Abstract
Cytoskeletal dysfunction has been proposed during the last decade as one of the main mechanisms involved in the aetiology of several neurodegenerative diseases. Microtubules are basic elements of the cytoskeleton and the dysregulation of microtubule stability has been demonstrated to be causative for axonal transport impairment, synaptic contact degeneration, impaired neuronal function leading finally to neuronal loss. Several pathways are implicated in the microtubule assembly/disassembly process. Emerging evidence is focusing on Notch as a microtubule dynamics regulator. We demonstrated that activation of Notch signalling results in increased microtubule stability and changes in axonal morphology and branching. By contrast, Notch inhibition leads to an increase in cytoskeleton plasticity with intense neurite remodelling. Until now, several microtubule-binding compounds have been tested and the results have provided proof of concept that microtubule-binding agents or compounds with the ability to stabilize microtubules may have therapeutic potential for the treatment of Alzheimer’s disease and other neurodegenerative diseases. In this review, based on its key role in cytoskeletal dynamics modulation, we propose Notch as a new potential target for microtubule stabilization.
Keywords: cytoskeleton, microtubule stabilization, neurodegeneration, Notch, Spastin, structural plasticity
The Cytoskeleton and diseases
Cytoskeletal dysfunction has been proposed during the last decade as one of the main mechanisms involved in the aetiology of several neurodegenerative diseases [Bunker et al. 2004; Cairns et al. 2004; Cartelli et al. 2010].
Microtubules (MTs) are basic elements of the cytoskeleton composed of α- and β-tubulin heterodimers; the dysregulation of MT stability has been demonstrated to be causative for axonal transport impairment, synaptic contact degeneration, impaired neuronal function leading finally to neuronal loss. Several neurodegenerative diseases have been linked to impaired MT dynamics [Cappelletti et al. 2005] and axonal transport [Trojanowski et al. 2005; Roy et al. 2005]: hereditary spastic paraplegia [Rainier et al. 1998; Errico et al. 2002; Reid et al. 2002], familial motor neuron disease [LaMonte et al. 2002; Jablonka et al. 2004], Charcot-Marie-tooth disease type2A [Zhao et al. 2001; Tanabe and Takei, 2009], Huntington’s disease [Trushina et al. 2003], familial amyotropic lateral sclerosis [Julien et al. 2005; Mórotz et al. 2012], Parkinson’s disease and related synucleinopathies [Wersinger and Sidhu, 2005; Cartelli et al. 2012], Alzheimer’s disease [Terry, 1998; Kanaan et al. 2012], progressive supranuclear palsy [Morfini et al. 2002], frontotemporal dementias [Ittner et al. 2008] and related tauopathies. All of these pathologies are triggered by different events that finally converge on MT disruption/destabilization.
Neuron cytoskeleton has to be maintained in a condition of ‘dynamics balance’ between stabilization/destabilization to achieve the right degree of wellbeing. Indeed, equilibrium loss is linked to pathologic conditions. For example, in hereditary spastic paraplegia MTs are hyperstabilized, cytoskeletal structure is too rigid and neurons lack plasticity, essential for neurite branching and new connections formation [Fassier et al. 2013; Tarrade et al. 2006]; by contrast, in Alzheimer’s disease, MTs are too destabilized, axonal trafficking is impaired and synaptic contacts collapse [Zhang et al. 2012].
MTs extend in all directions throughout the cell, forming a dynamic network that continuously grows, retracts, bends and breaks. Therefore, rather than providing cellular rigidity, they are important for enabling dynamic processes such as intracellular transport or mitotic spindle formation that heavily depend on their ability to be polymerized, depolymerized and severed. The tight regulation of their dynamics is pivotal to ensure efficient transport of cargoes along the axons. With a severely destabilized MT network and a disturbed axonal transport system, neurons are not able to function properly and consequently degenerate [Almeida-Souza et al. 2011]. Loss of normal regulation of MT dynamics could have deleterious effects on cell viability; they can be considered as ‘biosensors’ of cellular wellbeing [Bunker et al. 2004].
Despite the diverse etiopathogenesis, the different neurodegenerative pathologies are linked to progressive accumulation of abnormal filamentous proteins; and this, together with the absence of other disease-specific neuropathological abnormalities, provides evidence implicating neuronal filaments and MTs in disease onset and progression [Cairns et al. 2004].
Microtubule-stabilizing compounds
Until now, several MT-binding compounds have been tested and the studies carried out have provided proof of concept that MT-binding agents or compounds with the ability to stabilize MTs may have therapeutic potential for the treatment of Alzheimer’s disease and other neurodegenerative diseases [Michaelis et al. 2002, 2005; Silva et al. 2011; Nelson, 2005; Butler et al. 2007].
Paclitaxel, a complex diterpene obtained from the Pacific yew (Taxus brevifolia), was the first natural product shown to stabilize MTs [Schiff et al. 1979]. It is able to prevent the disassembly of MTs and to promote their assembly. Paclitaxel (or Taxol, Bristol-Myers Squibb Company, New York City, USA) was originally studied and finally approved by the US Food and Drug Administration for clinical use in 1992 as a chemotherapeutic agent due to its ability to stabilize MTs in the mitotic spindle and arrest mitosis in cancer fast proliferating cells. In addition, it was demonstrated to have a beneficial role in an animal model of multiple sclerosis. Treatment with paclitaxel resulted in amelioration of clinical disease, reduction of gliosis and even remyelination [Moscarello et al. 2002].
