Abstract
Long-term consequences of early developmental exposure to drugs of abuse may have deleterious effects on the proliferative plasticity of the brain. The purpose of this study was to examine the long-term effects of prenatal exposure to cocaine, using the IV route of administration and doses that mimic the peak arterial levels of cocaine use in humans, on the proliferative cell types of the subventricular zones (SVZ) in the adult (180 days-old) rat brain. Employing immunocytochemistry, the expression of GFAP+ (type B cells) and nestin+(GFAP−) (Type C and A cells) staining was quantified in the subcallosal area of the SVZ. GFAP+ expression was significantly different between the prenatal cocaine treated group and the vehicle (saline) control group. The prenatal cocaine treated group possessed significantly lower GFAP+ expression relative to the vehicle control group, suggesting that prenatal cocaine exposure significantly reduced the expression of type B neural stem cells of the SVZ. In addition, there was a significant sex difference in nestin+ expression with females showing approximately 8–13% higher nestin+ expression compared to the males. More importantly, a significant prenatal treatment condition (prenatal cocaine, control) by sex interaction in nestin+ expression was confirmed, indicating different effects of cocaine based on sex of the animal. Specifically, prenatal cocaine exposure eliminated the basal difference between the sexes. Collectively, the present findings suggest that prenatal exposure to cocaine, when delivered via a protocol designed to capture prominent features of recreational usage, can selectively alter the major proliferative cell types in the subcallosal area of the SVZ in an adult rat brain, and does so differently for males and females.
Keywords: Pregnancy, Rats, Cocaine, Drug abuse, Intravenous injection, Chronic administration, Vascular access port, Subventricular zone, Progenitor cells, Neural stem cells
1. INTRODUCTION
A high rate of drug use in the U.S. is consistently found among our youth; for 8th, 10th and 12th grade students; lifetime usage figures are of any illicit drug in the 12 months preceding the survey were 19%, 36%, and 47% (Johnston et al., 2010). In other words, just under half of American recent secondary school students have tried an illicit drug by the time they near high school graduation. In 2009, an estimated 21.8 million Americans aged 12 or older were current (past month) illicit drug users, meaning they had used an illicit drug during the month prior to the survey interview (SAMHSA, 2010). This estimate represents 8.7 percent of the population aged 12 years old or older. The rate of current illicit drug use among persons aged 12 or older in 2007 (8.7 percent) is higher than the rate in 2008 (8.0 percent). The population of young female drug users of childbearing age is of particular note. Among pregnant women aged 15 to 44 years, 4.5 percent used illicit drugs in the past month (SAMHSA, 2010).
Additional troublesome data on the specific abuse of cocaine in the U.S. is also available. Today, about one in seven young adults (14% in 2009) have tried cocaine, and 6% have tried it by their senior year of high school (i.e., by age 17 or 18). More than 1 in every 42 twelfth graders (2.4%) has tried crack. In the young adult sample, 1 in 28 (3.6%) has tried crack by age 29–30 (Johnston et al., 2010). Presently, there are 1.6 million current cocaine users aged 12 or older, comprising 0.7 percent of the population. These estimates were similar to the number and rate in 2008 (1.9 million or 0.7 percent). Clearly cocaine/crack use among adult and young women of childbearing age remains a significant societal concern, placing future generations at risk. Furthermore, the recent review of countries around the world has suggested high lifetime prevalence use of cocaine in Argentina (7.9%), Italy (6.6%), United Kingdom (6.5%), Chile (5.9%), and Ireland 5.3% (Degenhardt et al., 2011); the implication of cocaine abuse for future generations appears to be far more than a U.S. health issue. Thus, comprehending the effects of prenatal drug exposure, such as cocaine/crack, on the development of the fetal brain remains extremely important; understanding the long-term effects of prenatal cocaine exposure that are detectable in adulthood is even more essential.
