Abstract
The endothelium is often viewed solely as the barrier that prevents the penetration of circulating lipoproteins into the arterial wall. However, recent research has demonstrated that the endothelium both takes an important part in regulating circulating fatty acids and lipoproteins, and is in turn affected by these lipids/lipoproteins in ways that appear to have important repercussions for atherosclerosis. Thus, a number of potentially toxic lipids are produced during lipolysis of lipoproteins at the endothelial cell surface. Catabolism of triglyceride-rich lipoproteins creates free fatty acids that are readily taken up by endothelial cells, and, likely through the action of acyl-CoA synthetases, exacerbate inflammatory processes. In this article, we will review how endothelium participates in lipoprotein metabolism, how lipids alter endothelial functions, and how lipids are internalized, processed and transported into the subendothelial space. Finally, we will address the many endothelial changes that might promote atherogenesis, especially in the setting of diabetes.
Keywords: Acyl-CoA, Atherosclerosis, Diabetes, Endothelium, Fatty Acid, High-density lipoprotein, Lipoprotein lipase, Low-density lipoprotein, Metabolic Syndrome, Mouse models, Very low-density lipoprotein
Introduction
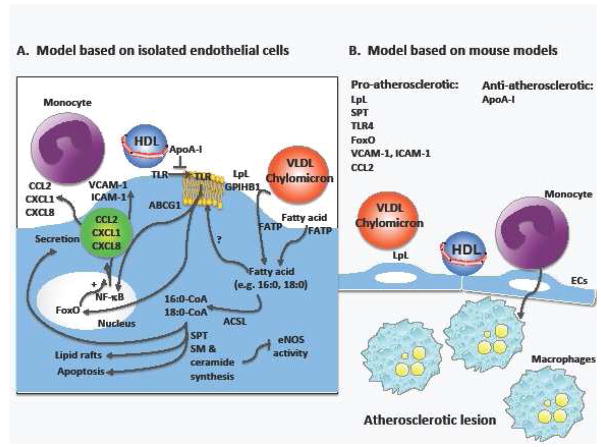
Cardiovascular (CVD) disease caused by atherosclerosis is the major cause of morbidity and mortality in subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [1]. It also is common in subjects with the metabolic syndrome [2]. Mouse models have demonstrated that diabetes promotes both atherosclerotic lesion initiation and progression into advanced lesions [3–5] and slows regression of lesions [6**]. At least some of these effects are likely to be mediated by effects on the endothelium [7]. In this review, we will focus on the endothelial cell and its roles in lipoprotein metabolism. In addition, we will discuss studies of endothelial dysfunction that relate to CVD in the setting of diabetes and the metabolic syndrome. Special emphasis is placed on recent discoveries related to the roles of lipoproteins and fatty acids, and the mechanisms whereby lipoproteins and fatty acids modulate endothelial function and subsequent atherosclerosis. The discussed mechanisms are summarized in Figure 1.
Figure 1. Effects of lipoprotein lipolysis and fatty acids in endothelial cells in relation to atherosclerosis.
Fatty acids are liberated from triglyceride-rich lipoproteins, such as VLDL or remnants, by the action of lipoprotein lipase (LpL) tethered to the luminal endothelial surface by proteoglycans and a specific LpL binding protein, glycosyl inositol HDL binding protein 1 (GPIHBP1). Fatty acids also circulate in blood bound to albumin. The fatty acids are taken up by endothelial cells through transport proteins, including the fatty acid transport proteins (FATP) FATP3 and FATP4. Some uptake is believed to occur through diffusion through the plasma membrane and through the scavenger receptor CD36. Once fatty acids enter the cell, they are bound to intracellular fatty acid binding proteins or are converted into hydrophilic acyl-CoAs by a group of enzymes with acyl-CoA synthetase activity. All members of the long-chain acyl-CoA synthetase (ACSL) family are expressed in endothelial cells, with the exception of ACSL6. The acyl-CoAs are used by the cell for membrane phospholipid synthesis and re-acylation, ceramide synthesis, beta-oxidation, protein modification, and several other processes. An unanswered question is whether saturated fatty acids have to be converted to acyl-CoAs in order to promote TLR4 movement to lipid rafts, whereas the pro-apoptotic effects of saturated fatty acids are dependent on ceramide synthesis and acyl-CoA formation. Ceramides have also been implicated in eNOS inhibition. Saturated fatty acid-mediated activation of TLR4 results in NF-kB activation and production of pro-atherosclerotic cytokines and chemokines, as well as adhesion molecules, which increase monocyte binding and recruitment into the artery wall. Finally, ApoA-I and recombinant HDL have the ability to inhibit palmitate-induced TLR4 translocation into lipid rafts in endothelial cells. Although much of this model is based on studies on cultured endothelial cells and addition of single fatty acids to these cells (A), mouse models of atherosclerosis are starting to provide a picture of important roles of several of the enzymes and proteins involved in fatty acid handling in atherosclerosis (B).
