Abstract
Headache is a common clinical feature in patients in the emergency room and in general neurology clinics. For physicians not experienced in headache disorders it might be difficult sometimes to decide in which patients neuroimaging is necessary to diagnose an underlying brain pathology and in which patients cerebral imaging is unnecessary. Most patients presenting to the primary-care physician with a nonacute headache and no further neurological signs or symptoms will not be suffering from an underlying serious condition. This review focuses on the main primary headache diseases, including migraine, tension-type headache and cluster headache, as well as frequent secondary headache entities with common clinical presentation and appropriate diagnostic and therapeutic algorithms to help guide the decision on the utilization of neuroimaging in the diagnostic workup.
Keywords: cluster headache, diagnosis, headache, migraine, neuroimaging, tension-type headache
Introduction
Headache is the most often reported neurological symptom. Physicians are regularly confronted with the question of whether or not it is necessary to perform neuroimaging in order to confirm a distinct headache diagnosis. Many patients are frightened that they are suffering from a severe disease and therefore request further diagnostics. In a resource-restricted medical environment this is sometimes difficult to justify without appropriate and accepted clinical evidence. To make a responsible clinical and economic decision it is important to differentiate between a primary headache without underlying cerebral abnormality and secondary headaches, which are often associated with brain pathology. However, in most cases when neuroimaging is performed in headache patients, especially when there are no further associated neurological symptoms, the results will be negative. A large review of 3026 scans of patients with headache showed that only a minority of patients suffered from a serious disease that could be diagnosed with cerebral imaging: (a) 0.8% brain tumours; (b) 0.2% arteriovenous malformations; (c) 0.3% hydrocephalus; (d) 0.1% aneurysm; (e) 0.2% subdural haematoma; (f) 1.2% strokes, including chronic ischaemic processes [Evans, 1996].
This review aims to aid the decision process to identify patients that require neuroimaging for proper diagnostic workup and indicates cases where this may be omitted. It will mainly focus on computed tomography (CT) imaging and magnetic resonance imaging (MRI) as these are the most commonly used methods in clinical practice.
Neuroimaging in the diagnosis of primary headache disorders
Migraine
Migraine is a very common primary headache disorder, which affects more women than men and usually starts around the age of 20. Headache attacks last between 4 and 72 h and are characterized by unilateral location, pulsating quality and moderate to severe pain intensity [ICHD, 2004]. Pain is aggravated by routine physical activity. Many patients, therefore, avoid these activities and feel the need to rest. Autonomic features such as nausea and/or vomiting, photophobia and phonophobia usually accompany a migraine attack. Most patients display an episodic course of disease; a few patients suffer from chronic migraine, which is characterized by ≥ 15 headache days/month over a period of more than 3 months.
Usually, neuroimaging is not required in patients with episodic migraine who present with typical headache features according to the classification of the International Headache Society (IHS) [ICHD, 2004], and normal neurological examination. A meta-analysis of studies with migraine patients showed that in cases with normal neurological examination and a typical clinical presentation significant brain pathology was only detected in 0.18% of the patients. Therefore, neuroimaging is usually unnecessary in these patients.
In some cases, patients might be frightened that their headache is caused by a brain tumour or another serious disorder. In these cases the strong patient wish for clarification might make it reasonable to perform cerebral imaging to eliminate these possible causes and to appease the patient. One study showed that 60% of patients in a British regional headache clinic were afraid that they were suffering from a serious illness because of the headache. Two-thirds of them were still afraid after the appointment, and expressed the wish for neuroimaging [Fitzpatrick and Hopkins, 1981].
In some patients that present with migrainous features, however, further diagnostic workup is necessary and helpful. Neuroimaging should be considered in patients presenting with atypical headache features, change of headache type, additional significant risk factors or chronic subtype.
Tension-type headache
Tension-type headache (TTH) has a life-time prevalence of up to 78% in the general population. Duration of TTH attack is variable and can last from 30 min to 7 days. It is characterized by bilateral location, pressing/tightening quality and is typically of mild to moderate intensity [ICHD, 2004]. In contrast to migraine, physical activity does not lead to aggravation of head pain. TTH is not accompanied by nausea or vomiting, and usually not by more than one of photophobia or phonophobia. In episodic TTH patients will only occasionally seek medical attention and neuroimaging will not be an issue. Patients with chronic TTH should receive cerebral MRI once to exclude further pathology.
