Summary
Background
Type 2M von Willebrand disease (VWD) is characterized by a qualitative defect in von Willebrand factor (VWF) and diagnosed by a disproportionate decrease in VWF ristocetin cofactor activity (VWF:RCo) as compared to VWF antigen (VWF:Ag).
Objective
We report here on the spurious diagnosis of VWD in a patient with a sequence variation in the ristocetin binding domain of VWF.
Patients/Methods
The index case had a VWF:RCo of 11 IU/dL, with VWF:RCo/VWF:Ag ratio of 0.09. DNA sequencing revealed a novel P1467S mutation in a known ristocetin-binding region of the A1 domain. Because of the discrepancy between the laboratory findings, consistent with type 2M VWD, and the patient’s lack of bleeding symptoms, further studies were performed to determine whether this mutation affected VWF function or merely reduced its ability to interact with ristocetin.
Results
Studies with recombinant VWF showed normal platelet binding with botrocetin, but a significant decrease in binding in response to ristocetin. Ristocetin-induced binding to recombinant GPIb was also absent, but normal binding was seen when a gain-of-function GPIb construct was used in the absence of ristocetin. VWF function under shear stress was normal when analyzed with a cone and plate(let) analyzer.
Conclusions
The decreased VWF:RCo seen with the P1467S sequence variation likely represents an artifact due to the use of ristocetin to measure VWF activity. The normal VWF function in other assays correlates with the lack of hemorrhagic symptoms, and suggests the need for more physiologically relevant assays of VWF function.
Keywords: bleeding, platelet glycoprotein Ib, ristocetin, von Willebrand disease, von Willebrand factor
Introduction
Von Willebrand factor (VWF) is a key hemostatic protein, but documenting its function through laboratory tests is not always straightforward. VWF serves as a carrier protein for factor VIII (FVIII), and also facilitates platelet adhesion through its interaction with platelet GPIb on the platelet surface and through its binding to the subendothelial matrix. This interaction is driven in vivo by shear stress, which induces a conformational change in VWF that allows it to bind platelet GPIb [1]. In vitro, however, this interaction is induced by the antibiotic ristocetin, which enables VWF and platelet GPIb to interact in the absence of shear forces [2]. Laboratory testing of VWF utilizes ristocetin in the VWF ristocetin cofactor activity assay (VWF:RCo), which is a measure of VWF binding to platelets, or by ristocetin-induced platelet aggregation (RIPA). Other common assays include the VWF antigen (VWF:Ag), which determines total protein present, and the collagen binding assay (VWF:CB), which measures VWF binding to collagen. Multimer analysis is also important to ensure presence of the high molecular weight VWF multimers.
Von Willebrand disease (VWD) is caused by either quantitative or qualitative defects in VWF. Type 1 and type 3 VWD are the result of partial or complete deficiency of VWF, respectively, with proportional decreases in VWF:Ag and VWF:RCo. The type 2 variants include those with qualitative defects in VWF function. The diagnostic hallmark of most type 2M variants is a disproportionate decrease in VWF:RCo compared to VWF:Ag. Type 2A and 2B are characterized by loss of high molecular weight multimers, while 2N VWD refers to those mutations that affect the ability of VWF to bind factor VIII. Type 2M includes those variants with loss of VWF function despite normal (or near normal) VWF multimer structure [3]. Many of these are thought to have a defect in the ability of VWF to bind platelet GPIb.
The vast majority of mutations associated with type 2M VWD are located in exon 28 of the VWF gene and affect the A1 loop of VWF [4–7]. Four mutations have been reported outside this region: one in exon 17, two in exon 27, and one in exon 52, as documented in a list collected by the ISTH VWF SSC and detailed at http://www.vwf.group.shef.ac.uk, last accessed May 6, 2009 [8]. Because of the significant decrease in functional VWF, patients with type 2M VWD typically have bleeding symptoms [4,6,7]. Epistaxis, menorrhagia, and easy bruising are common, as is post-surgical bleeding [1].
Diagnosis of VWD requires a personal and family history of bleeding as well as laboratory findings consistent with the diagnosis [9]. Racial differences in VWF assays have been previously described in healthy controls. One study showed a decrease in ristocetin-induced platelet aggregation in African Americans [10], while a more recent study has demonstrated higher VWF:Ag, and lower VWF:RCo/VWF:Ag ratio, in African Americans [11]. We present here a case of “2M VWD” in a patient with marked decrease in VWF:RCo but no bleeding symptoms. The low VWF:RCo levels appear to be due to a defect in VWF-ristocetin binding, but in vivo VWF function does not seem to be affected. Therefore, this patient likely has a spurious diagnosis of VWD. These This disparity in low “functional” VWF with absence of clinical bleeding does not only affect the case reported here, but also calls into question the reliance on VWF:RCo as a means of assessing VWF function.
Materials and Methods
Patient data
The proband was initially referred to hematology clinic due to a prolonged partial thromboplastin time (PTT). Further testing demonstrated low VWF:RCo with a normal VWF:Ag. Informed consent was obtained and the family enrolled in the Zimmerman Program for the Molecular and Clinical Biology of von Willebrand Disease (ZPMCB-VWD), a large multicenter study of VWD. Bleeding scores were obtained using the revised European Union questionnaire from the European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD study [12]. Data are also reported here from both Caucasian and African American control subjects as well as subjects enrolled in the ZPMCB-VWD with the diagnosis of type 2M VWD.
VWF testing
VWF measurements were performed by the clinical laboratory of the BloodCenter of Wisconsin. VWF:Ag was measured by ELISA using monoclonal antibodies to VWF for both capture and detection. VWF:RCo was measured by ristocetin-induced aggregation of formalin-fixed platelets using an automated BCS system (Dade-Behring, Newark, DE). Confirmatory testing on the index case was performed at 5 different national laboratories. Collagen binding (VWF:CB) was also measured by ELISA [13]. Multimers were analyzed by gel electrophoresis [14]. Platelet aggregation studies were performed by the BloodCenter of Wisconsin clinical laboratory using the agonists thrombin, arachidonic acid, ADP, collagen, and ristocetin. Ristocetin-induced platelet aggregation was measured at concentrations of 1.5 and 0.5 mg/mL ristocetin.
DNA sequencing
Initial sequencing was performed to identify potential mutations in exon 28 by the clinical laboratory at the Blood Research Institute. Full length gene sequencing of all 52 exons, including all intron-exon boundaries, was performed through the ZPMCB-VWD. In addition, 3.5 kb upstream of exon 1 and 1 kb downstream of the C-terminal stop codon were sequenced. Primer sequences are available upon request.