Knowledge of the mechanisms through which cocaine may cause adverse effects on the developing brain has been, and remains, an important quest. It is of interest that measures of head circumference have been reported as correlated with behavioral and psychological impairments associated with in utero cocaine exposure (Chiriboga et al., 1999; Bateman and Chiriboga, 2000; Singer et al., 2008; Eyler et al., 2009). However, even when prenatal cocaine effects on physical growth including head circumference are reported, they may not persist beyond infancy (Lumeng et al., 2007). Multiple comprehensive reviews covering several decades of research have failed to definitively identify any unique pathogenic consequence(s) of prenatal cocaine. For example, among children aged 6 years or younger, there is no convincing evidence that prenatal cocaine exposure is associated with developmental toxic effects that are different in severity, scope, or kind from the sequelae of multiple other risk factors (Frank et al., 2001). A similar recent reaffirmation noted that studies through 6 years have shown no long-term direct effects of prenatal cocaine exposure on children’s physical growth, developmental test scores, or language outcomes (Ackerman et al., 2010). Many findings once thought to be specific effects of in utero cocaine exposure are correlated with other factors, including prenatal exposure to tobacco, marijuana, or alcohol, and/or a host of social/environmental factors, such as poverty, caregiver education, placement stability, and quality of child-caregiver relationships that are known to affect the a child’s development.
Most recent data employing state-of-the-art neuroimaging techniques do suggest subtle differences between prenatal cocaine-exposed and nonexposed children on structural measures of the brain (Roussotte et al., 2010). For example, volumetric MRI suggested reductions in cortical gray matter and total parenchymal volume (and smaller head circumference), however, the decreases were no longer statistically significant after controlling for polydrug exposure, to alcohol, cigarettes, and marijuana (Rivkin et al., 2008). In an earlier symposium report, which suggested gray matter reductions in occipital and parietal lobes, the volume decreases remained significant after controlling for polydrug exposure, but the reductions were not dose-dependent (Singer et al., 2006).
Preclinical studies with various strains of animals have shown prenatal cocaine exposure may cause abnormalities in the developing cerebral cortex. In sub-human primates, administration of cocaine at the time of neocortical neurogenesis (the second trimester) reduced neocortex volume, disturbed lamination, altered positioning of cerebral cortical neurons, and reduced density and number of cortical neurons (Lidow, 1995; Lidow and Song, 2001). In the mouse, cocaine administered during corticogenesis disrupted horizontal lamination, impaired establishment of vertical columns, and markedly decreased the number of radially organized axonal-dendritic bundles (Gressens et al., 1992). Despite these demonstrations of the capability of prenatal cocaine to disturb corticogenesis, it is important to note that both of those models used very high drug doses; i.e., altered corticogenesis in the sub-human primate was found after maternal doses of cocaine sufficient to reduce the entire brain volume of the offspring by > 20% (Lidow, 1995). Similarly, cocaine-induced disturbances of corticogenesis in the developing mouse brain are reported under conditions in which the entire brain size is reduced by about 25% (Gressens et al., 1992). The translation of such findings to the clinical situation is challenging, what can we infer from such findings for the recreational usage of cocaine?
Several studies using a preclinical intravenous injection model, to more closely mimic the route and pharmacokinetics of the smoking of crack cocaine, have nevertheless discovered a more subtle disruption of cortical development may be observed (disruption of organization of anterior cingulate cortex in 20–25 day-old rabbits, Stanwood et al., 2001; irregularities in the formation of cortical minicolumns in weanling rats, Buxhoeveden et al., 2006). An inhibition of progenitor cell proliferation by acute cocaine treatment, as shown in cell culture and rat fetuses, affords one potential mechanism for such cortical architectural disruptions (Lee et al., 2008). Given the unambiguous demonstration that neurogenesis continues throughout life into adulthood [McKay, 1997, Temple and Alvarez-Buylla, 1999; Weiss et al., 1996], it is important to establish whether adult brain plasticity has been altered by prenatal cocaine exposure.
The subventricular zone (SVZ) harbors the largest population of proliferating cells in the adult brain (Altman, 1969; Lois & Alvarez-Buylla, 1994; including in humans, Sanai et al., 2004; Curtis et al., 2007); the focus of the present study was specifically on an examination of that brain region. The prevailing view (Petreanu and Alvarez-Buylla, 2002; Nissant and Pallotto, 2011) holds that neural stem cells (NSCs) of the SVZ are quiescent GFAP+ cells that share properties of astrocytes (referred to as type B cells). Type B cells give rise to transient amplifying type C cells that are GFAP−. The intermediate progenitor cells give rise to neuroblasts (referred to as type A cells) that migrate a significant distance via the rostral migratory stream (RMS) to the olfactory bulb where they differentiate and durably integrate (~50% of these nascent cells) into the existing neural circuitry. Thus, the most important function of NSCs is to generate neurons (but see elaboration of their functions, Zhao et al., 2008); however, adult NSCs of the SVZ are also involved in gliogenesis generating oligodendrocytes (Jackson et al., 2006).
