596 patients with AML (1.9%) had the A1062T variant in TERT compared with 13 of 1,110 controls (0.6%)(52). In their initial screen of 222 patients with AML, 9 had the A1062T variant and 5 had different TERT mutations.
Abbreviations: XLR, X-linked recessive; AD, autosomal dominant; AR autosomal recessive; DC, dyskeratosis congenita; SAA, severe aplastic anemia; IPF, idiopathic pulmonary fibrosis; AML, acute myelogenous leukemia; NR, none reported
Mutations in TERC or TERT were subsequently identified in the autosomal dominant form of DC (46;47;66). These patients may have classic DC with many complications, whereas others may be less severely affected. Functional analysis of several disease-associated TERC and TERT mutations revealed that they result in short telomeres due to haploinsufficiency of telomerase rather than a dominant negative effect, indicating that telomerase is limiting in normal cells. A small number of severely affected patients have also been shown to have DC due to autosomal recessive inheritance of TERT mutations which result in significantly decreased levels of telomerase (47;67). Patients with biallelic TERT mutations appear to be more severely affected (i.e., Hoyeraal-Hreidarsson variant) than those with the autosomal dominant TERT mutations.
Homozygosity mapping of consanguineous families with DC identified autosomal recessive inheritance of mutations in NOP10 (one family) or NHP2 (two families) as causative of DC (53;67). The NHP2 and NOP10 proteins associate with DKC1 and GAR1 in the H/ACA snoRNP structures. NHP2 and NOP10 mutations appear to result in reduced levels of TERC, similar to the effects of a DKC1 mutation. Individuals with these mutations have the more severe form of DC, Hoyeraal-Hreidarsson syndrome.
The sixth gene implicated in DC, TINF2, encodes not a component of telomerase, but rather a component of the telomeric shelterin complex, TIN2(2;19). In fact, mutations in TINF2 are responsible for a large proportion of autosomal dominant DC (19;68). Patients with TINF2 mutations may have severe forms of DC, including the Revesz syndrome variant, but silent carriers (i.e., individuals who have a TINF2 mutation and very short telomeres, but lack the clinical phenotype) have also been identified (19). In contrast to the molecular mechanism by which mutations in telomerase components lead to telomere shortening, the mechanism by which TIN2 mutations result in telomere shortening has not yet been identified.
Diagnosis
The ability to diagnose DC has improved over the last three to four years, in part, through studies of telomere length in individuals with inherited bone marrow failure syndromes (IBMFS) and other disorders. The diagnosis is quite straightforward in patients who present with the diagnostic triad of dysplastic nails, abnormal skin pigmentation and oral leukoplakia, with or without additional medical complications. However, the diagnosis is often challenging because the clinical manifestations vary widely in age of onset, even in individuals from the same family. The clinical complications of DC, including the diagnostic triad, can evolve over time. Clinical telomere length testing has helped improve our ability to diagnosis DC.
Measurement of telomere length peripheral white blood cells by flow-FISH was found to be both sensitive and specific for identifying patients with DC (40). In that study, telomere length in leukocyte subsets (granulocytes, total lymphocytes, naïve T-cells, memory T-cells, B-cells, and NK cells) was determined by flow-FISH on cells from patients with DC, their relatives, and patients with other IBMFS. Percentiles of telomere length were generated by comparison to a reference group of 400 healthy controls (newborn through 100 years of age)(35;40). Values below the 1st percentile for age were considered “very short.” The diagnostic sensitivity and specificity of very short telomeres was greater than 90% in total lymphocytes, naïve T-cells, and B-cells for the diagnosis of DC in comparison with healthy relatives of patients with DC or non-DC IBMFS patients (40). Rare apparently unaffected relatives with very short telomeres (silent carriers) were later shown to have mutations in the same DC genes as the affected probands (19). Figure 2 shows an example of telomere length results that could be expected from a patient with classic DC compared with patients with severe aplastic anemia and pulmonary fibrosis associated with mutations in TERC or TERT.
A subsequent study from a different laboratory used a modified flow-FISH method, which involved comparison of telomere signal intensity in peripheral blood mononuclear cells to that of an aneuploid (4N) cell line (69). Individual telomere length percentiles were determined based on the age-dependent distribution of telomere length found in a population of 234 healthy controls, ranging from 1 day to 94 years of age. That study found that telomere length was sensitive for DC, but not specific. The lack of specificity in that study may have been a consequence, in part, of a smaller number of controls which resulted in a wider extrapolated 1st percentile interval. The differences between the laboratory and analytic methods of the two studies suggest the results are not directly comparable.
Most studies to date have evaluated telomere length measurement in total WBCs, which has proven to be a useful surrogate for constitutional telomere length, even when a non-hematopoietic tissue (e.g., lung) is primarily affected. Although tissue-specific telomere factor(s), other than telomerase have yet to be described, it is possible that such factor(s) exists, for which testing of telomere length in WBC may lead to an erroneous conclusion that a particular condition is not a telomere biology disorder.