Mutant VWF expression
A DNA fragment containing the P1467S sequence variation was generated and cloned into a mammalian expression vector containing the full length VWF sequence. Both wild-type and mutant vectors were transfected into HEK293T cells as previously described [6]. A mock construct utilized the pCIneo vector alone without VWF DNA. A 2M control was also generated using a previously reported mutation found in a patient with severe type 2M VWD, Δ1392–1402 [7]. The VWF concentration of the resulting cell culture supernatants and lysates were assayed by ELISA. Cell culture supernatant was used for the platelet binding and GPIb binding studies described below.
Flow cytometry for platelet binding
Fixed, lyophilized platelets were obtained from Bio/data (Horsham, PA) and reconstituted in tris-buffered saline. Platelets (1 × 106 platelets per well) were incubated for 30 minutes with VWF and either ristocetin at concentrations from 0 to 2 mg/mL (American Biochemical and Pharmaceutical, Marlton, NJ) or botrocetin at concentrations from 0 to 1 mcg/mL (purified from snake venom (Sigma-Aldrich, St. Louis, MO) as previously described [15]). A polyclonal anti-VWF antibody (Dako, Carpenteria, CA) directly conjugated with Alexa Fluor 633 (Invitrogen, Carlsbad, CA) was used to detect the platelet-VWF complex. Fluorescence was measured on an LSR II flow cytometer (BD Biosciences, San Jose, CA). All fluorescence values were corrected for the mean fluorescence values of platelets incubated without additional VWF.
Flow cytometry for GPIb binding
A construct containing full-length GPIbα was made with 2 gain-of-function mutations, G233V and M239V, and expressed in HEK293T cells with GPIbβ and GPIX to improve surface expression. Cells were collected at 60 hours and equal numbers of cells (1.5 × 105 cells/well) aliquoted into a 96 well plate. Diluted citrate-anticoagulated plasma was added to the cells. A fluorescently labeled polyclonal antibody to VWF (Dako) was added and the resulting complex detected using an LSR II flow cytometer (BD Biosciences). A normal curve was constructed with lyophilized, reconstituted normal control plasma calibrated to the WHO standard. The normal range for this assay was established using 100 healthy Caucasian control subjects from the ZPMCB-VWD. The index case, family members, and 10 type 2M VWD subjects were also examined.
GPIb binding ELISAs
Two gain-of-function mutations, D235Y and M239V, were introduced into a GPIbα construct truncated at the transmembrane domain (ΔTM290). The resulting construct was expressed in S2 insect cells and purified over a nickel column (GE Healthcare, Piscataway, NJ). A wild-type construct without the gain-of-function mutations was also generated and expressed. For the GPIb-binding ELISAs, a monoclonal anti-GPIb antibody was used to capture either the mutant or wild-type GPIb. Cell culture supernatants were added and a mixture of monoclonal anti-VWF antibodies used to detect the presence of VWF. For the wild-type construct, ristocetin was added to achieve a final concentration of 1 mg/mL along with the VWF source. No ristocetin was used in the assays measuring binding to the mutant GPIb construct.
Platelet adhesion
Whole blood was drawn into tubes containing 3.2% sodium citrate. Samples were obtained from the index patient with the P1467S sequence variation and also from a group of ten healthy controls. The blood samples were incubated for 45 minutes at room temperature prior to study with a cone and plate(let) analyzer (Impact-R, Diamed, Cressier, Switzerland). For each assay, 130 µl of whole blood was added to a polystyrene well and subjected to a shear rate of 1800 s−1 for 2 minutes [16]. The wells were stained for 1 minute with May-Grünwald stain and visualized using the attached inverted light microscope. For each well, 9 different images are obtained and surface coverage and aggregate size calculated by the device from the 3 most representative images per well. Results were averaged over a minimum of 4 wells per subject.
Results
Case history
The proband was a twelve-year-old African American girl who had a prolonged PTT on pre-operative testing and was referred to hematology clinic for further evaluation. She had no history of epistaxis, easy bruising, menorrhagia, or other bleeding symptoms. There was no family history of bleeding on either side of the family. Her prothrombin time was 12.7 seconds and her thrombin time was 12.8 seconds, both within the normal range in our laboratory. Initial PTT was prolonged at 34.6 seconds (normal range 23.9–33.5 seconds). Repeat PTT was normal at 30.2 seconds. Bleeding time was 3 minutes, with normal <8.5 minutes.