It was subsequently studied in order to stabilize MTs of neuronal cells and to prevent axonal collapse and degeneration. To act as a neuroprotective compound, paclitaxel was used at doses far lower than those used in chemotherapy. It showed protective properties when used on neurons in vitro challenged with amyloid β [Michaelis et al. 2005] and it was able to enhance neurite outgrowth both in vitro and in vivo [Sengottuvel et al. 2011]. Paclitaxel also reduced deficits induced by mutant-tau transfection in cultured neurons [Shemesh and Spira, 2011], improved axonal transport rate, MTs number and motor function in a spinal cord tauopathy model [Zhang et al. 2005]. Unfortunately, in vivo studies demonstrated that it has a very poor entry in to the brain (less than 1% of the injected dose) [Moscarello et al. 2002], so the hypothesis of using paclitaxel for neuroprotection conflicts with the risk of peripheral accumulation and toxic side effects (neutropenia, unusual bruising or bleeding, gastrointestinal disease, fever, difficulty swallowing, ovarian damage and much more).
The same MT-stabilizing property emerged to be shared by other structurally complex natural products derived from microorganisms, plants and sponges. Among these, epothilone D (Epo D) displays interesting properties. Epo D derives from myxobacteria [Goodin et al. 2004] and besides being an efficacious MT-stabilizing agent, it is brain penetrant and has a good pharmacokinetics [Kolman, 2004; Andrieux et al. 2006; Brunden et al. 2010, 2012].
Another promising compound is NAP (generic name davunetide) (Allon Therapeutics Inc., Vancouver, BC, Canada), an MT protective agent [Gozes, 2011]. Gozes and colleagues tested NAP in several disease models and they found it to be a potent neuroprotective, memory-enhancing, neurotrophic agent and capable of inhibiting the aggregation of β-amyloid [Gozes et al. 2002; Ashur-Fabian et al. 2003; Matsuoka et al. 2007]. Surprisingly, even in human clinical trials, it was shown to increase memory scores in patients with amnestic mild cognitive impairment, a precursor of Alzheimer’s disease [Gozes et al. 2009] and to enhance functional daily behaviours in patients with schizophrenia [Javitt et al. 2012]. Furthermore, it has a good pharmacokinetics and can also be administered intranasally [Gozes et al. 2000, 2009].
Microtubule dynamics regulation
MT dynamics is spatially and temporally regulated by several pathways and MT-interacting proteins (see Table 1): tau, a MT-associated protein (MAP) that stabilizes MTs [Maccioni and Cambiazo, 1995], and katanin, the MT-severing enzyme [Roll-Mecak and McNally, 2010], both interact with the MT lattice; PAR-1 (also known as MARK) phosphorylates classical MAPs and detaches MAPs from MTs [Matenia and Mandelkow, 2009]; +TIPs, the MT plus-end-tracking proteins, specifically control the dynamic properties of the MT end [Gouveia and Akhmanova, 2010; Schuyler and Pellman, 2001]; ROCK pathway regulates MT dynamics via phosphorylation of the tubulin polymerization promoting protein 1 (TPPP1/p25) [Schofield et al. 2012]; (aPKC)-Aurora A-NDEL1 pathway is crucial for the regulation of MT organization during neurite extension [Mori et al. 2009]; Dishevelled (Dvl) pathway and the cooperation of Wnt-Dvl pathways increase MT stability though Gsk3β inhibition and c-Jun N-terminal kinase (JNK) activation [Ciani and Salinas, 2007]. These are only some of the known regulatory mechanisms that contribute to orchestrate MT dynamic remodelling.
Table 1.
Microtubule-interacting proteins.
| Protein | Function | References |
|---|---|---|
| Tau | Stabilizes MT; interacts with MT lattice | Maccioni and Cambiazo [1995] |
| Katanin | MT-severing enzyme; interacts with MT lattice | Roll-Mecak and McNally [2010] |
| PAR-1 (or MARK) | Phosphorylates and detaches MAP from MT | Matenia and Mandelkow [2009] |
| +TIPs (microtubule plus-end-tracking proteins) | Controls the dynamic properties of MT end | Gouveia and Akhmanova [2010]; Schuyler and Pellman [2001] |
| ROCK | Phosphorylates TPPP1/p25 and regulates MT dynamics | Schofield et al. [2012] |
| (aPKC)-Aurora A-NDEL1 | Organizes MT during neurite extension | Mori et al. [2009] |
| Dishevelled | Increases MT stability | Ciani and Salinas [2007] |
| Wnt | Increases MT stability | Ciani and Salinas [2007] |
| Spastin | MT-severing protein | Wood et al. [2006]; Trotta et al. [2004]; Sherwood et al. [2004] |
MAP, MT-associated protein; MT, microtubule; TPPP1, tubulin polymerization promoting protein 1.
Notch pathway
Notch pathway is recently emerged as a possible MT stability regulator. Notch is a heterodimer transmembrane receptor that, after binding with its ligands expressed on adjacent cells, goes through several proteolitic cleavages. The cytoplasmic cleavage by γ-secretase complex originates the active Notch intracellular domain; it translocates to the nucleus and triggers a transcriptional effects cascade. The best characterized target genes of Notch are the transcriptional repressors bHLH (basic helix loop helix) genes [the enhancer of split E(spl) complex in Drosophilia melanogaster and the hairy and enhancer of split (HES) and HES related (HESR/HEY) family genes in vertebrates].
In the past, Notch was considered a developmental protein that played a key role in cell fate decisions in uncommitted proliferative cells and in neurogenesis [Artavanis-Tsakonas and Simpson, 1991; Brennan et al. 1997; Go et al. 1998; Greenwald and Rubin, 1992; Hoppe and Greenspan, 1990]. In this context, Notch pathway activation results in inhibition of cellular differentiation and maintenance of a proliferative cellular pool [Louvi and Artavanis-Tsakonas, 2006]. For this reason, it started to be studied as a potential pharmacological target for several cancers [Nickoloff et al. 2003; Miele et al. 2006; Santos et al. 2006; Kunnimalaiyaan and Chen, 2007; Purow, 2012]. Finally, several Notch-targeting drugs (both inhibiting and activating, depending on the type of tumour) have been successfully chosen and tested in clinical trials for cancer therapies [Greenblatt et al. 2007; Fouladi et al. 2011; Sharma et al. 2012; Pinchot et al. 2011].