Genetic anticipation
The penetrance, severity and time of onset of the clinical features of DC are variable even among family members with the same mutation. The telomere length of paternal, more so than maternal, chromosomes in the zygote is thought to contribute to this variability in healthy individuals (24;27;28;70). Nonetheless, disease anticipation (increasing disease severity and earlier onset with succeeding generations) has been observed in several multi-generation families with autosomal dominant DC (71–74). Mechanistically, disease anticipation has been shown to be associated with progressive telomere shortening and increasing numbers of chromosomes with very short telomeres (71;73). Figure 4 shows the pedigree of a family with autosomal dominant inheritance of a TINF2 mutation. The importance of disease anticipation is that an affected individual may have a phenotypically unaffected, but mutation-positive parent, and their siblings may also be at risk. Additionally, carrier offspring may need to be screened for associated illnesses at a younger age than the presentation age of their parents.
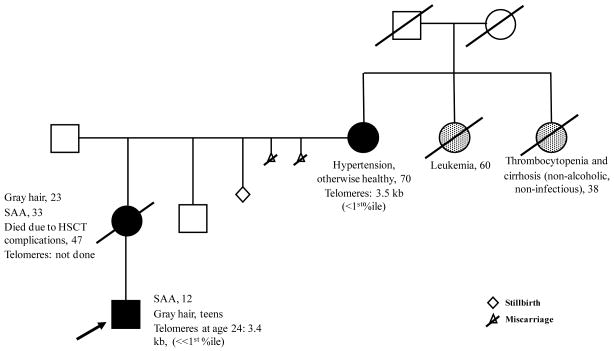
Figure 4. Example of a family with genetic anticipation due to a TINF2 mutation.
The arrow indicates the proband. Mutation carriers and the obligate mutation carrier are shaded black. The history of the proband’s paternal family is not available. Possible, but untested, affected relatives are shown in gray. The history of the proband’s maternal great-grandparents is unknown, but one of them was likely a mutation carrier. Specific medical complications, if present, are noted on the pedigree. The numbers indicate the age in years of the finding. The family is part of our IRB-approved study (132). Lymphocyte telomere lengths measured by flow-FISH are shown. The median lymphocyte telomere length for a healthy 70 year-old is 5.8 kb and for a healthy 24 year-old is 7.6 kb.
Abbreviations: SAA, severe aplastic anemia; HSCT, hematopoeitic stem cell transplantation; %ile, percentile for age.
Clinical management
The clinical management of DC is complicated due to the highly variable onset and progression of DC manifestations, even between family members (55;75). Individuals may have few physical findings and normal bone marrow function, and others may present at very young ages with the diagnostic triad, early-onset BMF, and other medical problems. Genetic counseling of the patient and family should be incorporated into the management as soon as the diagnosis of DC is considered (75).
BMF is often the complication that requires the most intensive management. Hematopoietic stem cell transplantation (HSCT) is the only curative treatment for BMF and leukemia, but historically it has had poor long-term efficacy, in part due to treatment-related morbidity and mortality (76–85). Pulmonary fibrosis, hepatic and gastro-intestinal complications are frequently reported (78). Recent clinical trials that employ nonmyeloablative conditioning regimens and exclude agents known to be associated with severe pulmonary and hepatic toxicity show promising results but have limited follow-up time (86).
If a suitable HSCT donor is not available, androgen therapy may be considered for BMF (75;87). Clinical reports indicate that patients with DC may have an improvement in cytopenias while on androgens, although efficacy data are lacking. Androgens have been shown to affect TERT gene expression and telomerase activity in hematopoietic cells, including TERT mutant cells, providing a potential mechanism of action (88). As with other individuals with an IBMFS, patients with DC on androgen therapy need to have careful monitoring for abnormalities in cholesterol, triglycerides, liver function, and for the development of liver adenomas. It is recommended that BMF patients taking androgens do not use concurrent growth factors, such as G-CSF or erythropoietin, due to reports of splenic peliosis and rupture (89).
Individuals with DC are at an 11-fold increased risk of cancer compared with the general population. The risk of certain head and neck cancers, for example tongue squamous cell cancer, is elevated by about 1000-fold (58). Annual cancer screening by a dermatologist, gynecologist for females, an otolaryngologist, as well as biannual dental exams, are important for early detection (75;90). Because the risk of tongue squamous cell cancer is so dramatically elevated in DC, DC should be carefully considered in any individual presenting with this cancer, particularly in young, nonsmoking, nondrinking adults.
APLASTIC ANEMIA
Aplastic anemia is diagnosed based on well established peripheral blood count and bone marrow cellularity criteria (91). Nonetheless, it is a complex, heterogeneous BMF disorder. It can present at any age and is now often subclassified into inherited and acquired forms, based on underlying etiology. Acquired aplastic anemia may be characterized by the presence of immune-mediated BMF (91). Exposures to putative environmental risk factors, chemicals such as benzene, idiosyncratic reactions to drugs such as chloramphenicol, and certain infections contribute to the etiology of acquired aplastic anemia (91), although in most cases an instigating factor cannot be identified. Immune-mediated T-cell destruction of the bone marrow has been shown both in vitro and in vivo, but the reason for T-cell activation in these patients is not clear. A combination of genetic risk factors, including SNPs in tumor necrosis factor-alpha (92;93) and gamma-interferon (94) may be contributory. Despite this knowledge, there is no diagnostic test to establish immune-mediated aplastic anemia.