How do endothelial cells modulate metabolism of triglyceride-rich lipoproteins?
Endothelial biology is almost certainly affected by circulating lipoproteins. T2DM and the metabolic syndrome are associated with abnormalities in circulating lipids, whereas T1DM does not usually result in lipid abnormalities, unless inadequately controlled. In poorly controlled subjects with T1DM, higher triglyceride levels are observed [8]. The most common lipid abnormalities in T2DM and the metabolic syndrome are hypertriglyceridemia, greater concentrations of small dense LDL, and changes in HDL cholesterol [8]. The relationship between circulating triglyceride levels and atherosclerosis is still unclear and the human epidemiologic and clinical intervention data has been reviewed recently [2,10]. In part, this controversy has continued because of a lack of definitive human intervention data and experimental animal models. Severe hypertriglyceridemic mice develop small amounts of atherosclerosis with aging [11–12]. However, these mice had defective lipolysis and therefore did not test whether local lipolysis is deleterious.
Endothelial cell toxicity can occur due to circulating lipids or lipolysis products that are generated at the endothelial surface. This latter process is due to the actions of lipoprotein lipase (LpL) anchored to the luminal surface of endothelial cells via its interactions with proteoglycans and a specific LpL binding protein, glycosyl inositol HDL binding protein 1 (GPIHBP1) [13] which also might allow transfer of LpL from the abluminal to luminal sides of endothelium [14]. Discovery of this protein by the Young laboratory was a major advance in our understanding of LpL metabolism as it showed that LpL binding to the endothelium was not a non-specific interaction with proteoglycans. It should be noted that humans with defects in GPIHPB1 have hypertriglyceridemia [15]. Aside from recent and on-going studies to understand the structure/function relationship of LpL-GPIHPB1 interaction, reviewed in [16], there are a number of still unanswered issues relating to this biology. Is part of the LpL binding to GPIHBP1 a necessary process to protect LpL from inactivation by angiopoietin-like protein 4, as recently suggested [17]? Are there alternative methods for LpL recruitment that are in play with high fat feeding or prolonged heparin administration, which has been shown to reduce triglyceride to normal in GPIHBP1 deficient patients [18]? Does the VLDL receptor, which is highly expressed in endothelial cells, also mediate LpL transport and is this the reason that VLDL receptor deficiency is associated with decreased LpL activity and hypertriglyceridemia in the setting of increased lipoprotein production [19–20]?
Lipolysis of triglyceride-rich lipoproteins liberates free fatty acids, lysolecithin and a number of epoxides and oxidized lipids [21]. Many of these lipids have been used in in vitro studies to mimic putative atherogenic events. These include increased expression of adhesion molecules by lysolecithin [22], and reduced activity of endothelial nitric oxide synthase (eNOS) and greater production of reactive oxygen species (ROS) [23]. However, it should be noted that increased LpL activity in humans is associates with lower circulating levels of triglycerides and higher HDL, which associates with less CVD [24], indicating that LpL has mixed actions. In agreement with a beneficial effect of LpL are studies demonstrating that local lipolysis provides ligands for activation of the transcription factors peroxisome proliferator-activated receptors (PPARs), which might have anti-inflammatory actions. These events appear to require local generation of lipolysis products. Experiments performed using medium that contains heparin, which leads to release of LpL from cells and lipolysis of triglyceride-rich lipoprotein at a distance from the cells, does not activate endothelial PPARs to the same extent as when triglyceride hydrolysis occurs at the endothelial cell surface [25]. Interestingly, when one of the PPARs, PPARγ, was deleted specifically in endothelial cells in a mouse model, the result was markedly increased circulating free fatty acids and triglycerides [26]. It is likely that the increase in circulating fatty acids and triglycerides was due to changes in lipolysis, which could derive from defective endothelial cell uptake of locally produced free fatty acids, as has been suggested to be one reason for decreased triglyceride clearance in mice deficient in the fatty acid transporter and scavenger receptor CD36 [27]. Free fatty acids inhibit LpL and also cause its dissociation from the endothelial surface. These findings demonstrate that the endothelium is a cell type that has a major impact on metabolism of triglyceride-rich lipoproteins.