Notch role in postmitotic neurons
The initial suggestion that Notch signalling might have a role in postmitotic neurons came from the clear detection of Notch1 immunoreactivity in the nuclei of terminally differentiated postmitotic neurons in the rat retina [Ahmad et al. 1995]. A few years later, it was demonstrated that Notch1, apart from being highly expressed in embryonal mouse and human brain, continued to be expressed, although at lower levels, in the adult brain [Berezovska et al. 1998]. Furthermore, the cytoplasmic domain of endogenous Notch1 translocated to the nucleus during neuronal differentiation [Redmond et al. 2000].
Therefore, Notch activation could also occur in postmitotic or quiescent cells in the absence of division. A new role for Notch emerged in regulating developmental events subsequent to specification of cell fate. It was demonstrated that Notch is present on the growth cones of developing axons in Drosophila and it is required for axon guidance in both the central and peripheral nervous system [Giniger et al. 1993; Menne and Klämbt, 1994]. An involvement of Notch, as well as Reelin and Wnt, known to be active participants in neuronal maturation, was also suggested in neurodegenerative events underlying Alzheimer’s disease [Grilli et al. 2003; Lathia et al. 2008; Woo et al. 2009]. Notch expression resulted, markedly induced by excitotoxic stimuli in hippocampal pyramidal neurons [Ferrari-Toninelli et al. 2003]; this event resembles what happens at the onset of neurodegeneration in the adult brain, probably as an attempt to compensate neuronal loss by promoting neuronal growth. However, in aged brains, Notch1 signalling is reduced [Tanveer et al. 2012] and a chronic decrease in Notch1 function results in learning and memory deficits [Costa et al. 2003].
Research into Notch function in fully differentiated cells and in the adult brain was initially hampered because of the embryonic lethality of Notch knockout mice [Yoon and Gaiano, 2005]. Currently, with the development of Cre/loxP and viral gene transduction technologies, it is possible to manipulate Notch expression in mature animals, thus circumventing its developmental requirement [Han et al. 2002; Johnson et al. 2009; Ehm et al. 2010].
The first studies on the role of Notch in postmitotic neurons were carried out by using Notch antagonists or ligands to modulate the Notch signal in primary neuronal cultures. Notably, several ex vivo studies in different species clearly showed that modulation of the signal had a significant influence on neuronal morphology by affecting the extension of existing neurites (that is, axons and dendrites) [Sestan et al. 1999; Redmond et al. 2000; Berezovska et al. 1999; Qi et al. 1999].
Therefore, Notch plays a role in determining the only possible ‘cell fate’ decisions in postmitotic mature neurons: synaptic remodelling or neurite extension/retraction.
Notch as a microtubule stabilizer
The mechanism through which Notch can act on neurite morphology regulation is still a matter of debate. A proposal that has yet to be fully explored is that Notch is capable of influencing neuronal cytoskeleton [Giniger, 1998; Major and Irvine, 2005]. It has been suggested that the role of the Notch pathway in maintaining neuronal arborization in the adult is linked to its capability to modulate cytoskeleton plasticity [Louvi and Artavanis-Tsakonas, 2006].
We investigated the possible influence of the Notch pathway on MT stability and actually confirmed this hypothesis [Ferrari-Toninelli et al. 2008]. We demonstrated that activation of the Notch pathway in primary cortical neurons resulted in reduction of neurite branches and loss of varicosities. Varicosities appear as membrane swellings of various size and are regarded as presynaptic, dynamic structures that are able to remodel their morphology in response to a variety of stimuli [De Paola et al. 2003; Nikonenko et al. 2003; Udo et al. 2005; Umeda et al. 2005; Ferrari-Toninelli et al. 2009].
The changes in neurite morphology induced by Jagged1, a Notch ligand, were comparable to those induced by a low concentration of the MT-stabilizing drug paclitaxel. Evidence of increased MT stabilization, suggesting that the Notch pathway could also act through cytoskeletal modifications, was provided by the analysis of post-translational modification of tubulin. These modifications, which include detyrosination/tyrosination, polyglutamilation, polyglycilation, palmytoilation, phosphorylation and acetylation [Verhey and Gaertig, 2007], may occur individually or in combination. In this way, tubulin post-translational modifications play an important role in regulating MTs properties, such as stability and structure [Hammond et al. 2008]. Following Notch pathway activation, we found an upregulation of two post-translationally modified tubulins, the acetylated α tubulin and the polyglutamylated tubulin, commonly used as markers of stability [Hammond et al. 2008].
Notch downstream effectors
Several studies imply that Notch signals may modulate the cytoskeleton through local signal transduction pathways that do not require activation of nuclear gene expression. Different players have been identified to act together with or downstream of Notch. For example, Giniger showed that Notch interacts synergistically with the Abl tyrosine kinase to regulate the pathfinding of specific axons in Drosophila [Giniger, 1998]. Both Notch and Abl are present in the axon and the binding of Notch to Disabled (Dab), a protein that interacts with Abl, may explain how Notch communicates with Abl. Sanpodo is another possible mediator of neurite development regulation by Notch through its cytoskeletal interactions [Skeath and Doe, 1998; Dye et al. 1998].