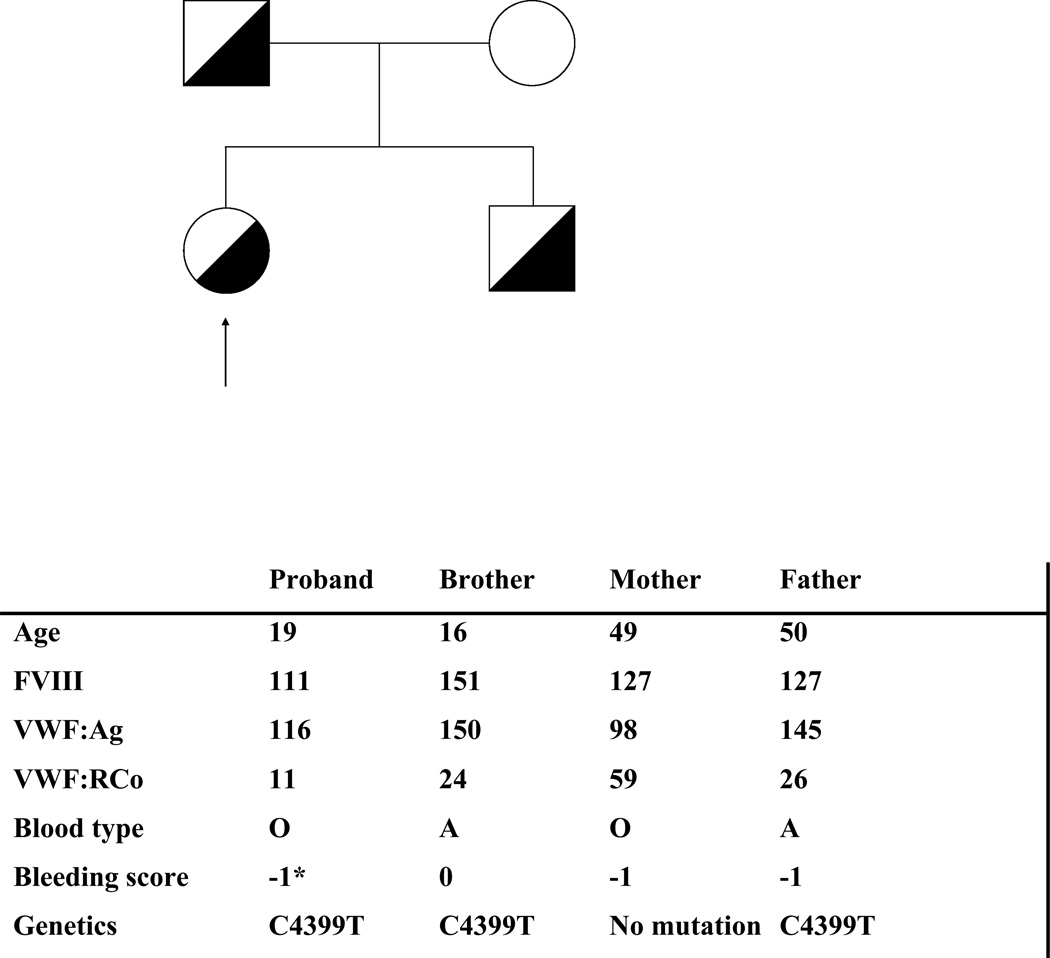
VWF testing revealed VWF:RCo of 11 IU/dL (normal 47–215), VWF:Ag of 116 IU/dL (normal 45–203), and a factor VIII activity of 111 IU/dL (normal 55–170). VWF:RCo/VWF:Ag ratio was 0.09. Multimer analysis was normal. VWF:CB was 130 U/dL (normal 59– 249). Platelet aggregation studies revealed absent aggregation at a ristocetin concentration of 1.5 mg/mL. A result of this severity is, in general, only seen with severe type 3 VWD or Bernard-Soulier syndrome. Due to the significant discrepancy between VWF:RCo and VWF:Ag, DNA sequencing was performed. Sequencing analysis revealed heterozygosity for a novel sequence variation, C4399T, leading to a substitution of serine for the wild-type proline at amino acid 1467, just outside the A1 loop and in a region previously implicated in VWF-ristocetin interactions [17]. The proband was also heterozygous for the common African American polymorphism G4414C (D1472H) and homozygous for the T4641C polymorphism. The patient’s brother and father were also heterozygous for the P1467S sequence variation, as seen in the pedigree shown in figure 1. Neither has experienced any clinically significant bleeding. The proband’s mother has the D1472H polymorphism. Five major North American laboratories all identified the same discrepancy between VWF:RCo and VWF:Ag when testing a plasma sample from the proband’s brother, with a mean ratio of 0.31 (range 0.18 to 0.61) when corrected to a standard reference plasma.
Figure 1.
The family pedigree is shown here with those members of the family who were heterozygous for the P1467S sequence variation marked here by a half-filled circle (females) or square (males). The arrow denotes the proband. Age in years, VWF testing, and bleeding score values obtained for each family member are provided below.
The index case has had an uneventful clinical course. She underwent tonsillectomy with less than 5 mL reported blood loss. One dose of DDAVP was administered as prophylaxis following surgery after her platelet aggregation study done that day showed a possible mild platelet function defect with ADP along with the decrease in ristocetin-induced aggregation, although later platelet aggregation studies with ADP were normal. She has subsequently experienced childbirth as well as dental surgeries without treatment or hemorrhage. No significant bleeding has been observed, either before or since diagnosis.
When a bleeding score was obtained using a modified version of the European Union questionnaire [12], the index case had a bleeding score of -1, excluding the treatment she received for the possible platelet defect. Her family members all had bleeding scores of ≤0. These bleeding scores were compared to those obtained in subjects from the ZPMCB-VWD, a large multicenter US study on VWD. The mean bleeding score for the ZPMCB-VWD subjects with type 2M VWD was 5 ± 6 (mean ± SD), with a range of -1 to 13. Healthy controls, on the other hand, had a mean bleeding score of 0 ± 2, with range -3 to 9.
P1467S plasma VWF-platelet binding assay
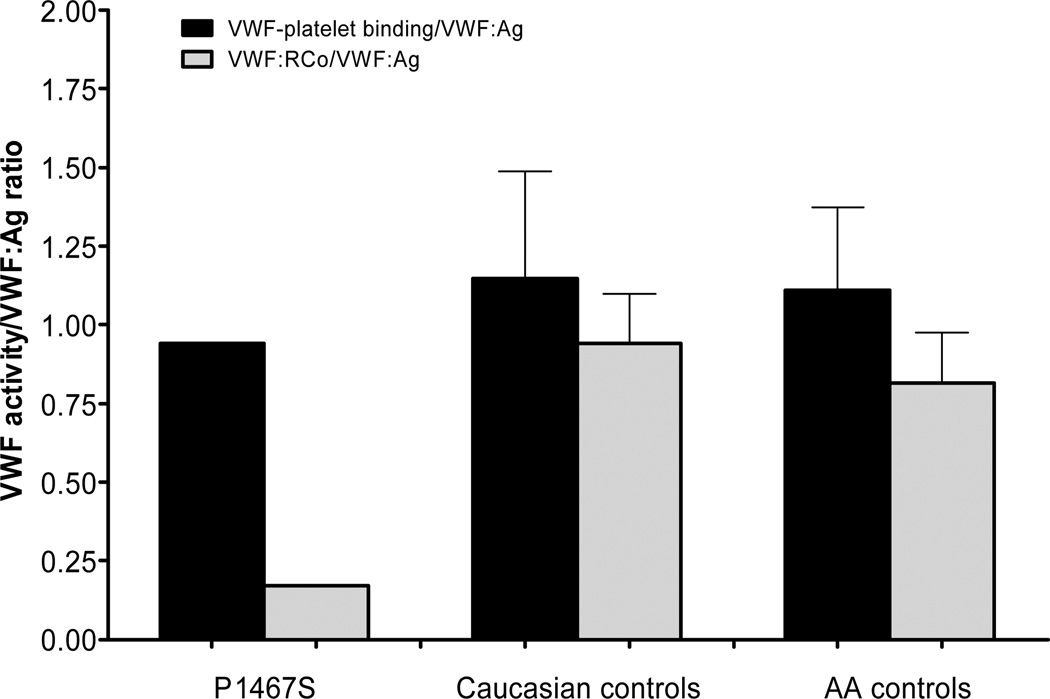
As the functional testing performed in our clinical laboratory relies on the use of ristocetin, alternate assays of VWF function may be required in the case of mutations that affect ristocetin binding. One such assay, referred to here as the VWF-platelet binding assay, utilizes gain-of-function mutations in GPIb to enable spontaneous VWF-GPIb interactions in the absence of ristocetin. Analysis of normal control subjects from the ZPMCB-VWD has shown that the racial differences seen with the VWF:RCo assay are reduced with the VWF-platelet binding assay (Flood and Montgomery, unpublished data). Plasma from the index case with the P1467S sequence variation demonstrated normal VWF-platelet binding when compared to results obtained from 66 Caucasian and 59 African American controls (figure 2). The mean VWF-platelet binding/VWF:Ag ratio for the subject with the P1467S sequence variation was 0.94. The Caucasian controls in the ZPMCB-VWD had a mean of 1.11 ± 0.34 (mean ± SD) and the African American controls had a mean of 1.15 ± 0.26 for the ratio of VWF-platelet binding/VWF:Ag.