Thus, prenatal cocaine exposure was hypothesized to have long-term consequences for proliferative plasticity in the adult brain. Using immunocytochemistry, we determined whether or not prenatal cocaine treatment altered glial fibrillary acidic protein (GFAP+) (type B cells) and nestin+/GFAP− (type A and type C cells) expression in the offspring at 6 months of age. Potential sex differences were also examined.
2. MATERIALS AND METHODS
2.1 Animals
Nulliparous female Long-Evans rats were obtained from Harlan Sprague-Dawley, Inc (Indianapolis, IN) at approximately 10–12 weeks of age (225–249g), placed into quarantine for one week, and subsequently moved to the animal vivarium. The animals were maintained according to NIH guidelines in AAALAC accredited facilities. Food (Pro-Lab Rat, Mouse, Hamster Chow No. 3000) and water were available ad libitum. The animal facility was maintained at 21° ± 2°C, 50% ± 10% relative humidity and had a 12 hr light:12 hr dark cycle with lights on at 07:00 h (EST). The protocols for the use of rats in this research were approved by the IACUC of the University of South Carolina.
2.2 Chemicals
Cocaine HCl (Sigma Chemical Co., St. Louis, MO), was dissolved in sterile isotonic saline based on the weight of the salt. Immediately prior to use, the drug solution was prepared in a volume of 1 ml/kg.
2.3 Experimental Design
Half of the adult female animals were randomly selected and surgically implanted with vascular catheters (as described below); the remaining half served as surrogate dams and received neither drug treatment nor the daily handling associated with drug. On GD8, pregnant catheterized females were randomly assigned to either a cocaine (3.0 mg/kg) or saline vehicle group and treated on GD8-21, as described below. At six months of age, one male and one female from each litter were sacrificed for use in the present study.
2.4 Surgery
Catheterization was performed as previously described (Mactutus et al., 1994). Briefly, the animals were anesthetized with a mixture of ketamine hydrochloride (100 mg/kg/ml) and xylazine (3.3 mg/kg/ml) and a sterile Intracath IV catheter (Becton, Dickinson and Co., Franklin Lakes, NJ) with a Luer-lock injection cap (Medex, Inc, Carlsbad, CA) was implanted dorsally in a subcutaneous pouch. The ketamine/xylazine mixture was chosen over pentobarbital because of the potent effect of the latter agent on cytochrome P450 activity (LaBella and Queen, 1993; Loch et al., 1995). The distal end of the catheter was inserted into the left jugular vein and advanced toward the heart. Animals were kept under periodic postoperative observation and returned to the vivarium upon recovery from anesthesia. Beginning on the day following surgery, the catheters were flushed with approximately 0.15 ml of 2.5% heparinized saline and the animals were observed for any signs of discomfort or behavioral distress on a daily basis. Body weights were recorded prior to surgery and periodically throughout the experiment.
2.5 Drug Treatment
The dose of cocaine (3.0 mg/kg), delivered IV, was chosen based on the observations that 1) it produces peak arterial plasma levels in the male rat which are not significantly different from peak arterial levels in humans administered 32 mg of cocaine IV (Booze et al., 1997; Evans et al., 1996); 2) the acute heart rate and blood pressure responses in the late gestation pregnant rat are similar to those produced in a variety of other species (Mactutus et al., 2000); and 3) under experimental conditions, this dose is self-administered by “users” multiple times in a 2.5 hr session (Fischman and Schuster, 1982) thus, representing a low or recreational dose. Maternal IV cocaine also produces an appreciable tissue uptake of cocaine by fetal brain, as well as a relatively rapid elimination (Robinson et al., 1994). The cocaine was administered as an IV bolus injection delivered in a volume of 1.0 ml/kg (15 sec) followed by a 15 sec flushing of the catheter with 0.2 ml heparinized (2.5%) saline, which is the approximate volume of the catheter. Cocaine and saline injections were administered 1 × per day from GD8–GD14, and 2 × per day from GD15–GD21 (Mactutus et al., 1994; Mactutus, 1999). Dams were weighed on gestational day (GD) 0, GD8, GD15, and GD21.