Treatment approaches, long term prognosis, disease surveillance, and counseling differ substantially for patients with acquired aplastic anemia versus those with an IBMFS. For example, patients with telomere biology disorders do not respond to immunosuppressive therapy for BMF (87), may be at increased risk of HSCT complications, require additional molecular or genetic screening of related HSCT donors, and have an increased risk of certain cancers. Thus, it is crucial to carefully consider a telomere biology disorder as an underlying cause of aplastic anemia. Importantly, BMF may be the first sign of an inherited telomere biology disorder and, historically, these patients were often initially diagnosed as having acquired aplastic anemia. Today, studies of telomere length by flow-FISH in WBC subsets and mutation analyses of telomere biology genes can be instrumental in differentiating inherited aplastic anemia due to a telomere biology disorder from acquired aplastic anemia and other IBMFS.
Telomere length in patients with acquired aplastic anemia may be relatively shorter than normal, but it is not as short as in patients with telomere biology disorders. Early studies of aplastic anemia suggested that telomeres were shorter than average in mononuclear cells or granulocytes in about one-third of patients compared with controls (41–43). Telomerase activity was shown to be up-regulated in acquired aplastic anemia, possibly to counteract telomere loss (95). Patients with acquired aplastic anemia may have short telomeres because of the rapid turnover of hematopoietic progenitor cells in order to compensate for a failing bone marrow (43;96). This effect may be more notable in granulocytes which turnover rapidly and often have shorter telomeres than mononuclear cells in controls (97).
Individuals with apparently acquired aplastic anemia who did not respond to immunosuppression had the shortest telomeres in several studies (41–43). This led to the hypothesis that some individuals with apparently acquired aplastic anemia, may have an underlying genetic defect, manifested by short telomeres. This was confirmed by the identification of mutations in TERC (44;45) or TERT (46;47) in patients with what had been previously diagnosed as having acquired aplastic anemia. The TERC and TERT mutations reported in aplastic anemia do affect enzyme function and thus are likely the cause of the short telomeres. Similarly, TINF2 exon 6 mutations were identified in six of 106 pediatric anonymous registry patients who underwent unrelated donor HSCT for SAA (98), a cohort for whom immunosuppressive therapy presumably failed. Four of the mutations had been previously reported to be associated with early-onset DC.
These mutations are rare but, taken together, do explain the presence of short telomeres in up to 10% of patients with aplastic anemia. Some of these patients may have a family history of cancer, mild hematopoietic abnormalities, pulmonary fibrosis, or liver disease. Telomere length testing by flow-FISH in WBC subsets is recommended for all patients with aplastic anemia or BMF. This will identify patients with a telomere biology disorder and has major implications for their treatment and long-term outcome, as reviewed above.
HEMATOPOIETIC MALIGNANCIES
A number of reports have suggested a pathologic role for telomerase mutations in the development of certain hematologic malignancies. Hypomorphic TERC or TERT mutations were identified in four families in a study of familial MDS/AML (51). The mucocutaneous features of DC were absent in these families, although bone marrow failure, gastric cancer and pulmonary fibrosis were reported in some mutation carriers. Constitutional TERT mutations were also reported in cases of sporadic AML (52), although the most frequently associated gene variant, TERT A1062T, was also found in unaffected controls, albeit at a significantly lower frequency (the frequency was increased three-fold in patients). In a separate study, the hypomorphic A1062T TERT allele was also found at a higher frequency in individuals with chronic lymphocytic lymphoma and diffuse large B-cell lymphoma relative to normal controls (99). Thus, TERT A1062T status is emerging, at the very least, as a risk factor for these particular hematologic malignancies.
IDIOPATHIC PULMONARY FIBROSIS
Idiopathic pulmonary fibrosis (IPF) is a complex disorder characterized by progressive and irreversible lung scarring that ultimately leads to respiratory failure. Most cases are sporadic, although 2 to 20% appear to be familial with autosomal dominant inheritance and variable penetrance (100). A number of environmental and genetic factors have been implicated, including defective telomere maintenance. In a study of 73 probands from families with IPF, six were found to be heterozygous for mutations in TERT or TERC (48). They also had short telomeres compared with related, wild-type family members as determined in lymphocytes by flow-FISH. Although IPF was the dominant phenotype, one of these probands had other relatives with aplastic anemia. Additional TERT and/or TERC mutations were identified in a separate study of familial and sporadic IPF (49). Again, some family members in these IPF pedigrees had clinical features similar to DC, including anemia, cancer, and osteoporosis/osteopenia. Consistent with a role of defective telomere maintenance in IPF, defective telomere maintenance, pulmonary fibrosis and other pulmonary diseases have been noted in 20% of individuals with DC (101). Patients with DC who undergo HCST are at increased risk of pulmonary complications as well (85). IPF was among the variable clinical phenotypes present in a study of subjects with novel TERC mutations (102). Thus, patients with IPF should be evaluated for germline mutations in TERC and TERT. Those that harbor such a mutation should be considered to be within the DC spectrum, and should be managed and counseled in the same way as patients with classical DC.