The exposure of the arterial wall to lipolysis products is dependent on a number of factors: the amount of LpL on the endothelial surface, the amount of substrate (i.e. the circulating triglyceride concentration) and the approximation of those lipoproteins to the luminal side of the blood vessel. In vitro and in vivo data suggest that LpL is saturated at a substrate concentration of 5 μM (~500 mg/dL) [28], therefore under most physiologic conditions local LpL actions are highly dependent on the circulating triglyceride level. Moreover, larger lipoproteins have a greater chance of contacting the vessel wall, are likely better LpL substrates, and create more lipolysis products. Besides production of atherogenic remnants [29], chylomicrons are potentially the source of more vascular damage as they create more products of lipolysis.
Another enzyme that produces fatty acids and lysolecithin is endothelial lipase (EL). Although EL was initially cloned from endothelial cells, its major sites of expression are the liver and thyroid gland [30–31]. EL is primarily a phospholipase and its preferred lipoprotein substrate is HDL. Although reduced EL activity is associated with higher HDL levels, EL gene variants do not correlate with CVD risk [32].
Together, these studies demonstrate that the endothelium takes an important part in regulating circulating levels of triglyceride-rich lipoproteins and fatty acids. The endothelium also reacts to these lipids in different ways that may contribute to vascular disease.
How do fatty acids interact with and cross the endothelium?
Locally produced fatty acids must cross the endothelial barrier to enable tissue uptake in muscle and adipose, and likely allow fatty acids to penetrate into the arterial wall. This trans-endothelial passage could occur via movement between or around endothelial cells. High local concentrations of free fatty acids disrupt the endothelial barrier as does active lipolysis, which can also enhance LDL movement into the artery [33]. Fatty acid uptake by endothelial cells is not completely understood; it might involve both receptor mediated uptake and non-specific uptake [34], which might occur in the presence of high local fatty acid concentrations, e.g. those that occur during chylomicron but not VLDL lipolysis [35].
The best characterized free fatty acid transporters are CD36 and members of the FATP family [34]. A recent report has localized the fatty acid binding site on CD36 [36]. Most importantly for our understanding of atherosclerosis, this binding site overlaps with that of oxidized LDL. These data suggest that fatty acids, which are likely to always be in higher molar concentration than oxidized LDL, are likely to compete for binding to CD36 and discourage uptake of some forms of atherogenic lipoproteins.
Regulation of endothelial fatty acid transport appears to involve vascular endothelial growth factor B (VEGF-B). This molecule promotes fatty acid uptake in endothelial cells by stimulating expression of the fatty acid transport proteins FATP3 (Slc27a3) and FATP4 (Slc27a4) [37**]. Accordingly, Vegfb−/− mice have defective fatty acid uptake into heart, muscle and brown adipose tissue after oral gavage of [3H]-oleic acid, as compared to wildtype controls [38]. The fatty acid transport protein family consists of six members, as reviewed in [34]. Endothelial cells express primarily FATP1 and FATP4, but other fatty acid transport proteins are expressed at lower levels [39]. These fatty acid transport proteins are believed to increase uptake of fatty acids, at least in part, through a process termed “vectorial acylation” [40] mediated by the linking of a FATP and an acyl-CoA synthetase (ACSL – see below). Once the fatty acid has reached the intracellular space, the ACSL links the free fatty acid to a hydrophilic CoA moiety, thereby “trapping” the fatty acid in the cell. Excess fatty acid uptake, as discussed below, could have untoward effects.
How do fatty acids affect endothelial biology?
In vitro, saturated fatty acids, such as palmitate (C16:0) and stearate (C18:0) induce generation/secretion of a number of pro-inflammatory molecules in endothelial cells that may be involved in recruitment of monocytes and other leukocytes into lesions of atherosclerosis. These include CCL2, CXCL1, CXCL8 [41*], IL-6 and CXCL3 [42]. This response is most likely mediated by an increased activation of NF-kB signaling and ER stress [42]. Saturated fatty acids have been proposed to generate inflammatory effects through extracellular actions in cells, by acting as ligands of toll-like receptor 2 (TLR2) and TLR4 [43]. More recently however, the ability of saturated fatty acids to activate TLR signaling has been attributed to an increased recruitment of TLRs into membrane lipid raft domains [44]. This process has been observed also in endothelial cells [45]. Thus, it is now generally thought that saturated fatty acids alter cellular membrane properties to promote TLR activation, rather than acting as TLR ligands. Moreover, in many systems the potentially harmful effects of saturated fatty acids have been neutralized by the addition of oleic acid (a monounsaturated fatty acid), perhaps due to greater esterification of intracellular palmitate to triglyceride [46].