2.6 Animal Mating
At 4–7 days following surgery, the females were group housed with males for breeding. Vaginal cytology and the presence of sperm were checked daily in the females. A sperm positive female was considered pregnant (GD0) and subsequently individually housed in plastic cages with Sani-chip™ bedding throughout pregnancy and lactation.
2.7 Offspring Treatment
Pregnant rats were checked twice daily for pups. Day of birth was defined as postnatal day 0 (PD0). On PD1, pups were weighed and culled to 8, with an equal number of males (M) and females (F) when possible. Each pup was tattooed for identification and the culled litters of all catheterized dams were fostered to surrogate dams that had delivered within 24 h. Thus, no pups were raised by their biological mother or exposed to drug during the postnatal period. Pups were reared in their surrogate mothers’ cage until weaning at P21, at which time offspring were group housed in same sex pairings. Animals were subsequently group-housed and then same-sex pair-housed until use in present experiment at 180 days of age. The total sample size was n = 32, with 10 M and 6 F in the control group, and 8 M and 8 F in the treatment group.
2.8 Tissue Preparation
The animals were anesthetized and transcardially perfused with 100 ml saline solution at room temperature followed by 200 ml of cold 4% paraformaldehyde in 0.1M PBS containing 0.2% picric acid at pH 6.9 for 10 minutes. The brains were carefully removed and post-fixed for 2 hours in the same fixative in a 4oC refrigerator. Subsequently, the brains were cryoprotected in buffered sucrose for at least 24 hours at 4oC.
2.9 Sections
The cryoprotected brains were sectioned in the coronal plane with a cryostat (50 μm thick sections), and tissue sections were systematically collected throughout the forebrain from Bregma +1.70 through Bregma 0.0 (Paxinos and Watson, 2006). Two sections (one rostral and one caudal) in a specific rostral-caudal order were chosen from each rat for density measurements. The sections chosen were consistent between rats by selecting sections that possessed clear anatomical landmarks (Figure 1). The rostral section was selected at the initial genu of the corpus callosum and the difference between the rostral and the caudal section was 7 × 50μm sections (350μm). This strategy ensured that the density in the same SVZ area was measured for every rat.
Figure 1.
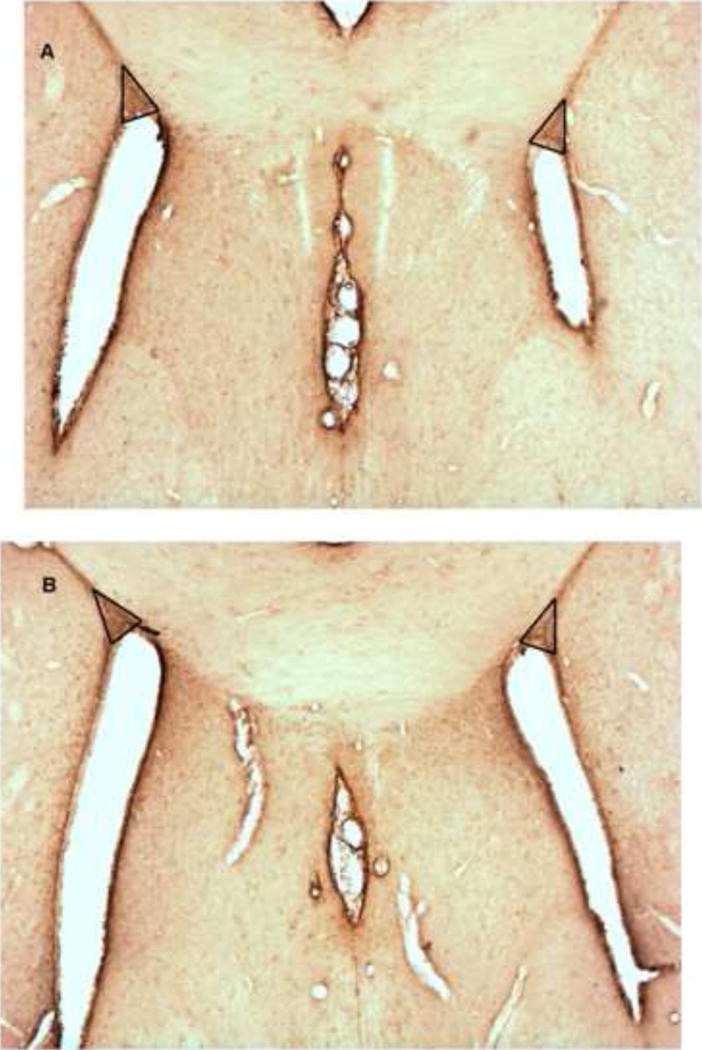
Coronal rostral (A) and caudal (B) Nestin-immunostained tissue sections indicating the corpus callosal/ventricular anatomical landmarks for selection of tissue sections and the SVZ (triangles). The quantification of immunostaining of the subcallosal area of the SVZ occurred anteriorly at the genu of the corpus callosum (approximately bregma 1.60) and 350 μm caudally at the septohippocampal nucleus (approximately bregma 1.20).