Cluster headache
Cluster headache (CH) is a rare disease, but the most frequent headache entity in the group of trigeminal autonomic cephalagias (TACs). In addition to CH, a few other headaches, for example, paroxysmal hemicrania and short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT), are assigned to the group of TACs, but are even less common. Therefore, this section will primarily focus on CH.
More men than women are affected, usually around the age of 40 years. CH is characterized by severe unilateral orbital, supraorbital and/or temporal pain lasting between 15 min and 180 min. Headache is associated with ipsilateral trigeminal autonomic symptoms such as conjunctival injection, lachrymation, nasal congestion, rhinorrhoea, forehead and facial sweating, myosis, ptosis and eyelid oedema. Patients usually suffer from one attack every other day up to eight attacks per day. Typically, CH is characterized by a striking circannual and circadian rhythm with episodes (or bouts) during spring and autumn, and remission periods in summer and winter. Due to the characteristic clinical presentation diagnosis can be based mainly on the clinical presentation. However, as there are some differential diagnoses, for example, cerebral tumours such as prolactinoma [Porta-Etessam et al. 2001], ateriovenous malformation [Muñoz et al. 1996], carotid dissection [Rigamonti et al. 2008], cerebral infarctions [Khatri and Saeed, 2011], and multiple sclerosis plaques [González-Quintanilla et al. 2012], MRI should be performed once in each patient for accurate elimination of these secondary causes. This is also true for other trigeminal autonomic syndromes such as SUNCT, paroxysmal hemicrania and hemicrania continua.
Which neuroimaging should be performed in nonacute headache?
Data are insufficient regarding the relative sensitivity of MRI compared with CT in nonacute headaches. However, MRI offers a greater resolution and discrimination and might therefore be the preferred method of choice in nonacute headache. In addition, radiation due to CT scanning may be avoided.
Neuroimaging in the emergency room
Patients often present to the emergency room with symptoms of headache. First it is important to decide whether this is a primary headache that only demands the appropriate therapy or whether it is a secondary headache that requires special attention and further diagnostic workup as this patient might be suffering from an underlying life-threatening condition. A distinct history evaluation is imperative. The most important question is whether the headache is occurring for the first time, followed by the question as to whether it is the worst headache of the patients’ life time. Additional ‘red flags’ that should be considered are summarized in Table 1. Usually, in most secondary headaches pain is not the only symptom. Physical examination often produces further findings that help to find the correct diagnosis (Table 2). Patients with abnormal neurological examination are much more likely to have a significant brain pathology as detected by MRI or CT, whereas a normal neurological examination indicates a decreased likelihood of a significant cerebral lesion [Cala and Mastaglia, 1976; Carrera et al. 1977; Duarte et al. 1996; Larson et al. 1980; Mitchell et al. 1993].
Table 1.
Red flags in headache.
| • Abnormal neurological examination (others than typical aura) |
| • New headache in older patients |
| • Headache increasing in frequency and severity |
| • Worst headache ever |
| • Sudden onset of headache |
| • New-onset headache in a patient with risk factors for HIV infection or cancer |
| • Headache with signs of systemic illness (e.g. fever, stiff neck, rash) |
| • Papilloedema |
| • Headache subsequent to head trauma |
| • History of dizziness or lack of coordination |
| • Progressively worsening headache |
| • Headache worsening with Valsalva manoeuvre |
Table 2.
Features on which physical examination should focus.
| • Level of consciousness |
| • Cranial nerve testing (especially II, III, IV, VI) |
| • Motor strength testing and sensation |
| • Deep tendon reflexes and pathological reflexes |
| • Signs of meningeal irritation (Kernig’s and Brudzinski’s signs) |
| • Coordination and gait |
Important secondary headache entities that might present in the emergency room are as follows.
Temporal arteritis
Temporal arteritis (giant cell arteritis) is an inflammatory disease of blood vessels most commonly involving the large and medium arteries of the head, predominantly the branches of the external carotid artery with inflammation of the vessel wall. Elderly patients above the age of 50 years are typically affected. They report severe unilateral or bilateral headache. Commonly, a temporal artery tenderness or decreased temporal artery pulse can be observed. Additionally, an increased erythrocyte sedimentation rate ≥ 60 mm/h can be found. An early diagnosis is warranted to avoid irreversible complications such as blindness and stroke. MRI can be used to investigate mural thickness, contrast enhancement and lumen diameter of the temporal artery [Bley et al. 2005a, 2005b]. Increasing field strengths of 3 Tesla and above might increase sensitivity and specificity for diagnosing vascular inflammation [Blockmans et al. 2009]. However, results are often ambiguous and evaluation requires experience. Therefore, MRI should primarily be used to exclude alternative causes for secondary headache. Diagnosis should be based mainly on typical clinical presentation, ultrasound of the temporal vessels yielding a halo sign and biopsy, if needed.