Figure 2.
Two different tests of VWF function are shown. The VWF-platelet binding assay uses a gain-of-function GPIb to induce binding to plasma VWF. The black bars represent the mean ratio of VWF-platelet binding/VWF:Ag for the P1467S index case, Caucasian, and African American (AA) controls from the ZPMCB-VWD. The grey bars represent the mean VWF:RCo/VWF:Ag ratio, as measured in the clinical laboratory, for each group. Error bars denote 1 SD. While the VWF-platelet binding/VWF:Ag ratios were similar for the P1467S index case and controls, the P1467S index case had a markedly reduced VWF:RCo/VWF:Ag ratio.
P1467S expression
To evaluate VWF synthesis and to produce recombinant VWF for further functional studies, the P1467S sequence variation was placed into an expression vector containing full length VWF and expressed in HEK293T cells as previously described [6]. There was no difference in expression when the P1467S VWF construct was compared to wild-type VWF, suggesting that this sequence variation does not affect VWF expression (data not shown). In support of the data with recombinant VWF, the index case and affected family members all had VWF antigen levels >100 IU/dL, well within the normal range.
P1467S-platelet binding
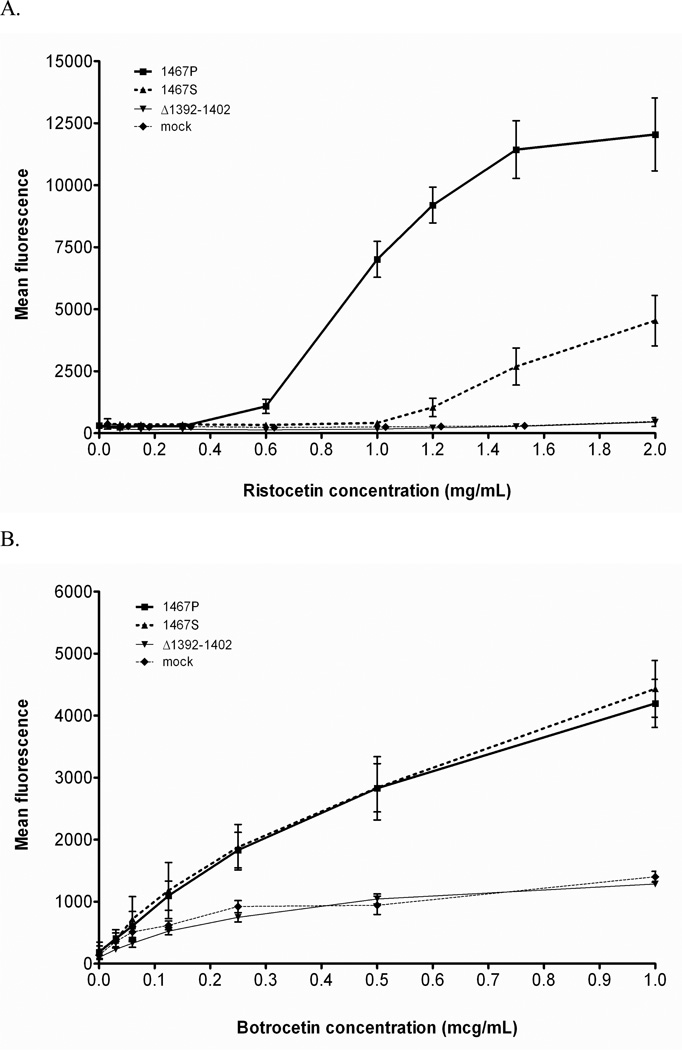
Platelet-VWF interactions were assessed with the recombinant VWF constructs and fixed platelets. The P1467S VWF construct demonstrated no binding in the presence of low dose ristocetin. This correlates well with the low VWF:RCo/VWF:Ag ratios seen in the index case and family members with this sequence variation, whose ratios ranged from 0.09 to 0.18. At concentrations of 1 mg/mL or less, binding was at or below background, and even at higher concentrations, binding was significantly less than that seen with wild-type VWF (figure 3A). Binding with botrocetin was normal when compared to wild-type VWF (figure 3B). A construct containing the known 2M deletion mutant Δ1392–1402 demonstrated complete lack of binding with both ristocetin and botrocetin.
Figure 3.
Flow cytometry was performed using fixed platelets and wild-type or mutant P1467S VWF with either ristocetin (panel A) or botrocetin (panel B) as the agonist. While the mutant VWF showed absent binding in response to ristocetin, botrocetin-induced binding was essentially normal. A control 2M mutation, Δ1392–1402, showed absent binding with both ristocetin and botrocetin as did the supernatant from a transfection with an empty vector, denoted here as “mock”.