The hypothesis of Notch-mediated neurite regulation by means of local modulators was interesting, especially considering activated Notch fragments that travel potentially great distances from growth cones to the nucleus. We explored this possibility, but our studies demonstrated that transcription and translation processes are necessary for the Notch-mediated morphological effect [Ferrari-Toninelli et al. 2009]. Alternatively, nuclear and local effects of Notch signalling may be integrated to regulate neurite development.
One of the links between Notch and a morphology-regulation-related protein was found by Hassan and colleagues: they reported a link with atonal, a proneural gene in the Drosophila nervous system. Characterization of atonal mutants indicated that in the brain atonal did not act as a proneural gene, but it was required for the proper axonal arborization of a subpopulation of neurons that innervate the optic lobe. Overexpression studies indicated that atonal and notch acted antagonistically in this population of neurons, with Atonal increasing axonal arborization and Notch decreasing it [Hassan et al. 2000].
Another possible player identified is neurogenin 3 (NGN3): Notch activation leads to expression of HES genes that inhibit NGN3 expression and finally reduces neurite outgrowth in the hippocampus. Therefore NGN3 acts to promote neurite outgrowth [Simon-Areces et al. 2010; Salama-Cohen et al. 2006].
In neocortical cells and in sensory neurons, Numb and numb-like (Numbl) are able to regulate axonal arborization acting as Notch antagonists [Huang et al. 2005].
We also identified a novel mechanism through which Notch is able to modulate neuronal cytoskeleton plasticity: by acting on the MT-severing protein Spastin. Stimulation of the Notch pathway by Jagged1 inhibited both the transcription and the expression levels of Spastin and induced MT stabilization and changes in axonal morphology [Ferrari-Toninelli et al. 2008]. Spastin gene mutation has been associated with axonal degeneration, leading to hereditary spastic paraplegia [Errico et al. 2002]. Further studies showed that Spastin is a MT-severing protein and its MT-destabilizing properties are fundamental for axon outgrowth and synaptic modulation in long motor neurons [Wood et al. 2006; Trotta et al. 2004; Sherwood et al. 2004]; interestingly, Yu and colleagues showed that in cultured neurons Spastin is more concentrated at the sites of branches formation and that protein downregulation resulted in neurite morphology changes, with a dramatic reduction of axonal branches [Yu et al. 2008]. Furthermore, it has been demonstrated that Spastin protein downregulation led to increased levels of acetylated and polyglutamylated tubulin, whereas Spastin overexpression caused a reduction of these post-translationally modified proteins [Trotta et al. 2004].
Therefore, we first established a link between the Notch signalling pathway and MT stabilization in postmitotic neurons, suggesting a novel endogenous pathway involved in modulating MT plasticity.
Notch microtubule-stabilizing effect is reversible
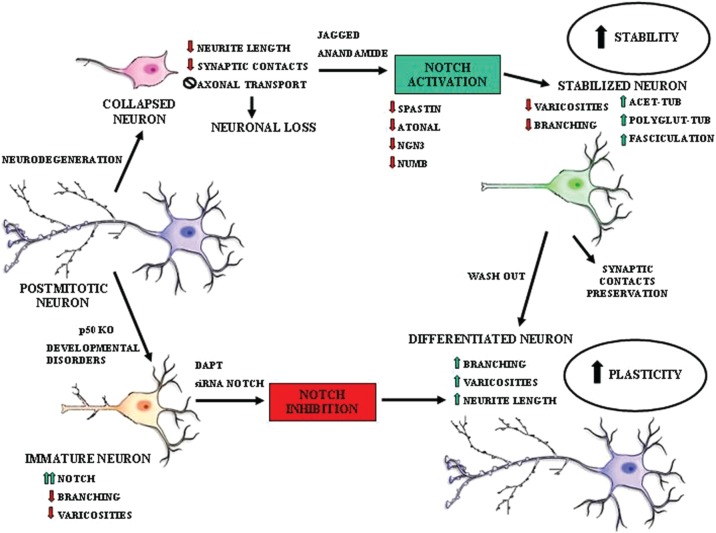
We found that Notch pathway activation acts as a MT stabilizer, and interestingly we demonstrated that this is a dynamic event that can be reversed. As evaluated by time lapse digital imaging, dynamic changes in cell morphology were rapidly reversible and dependent on the activation of the Notch signalling pathway [Ferrari-Toninelli et al. 2009]. Indeed, if Notch pathway activation causes MT stabilization followed by both neurite branching reduction and loss of varicosity, Notch pathway inhibition reverts such morphological effects and increases cytoskeletal plasticity. In particular, we observed morphological alterations (reduced neurite branching and loss of varicosities) in cortical neurons from transgenic mice characterized by Notch1 signalling hyperactivation (mice lacking the nuclear factor κB p50 subunit). The neuronal morphological effects found in p50–/– cortical cells were reversed after treatment with the γ-secretase inhibitor DAPT or Notch RNA interference [Bonini et al. 2011]. This means that, modulating the Notch pathway activation state, it is possible to act on MT dynamics hence cytoskeletal plasticity increasing or decreasing them depending on the necessity (see Figure 1).
Figure 1.
Notch pathway effects on cytoskeletal plasticity.
Conclusion
In light of the growing evidence that MT dynamic balance maintenance could be beneficial in several neurodegenerative pathologies and even prevent them, we propose Notch pathway as a new possible pharmacological target for cytoskeletal protection.