Saturated fatty acids also induce apoptosis in endothelial cells [41*,47]. The effects of saturated fatty acids are counteracted by unsaturated fatty acids [47], and overexpression of stearoyl-CoA desaturase, which results in increased conversion of C16:0 and C18:0 to the monounsaturated C16:1 and C18:1 protects endothelial cells from the apoptotic effects of saturated fatty acids [48]. Saturated fatty acid-induced endothelial cell apoptosis appears to be largely independent of TLR4 [41*].
Whole-body TLR4-deficiency protects against atherosclerosis [49–50]. It is possible that the atherogenic effect of TLR4 is due to at least some extent to endothelial expression of TLR4, because TLR4-deficiency restricted to the hematopoietic cells had only minor effects on atherosclerosis [51]. In support of this concept are findings from mice deficient in MyD88, an adapter protein that mediates some of TLR4 signaling, but also signaling from other TLRs. MyD88-deficienct mice, like whole-body TLR4-deficient mice, exhibit reduced atherosclerosis [49]. Endothelial cells from these Myd88−/− mice show reduced binding of leukocytes [49], suggesting that increased monocyte recruitment into lesions due to exacerbated endothelial cell binding of these cells could be an important mechanism for how TLR4 and MyD88 promotes atherosclerosis through endothelial effects. Endothelium-specific TLR4-deficient mice have not yet been reported. It is important to emphasize that TLR4 binds and is activated by a number of ligands, so that an effect of TLR4-deficiency on atherosclerosis is not necessarily due to reduced fatty acid-mediated TLR4 activation.
Another possible inflammatory mechanism of saturated fatty acids is the generation of toxic ceramides. Serine palmitoyltransferase (SPT) is the first enzyme of the de novo biosynthetic pathway of sphingomyelin, which combines palmitoyl-CoA and serine to form 3-ketoshinganine and leads to downstream synthesis of ceramide, sphingomyelin, and sphingosine 1 phosphate (S1P). SPT is composed of 2 subunits and a recent study showed that SPT subunit 2 haploinsufficiency in myeloid cells reduced atherosclerosis [52*]. In contrast, despite beneficial effects of SPT deficiency in heart lipid toxicity [53], cardiomyocyte SPT deletion — which reduces heart ceramide content — leads to heart dysfunction and ER stress as ER phospholipids become palmitate enriched [54]. Whether SPT-deficiency has the same effect in endothelial cells is an important question, which can now be addressed by generating endothelium-specific SPT-deficient mice. In this context, it is interesting that inhibition of SPT protects endothelial cells from the deleterious effects of palmitate on endothelium-dependent vasorelaxation [55]. Reduced levels of SPT may decrease levels of sphingomyelin in lipid rafts and reduce function of lipid raft-associated proteins, including TLR4 [52*]. Thus, SPT-deficient macrophages exhibited reduced cytokine production following stimulation with lipopolysaccharide (LPS) or palmitate, and increased cholesterol efflux through the cholesterol exporters ABCA1 and ABCG1. These studies suggest that the function of lipid rafts has important downstream effects in vivo, including stimulation of atherosclerosis.
A recent study revealed another mechanism whereby saturated fatty acids might exert inflammatory effects in endothelial cells. Both saturated fatty acids (C16:0) and LPS (a TLR4 ligand) inhibit insulin-induced phosphorylation of the transcription factor FoxO1, thereby increasing its activity [56]. Deletion of the three genes encoding isoforms of FoxO specifically in endothelial cells protected the endothelial cells from inflammatory activation by LPS, and also protected low-density lipoprotein receptor-deficient (Ldlr−/−) mice from atherosclerosis induced by high fat feeding [56]. Thus, FoxO transcription factors might provide an additional important link between saturated fatty acid-induced endothelial cell dysfunction and atherosclerosis.
Whereas a lot of attention has been given to the adverse effects of saturated fatty acids in endothelial cells, unsaturated and polyunsaturated fatty acids also affect these cells. For example, the literature contains a number of examples of in vitro effects (usually beneficial) of omega 3 fatty acids [57]. The role(s) of omega 3 fatty acids as anti-atherogenic molecules are in controversy as recent studies have failed to show beneficial effects in clinical outcome studies [58].
Further studies are required to establish whether saturated fatty acids indeed induce endothelial dysfunction in vivo, and whether this contributes to atherosclerosis in mice and humans. It is important to consider that cells are never exposed to a single saturated fatty acid in vivo, and that combinations of saturated fatty acids and unsaturated fatty acids that mimic those seen in plasma of fat-fed animals or humans do not induce the same inflammatory and apoptotic effects as single saturated fatty acids in isolated endothelial cells [41*].
To what extent are the fatty acid effects mediated through acyl-CoA formation?