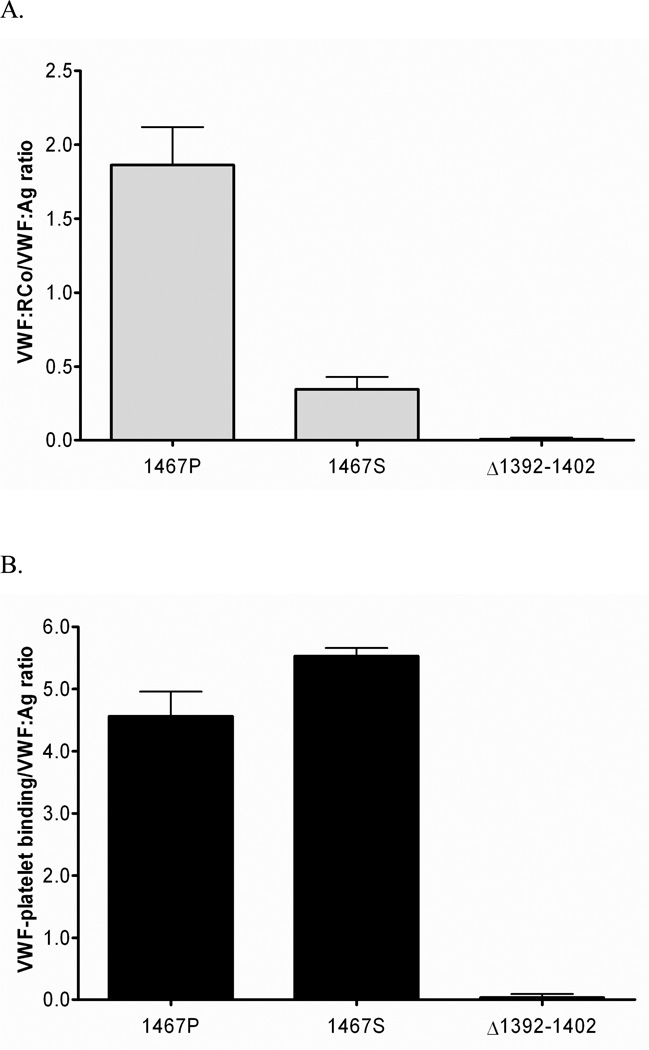
P1467S-GPIb binding
An ELISA-based assay was developed to detect recombinant VWF binding to platelet GPIb. Previous investigators have reported similar assays utilizing ristocetin [18,19]. Ristocetin, at a dose of 1 mg/mL, was used in our assay to induce binding to wild-type GPIb. We also utilized a gain-of-function GPIb construct to assess VWF binding to GPIb in the absence of ristocetin. Ristocetin-induced binding to wild-type GPIb was markedly decreased, as expected, for the P1467S construct, to an average of 18% of the wild-type VWF binding. In contrast, there was no difference in GPIb binding when the P1467S construct was compared to wild-type VWF using gain-of-function mutant GPIb (figure 4).
Figure 4.
Expressed wild-type and mutant recombinant VWF ELISA binding. (A) An ELISA-based binding assay using wild-type GPIb and added ristocetin shows lack of binding with the P1467S sequence variation compared to wild-type VWF. A control 2M mutation, Δ1392–1402, also shows lack of binding. (B) An ELISA-based binding assay using a gain-of-function GPIb in the absence of ristocetin shows normal binding for the P1467S construct, but lack of binding is again observed for the control 2M mutation, Δ1392–1402.
Similar results were seen with the GPIb binding assays without ristocetin when plasma from the index case was compared to plasma from normal control subjects enrolled in the ZPMCB-VWD. In contrast, plasma from ten subjects with previously diagnosed type 2M VWD showed minimal binding to the gain-of-function GPIb in this assay.
P1467S whole blood platelet adhesion
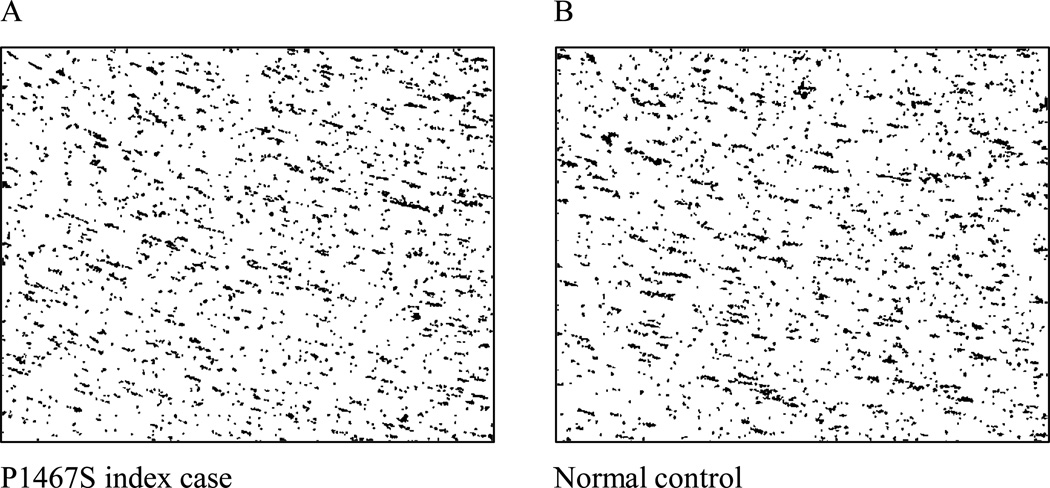
In order to test the ability of VWF to bind GPIb under shear conditions, a cone and plate(let) analyzer was used to visualize VWF-platelet interactions. The cone and plate(let) analyzer is an instrument for testing platelet adhesion and aggregation in response to shear of 1800 s−1 [16]. Testing occurs in whole blood, thus simulating in vivo conditions to provide an estimate of platelet function. VWF adheres to the surface of the polystyrene wells used in this assay, leading to deposition of platelets which are visualized by staining the wells. Previous work has shown that patients with severe VWD have abnormal results using this assay [16,20]. A blood sample from the index patient was analyzed and the results compared to those from a group of healthy controls (figure 5). The mean surface coverage, a measure of platelet adhesion, was 6.4 ± 1%, as compared to the normal control mean of 8.3 ± 2.2% (mean ± SD). Mean aggregate size was 28 ± 0.5 µm2, also well within the normal range of 31± 7 µm2. This demonstrates that blood from the index patient was able to adhere to the polystyrene well and bind platelets in a comparable manner to healthy controls.
Figure 5.
Using a cone and plate(let) analyzer, the Impact-R, VWF-platelet interactions were tested under shear conditions. A representative sample from the index patient is shown in panel A, as compared to a healthy control in panel B. No significant difference in platelet adhesion was seen.
Discussion
Our results suggest that the P1467S sequence variation results in an apparent decrease in binding to ristocetin but does not indicate a dysfunctional VWF. Platelet binding appears to be normal under both shear conditions and static conditions that do not rely on ristocetin. Only in the presence of ristocetin is a difference observed. In this case, diagnostic use of a VWF activity assay that depends on in vitro binding to ristocetin led to a spurious diagnosis of type 2M VWD. This diagnosis was further suggested by an absence of RIPA at 1.5 mg/mL ristocetin. Although the VWF:RCo assay has proven useful in the diagnosis and classification of many patients with VWD, ristocetin-induced binding to platelet GPIb reflects a pharmacologic rather than a physiologic effect.
Ristocetin was originally developed as an antibiotic, but thrombocytopenia, secondary to VWF-platelet agglutination and clearance, led to its removal from clinical practice [21]. Studies in VWD patients subsequently demonstrated that ristocetin could be useful in the classification of VWD patients [22–24]. At concentrations of 1 mg/mL or greater, dimeric ristocetin leads to platelet agglutination in the presence of VWF [2]. This led to the use of ristocetin for quantitative assessment of VWF activity [25]. Current laboratory testing for VWD relies heavily on the VWF:RCo as a surrogate measure of VWF function [9].