Thinking about Notch as a possible target to protect the cytoskeleton presents several advantages. Notch can be modulated with activating or inhibiting compounds depending on the context and the effect required: Notch signalling activation results in increased MT stability and changes in axonal morphology and branching; Notch signalling inhibition leads to an increase in cytoskeleton plasticity with intense neurite remodelling. Some Notch-modulating compounds (such as the γ-secretase inhibitors) are already used in clinical trials for Alzheimer’s disease as β-amyloid reducing agents; it is well known that the Notch pathway and APP (Amyloid Precursor Protein) metabolism converge on γ-secretase proteolitic enzyme and that γ-secretase-inhibiting compounds, other than decreasing β-amyloid formation, also act on Notch pathway activation. Furthermore, it has recently been demonstrated that endogenous modulators of Notch signalling, as the endocannabinoid anandamide, can promote a shift in γ-secretase substrate processing to favour processing of Notch1 over APP, and this can confer neuroprotection [Tanveer et al. 2012].
Unfortunately, Notch may also present disadvantages: it is ubiquitously expressed, so the main challenge will be to find a compound able to pass the blood–brain barrier avoiding peripheral Notch side effects.
We suggest that a fine-tuned manipulation of Notch signalling may represent a novel approach to modulate neuronal cytoskeleton plasticity in order to guarantee neurons both the stability required to maintain their axonal architecture and the structural plasticity necessary to create new synaptic connections.
Finally, considering the relevant role of Notch in structural plasticity regulation and the importance of acting on MTs to protect neurons, we propose Notch as a new potential target for MT stabilization.
Footnotes
Funding: This work was supported by the Italian Ministry of Education, University and Research (Progetto di Ricerca di Rilevante Interesse Nazionale, PRIN 2009); and Fondo NEDD (Network-Enabled Drug Design), Accordi Istituzionali Regione Lombardia.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Sara Anna Bonini, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy.
Giulia Ferrari-Toninelli, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy.
Mery Montinaro, Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy.
Maurizio Memo, Department of Molecular and Translational Medicine, University of Brescia, Viale Europa 11, Brescia 25123, Italy.
References
- Ahmad I., Zaqouras P., Artavanis-Tsakonas S. (1995) Involvement of Notch-1 in mammalian retinal neurogenesis: association of Notch-1 activity with both immature and terminally differentiated cells. Mech Dev 53:73–85 [DOI] [PubMed] [Google Scholar]
- Almeida-Souza L., Timmerman V., Janssens S.(2011) Microtubule dynamics in the peripheral nervous system: a matter of balance. Bioarchitecture 1: 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieux A., Salin P., Schweitzer A., Bégou M., Pachoud B., Brun P., et al. (2006) Microtubule stabilizer ameliorates synaptic function and behavior in a mouse model for schizophrenia. Biol Psychiatry 60: 1224–1230 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Simpson P. (1991) Choosing a cell fate: a view from the Notch locus. Trends Genet 7: 403–408 [DOI] [PubMed] [Google Scholar]
- Ashur-Fabian O., Segal-Ruder Y., Skutelsky E., Brenneman D., Steingart R., Giladi E., et al. (2003) The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides 24: 1413–1423 [DOI] [PubMed] [Google Scholar]
- Berezovska O., McLean P., Knowles R., Frosh M., Lu F., Lux S., et al. (1999) Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience 93: 433–439 [DOI] [PubMed] [Google Scholar]
- Berezovska O., Xia M., Hyman B. (1998) Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol 57: 738–745 [DOI] [PubMed] [Google Scholar]
- Bonini S., Ferrari-Toninelli G., Uberti D., Montinaro M., Buizza L., Lanni C., et al. (2011) Nuclear factor κB-dependent neurite remodeling is mediated by Notch pathway. J Neurosci 31: 11697–11705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K., Tateson R., Lewis K., Arias A. (1997) A functional analysis of Notch mutations in Drosophila. Genetics 147: 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K., Ballatore C., Lee V., Smith A., 3rd, Trojanowski J. (2012) Brain-penetrant microtubule-stabilizing compounds as potential therapeutic agents for tauopathies. Biochem Soc Trans 40: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K., Zhang B., Carroll J., Yao Y., Potuzak J., Hogan A., et al. (2010) Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci 30: 13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker J., Wilson L., Jordan M., Feinstein S. (2004) Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration. Mol Biol Cell 15: 2720–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D., Bendiske J., Michaelis M., Karanian D., Bahr B. (2007) Microtubule-stabilizing agent prevents protein accumulation-induced loss of synaptic markers. Eur J Pharmacol 562: 20–27 [DOI] [PubMed] [Google Scholar]
- Cairns N., Lee V., Trojanowski J. (2004) The cytoskeleton in neurodegenerative diseases. J Pathol 204: 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti G., Surrey T., Maci R. (2005) The parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as a destabilising factor. FEBS Lett 579: 4781–4786 [DOI] [PubMed] [Google Scholar]
- Cartelli D., Goldwurm S., Casagrande F., Pezzoli G., Cappelletti G. (2012) Microtubule destabilization is shared by genetic and idiopathic Parkinson’s disease patient fibroblasts. PLoS One 7: e37467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartelli D., Ronchi C., Maggioni M., Rodighiero S., Giavini E., Cappelletti G. (2010) Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+ -induced neurodegeneration. J Neurochem 115: 247–258 [DOI] [PubMed] [Google Scholar]