As discussed above, a majority of the effects of saturated fatty acids in endothelial cells would be expected to be mediated by processes that require the conversion of free fatty acids into their acyl-CoA derivatives, such as incorporation into membrane phospholipids that might govern the activity of TLR4 in lipid rafts, ceramide synthesis and ER stress. The synthesis of acyl-CoAs from free fatty acids is catalyzed by the ACSL gene family, reviewed in [59]. Endothelial cells express ACSL1, ACSL3, ACSL4, and ACSL5, but not ACSL6 [39,41*]. Early studies took advantage of a pharmacological ACSL inhibitor (triacsin C, a fungal metabolite) to block activity of many of the ACSL isoforms in endothelial cells. These studies suggested that the effect of palmitate on apoptosis (caspase 3 activity) in endothelial cells is dependent on ACSL activity, but that palmitate-induced NF-kB nuclear translocation is not [60]. Recently, several mouse models deficient in specific ACSL isoforms in selected cells types have been generated [61–63], including an endothelium-targeted ACSL1-deficient mouse [41*]. Interestingly, endothelial cells isolated from these mice did not show any protection against pro-inflammatory and pro-apoptotic effects of saturated fatty acids, nor did these mice demonstrate reduced recruitment of macrophages to adipose tissue during high fat feeding [41*]. These studies suggest that other ACSL isoforms are more important than ACSL1 in mediating detrimental effects of saturated fatty acids in endothelial cells, or perhaps, that some effects of saturated fatty acids are mediated by the free fatty acid rather than an acyl-CoA-dependent lipid mediator, or that saturated fatty acids are not detrimental in vivo in the presence of unsaturated fatty acids and other lipids. It will be important to address the role of other ACSL isoforms in endothelial cells.
Does the endothelium regulate HDL and vice versa?
Endothelial surfaces are the stage for most intravascular lipoprotein metabolism. This highly regulated process creates atherogenic lipoproteins; chylomicrons are converted to remnants and VLDL to LDL, as discussed above. In addition, catabolism of triglyceride-rich lipoproteins creates HDL and reduces the cholesteryl ester transfer protein (CETP) reaction. The CETP reaction reduces HDL cholesterol due to transfer of cholesteryl ester for triglyceride, lipolysis of HDL by hepatic and endothelial lipases, and greater clearance of small HDL by the kidney. It is unknown whether reduced lipolysis changes HDL composition and allows more rapid HDL removal from the circulation. It is also unknown whether the reduction in HDL cholesterol affects HDL’s function, such as its ability to remove cholesterol from macrophages. Together, these observations suggest that the endothelium may contribute to regulation of HDL levels and possibly function.
The role of HDL as a mediator of reverse cholesterol transport is well known, as is the inverse putative relationship between HDL efflux capacity and CVD risk [64**]. In addition, HDL may function as an anti-inflammatory molecule, as recently shown in a study of cytokine production by adipocytes [65]. HDL has been reported to exert protective effects in endothelial cells. Thus, S1P associated with HDL promotes endothelial barrier function [66], ApoA-I, the structural protein in HDL, protects endothelial cells from palmitate-induced TLR4 recruitment into lipid rafts [45], and HDL protects eNOS activity in cholesterol-loaded endothelial cells [67]. Thus, HDL modulates endothelial cell function.
Although HDL cholesterol levels are often reduced in patients with metabolic syndrome and T2DM, they are sometimes strikingly increased in well-insulinized patients with T1DM [68]. There might be issues both of HDL quantity and function that differ between T1DM and T2DM. HDL from patients with diabetes has been shown to contain more oxidation products and to be less able to reduce endothelial cell adhesion molecule expression, as compared with HDL from non-diabetic controls [69]. It is interesting to speculate that diabetes might render HDL less able to suppress inflammatory processes in endothelial cells.
How is the endothelium affected by diabetes and the metabolic syndrome?
A number of cardiovascular risk factors are altered by diabetes and the metabolic syndrome, each of which might negatively impact the endothelium. Three different factors might affect endothelial biology in the setting of diabetes: hyperglycemia, reduced (or increased) insulin actions, and exposure to lipids or other circulating factors such as advanced glycation endproducts (AGEs). Brownlee has suggested that a characteristic of endothelial cells is their failure to reduce glucose uptake in the presence of increased glucose exposure [70]. While most cells obtain glucose via insulin-regulated transporters, such as GLUT4, endothelial cell glucose uptake appears to be via non-insulin regulated processes such as via GLUT1 [71]. Support for a role of hyperglycemia in a type 1 diabetes mouse model was recently illustrated in a study showing that glucose reduction via inhibition of the sodium glucose co-transporter 2 (SGLT2) improves regression of aortic lesions in diabetic mice [6].