LIVER DISEASE
Liver disease, peptic ulceration, or enteropathy has also been noted in approximately 7% of individuals with DC (103). The clinicopathologic features of these gastrointestinal manifestations of defective telomere maintenance, however, remain poorly defined. Most information comes from single case reports or small case series. Non-cirrhotic portal hypertension (104–106) and hepatopulmonary syndrome (107) have been reported in individuals with DC. Similar to IPF, liver disease may be the presenting clinical manifestation of a telomere biology disorder. In a study of family members of five unrelated individuals with bone marrow failure and TERT or TERC mutations, severe liver disease was found to occur in some mutation carriers independently of bone marrow failure or other apparent organ involvement (50). Although the hepatic pathology findings were variable in the seven liver biopsies available for review, combined inflammation and fibrosis were common and nodular regeneration were common. Again, as recommended for individuals with IPF, a telomere biology disorder should be considered in individuals with unexplained severe liver disease and family histories with clinical features found in DC.
TELOMERIC REPEAT CONTAINING RNA AND ICF SYNDROME TYPE 1
Once thought to be transcriptionally silent, it is now clear that human telomeres are transcribed to produce telomeric repeat containing RNA (TERRA)(108;109). TERRA, which is noncoding RNA, localizes to telomeric foci and has been implicated in the formation of telomeric heterochromatin and telomerase inhibition. Recently, changes in TERRA levels and other aspects of telomere biology were described in immunodeficiency-centromeric instability-facial anomalies syndrome (ICF Syndrome Type 1, MIM #242860). ICF Type 1 is caused by biallelic mutations in DNA methyltransferase 3b (DNMT3B) (110–112). This defect results in abnormal subtelomeric methylation and abnormally short or undetectable telomeres on some chromosome arms (113). Transcription of TERRA is significantly increased in these individuals, possibly due to defects in the telomeric structure caused by aberrant methylation. Thus, ICF Type 1 represents the first syndrome in which changes in TERRA have been described. Whether any of the clinical manifestations of ICF Type 1 are the result of changes in TERRA remains to be determined.
TELOMERES IN EPIDEMIOLOGY STUDIES
The association of telomere length in surrogate tissues, such as blood or buccal cell DNA, and disease has been evaluated in numerous epidemiologic studies. Several case-control studies have suggested that individuals with cancer may have shorter leukocyte or buccal cell telomere length. These include studies of bladder (114–116), head and neck (116), lung (117), gastric (118;119), and ovarian (120) cancer. Case-control and cohort studies of individuals with breast cancer have yielded inconsistent results (121;122). One cohort study of prostate cancer which evaluated pre-cancer diagnosis specimens did not find an association between telomere length and prostate cancer (123). Similar null results were found in skin cancer studies (124).
Numerous association studies between telomere length and non-malignant diseases or conditions have been reported as well, including cardiovascular disease (125;126), diabetes (127), obesity (128), and perception of stress (129). A number of these studies have suggested that affected individuals have shorter telomeres than unaffected individuals. Some of these and other studies have evaluated lifestyle factors, such as diet, exercise, and smoking, and telomere length (123;130). Telomere length does appear to be shorter in smokers than non-smokers, but the effect may be small. Studies of healthy (i.e. normal body mass index, more exercise, higher intakes of fruits and vegetables, non-smokers) compared with unhealthy lifestyles have suggested that individually these variables to do not significantly contribute to telomere length, but that in combination, there is an association between longer telomeres and a healthier lifestyle (123;130).
Telomere epidemiology studies seek to find statistically significant associations between relatively small changes in telomere length between cases and controls. This is in contrast to the very short telomeres found in individuals with telomere biology disorders. These combined approaches will advance understanding of the complex mechanisms of telomere length regulation in inherited disorders and common diseases.
SUMMARY AND FUTURE DIRECTIONS
A broad spectrum of clinical phenotypes is now appreciated in telomere biology disorders (Figure 5, Box 1). We propose the grouping schema listed in Box 1 in an effort to better define the overlap of telomere biology disorders. DC is the first disease identified to be caused by abnormalities in genes important in telomere biology. It also has the most severe phenotype. Individuals with isolated SAA or IPF may also have a genetic defect in telomere biology. The clinical manifestations of DC develop at highly variable rates, even within family members with the same mutation. Individuals with apparently isolated SAA, IPF, or liver disease warrant consideration aimed at identifying a mutation in a telomere biology gene. Genetic counseling and a detailed family history should be obtained. Individuals with mutations in a telomere biology gene should be counseled for a potential increased risk of cancer since patients with DC have very high cancer rates. The rates of cancer in individuals with isolated SAA or IPF due to TERT or TERC mutations are not known; future studies are needed to better understand cancer incidence in these individuals.