Subarachnoid haemorrhage
In general, patients with subarachnoid haemorrhage will report a rapid-onset headache that is often referred to as a ‘thunderclap headache’. This severe headache is often accompanied by an alteration of the level of consciousness, and may lead to nausea and vomiting. The most common underlying pathophysiology is bleeding into the subarachnoid space from a cerebral aneurysm, which can occur spontaneously or be caused by head trauma. A thunderclap headache always needs further diagnostic evaluation, although a primary benign thunderclap headache also exists.
To differentiate one condition from the other a CT scan has to be performed as soon as possible because the sensitivity of the CT to detect subarachnoid blood decreases rapidly within the first 24 h. A large Canadian study enrolled 3132 patients from 11 tertiary-care emergency departments across Canada that presented with thunderclap headache [Perry et al. 2011]. A total of 240 patients had subarachnoid haemorrhage. The sensitivity for CT-based diagnosis of subarachnoid haemorrhage was 92.9%, the specificity was 100%. When the CT was acquired within 6 h of disease onset (121/953), sensitivity was increased to 100%. MRI might show an increased sensitivity a few days after bleeding [Van Gijn et al. 2007]. In contrast, a benign thunderclap headache is not attributed to another disorder, but can last from 1 h to 10 days.
If cerebral imaging does not show subarachnoid haemorrhage but the clinical presentation is typical, a lumbar puncture should be performed as in 3% of patients a diagnosis of subarachnoid haemorrhage can be made despite unremarkable CT or MRI [Van Gijn et al. 2007]. Angiography is required for further diagnostic workup to identify aneurysms and treat them appropriately (i.e. with coiling).
Another important differential diagnosis in these patients is the reversible cerebral vasoconstriction syndrome, which is characterized by intracranial vasospasms that can also be detected by CT angiography.
Other intracranial haemorrhage
Intracranial haemorrhage in terms of epidural haematoma, subdural haematoma and intraventricular haemorrhage are often seen in the neurological emergency room. Usually, these patients do not present primarily with headache as their leading symptom but have other focal neurological deficits, however, sometimes headache can be the most pressing complaint. CT scans should be performed to diagnose intracranial haemorrhage.
Cerebral tumour/metastasis
Sometimes headache is the sole symptom of cerebral tumours or metastasis. Lee and Ho presented a study of 14 cases with nasopharyngeal carcinoma who initially suffered only from headaches [Lee and Ho, 2012]. However, this is only rarely the case. Usually, headache is accompanied by further neurological deficits that suggest a secondary cause of headache.
Meningitis, encephalitis and brain abscess
Infectious diseases of the brain are often accompanied by headache. However, only in rare cases is it the sole symptom of disease. The true prevalence of secondary headache due to infection remains unknown and current knowledge is restricted to case reports. Characteristically, headache due to infection is accompanied by fever and laboratory signs of infection, which should not be overlooked in the emergency room and neuroimaging is always necessary.
Idiopathic intracranial hypertension
Although idiopathic intracranial hypertension (IIH) or pseudotumour cerebri generally leads to chronic headache, some patients may present with an acute or subacute headache exacerbation accompanied by nausea and vomiting often reported on top of frequent headache. In most cases the headache is associated with transient visual disturbances; about 70% have a permanent visual impairment and about 5% even report a complete loss of vision. Papilloedema and obesity are the characteristic clinical features in these patients who are mainly young women [Durcan et al. 1988]. The underlying pathophysiology is suspected increased intracranial cerebrospinal fluid pressure, although the distinct mechanism for the development of this increase in IIH remains unknown [Walker, 2001]. In the past, imaging in IIH was only used to exclude other secondary pathology, such as intracranial mass lesion that has to be ruled out before performing a lumbar puncture. More recent studies determined additional MRI-based radiological features in IIH. In affected patients the following observations can be made: (a) empty sella; (b) flattened posterior globe/sclera; (c) enlarged perioptic subarachnoid space; (d) increased tortuosity and enhancement of the optic nerve; (e) intraocular protrusion of the optic nerve head; (f) slit-like ventricles (for review, see Degnan and Levy [2011]). In MR venography a transverse sinus narrowing can be detected.