What atherogenic processes are altered in diabetic endothelium? Diabetic mice exhibit increased monocyte adhesion to endothelial cells through mechanisms that are intrinsic to the endothelial cells themselves. It has been suggested that endothelial cells exposed to a diabetic environment become “primed” to bind monocytes [72]. The increased endothelial cell ability to bind monocytes under conditions of hyperglycemia and diabetes has been explained by several different mechanisms, which are not mutually exclusive. These mechanisms, which will be mentioned only briefly, include increased levels of AGEs associated with diabetes [73], increased production of reactive oxygen species through mitochondria [74] and NADPH oxidase 1 [75], aldose reductase activity [4,7], activation of protein kinase C by hyperglycemia [76], and lack of proper insulin signaling [77–78]. Creation of a double heterozygous knockout of insulin receptors and IRS1 on the apoE-deficient background increased atherosclerosis, an effect associated with defective endothelial cell function [78]. Insulin receptor deficiency specifically in endothelial cells accelerates atherosclerosis in Apoe−/− mice [77], suggesting that insulin has beneficial effects in endothelial cells. In contrast, endothelial cell deletion of the three FoxO transcription factors, which act downstream of Akt, resulted in reduced atherosclerosis [56]. Loss of FoxOs improved NO production and reduced ROS and adhesion molecule expression; these protective effects occurred in the setting of reduced IRS phosphorylation and Akt phosphorylation. Thus, the relationship between vascular disease and insulin signaling in endothelial cells is multifactorial.
Diabetes also results in impaired vasodilation, mediated, at least in part, through a reduced ability of eNOS to generate normal levels of the vasodilator NO. This might be due to ROS generation secondary to greater uptake of glucose [79] and fatty acids [24]. Thus, acetylcholine-mediated endothelium-dependent vasorelaxation is impaired in mouse models of diabetes [80–81]. Part of the detrimental effect of diabetes on vasodilatation may also be mediated by a reduced expression of stromal interaction molecule 1 (STIM1) and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 3 (SERCA3), which leads to an impaired ability to mobilize Ca2+ from the ER [82]. It is unclear if reduced vasodilatation leads to increased atherosclerosis, although it is well established that hypertension is a risk factor for cardiovascular disease.
Thus, so far much of the research on effects of diabetes on the endothelium has focused on glucose-mediated adverse effects. With the development of an increasing number of mouse models with endothelial-targeted deletion or overexpression of molecules involved in lipid metabolism, the next few years are likely to see an increase in studies on diabetes-mediated changes in lipids in endothelial cells, and the relationship to atherosclerosis.
Translational relevance and conclusions
More studies are needed to evaluate to what extent the studies based on isolated endothelial cells hold true in vivo. Although numbers of in vitro studies have shown toxic effects of various lipids on endothelial cells, in vivo models to modulate lipolysis along the artery are missing. Even identification of the putative toxic lipids is tenuous as lipid metabolic pathways are very interconnected and addition of a single species to cultured cells is likely to lead to changes in many intracellular species. The interactions between changes in circulating triglyceride and other lipoproteins, especially HDL levels and function, are still uncertain. Basic mechanisms of lipid uptake and transport by the endothelium are not understood. Similarly, very few studies have investigated the roles of fatty acid-handling proteins specifically in endothelial cells in relation to atherosclerosis. Even more importantly, human studies are needed before we know if these mechanisms are of relevance in humans and to what extent they might contribute to cardiovascular events.
Acknowledgments
Research in the authors’ laboratories is supported by National Institutes of Health grants HL062887, HL092969, HL097365, DK017047 and Novo Nordisk A/S (KEB) and HL45095 and HL73029 (IJG).
Footnotes
Conflict of Interest
Ira J. Goldberg and Karin E. Bornfeldt declare that they have no conflict of interest.
Contributor Information
Ira J. Goldberg, Email: ijg3@columbia.edu, Department of Medicine, Division of Preventive Medicine & Nutrition, Columbia University College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032. Tel: (212) 305-5961; Fax: (212) 305-3213.