Figure 5. Telomere biology disorders overlap phenotypically and genetically.
Patients with DC are the most severely affected and have extremely short telomeres. Telomeres are shorter than normal, although not as short as in DC, in some individuals with aplastic anemia, pulmonary fibrosis, leukemia, fibrotic liver disorders, and possibly others. Genes that are mutated in these disorders are shown in the gray boxes.
Although mutations in six genes have been shown to cause DC and/or related telomere biology disorders, approximately 40–50% of patients with DC lack a detectable mutation in one of these genes, which suggests that there are other genes to be identified. DC inheritance follows essentially all inheritance patterns (X-linked recessive, autosomal dominant, and autosomal recessive), but many cases appear to occur sporadically (i.e., with a negative family history). This could be due to new mutations in a family, incomplete disease penetrance, or variable expressivity of the consequences of the mutation. Thus, the absence of a positive family history should not preclude further evaluation of a suspected case.
The complexities of telomere length regulation suggest that there may be many more genes that contribute to DC and related telomere biology disorders. Numerous genes that regulate telomere length are still being identified and further characterized. In addition, there is an increasing body of research literature that supports nontelomeric functions of TERT such as in the WNT/β-catenin signaling pathway (131). Findings such as these lead to the possibility that certain aspects of human disease associated with TERT mutations or SNPs may be secondary to loss of one or more of these extracurricular activities.
With the availability of telomere length testing, and an increasing awareness of the variable presentations, it is likely we will appreciate a greater frequency of telomere biology disorders than previously thought. As laboratory research leads to a greater understanding of the factors that control telomere length, directed therapies for telomere biology disorders may be at hand in the future.
Acknowledgments
We thank the patients and families who have generously contributed to our understanding of dyskeratosis congenita and telomere biology disorders. We also thank Drs. Blanche Alter, and Neelam Giri, National Cancer Institute, for helpful discussions. This work was funded, in part, by the intramural research program of the National Cancer Institute, National Institutes of Health.
Funding disclosures:
Sharon A. Savage, M.D.: This work was funded by the intramural research program of the National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of Interest Notification
Sharon A. Savage, M.D.: No commercial associations to disclose.
Alison A. Bertuch, M.D., Ph.D.: No commercial associations to disclose
Reference List
- 1.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 2.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Robertson JD, Gale RE, Wynn RF, Dougal M, Linch DC, Testa NG, et al. Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. Br J Haematol. 2000;109(2):272–279. doi: 10.1046/j.1365-2141.2000.01970.x. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 6.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58(18):4168–4172. [PubMed] [Google Scholar]
- 7.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996;93(13):6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155(8):3711–3715. [PubMed] [Google Scholar]
- 9.Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14(2):239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 10.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315(5820):1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402(6761):551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 13.Hamma T, Ferre-D’Amare AR. The box H/ACA ribonucleoprotein complex: interplay of RNA and protein structures in post-transcriptional RNA modification. J Biol Chem. 2010;285(2):805–809. doi: 10.1074/jbc.R109.076893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114(1):1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trahan C, Martel C, Dragon F. Effects of dyskeratosis congenita mutations in dyskerin, NHP2 and NOP10 on assembly of H/ACA pre-RNPs. Hum Mol Genet. 2010;19(5):825–836. doi: 10.1093/hmg/ddp551. [DOI] [PubMed] [Google Scholar]
- 16.Leulliot N, Godin KS, Hoareau-Aveilla C, Quevillon-Cheruel S, Varani G, Henry Y, et al. The box H/ACA RNP assembly factor Naf1p contains a domain homologous to Gar1p mediating its interaction with Cbf5p. J Mol Biol. 2007;371(5):1338–1353. doi: 10.1016/j.jmb.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132(6):945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323(5914):644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82(2):501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummendorf TH, Rufer N, Holyoake TL, Maciejewski J, Barnett MJ, Eaves CJ, et al. Telomere length dynamics in normal individuals and in patients with hematopoietic stem cell-associated disorders. Ann N Y Acad Sci. 2001;938:293–303. doi: 10.1111/j.1749-6632.2001.tb03598.x. [DOI] [PubMed] [Google Scholar]