2.10 Immunohistochemistry
The free-floating tissue sections were thoroughly rinsed in 3 × (10 minutes each) in 0.1 M PBS. After rinsing in 0.1M PBS, the sections were incubated overnight in 5°C refrigerator in the primary antibody diluted in a solution comprised of 0.1M PBS, 0.75% BSA (Albumin, Bovine) (A-2153, Sigma Chemical CO, St. Louis, MO 63178), and 0.5% Triton X-100 (T-9284, Sigma Chemical CO, St. Louis, MO 63178). The following primary antibodies were used: mouse anti-nestin (MAB353, 1:400; Chemicon International, Temecula, CA 92590), and rabbit anti-GFAP (ZO334, 1:1500; Dako Corporation, Carpinteria, CA 93013). Nestin is a neuroepithelial stem cell marker (Lendahl et al., 1990) whereas GFAP is a well-accepted marker of astrocytes (Bignami and Dahl, 1974); both have great utility in the immunocytochemical characterization of the SVZ (Doetsch et al., 1997). GFAP and Nestin expression can be used to indicate the “capacity” for proliferation as they are markers for early stages of differentiation (von Bohlen und Halbach, 2010). After the primary antibody incubation, the tissue sections were thoroughly rinsed 3 × (10 minutes each) in 0.1M PBS. The sections were further incubated in the secondary antibody diluted in a solution comprised of 0.1M PBS, 0.75% BSA, and 0.5% Triton X-100 for 1 hour at room temperature. The following secondary antibodies were used: biotinylated anti-mouse IgG (BA-2000, 1:200; Vector Labs Inc., Burlingame, CA 94010), and biotinylated anti-rabbit IgG (BA-1000, 1:500; Vector Labs Inc., Burlingame, CA 94010). The sections were again rinsed 3 × (10 minutes each) in 0.1M PBS and were further incubated in Avidin: Biotin: Peroxidase complex (Vectastain ABC Kit, 1:100 for Nestin and 1:200 for GFAP, Vector Laboratories, Inc., Burlingame, CA 94010) diluted in 0.1M PBS for 60 minutes at room temperature. The tissue sections were thoroughly rinsed 1 × (10 minutes) in 0.1M PBS and 2 × (10 minutes each) in 0.05M Tris buffer (pH 7.2–7.4). The sections were then incubated in DAB (Sigma, St. Louis, MO) for 5 minutes.
After the DAB reaction, the tissue sections were rinsed 3 × (10 minutes each) in 0.05 M Tris buffer, and were carefully mounted on slides. All the tissue sections were stained at once to ensure consistency in the solutions used for immunohistochemistry. Sections uneven in staining were not included in the data collection.
The sections were mounted on Fisher Superfrost Plus microscope slides and dehydrated in ethanol before being coverslipped and mounted in DPX Mounting Media (Sigma-Aldrich).