Karin E. Bornfeldt, Email: bornf@u.washington.edu, Departments of Medicine and Pathology, Diabetes and Obesity Center of Excellence, 850 Republican Street, University of Washington, Seattle, WA 98109-8055. Tel: (206) 543-1681; Fax: (206) 543-3567.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Sarwar N, Gao P, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renard CB, Kramer F, Johansson F, et al. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114(5):659–68. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vikramadithyan RK, Hu Y, Noh HL, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115(9):2434–43. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson F, Kramer F, Barnhart S, et al. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105(6):2082–7. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Nagareddy PR, Murphy AJ, Hewing B, et al. Hyperglycemia enhances myeloid cell proliferation and impairs atherosclerosis regression in diabetes. Cell Metab. 2013 In Press This article provides evidence that hyperglycemia contributes to the impaired regression of atherosclerotic lesions associated with diabetes, and that the mechanism is due to glucose stimulation of neutrophils. [Google Scholar]
- 7.Vedantham S, Noh H, Ananthakrishnan R, et al. Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol. 2011;31(8):1805–13. doi: 10.1161/ATVBAHA.111.226902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care. 1991;14(9):839–55. doi: 10.2337/diacare.14.9.839. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31(8):1716–25. doi: 10.1161/ATVBAHA.111.226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Qi R, Xian X, et al. Spontaneous atherosclerosis in aged lipoprotein lipase-deficient mice with severe hypertriglyceridemia on a normal chow diet. Circ Res. 2008;102(2):250–6. doi: 10.1161/CIRCRESAHA.107.156554. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein MM, Yin L, Tu Y, et al. Chylomicronemia elicits atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol. 2009;30(1):20–3. doi: 10.1161/ATVBAHA.109.196329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigneux AP, Davies BS, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5(4):279–91. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young SG, Davies BS, Voss CV, et al. GPIHBP1, an endothelial cell transporter for lipoprotein lipase. J Lipid Res. 2011;52(11):1869–84. doi: 10.1194/jlr.R018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigneux AP, Franssen R, Bensadoun A, et al. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29(6):956–62. doi: 10.1161/ATVBAHA.109.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes & development. 2013;27(5):459–84. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenburg WK, Yu D, Lee EC, et al. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J Lipid Res. 2009;50(12):2421–9. doi: 10.1194/jlr.M900145-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franssen R, Young SG, Peelman F, et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ Cardiovasc Genet. 2010;3(2):169–78. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagyu H, Lutz EP, Kako Y, et al. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem Mar. 2002;277(12):10037–43. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 20.Tacken PJ, Teusink B, Jong MC, et al. LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J Lipid Res. 2000;41(12):2055–62. [PubMed] [Google Scholar]
- 21.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50(2):204–13. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90(3):1138–44. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–80. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31(8):1716–25. doi: 10.1161/ATVBAHA.111.226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruby MA, Goldenson B, Orasanu G, Johnston TP, Plutzky J, Krauss RM. VLDL hydrolysis by LPL activates PPAR-alpha through generation of unbound fatty acids. J Lipid Res. 2010;51(8):2275–81. doi: 10.1194/jlr.M005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda T, Brown JD, Orasanu G, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119(1):110–24. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drover VA, Ajmal M, Nassir F, et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115(5):1290–7. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52(7):1578–85. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilversmit DB. Mechanisms of cholesterol accumulation in the arterial wall. Am J Cardiol. 1975;35(4):559–66. doi: 10.1016/0002-9149(75)90840-1. [DOI] [PubMed] [Google Scholar]
- 30.Hirata K, Dichek HL, Cioffi JA, Choi SY, Leeper NJ, Quintana L, Kronmal GS, Cooper AD, Quertermous T. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J Biol Chem. 1999;274(20):14170–5. doi: 10.1074/jbc.274.20.14170. [DOI] [PubMed] [Google Scholar]
- 31.Jaye M, Lynch KJ, Krawiec J, Marchadier D, Maugeais C, Doan K, South V, Amin D, Perrone M, Rader DJ. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21(4):424–8. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 32.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol. 2007;292(6):H2745–53. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- 34.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821(5):852–7. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharadwaj KG, Hiyama Y, Hu Y, et al. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285(49):37976–86. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuda O, Pietka T, Demianova Z, et al. Sulfo-N-succinimidyl Oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164. SSO also Inhibits oxLDL uptake by macrophages. J Biol Chem. 2013 Apr 18; doi: 10.1074/jbc.M113.473298. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Hagberg CE, Falkevall A, Wang X, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464(7290):917–21. doi: 10.1038/nature08945. This manuscript described a new mechanism that controls endothelial fatty acid uptake. [DOI] [PubMed] [Google Scholar]
- 38.Hagberg C, Mehlem A, Falkevall A, Muhl L, Eriksson U. Endothelial fatty acid transport: role of vascular endothelial growth factor B. Physiology (Bethesda) 2013;28(2):125–34. doi: 10.1152/physiol.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandoval A, Fraisl P, Arias-Barrau E, et al. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys. 2008;477(2):363–71. doi: 10.1016/j.abb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found Symp. 2007;286:127–38. doi: 10.1002/9780470985571.ch11. [DOI] [PubMed] [Google Scholar]