- 21.Graakjaer J, Pascoe L, Der-Sarkissian H, Thomas G, Kolvraa S, Christensen K, et al. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3(3):97–102. doi: 10.1111/j.1474-9728.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 22.Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, et al. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37(4):523–531. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- 23.Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, et al. Mapping genetic Loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78(3):480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- 26.Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson JR, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76(1):147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordfjall K, Larefalk A, Lindgren P, Holmberg D, Roos G. Telomere length and heredity: Indications of paternal inheritance. Proc Natl Acad Sci U S A. 2005;102(45):16374–16378. doi: 10.1073/pnas.0501724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4(2):97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 29.Mirabello L, Yu K, Kraft P, De VI, Hunter DJ, Prescott J, et al. The association of telomere length and genetic variation in telomere biology genes. Hum Mutat. 2010 doi: 10.1002/humu.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangino M, Richards JB, Soranzo N, Zhai G, Aviv A, Valdes AM, et al. A genome-wide association study identifies a novel locus on chromosome 18q12. 2 influencing white cell telomere length. J Med Genet. 2009;46(7):451–454. doi: 10.1136/jmg.2008.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rog O, Smolikov S, Krauskopf A, Kupiec M. The yeast VPS genes affect telomere length regulation. Curr Genet. 2005;47(1):18–28. doi: 10.1007/s00294-004-0548-y. [DOI] [PubMed] [Google Scholar]
- 32.Codd V, Mangino M, van der HP, Braund PS, Kaiser M, Beveridge AJ, et al. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42(3):197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baird DM. New developments in telomere length analysis. Exp Gerontol. 2005;40(5):363–368. doi: 10.1016/j.exger.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Lin KW, Yan J. The telomere length dynamic and methods of its assessment. J Cell Mol Med. 2005;9(4):977–989. doi: 10.1111/j.1582-4934.2005.tb00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baerlocher GM, Lansdorp PM. Telomere length measurements in leukocyte subsets by automated multicolor flow-FISH. Cytometry A. 2003;55(1):1–6. doi: 10.1002/cyto.a.10064. [DOI] [PubMed] [Google Scholar]
- 36.Bendix L, Horn PB, Jensen UB, Rubelj I, Kolvraa S. The load of short telomeres, estimated by a new method, Universal STELA, correlates with number of senescent cells. Aging Cell. 2010;9(3):383–397. doi: 10.1111/j.1474-9726.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- 37.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33(2):203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 38.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JJ, Kook H, Chung IJ, Na JA, Park MR, Hwang TJ, et al. Telomere length changes in patients with aplastic anaemia. Br J Haematol. 2001;112(4):1025–1030. doi: 10.1046/j.1365-2141.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 42.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91(10):3582–3592. [PubMed] [Google Scholar]
- 43.Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97(4):895–900. doi: 10.1182/blood.v97.4.895. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102(3):916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 45.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359(9324):2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352(14):1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 47.Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2005;34(3):257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 49.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS ONE. 2009;4(11):e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirwan M, Vulliamy T, Marrone A, Walne AJ, Beswick R, Hillmen P, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat. 2009;30(11):1567–1573. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 52.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106(4):1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105(23):8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walne AJ, Marrone A, Dokal I. Dyskeratosis congenita: a disorder of defective telomere maintenance? Int J Hematol. 2005;82(3):184–189. doi: 10.1532/IJH97.05067. [DOI] [PubMed] [Google Scholar]
- 55.Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23(2):215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsilou ET, Giri N, Weinstein S, Mueller C, Savage SA, Alter BP. Ocular and orbital manifestations of the inherited bone marrow failure syndromes: Fanconi anemia and dyskeratosis congenita. Ophthalmology. 2010;117(3):615–622. doi: 10.1016/j.ophtha.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkinson JC, Harvey KE, Domingo DL, Trujillo MI, Guadagnini JP, Gollins S, et al. Oral and dental phenotype of dyskeratosis congenita. Oral Dis. 2008;14(5):419–427. doi: 10.1111/j.1601-0825.2007.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009 doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoyeraal HM, Lamvik J, Moe PJ. Congenital hypoplastic thrombocytopenia and cerebral malformations in two brothers. Acta Paediatr Scand. 1970;59(2):185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 60.Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, et al. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162(12):863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 61.Revesz T, Fletcher S, al Gazali LI, DeBuse P. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? J Med Genet. 1992;29(9):673–675. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riyaz A, Riyaz N, Jayakrishnan MP, Mohamed Shiras PT, Ajith KV, Ajith BS. Revesz syndrome. Indian J Pediatr. 2007;74(9):862–863. doi: 10.1007/s12098-007-0155-2. [DOI] [PubMed] [Google Scholar]