Posttraumatic headache
Headaches as a result of head and neck trauma are one of the most common secondary headache types. Headache and neck pain are the cardinal symptoms of trauma following motor vehicle accidents, sports accidents and rarer causes of mild, moderate or severe head trauma. Patients often present to the emergency room for fear of permanent or lethal damage and for legal compensation. Posttraumatic headache (PTH) can occur after a mild, moderate or severe trauma. Interestingly the relationship between the severity of the injury and the severity of resulting PTH has never been conclusively established. Much to the contrary, it was repeatedly demonstrated by different investigations that PTH is most common and, indeed most severe, in patients with the least injury [Hines, 1999]. According to the IHS classification acute PTH after moderate or severe head injury should fulfil at least one of the following symptoms or alterations: (a) loss of consciousness for >30 min; (b) Glasgow coma scale <13; (c) posttraumatic amnesia for >48 h; (d) imaging demonstration of a traumatic brain lesion (e.g. cerebral haematoma, intracerebral and/or subarachnoid haemorrhage, brain contusion and/or skull fracture). In these cases cerebral imaging is warranted to evaluate the cerebral injury and start appropriate therapy. The PTH usually develops within 7 days of head trauma or after regaining consciousness following head trauma. Usually, the headache resolves within 3 months. A mild head trauma is considered when patients did not lose consciousness or loss of consciousness was less than 30 min, Glasgow coma scale above 13, and symptoms and/or signs of concussion can be found. Some patients report a chronification of headache symptoms with persisting pain lasting longer than 3 months. Cerebral imaging should be performed in all patients that suffer head injury and complain about headache: firstly to diagnose traumatic brain lesions, and secondly to satisfy legal aspects of the accident (e.g. insurance matters, working ability or early retirement) as well as patients’ concerns.
Summary
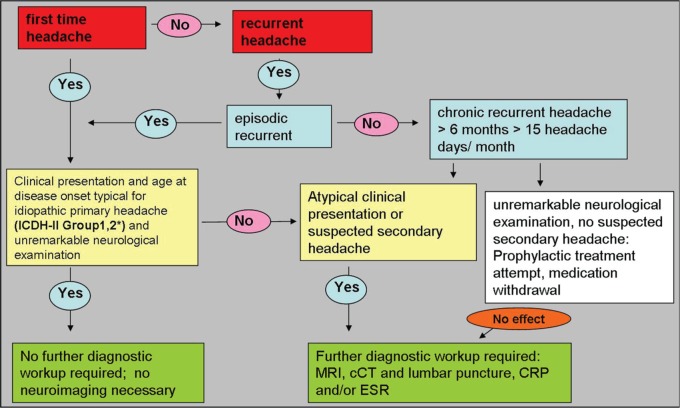
Headache is a common clinical feature in neurological patients. Patients with classic episodic migraine and TTH need no further neuroimaging as part of their diagnostic workup. These patients do not have a higher rate of relevant cerebral pathology than anyone else in the general population. Sometimes, however, it might be reasonable to perform neuroimaging in patients frightened that they are suffering from severe illness or who present with uncommon clinical features. Distinct ‘red flags’ in clinical neurological examination point to a secondary cause of the headache and require further neuroimaging to detect treatable causes and severe disease of this secondary headache (Figure 1).
Figure 1.
Decision tree for neuroimaging in headache.
*ICHD-II Group 1: migraine; Group 2: tension-type headache. CRP, C-reactive protein; cCT, cerebral computed tomography; ESR, erythrocyte sedimentation rate; ICHD, International Classification of Headache Disorders; MRI, magnetic resonance imaging.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Dagny Holle, Department of Neurology, University of Duisburg-Essen, Hufelandstrasse, 55, 45122 Essen, Germany.
Mark Obermann, Department of Neurology, University of Duisburg-Essen, Essen, Germany.