- Ciani L., Salinas P. (2007) c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability. BMC Cell Biol 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R., Honjo T., Silva A. (2003) Learning and memory deficits in Notch mutant mice. Curr Biol 13: 1348–1354 [DOI] [PubMed] [Google Scholar]
- De Paola V., Arber S., Caroni P. (2003) AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci 6: 491–500 [DOI] [PubMed] [Google Scholar]
- Dye C., Lee J., Atkinson R., Brewster R., Han P., Bellen H. (1998) The Drosophila sanpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development 125: 1845–1856 [DOI] [PubMed] [Google Scholar]
- Ehm O., Göritz C., Covic M., Schäffner I., Schwarz T., Karaca E., et al. (2010) RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30: 13794–13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A., Ballabio A., Rugarli E. (2002) Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum Mol Genet 11: 153–163 [DOI] [PubMed] [Google Scholar]
- Fassier C., Tarrade A., Peris L., Courageot S., Mailly P., Dalard C., et al. (2013) Microtubule- targeting drugs rescue axonal swellings in cortical neurons from Spastin knockout mice. Dis Model Mech 6: 72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari-Toninelli G., Bernardi C., Quarto M., Lozza G., Memo M., Grilli M. (2003) Long-lasting induction of Notch2 in the hippocampus of kainate-treated adult mice. Neuroreport 14: 917–921 [DOI] [PubMed] [Google Scholar]
- Ferrari-Toninelli G., Bonini S., Bettinsoli P., Uberti D., Memo M. (2008) Microtubule stabilizing effect of notch activation in primary cortical neurons. Neuroscience 154: 946–952 [DOI] [PubMed] [Google Scholar]
- Ferrari-Toninelli G., Bonini S., Uberti D., Napolitano F., Stante M., Santoro F., et al. (2009) Notch activation induces neurite remodeling and functional modifications in SH-SY5Y neuronal cells. Dev Neurobiol 69: 378–391 [DOI] [PubMed] [Google Scholar]
- Fouladi M., Stewart C., Olson J., Wagner L., Onar-Thomas A., Kocak M., et al. (2011) Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol 29: 3529–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E. (1998) A role for Abl in Notch signaling. Neuron 20: 667–681 [DOI] [PubMed] [Google Scholar]
- Giniger E., Jan L., Jan Y. (1993) Specifying the path of the intersegmental nerve of the Drosophila embryo: a role for Delta and Notch. Development 117: 431–440 [DOI] [PubMed] [Google Scholar]
- Go M., Eastman D., Artavanis-Tsakonas S. (1998) Cell proliferation control by Notch signaling in Drosophila development. Development 125: 2031–2040 [DOI] [PubMed] [Google Scholar]
- Goodin S., Kane M., Rubin E. (2004) Epothilones: mechanism of action and biologic activity. J Clin Oncol 22: 2015–2025 [DOI] [PubMed] [Google Scholar]
- Gouveia S., Akhmanova A. (2010) Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol 285: 1–74 [DOI] [PubMed] [Google Scholar]
- Gozes I. (2011) Microtubules (tau) as an emerging therapeutic target: NAP (davunetide). Curr Pharm Des 17: 3413–3417 [DOI] [PubMed] [Google Scholar]
- Gozes I., Alcalay R., Giladi E., Pinhasov A., Furman S., Brenneman D. (2002) NAP accelerates the performance of normal rats in the water maze. J Mol Neurosci 19(1-2): 167-170 [DOI] [PubMed] [Google Scholar]
- Gozes I., Giladi E., Pinhasov A., Bardea A., Brenneman D. (2000) Activity-dependent neurotrophic factor: intranasal administration of femtomolar-acting peptides improve performance in a water maze. J Pharmacol Exp Ther 293: 1091–1098 [PubMed] [Google Scholar]
- Gozes I., Stewart A., Morimoto B., Fox A., Sutherland K., Schmeche D. (2009) Addressing Alzheimer’s disease tangles: from NAP to AL-108. Curr Alzheimer Res 6: 455–460 [DOI] [PubMed] [Google Scholar]
- Greenblatt D., Vaccaro A., Jaskula-Sztul R., Ning L., Haymart M., Kunnimalaiyaan M., et al. (2007) Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 12: 942–951 [DOI] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. (1992) Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell 68: 271–281 [DOI] [PubMed] [Google Scholar]
- Grilli M., Ferrari-Toninelli G., Uberti D., Spano P., Memo M. (2003) Alzheimer’s disease linking neurodegeneration with neurodevelopment. Funct Neurol 18: 145–148 [PubMed] [Google Scholar]
- Hammond J., Cai D., Verhey K. (2008) Tubulin modifications and their cellular functions. Curr Opin Cell Biol 20: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Tanigaki K., Yamamoto N., Kuroda K., Yoshimoto M., Nakahata T., et al. (2002) Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol 14: 637–645 [DOI] [PubMed] [Google Scholar]
- Hassan B., Bermingham N., He Y., Sun Y., Jan Y., Zoghbi H., et al. (2000) Atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron 25: 549–561 [DOI] [PubMed] [Google Scholar]
- Hoppe P., Greenspan R. (1990) The Notch locus of Drosophila is required in epidermal cells for epidermal development. Development 109: 875–885 [DOI] [PubMed] [Google Scholar]
- Huang E., Li H., Tang A., Wiggins A., Neve R., Zhong W., et al. (2005) Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization. Genes Dev 19: 138–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner L., Fath T., Ke Y., Bi M., van Eersel J., Li K., et al. (2008) Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc Natl Acad Sci U S A 105: 15997–16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka S., Wiese S., Sendtner M. (2004) Axonal defects in mouse models of motoneuron disease. J Neurobiol 58: 272–286 [DOI] [PubMed] [Google Scholar]
- Javitt D., Buchanan R., Keefe R., Kern R., McMahon R., Green M., et al. (2012) Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res 136: 25–31 [DOI] [PubMed] [Google Scholar]