- 63.Knight SW, Heiss NS, Vulliamy TJ, Aalfs CM, McMahon C, Richmond P, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107(2):335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 64.Vulliamy TJ, Dokal I. Dyskeratosis congenita: The diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2007 doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65(1):50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413(6854):432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 67.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16(13):1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112(9):3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113(2):309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102(44):15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102(47):17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36(5):447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 74.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107(7):2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 75.Savage SA, Dokal I, Armanios M, Aubert G, Cowen EW, Domingo DL, et al. Dyskeratosis congenita: The first NIH clinical research workshop. Pediatr Blood Cancer. 2009 doi: 10.1002/pbc.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berthou C, Devergie A, D’Agay MF, Sonsino E, Scrobohaci ML, Loirat C, et al. Late vascular complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1991;79(2):335–336. doi: 10.1111/j.1365-2141.1991.tb04543.x. [DOI] [PubMed] [Google Scholar]
- 77.Brazzola P, Duval M, Fournet JC, Gauvin F, Dalle JH, Champagne J, et al. Fatal diffuse capillaritis after hematopoietic stem-cell transplantation for dyskeratosis congenita despite low-intensity conditioning regimen. Bone Marrow Transplant. 2005;36(12):1103–1105. doi: 10.1038/sj.bmt.1705171. [DOI] [PubMed] [Google Scholar]
- 78.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11(6):584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 79.Dror Y, Freedman MH, Leaker M, Verbeek J, Armstrong CA, Saunders FE, et al. Low-intensity hematopoietic stem-cell transplantation across human leucocyte antigen barriers in dyskeratosis congenita. Bone Marrow Transplant. 2003;31(10):847–850. doi: 10.1038/sj.bmt.1703931. [DOI] [PubMed] [Google Scholar]
- 80.Gungor T, Corbacioglu S, Storb R, Seger RA. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for treatment of Dyskeratosis congenita. Bone Marrow Transplant. 2003;31(5):407–410. doi: 10.1038/sj.bmt.1703844. [DOI] [PubMed] [Google Scholar]
- 81.Langston AA, Sanders JE, Deeg HJ, Crawford SW, Anasetti C, Sullivan KM, et al. Allogeneic marrow transplantation for aplastic anaemia associated with dyskeratosis congenita. Br J Haematol. 1996;92(3):758–765. doi: 10.1046/j.1365-2141.1996.424984.x. [DOI] [PubMed] [Google Scholar]
- 82.Lau YL, Ha SY, Chan CF, Lee AC, Liang RH, Yuen HL. Bone marrow transplant for dyskeratosis congenita. Br J Haematol. 1999;105(2):571. doi: 10.1111/j.1365-2141.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 83.Nobili B, Rossi G, De Stefano P, Zecca M, Giorgiani G, Perrotta S, et al. Successful umbilical cord blood transplantation in a child with dyskeratosis congenita after a fludarabine-based reduced-intensity conditioning regimen. Br J Haematol. 2002;119(2):573–574. doi: 10.1046/j.1365-2141.2002.03835_2.x. [DOI] [PubMed] [Google Scholar]
- 84.Rocha V, Devergie A, Socie G, Ribaud P, Esperou H, Parquet N, et al. Unusual complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1998;103(1):243–248. doi: 10.1046/j.1365-2141.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 85.Yabe M, Yabe H, Hattori K, Morimoto T, Hinohara T, Takakura I, et al. Fatal interstitial pulmonary disease in a patient with dyskeratosis congenita after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19(4):389–392. doi: 10.1038/sj.bmt.1700674. [DOI] [PubMed] [Google Scholar]
- 86.Dietz AC, Orchard PJ, Baker KS, Giller RH, Savage SA, Alter BP, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al Rahawan MM, Giri N, Alter BP. Intensive immunosuppression therapy for aplastic anemia associated with dyskeratosis congenita. Int J Hematol. 2006;83(3):275–276. doi: 10.1532/IJH97.06030. [DOI] [PubMed] [Google Scholar]
- 88.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giri N, Pitel PA, Green D, Alter BP. Splenic peliosis and rupture in patients with dyskeratosis congenita on androgens and granulocyte colony-stimulating factor. Br J Haematol. 2007;138(6):815–817. doi: 10.1111/j.1365-2141.2007.06718.x. [DOI] [PubMed] [Google Scholar]
- 90.Savage SA. Dyskeratosis Congenita. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Gene Reviews [Internet] Seattle (WA): University of Washington, Seattle; 2009. [Google Scholar]
- 91.Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15(3):162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Demeter J, Messer G, Schrezenmeier H. Clinical relevance of the TNF-alpha promoter/enhancer polymorphism in patients with aplastic anemia. Ann Hematol. 2002;81(10):566–569. doi: 10.1007/s00277-002-0544-6. [DOI] [PubMed] [Google Scholar]
- 93.Peng J, Liu C, Zhu K, Zhu Y, Yu Y, Li J, et al. The TNF2 allele is a risk factor to severe aplastic anemia independent of HLA-DR. Hum Immunol. 2003;64(9):896–901. doi: 10.1016/s0198-8859(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 94.Dufour C, Capasso M, Svahn J, Marrone A, Haupt R, Bacigalupo A, et al. Homozygosis for (12) CA repeats in the first intron of the human IFN-gamma gene is significantly associated with the risk of aplastic anaemia in Caucasian population. Br J Haematol. 2004;126(5):682–685. doi: 10.1111/j.1365-2141.2004.05102.x. [DOI] [PubMed] [Google Scholar]
- 95.Shen JB, Tang JY, Zhao JC, Pan C, Chen J, Zhou X, et al. Telomerase activity and its correlation with the proliferative potential of bone marrow in aplastic anemia in children. Acta Haematol. 2002;107(4):208–212. doi: 10.1159/000058316. [DOI] [PubMed] [Google Scholar]