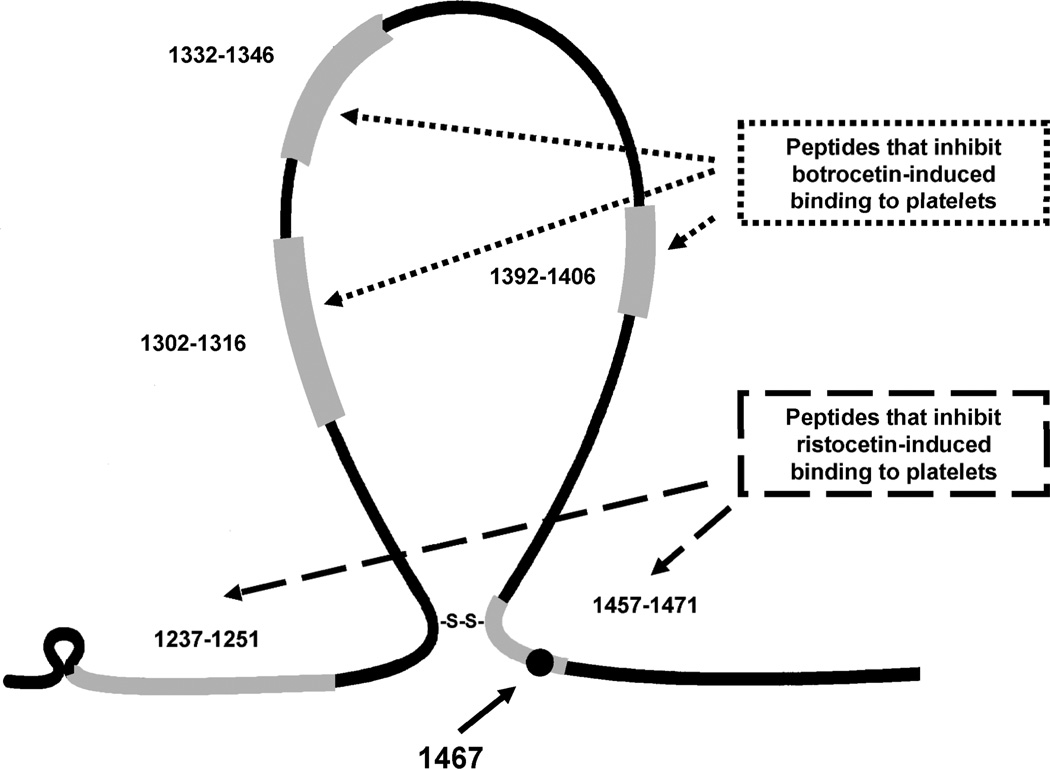
Several binding sites in the VWF A1 domain have been implicated in ristocetin binding, including C1237-P1251 and L1457-P1471 [17,26]. The P1467S sequence variation is located just beyond the A1 loop, as diagrammed in figure 6, and is located within one of these VWF regions known to specifically involve ristocetin binding rather than botrocetin [27,28]. Prior work has shown that substitution of the VWF proline triplet with either arginine or aspartic acid at position 1465–1467 disrupts ristocetin-induced GPIb binding [29]. Structural changes in the A1 domain at this ristocetin recognition site may explain the decreased VWF:RCo seen with the P1467S sequence variation. Botrocetin induces change in VWF affinity for GPIb through interactions with the A1 domain as well, but does not require shear forces for its interaction and does not change the A1 domain conformation [30]. These differences may explain why the P1467S VWF reacts normally in the presence of botrocetin.
Figure 6.
The A1 domain of the VWF protein is diagrammed here, with the location of two known ristocetin-binding domains and three botrocetin-binding domains marked in grey. The circle denotes the approximate location of the P1467S sequence variation. The disulfide bond is between Cys 1272 and Cys 1458.
Racial differences have been reported previously in VWF. Buchanan and colleagues noted that African Americans had decreased ristocetin-induced platelet aggregation when compared to Caucasians [10]. More recently, Miller et al reported increased VWF:Ag in African Americans with no apparent difference in VWF:RCo [11]. One possible explanation of these findings is that African Americans may have polymorphisms that could affect ristocetin-based assays, such that the VWF:RCo assay underestimates VWF function in this group. Our research in the ZPMCB-VWD has noted a common polymorphism in African American controls, D1472H (Flood and Montgomery, unpublished data). Studies of this relationship are in progress, although it is interesting to note that the proband’s mother does have the D1472H polymorphism and shows a decreased VWF:RCo/VWF:Ag ratio, although not as profound as the decrease seen in the family members with the P1467S polymorphism.
The use of screening tests for diagnosis of VWD is problematic. The PTT may be decreased if a low FVIII level is present, but is usually normal. Both bleeding time and the platelet function analyzer (PFA-100) have been promoted as screening tests, but neither is sufficiently sensitive [31,32]. Specific tests of VWF are generally recommended if a diagnosis of VWD is under consideration [9]. In this case, however, pre-operative testing might not have been indicated, as the proband was a healthy child with neither personal nor family history of bleeding. Use of the PTT as a pre-tonsillectomy screening test is not recommended in patients without a bleeding history [33,34].
VWD is a heterogeneous condition, with a great deal of variation in symptoms experienced by patients, especially those with only mild decreases in VWF levels. Recent data from Canada on a group of patients with type 2M VWD demonstrated that VWF mutations were more likely to be found in those patients with VWF:RCo/VWF:Ag ratios <0.5. All of their index cases were characterized by bleeding symptoms, although some had normal VWF:Ag [35]. Our patient was unusual in that she had an extremely low VWF:RCo (11 IU/dL), a level most clinicians would predict to be associated with major bleeding. This individual, however, did not display any clinical bleeding with surgeries, menses, or childbirth. Other tests of VWF function failed to demonstrate any defect inherent to the P1467S sequence variation. In the presence of normal multimers and normal collagen binding, the decreased VWF:RCo is the only marker in this patient of possible VWF dysfunction.