- Johnson M., Ables J., Eisch A. (2009) Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep 42: 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J., Millecamps S., Kriz J. (2005) Cytoskeletal defects in amyotrophic lateral sclerosis (motor neuron disease). Novartis Found Symp 264: 183–192; discussion 192–196, 227–230. [PubMed] [Google Scholar]
- Kanaan N., Pigino G., Brady S., Lazarov O., Binder L., Morfini G. (2012) Axonal degeneration in Alzheimer’s disease: when signaling abnormalities meet the axonal transport system. Exp Neurol 19 June (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman A. (2004) Epothilone D (Kosan/Roche). Curr Opin Investig Drugs 5: 657–667 [PubMed] [Google Scholar]
- Kunnimalaiyaan M., Chen H. (2007) Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 12: 535–542 [DOI] [PubMed] [Google Scholar]
- LaMonte B., Wallace K., Holloway B., Shelly S., Ascaño J., Tokito M., et al. (2002) Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 34: 715–727 [DOI] [PubMed] [Google Scholar]
- Lathia J., Mattson M., Cheng A. (2008) Notch: from neural development to neurological disorders. J Neurochem 107: 1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker R., Teichner A., Grigoriadis N., Ovadia H., Brenneman D., Fridkin M., et al. (2002) NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke 33: 1085–1092 [DOI] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2006) Notch signalling in vertebrate neural development. Nat Rev Neurosci 7: 93–102 [DOI] [PubMed] [Google Scholar]
- Maccioni R., Cambiazo V. (1995) Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev 75: 835–864 [DOI] [PubMed] [Google Scholar]
- Major R., Irvine K. (2005) Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development 132: 3823–3833 [DOI] [PubMed] [Google Scholar]
- Matenia D., Mandelkow E. (2009) The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci 34: 332–342 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Gray A., Hirata-Fukae C., Minami S., Waterhouse E., Mattson M., et al. (2007) Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer’s disease at early pathological stage. J Mol Neurosci 31: 165–170 [DOI] [PubMed] [Google Scholar]
- Menne T., Klämbt C. (1994) The formation of commissures in the Drosophila CNS depends on the midline cells and on the Notch gene. Development 120: 123–133 [DOI] [PubMed] [Google Scholar]
- Michaelis M., Ansar S., Chen Y., Reiff E., Seyb K., Himes R., et al. (2005) {beta}-Amyloid-induced neurodegeneration and protection by structurally diverse microtubule-stabilizing agents. J Pharmacol Exp Ther 312: 659–668 [DOI] [PubMed] [Google Scholar]
- Michaelis M., Chen Y., Hill S., Reiff E., Georg G., Rice A., et al. (2002) Amyloid peptide toxicity and microtubule-stabilizing drugs. J Mol Neurosci 19: 101–105 [DOI] [PubMed] [Google Scholar]
- Michaelis M., Seyb K., Ansar S. (2005) Cytoskeletal integrity as a drug target. Curr Alzheimer Res 2: 227–229 [DOI] [PubMed] [Google Scholar]
- Miele L., Miao H., Nickoloff B. (2006) NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets 6: 313–323 [DOI] [PubMed] [Google Scholar]
- Morfini G., Pigino G., Beffert U., Busciglio J., Brady S. (2002) Fast axonal transport misregulation and Alzheimer’s disease. Neuromolecular Med 2: 89–99 [DOI] [PubMed] [Google Scholar]
- Mori D., Yamada M., Mimori-Kiyosue Y., Shirai Y., Suzuki A., Ohno S., et al. (2009) An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol 11: 1057–1068 [DOI] [PubMed] [Google Scholar]
- Mórotz G., De Vos K., Vagnoni A., Ackerley S., Shaw C., Miller C. (2012) Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum Mol Genet 21: 1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello M., Mak B., Nguyen T., Wood D., Mastronardi F., Ludwin S. (2002) Paclitaxel (Taxol) attenuates clinical disease in a spontaneously demyelinating transgenic mouse and induces remyelination. Mult Scler 8: 130–138 [DOI] [PubMed] [Google Scholar]
- Nelson R. (2005) Microtubule-stabilising drugs may be therapeutic in AD. Lancet Neurol 4: 83–84 [DOI] [PubMed] [Google Scholar]
- Nickoloff B., Osborne B., Miele L. (2003) Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 22: 6598–6608 [DOI] [PubMed] [Google Scholar]
- Nikonenko I., Jourdain P., Muller D. (2003) Presynaptic remodeling contributes to activity-dependent synaptogenesis. J Neurosci 23: 8498–8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchot S., Jaskula-Sztul R., Ning L., Peters N., Cook M., Kunnimalaiyaan M., et al. (2011) Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer 117: 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purow B. (2012) Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol 727: 305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Rand M., Wu X., Sestan N., Wang W., Rakic P., et al. (1999) Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science 283: 91–94 [DOI] [PubMed] [Google Scholar]
- Rainier S., Jones S., Esposito C., Guice E., Otterud B., Leppert M., et al. (1998) Analysis of microtubule-associated protein 1a gene in hereditary spastic paraplegia. Neurology 51: 1509–1510 [DOI] [PubMed] [Google Scholar]
- Redmond L., Oh S., Hicks C., Weinmaster G., Ghosh A. (2000) Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci 3: 30–40 [DOI] [PubMed] [Google Scholar]
- Reid E., Kloos M., Ashley-Koch A., Hughes L., Bevan S., Svenson I., et al. (2002) A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am J Hum Genet 71: 1189–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A., McNally F. (2010) Microtubule-severing enzymes. Curr Opin Cell Biol 22: 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Zhang B., Lee V., Trojanowski J. (2005) Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol 109: 5–13 [DOI] [PubMed] [Google Scholar]
- Salama-Cohen P., Arévalo M., Grantyn R., Rodríguez-Tébar A. (2006) Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J Neurochem 97: 1269–1278 [DOI] [PubMed] [Google Scholar]