- 96.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72–77. doi: 10.1182/asheducation-2006.1.72. [DOI] [PubMed] [Google Scholar]
- 97.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1(5):2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 98.Du HY, Mason PJ, Bessler M, Wilson DB. TINF2 mutations in children with severe aplastic anemia. Pediatr Blood Cancer. 2009;52(5):687. doi: 10.1002/pbc.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hills M, Lansdorp PM. Short telomeres resulting from heritable mutations in the telomerase reverse transcriptase gene predispose for a variety of malignancies. Ann N Y Acad Sci. 2009;1176:178–190. doi: 10.1111/j.1749-6632.2009.04565.x. [DOI] [PubMed] [Google Scholar]
- 100.Wise AL, Schwartz DA. Pulmonary Fibrosis, Familial. 1993 [Google Scholar]
- 101.Marrone A, Walne A, Dokal I. Dyskeratosis congenita: telomerase, telomeres and anticipation. Curr Opin Genet Dev. 2005;15(3):249–257. doi: 10.1016/j.gde.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 102.Marrone A, Sokhal P, Walne A, Beswick R, Kirwan M, Killick S, et al. Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations. Haematologica. 2007;92(8):1013–1020. doi: 10.3324/haematol.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walne AJ, Dokal I. Telomerase dysfunction and dyskeratosis congenita. Cytotechnology. 2004;45(1–2):13–22. doi: 10.1007/s10616-004-5121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abramowsky C, Romero R, Heffron T. Pathology of noncirrhotic portal hypertension: clinicopathologic study in pediatric patients. Pediatr Dev Pathol. 2003;6(5):421–426. doi: 10.1007/s10024-003-1002-8. [DOI] [PubMed] [Google Scholar]
- 105.Brown KE, Kelly TE, Myers BM. Gastrointestinal involvement in a woman with dyskeratosis congenita. Dig Dis Sci. 1993;38(1):181–184. doi: 10.1007/BF01296794. [DOI] [PubMed] [Google Scholar]
- 106.Kawaguchi K, Sakamaki H, Onozawa Y, Koike M. Dyskeratosis congenita (Zinsser-Cole-Engman syndrome). An autopsy case presenting with rectal carcinoma, non-cirrhotic portal hypertension, and Pneumocystis carinii pneumonia. Virchows Arch A Pathol Anat Histopathol. 1990;417(3):247–253. doi: 10.1007/BF01600141. [DOI] [PubMed] [Google Scholar]
- 107.Renoux MC, Mazars N, Tichit R, Counil F. Cyanosis revealing hepatopulmonary syndrome in a child with dyskeratosis congenita. Pediatr Pulmonol. 2010;45(1):99–102. doi: 10.1002/ppul.21128. [DOI] [PubMed] [Google Scholar]
- 108.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35(4):403–413. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luke B, Lingner J. TERRA: telomeric repeat-containing RNA. EMBO J. 2009;28(17):2503–2510. doi: 10.1038/emboj.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagleitner MM, Lankester A, Maraschio P, Hulten M, Fryns JP, Schuetz C, et al. Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome) J Med Genet. 2008;45(2):93–99. doi: 10.1136/jmg.2007.053397. [DOI] [PubMed] [Google Scholar]
- 111.Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, et al. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1999;96(25):14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu GL, Bestor TH, Bourc’his D, Hsieh CL, Tommerup N, Bugge M, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402(6758):187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 113.Yehezkel S, Segev Y, Viegas-Pequignot E, Skorecki K, Selig S. Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions. Hum Mol Genet. 2008;17(18):2776–2789. doi: 10.1093/hmg/ddn177. [DOI] [PubMed] [Google Scholar]
- 114.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26(7):1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 115.McGrath M, Wong JY, Michaud D, Hunter DJ, De VI. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 116.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 117.Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hou L, Savage SA, Blaser MJ, Perez-Perez G, Hoxha M, Dioni L, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X, Bao G, Huo T, Wang Z, He X, Dong G. Constitutive telomere length and gastric cancer risk: case-control analysis in Chinese Han population. Cancer Sci. 2009;100(7):1300–1305. doi: 10.1111/j.1349-7006.2009.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mirabello L, Garcia-Closas M, Cawthon R, Lissowska J, Brinton LA, Peplonska B, et al. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De VI, Prescott J, Wong JY, Kraft P, Hankinson SE, Hunter DJ. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–1156. doi: 10.1158/1055-9965.EPI-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Svenson U, Nordfjall K, Stegmayr B, Manjer J, Nilsson P, Tavelin B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res. 2008;68(10):3618–3623. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- 123.Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129(2):415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 127.Uziel O, Singer JA, Danicek V, Sahar G, Berkov E, Luchansky M, et al. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp Gerontol. 2007;42(10):971–978. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 128.Nordfjall K, Eliasson M, Stegmayr B, Lundin S, Roos G, Nilsson PM. Increased abdominal obesity, adverse psychosocial factors and shorter telomere length in subjects reporting early ageing; the MONICA Northern Sweden Study. Scand J Public Health. 2008;36(7):744–752. doi: 10.1177/1403494808090634. [DOI] [PubMed] [Google Scholar]
- 129.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 131.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4(1):e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010 doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]