- 41*.Li X, Gonzalez O, Shen X, et al. Endothelial acyl-CoA synthetase 1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment. Arterioscler Thromb Vasc Biol. 2013;33(2):232–40. doi: 10.1161/ATVBAHA.112.252239. This study is the first to study deletion of an ACSL in endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krogmann A, Staiger K, Haas C, et al. Inflammatory response of human coronary artery endothelial cells to saturated long-chain fatty acids. Microvasc Res. 2011;81(1):52–9. doi: 10.1016/j.mvr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87(6):989–99. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 44.Wong SW, Kwon MJ, Choi AM, et al. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284(40):27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng AM, Handa P, Tateya S, et al. Apolipoprotein A-I attenuates palmitate-mediated NF-κB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS One. 2012;7(3):e33917. doi: 10.1371/journal.pone.0033917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100(6):3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staiger K, Staiger H, Weigert C, et al. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55(11):3121–6. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 48.Peter A, Weigert C, Staiger H, et al. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab. 2008;295(2):E339–49. doi: 10.1152/ajpendo.00022.2008. [DOI] [PubMed] [Google Scholar]
- 49.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Y, Subramanian S, Montes VN, et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1596–604. doi: 10.1161/ATVBAHA.112.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coenen KR, Gruen ML, Lee-Young RS, et al. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52(2):318–28. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Chakraborty M, Lou C, Huan C, et al. Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis. J Clin Invest. 2013;123(4):1784–97. doi: 10.1172/JCI60415. This study demonstrates that the sphingomyelin pathway contributes in important ways to atherosclerosis in mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park TS, Hu Y, Noh HL, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49(10):2101–12. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SY, Kim JR, Hu Y, et al. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem. 2012;287(22):18429–39. doi: 10.1074/jbc.M111.296947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang QJ, Holland WL, Wilson L, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61(7):1848–59. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Tsuchiya K, Tanaka J, Shuiqing Y, et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15(3):372–81. doi: 10.1016/j.cmet.2012.01.018. This article provides the first demonstration that endothelial expression of FoxO transcription factors are pro-atherogenic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005;206(2):103–16. doi: 10.1007/s00232-005-0783-2. [DOI] [PubMed] [Google Scholar]
- 58.Investigators OT, Bosch J, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 59.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21(3):212–7. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciapaite J, van Bezu J, van Eikenhorst G, et al. Palmitate and oleate have distinct effects on the inflammatory phenotype of human endothelial cells. Biochim Biophys Acta. 2007;1771(2):147–54. doi: 10.1016/j.bbalip.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Ellis JM, Li LO, Wu PC, et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12(1):53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109(12):E715–24. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellis JM, Mentock SM, Depetrillo MA, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs Fatty Acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31(6):1252–62. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689. This work demonstrates that reduced cholesterol efflux capacity of HDL is a better predictor of coronary artery disease than HDL cholesterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein A-I and HDL have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-Binding Cassette (ABC) A-1, ABCG-1 and Scavenger Receptor B-1(SRB-1) Circ Res. 2013;112(10):1345–54. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem. 2012;287(53):44645–53. doi: 10.1074/jbc.M112.423426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terasaka N, Westerterp M, Koetsveld J, et al. ATP-binding cassette transporter G1 and high-density lipoprotein promote endothelial NO synthesis through a decrease in the interaction of caveolin-1 and endothelial NO synthase. Arterioscler Thromb Vasc Biol. 2010;30(11):2219–25. doi: 10.1161/ATVBAHA.110.213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cleland SJ. Cardiovascular risk in double diabetes mellitus--when two worlds collide. Nat Rev Endocrinol. 2012;8(8):476–85. doi: 10.1038/nrendo.2012.47. [DOI] [PubMed] [Google Scholar]
- 69.Morgantini C, Natali A, Boldrini B, et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60(10):2617–23. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 71.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219(3):713–25. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 72.Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem. 2003;278(28):25369–75. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96(3):1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 76.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11(5):379–89. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galkina EV, Butcher M, Keller SR, et al. Accelerated atherosclerosis in Apoe−/− mice heterozygous for the insulin receptor and the insulin receptor substrate-1. Arterioscler Thromb Vasc Biol. 2012;32(2):247–56. doi: 10.1161/ATVBAHA.111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97(22):12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia Soriano F, Virág L, Jagtap P, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7(1):108–13. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 81.Ding H, Hashem M, Wiehler WB, et al. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol. 2005;146(8):1110–8. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Estrada IA, Donthamsetty R, Debski P, et al. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res. 2012;111(9):1166–75. doi: 10.1161/CIRCRESAHA.112.275743. [DOI] [PMC free article] [PubMed] [Google Scholar]