- Santos L., León-Galván M., Marino-Marmolejo E. (2006) Notch signaling pathway and new strategies in cancer treatment. Salud Publica Mex 48: 155–165 [DOI] [PubMed] [Google Scholar]
- Schiff P., Fant J., Horwitz S. (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277: 665–667 [DOI] [PubMed] [Google Scholar]
- Schofield A., Steel R., Bernard O. (2012) ROCK controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J Biol Chem 287: 43620–43629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler S., Pellman D. (2001) Microtubule ‘plus-end-tracking proteins’: the end is just the beginning. Cell 105: 421–424 [DOI] [PubMed] [Google Scholar]
- Sengottuvel V., Leibinger M., Pfreimer M., Andreadaki A., Fischer D. (2011) Taxol facilitates axon regeneration in the mature CNS. J Neurosci 31: 2688–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestan N., Artavanis-Tsakonas S., Rakic P. (1999) Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 286: 741–746 [DOI] [PubMed] [Google Scholar]
- Sharma A., Paranjape A., Rangarajan A., Dighe R. (2012) A monoclonal antibody against human Notch1 ligand-binding domain depletes subpopulation of putative breast cancer stem-like cells. Mol Cancer Ther 11: 77–86 [DOI] [PubMed] [Google Scholar]
- Shemesh O., Spira M. (2011) Rescue of neurons from undergoing hallmark tau-induced Alzheimer’s disease cell pathologies by the antimitotic drug paclitaxel. Neurobiol Dis 43: 163–175 [DOI] [PubMed] [Google Scholar]
- Sherwood N., Sun Q., Xue M., Zhang B., Zinn K. (2004) Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol 2: e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D., Esteves A., Arduino D., Oliveira C., Cardoso S. (2011) Amyloid-β-induced mitochondrial dysfunction impairs the autophagic lysosomal pathway in a tubulin dependent pathway. J Alzheimers Dis 26: 565–581 [DOI] [PubMed] [Google Scholar]
- Simon-Areces J., Membrive G., Garcia-Fernandez C., Garcia-Segura L., Arevalo M. (2010) Neurogenin 3 cellular and subcellular localization in the developing and adult hippocampus. J Comp Neurol 518: 1814–1824 [DOI] [PubMed] [Google Scholar]
- Skeath J., Doe C. (1998) Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development 125: 1857–1865 [DOI] [PubMed] [Google Scholar]
- Tanabe K., Takei K. (2009) Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J Cell Biol 185: 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanveer R., Gowran A., Noonan J., Keating S., Bowie A., Campbell V. (2012) The endocannabinoid, anandamide, augments Notch-1 signaling in cultured cortical neurons exposed to amyloid-β and in the cortex of aged rats. J Biol Chem 287: 34709–34721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A., Fassier C., Courageot S., Charvin D., Vitte J., Peris L., et al. (2006) A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum Mol Genet 15: 3544–3558 [DOI] [PubMed] [Google Scholar]
- Terry R. (1998) The cytoskeleton in Alzheimer disease. J Neural Transm Suppl 53: 141–145 [DOI] [PubMed] [Google Scholar]
- Trojanowski J., Smith A., Huryn D., Lee V. (2005) Microtubule-stabilising drugs for therapy of Alzheimer’s disease and other neurodegenerative disorders with axonal transport impairments. Expert Opin Pharmacother 6: 683–686 [DOI] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M., Daga A., Broadie K.(2004) The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol 14: 1135–1147 [DOI] [PubMed] [Google Scholar]
- Trushina E., Heldebrant M., Perez-Terzic C., Bortolon R., Kovtun I., Badger J., 2nd, et al. (2003) Microtubule destabilization and nuclear entry are sequential steps leading to toxicity in Huntington’s disease. Proc Natl Acad Sci U S A 100: 12171–12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo H., Jin I., Kim J., Li H., Youn T., Hawkins R., et al. (2005) Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron 45: 887–901 [DOI] [PubMed] [Google Scholar]
- Umeda T., Ebihara T., Okabe S. (2005) Simultaneous observation of stably associated presynaptic varicosities and postsynaptic spines: morphological alterations of CA3-CA1 synapses in hippocampal slice cultures. Mol Cell Neurosci 28: 264–274 [DOI] [PubMed] [Google Scholar]
- Verhey K., Gaertig J. (2007) The tubulin code. Cell Cycle 6: 2152–2160 [DOI] [PubMed] [Google Scholar]
- Wersinger C., Sidhu A. (2005) Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry 44: 13612–13624 [DOI] [PubMed] [Google Scholar]
- Woo H., Park J., Gwon A., Arumugam T., Jo D. (2009) Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun 390: 1093–1097 [DOI] [PubMed] [Google Scholar]
- Wood J., Landers J., Bingley M., McDermott C., Thomas-McArthur V., Gleadall L., et al. (2006) The microtubule-severing protein Spastin is essential for axon outgrowth in the zebrafish embryo. Hum Mol Genet 15: 2763–2771 [DOI] [PubMed] [Google Scholar]
- Yenjerla M., LaPointe N., Lopus M., Cox C., Jordan M., Feinstein S., et al. (2010) The neuroprotective peptide NAP does not directly affect polymerization or dynamics of reconstituted neural microtubules. J Alzheimers Dis 19: 1377–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Gaiano N. (2005) Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 8: 1411. [DOI] [PubMed] [Google Scholar]
- Yu W., Qiang L., Solowska J., Karabay A., Korulu S., Baas P. (2008) The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell 19: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Carroll J., Trojanowski J., Yao Y., Iba M., Potuzak J., et al. (2012) The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 32: 3601–3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Maiti A., Shively S., Lakhani F., McDonald-Jones G., Bruce J., et al. (2005) Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci U S A 102: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., et al. (2001) Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell 105: 587–597 [DOI] [PubMed] [Google Scholar]