The current use of VWF:RCo as a measure of VWF function may not always provide an accurate assessment of in vivo VWF function. Collagen binding has also been recommended as an additional test of VWF function [36,37]. While the VWF:CB assay measures VWF binding to matrix proteins such as collagen, it does not measure VWF binding to platelets. Regardless of the ligand, be it GPIb, platelets, or collagen, ELISA-based studies only measure static VWF interactions. Evaluation of VWF interactions under shear stress is technically more difficult, but might provide additional, qualitative, demonstration of VWF function. The International Society on Thrombosis and Haemostasis has recommended development of flow based assays in order to include shear [38]. This might include techniques such as the cone and plate(let) analyzer mentioned above, but preferably limited to examination of VWF function. No such assays are, as yet, readily available or validated for diagnosis of VWD. A combination of static and flow based assays may ultimately be required for accurate diagnosis.
Our data suggest that this patient’s diagnosis of VWD is spurious, as it is based on binding of VWF to the non-physiologic agonist ristocetin. The P1467S sequence variation confers a decreased ability to interact with ristocetin, but other assays of VWF function, as well as the patient’s clinical history, suggest that there is no actual functional defect. While the lack of bleeding seen in the proband and family might also be the result of a co-existing thrombophilic trait, in vitro testing of the P1467S sequence variation does not support its inclusion as a causative mutation in VWD. Other VWF A1 domain mutations and polymorphisms may result in similar laboratory profiles. It is therefore critical to assess patients’ symptoms along with laboratory assays in diagnosis of VWD. This case demonstrates the limitations of the current assay for VWF activity and the importance of basing diagnosis and treatment on biologically relevant assays.
Acknowledgements
This work was supported by program project grant P01 HL081588 from the National Institutes of Health. VHF was also supported by a Career Development Award from the National Hemophilia Foundation and a Mentored Research Award from the Hemophilia and Thrombosis Research Society. RRM was also supported by National Institutes of Health grants HL33721 and HL044612.
Bibliography
- 1.Sadler JE. Biochemistry and genetics of von willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Scott JP, Montgomery RR, Retzinger GS. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991 May 5;266(13):8149–8155. [PubMed] [Google Scholar]
- 3.Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB. Working Party on von Willebrand Disease Classification. Update on the pathophysiology and classification of von willebrand disease: A report of the subcommittee on von willebrand factor. J Thromb Haemost. 2006 Oct;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz I, Tuley EA, Mancuso DJ, Randi AM, Firkin BG, Howard MA, Sadler JE. Von willebrand disease type B: A missense mutation selectively abolishes ristocetin-induced von willebrand factor binding to platelet glycoprotein ib. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9846–9849. doi: 10.1073/pnas.89.20.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilbert L, Gaucher C, Mazurier C. Identification of two mutations (Arg611Cys and Arg611His) in the A1 loop of von willebrand factor (vWF) responsible for type 2 von willebrand disease with decreased platelet-dependent function of vWF. Blood. 1995 Aug 1;86(3):1010–1018. [PubMed] [Google Scholar]
- 6.Mancuso DJ, Kroner PA, Christopherson PA, Vokac EA, Gill JC, Montgomery RR. Type 2M:Milwaukee-1 von willebrand disease: An in-frame deletion in the Cys509-Cys695 loop of the von willebrand factor A1 domain causes deficient binding of von willebrand factor to platelets. Blood. 1996 Oct 1;88(7):2559–2568. [PubMed] [Google Scholar]
- 7.Hillery CA, Mancuso DJ, Evan Sadler J, Ponder JW, Jozwiak MA, Christopherson PA, Cox Gill J, Paul Scott J, Montgomery RR. Type 2M von willebrand disease: F606I and I662F mutations in the glycoprotein ib binding domain selectively impair ristocetin- but not botrocetinmediated binding of von willebrand factor to platelets. Blood. 1998 Mar 1;91(5):1572–1581. [PubMed] [Google Scholar]
- 8. [Accessed May 6];International Society on Thrombosis and Haemostasis Scientific and Standardization Committee VWF Information Homepage. 2009 http://www.vwf.group.shef.ac.uk. [Google Scholar]
- 9.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. Von willebrand disease (VWD): Evidence-based diagnosis and management guidelines, the national heart, lung, and blood institute (NHLBI) expert panel report (USA) Haemophilia. 2008 Mar;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan GR, Holtkamp CA, Levy EN. Racial differences in ristocetin-induced platelet aggregation. Br J Haematol. 1981 Nov;49(3):455–464. doi: 10.1111/j.1365-2141.1981.tb07249.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller CH, Haff E, Platt SJ, Rawlins P, Drews CD, Dilley AB, Evatt B. Measurement of von willebrand factor activity: Relative effects of ABO blood type and race. J Thromb Haemost. 2003 Oct;1(10):2191–2197. doi: 10.1046/j.1538-7836.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. A quantitative analysis of bleeding symptoms in type 1 von willebrand disease: Results from a multicenter european study (MCMDM-1 VWD) J Thromb Haemost. 2006 Apr;4(4):766–773. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown JE, Bosak JO. An ELISA test for the binding of von willebrand antigen to collagen. Thromb Res. 1986 Aug 1;43(3):303–311. doi: 10.1016/0049-3848(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery RR, Hathaway WE, Johnson J, Jacobson L, Muntean W. A variant of von willebrand's disease with abnormal expression of factor VIII procoagulant activity. Blood. 1982 Jul;60(1):201–207. [PubMed] [Google Scholar]
- 15.Andrews RK, Booth WJ, Gorman JJ, Castaldi PA, Berndt MC. Purification of botrocetin from bothrops jararaca venom. analysis of the botrocetin-mediated interaction between von willebrand factor and the human platelet membrane glycoprotein ib-IX complex. Biochemistry. 1989 Oct 17;28(21):8317–8326. doi: 10.1021/bi00447a009. [DOI] [PubMed] [Google Scholar]