References
- Bley T., Wieben O., Uhl M., Thiel J., Schmidt D., Langer M. (2005a) High-resolution MRI in giant cell arteritis: imaging of the wall of the superficial temporal artery. AJR Am J Roentgenol 184: 283–287 [DOI] [PubMed] [Google Scholar]
- Bley T., Weiben O., Uhl M., Vaith P., Schmidt D., Warnatz K., et al. (2005b) Assessment of the cranial involvement pattern of giant cell arteritis with 3T magnetic resonance imaging. Arthritis Rheum 52: 2470–2477 [DOI] [PubMed] [Google Scholar]
- Blockmans D., Bley T., Schmidt W. (2009) Imaging for large-vessel vasculitis. Curr Opin Rheumatol 21: 19–28 [DOI] [PubMed] [Google Scholar]
- Cala L., Mastaglia F. (1976) Computerized axial tomography findings in a group of patients with migrainous headaches. Proc Aust Assoc Neurol 13: 35–41 [PubMed] [Google Scholar]
- Carrera G., Gerson D., Schnur J., McNeil B. (1977) Computed tomography of the brain in patients with headache or temporal lobe epilepsy: findings and cost-effectiveness. J Comput Assist Tomogr 1: 200–203 [PubMed] [Google Scholar]
- Degnan A., Levy L. (2011) Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol 32: 1986–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J., Sempere A., Delgado J., Naranjo G., Sevillano M., Clavería L. (1996) Headache of recent onset in adults: a prospective population-based study. Acta Neurol Scand 94: 67–70 [DOI] [PubMed] [Google Scholar]
- Durcan F., Corbett J., Wall M. (1988) The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 45: 875–877 [DOI] [PubMed] [Google Scholar]
- Evans R. (1996) Diagnostic testing for the evaluation of headaches. Neurol Clin 14: 1–26 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R., Hopkins A. (1981) Referrals to neurologists for headaches not due to structural disease. J Neurol Neurosurg Psychiatry 44: 1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Quintanilla V., Oterino A., Toriello M., De Pablos C., Wu Y., De Marco E., et al. (2012) Cluster-tic syndrome as the initial manifestation of multiple sclerosis. J Headache Pain 13: 425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. (1999) Posttraumatic Headaches. Mahwah, NJ: Lawrence Erlbaum Associates [Google Scholar]
- ICHD (2004) The International Classification of Headache Disorders: 2nd edition Cephalalgia 24(Suppl 1): 9–160 [DOI] [PubMed] [Google Scholar]
- Khatri I., Saeed U. (2011) Cluster like headache in an elderly patient with lateral medullary infarct – does the clue lie somewhere else? J Pak Med Assoc 61: 1022–1024 [PubMed] [Google Scholar]
- Larson E., Omenn G., Lewis H. (1980) Diagnostic evaluation of headache. Impact of computerized tomography and cost-effectiveness. JAMA 243: 359–362 [PubMed] [Google Scholar]
- Lee Y., Ho C. (2012) Headache as the sole symptom of nasopharyngeal carcinoma and its clinical implications. ScientificWorldJournal 2012: 143829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Osborn R., Grosskreutz S. (1993) Computed tomography in the headache patient: is routine evaluation really necessary? Headache 33: 82–86 [DOI] [PubMed] [Google Scholar]
- Muñoz C., Díez-Tejedor E., Frank A., Barreiro P. (1996) Cluster headache syndrome associated with middle cerebral artery arteriovenous malformation. Cephalalgia 16: 202–205 [DOI] [PubMed] [Google Scholar]
- Perry J., Stiell I., Sivilotti M., Bullard M., Emond M., Symington C., et al. (2011) Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 343: d4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-Etessam J., Ramos-Carrasco A., Berbel-García A., Martínez-Salio A., Benito-León J. (2001) Clusterlike headache as first manifestation of a prolactinoma. Headache 41: 723–725 [DOI] [PubMed] [Google Scholar]
- Rigamonti A., Iurlaro S., Reganati P., Zilioli A., Agostoni E. (2008) Cluster headache and internal carotid artery dissection: two cases and review of the literature. Headache 48: 467–470 [DOI] [PubMed] [Google Scholar]
- Van Gijn J., Kerr R., Rinkel G. (2007) Subarachnoid haemorrhage. Lancet 369: 306–318 [DOI] [PubMed] [Google Scholar]
- Walker R. (2001). Idiopathic intracranial hypertension: any light on the mechanism of the raised pressure? J Neurol Neurosurg Psychiatry 71: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]