- 16.Varon D, Dardik R, Shenkman B, Kotev-Emeth S, Farzame N, Tamarin I, Savion N. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb Res. 1997 Feb 15;85(4):283–294. doi: 10.1016/s0049-3848(97)00014-5. [DOI] [PubMed] [Google Scholar]
- 17.Mohri H, Fujimura Y, Shima M, Yoshioka A, Houghten RA, Ruggeri ZM, Zimmerman TS. Structure of the von willebrand factor domain interacting with glycoprotein ib. J Biol Chem. 1988 Dec 5;263(34):17901–17904. [PubMed] [Google Scholar]
- 18.Vanhoorelbeke K, Cauwenberghs N, Vauterin S, Schlammadinger A, Mazurier C, Deckmyn H. A reliable and reproducible ELISA method to measure ristocetin cofactor activity of von willebrand factor. Thromb Haemost. 2000 Jan;83(1):107–113. [PubMed] [Google Scholar]
- 19.Federici AB, Canciani MT, Forza I, Mannucci PM, Marchese P, Ware J, Ruggeri ZM. A sensitive ristocetin co-factor activity assay with recombinant glycoprotein ibalpha for the diagnosis of patients with low von willebrand factor levels. Haematologica. 2004 Jan;89(1):77–85. [PubMed] [Google Scholar]
- 20.Shenkman B, Savion N, Dardik R, Tamarin I, Varon D. Testing of platelet deposition on polystyrene surface under flow conditions by the cone and plate(let) analyzer: Role of platelet activation, fibrinogen and von willebrand factor. Thromb Res. 2000 Aug 15;99(4):353–361. doi: 10.1016/s0049-3848(00)00255-3. [DOI] [PubMed] [Google Scholar]
- 21.Gangarosa EJ, Landerman NS, Rosch PJ, Herndon EG., Jr Hematologic complications arising during ristocetin therapyl; relation between dose and toxicity. N Engl J Med. 1958 Jul 24;259(4):156–161. doi: 10.1056/NEJM195807242590402. [DOI] [PubMed] [Google Scholar]
- 22.Howard MA, Firkin BG. Ristocetin--a new tool in the investigation of platelet aggregation. Thromb Diath Haemorrh. 1971 Oct 31;26(2):362–369. [PubMed] [Google Scholar]
- 23.Howard MA, Sawers RJ, Firkin BG. Ristocetin: A means of differentiating von willebrand's disease into two groups. Blood. 1973 May;41(5):687–690. [PubMed] [Google Scholar]
- 24.Weiss HJ, Rogers J, Brand H. Defective ristocetin-induced platelet aggregation in von willebrand's disease and its correction by factor VIII. J Clin Invest. 1973 Nov;52(11):2697–2707. doi: 10.1172/JCI107464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss HJ, Hoyer LW, Rickles FR, Varma A, Rogers J. Quantitative assay of a plasma factor deficient in von willebrand's disease that is necessary for platelet aggregation. relationship to factor VIII procoagulant activity and antigen content. J Clin Invest. 1973 Nov;52(11):2708–2716. doi: 10.1172/JCI107465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berndt MC, Ward CM, Booth WJ, Castaldi PA, Mazurov AV, Andrews RK. Identification of aspartic acid 514 through glutamic acid 542 as a glycoprotein ib-IX complex receptor recognition sequence in von willebrand factor. mechanism of modulation of von willebrand factor by ristocetin and botrocetin. Biochemistry. 1992 Nov 17;31(45):11144–11151. doi: 10.1021/bi00160a027. [DOI] [PubMed] [Google Scholar]
- 27.Girma JP, Takahashi Y, Yoshioka A, Diaz J, Meyer D. Ristocetin and botrocetin involve two distinct domains of von willebrand factor for binding to platelet membrane glycoprotein ib. Thromb Haemost. 1990 Oct 22;64(2):326–332. [PubMed] [Google Scholar]
- 28.De Luca M, Facey DA, Favaloro EJ, Hertzberg MS, Whisstock JC, McNally T, Andrews RK, Berndt MC. Structure and function of the von willebrand factor A1 domain: Analysis with monoclonal antibodies reveals distinct binding sites involved in recognition of the platelet membrane glycoprotein ib-IX-V complex and ristocetin-dependent activation. Blood. 2000 Jan 1;95(1):164–172. [PubMed] [Google Scholar]
- 29.Azuma H, Sugimoto M, Ruggeri ZM, Ware J. A role for von willebrand factor proline residues 702–704 in ristocetin-mediated binding to platelet glycoprotein ib. Thromb Haemost. 1993 Feb 1;69(2):192–196. [PubMed] [Google Scholar]
- 30.Fukuda K, Doggett TA, Bankston LA, Cruz MA, Diacovo TG, Liddington RC. Structural basis of von willebrand factor activation by the snake toxin botrocetin. Structure. 2002 Jul;10(7):943–950. doi: 10.1016/s0969-2126(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 31.Dean JA, Blanchette VS, Carcao MD, Stain AM, Sparling CR, Siekmann J, Turecek PL, Lillicrap D, Rand ML. Von willebrand disease in a pediatric-based population--comparison of type 1 diagnostic criteria and use of the PFA-100 and a von willebrand factor/collagen-binding assay. Thromb Haemost. 2000 Sep;84(3):401–409. [PubMed] [Google Scholar]
- 32.Quiroga T, Goycoolea M, Munoz B, Morales M, Aranda E, Panes O, Pereira J, Mezzano D. Template bleeding time and PFA-100 have low sensitivity to screen patients with hereditary mucocutaneous hemorrhages: Comparative study in 148 patients. J Thromb Haemost. 2004 Jun;2(6):892–898. doi: 10.1111/j.1538-7836.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- 33.Howells RC,2nd, Wax MK, Ramadan HH. Value of preoperative prothrombin time/partial thromboplastin time as a predictor of postoperative hemorrhage in pediatric patients undergoing tonsillectomy. Otolaryngol Head Neck Surg. 1997 Dec;117(6):628–632. doi: 10.1016/S0194-59989770044-5. [DOI] [PubMed] [Google Scholar]
- 34.Kitchens CS. To bleed or not to bleed? is that the question for the PTT? J Thromb Haemost. 2005 Dec;3(12):2607–2611. doi: 10.1111/j.1538-7836.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 35.James PD, Notley C, Hegadorn C, Poon MC, Walker I, Rapson D, Lillicrap D. Association of Hemophilia Clinic Directors of Canada. Challenges in defining type 2M von willebrand disease: Results from a canadian cohort study. J Thromb Haemost. 2007 Sep;5(9):1914–1922. doi: 10.1111/j.1538-7836.2007.02666.x. [DOI] [PubMed] [Google Scholar]
- 36.Favaloro EJ. Collagen binding assay for von willebrand factor (VWF:CBA): Detection of von willebrands disease (VWD), and discrimination of VWD subtypes, depends on collagen source. Thromb Haemost. 2000 Jan;83(1):127–135. [PubMed] [Google Scholar]
- 37.Favaloro EJ. An update on the von willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007 Nov;33(8):727–744. doi: 10.1055/s-2007-1000364. [DOI] [PubMed] [Google Scholar]
- 38.Zwaginga JJ, Sakariassen KS, King MR, Diacovo TG, Grabowski EF, Nash G, Hoylaerts M, Heemskerk JW. Can blood flow assays help to identify clinically relevant differences in von willebrand factor functionality in von willebrand disease types 1–3? J Thromb Haemost. 2007 Dec;5(12):2547–2549. doi: 10.1111/j.1538-7836.2007.02807.x. [DOI] [PubMed] [Google Scholar]