2.11 Quantification
The data were acquired using a MCID system with a MCID BASIC program. All the parameters such as light exposure and magnification (20x) were kept constant across all measurements to avoid confounding variables. The quantification of GFAP and nestin immunocytochemical markers were measured in the subcallosal area of the SVZ by taking a background reading and five repeated density measurements at 20x. Fiber density (number of pixels above threshold) was measured in the field of view relative to the background reference staining. The immunocytochemical markers were segmented from background by thresholding. Due to variations in staining intensities, the background threshold level was measured on a per-section basis. The background reading was subtracted from the five SVZ density readings. The five density readings were averaged to perform statistical analysis. The same procedure was employed for both rostral and caudal sections.
2.12 Statistics
The litter parameters of number of male and female pups per litter, mean D1 body weights of male and female pups and sex ratio of the litter were compared based on litter means. All data were analyzed by ANOVA techniques (Winer, 1971) with BMDP software (Statistical Solutions Ltd, 2009). Repeated-measures ANOVA (BMDP-P4V) was used to analyze the GFAP and nestin expression in the subcallosal area of the SVZ. Data imputation for missing data (BMDP-PAM) was employed (6% loss) so that entire subjects were not deleted from the repeated-measures data matrix. A similar pattern of results was found with or without data imputation. The graphs utilized the group SEMs from the nonimputed data matrix. An α level of p ≤ 0.05 was considered to be significant for the rejection of null hypothesis.
3. RESULTS
3.1 Offspring Growth
Prenatal IV cocaine, administered from GD8–20, had no detectable adverse effect on any of the litter parameters measured, including birth weight of male or female pups, number of male or female pups, and the sex ratio of the litter (Table 1 ; Fs < 1.0).
Table 1.
Prenatal Effects of Cocaine on Litter Parameters.
| Treatment | D1 Male Wt | # of Males | D1 Female Wt | # of Females | Sex Ratio |
|---|---|---|---|---|---|
| Saline | 7.2 ± 0.2 | 5.9 ± 0.6 | 6.8 ± 0.2 | 5.0 ± 0.6 | 0.55 ± 0.1 |
| Cocaine | 7.5 ± 0.1 | 5.4 ± 0.4 | 7.1 ± 0.1 | 5.0 ± 0.4 | 0.52 ± 0.1 |
No significant prenatal treatment effects were detected on the litter parameters measured (Fs < 1.0).
3.2 Immunohistochemistry
An overall repeated-measures ANOVA conducted on the GFAP and nestin expression in the subcallosal area of the SVZ revealed, most importantly, a significant prenatal treatment by sex interaction, [F(1, 28) = 7.9, p ≤ 0.009]. Specific contrasts indicated that the prenatal cocaine effect was significant in the female [F(1, 28) = 10.0, p ≤ 0.004], but not male offspring [F < 1.0)] as well as that the significant overall sex difference in the vehicle control animals [F(1, 28) = 18.3, p ≤ 0.001] was not present in the prenatal cocaine-exposed offspring [F < 1.0)]. Given the differential caudal-rostral expression of the two immunocytochemical markers in this overall analysis [F(1, 28) = 7.0, p ≤ 0.013] and the presence of higher order interactions, the interpretation of the results was guided by subsequent analyses of each expression marker.
3.2.1 GFAP Expression
A differential expression of GFAP expression in the subcallosal area of the SVZ was noted across caudal and rostral sections [F(1, 28) = 6.8, p ≤ 0.015]. The overall prenatal treatment by sex interaction noted above was evident in the caudal sections with the prenatal vehicle treated adults showing a significant sex difference in GFAP expression [F(1, 28) = 6.8, p ≤ 0.015], with the females displaying the significantly greater expression of GFAP. No sex difference was detected in the prenatal cocaine treated animals [F < 1.0]. The overall prenatal cocaine treatment effect for the female offspring noted above was suggested for GFAP expression in the caudal sections [F(1,28) = 4.0, p ≤ 0.056]. In contrast to the results of the caudal section, GFAP expression in the rostral section revealed a significant overall prenatal treatment effect [F (1, 28) = 6.1, p ≤ 0.02] with, as shown in Figure 2, the prenatal cocaine-treated animals displaying a significant reduction of GFAP expression compared to the vehicle group. However, again the overall prenatal cocaine treatment effect for the female offspring noted above was suggested for GFAP expression in the rostral sections [F(1,28) = 4.1, p ≤ 0.052].
Figure 2.
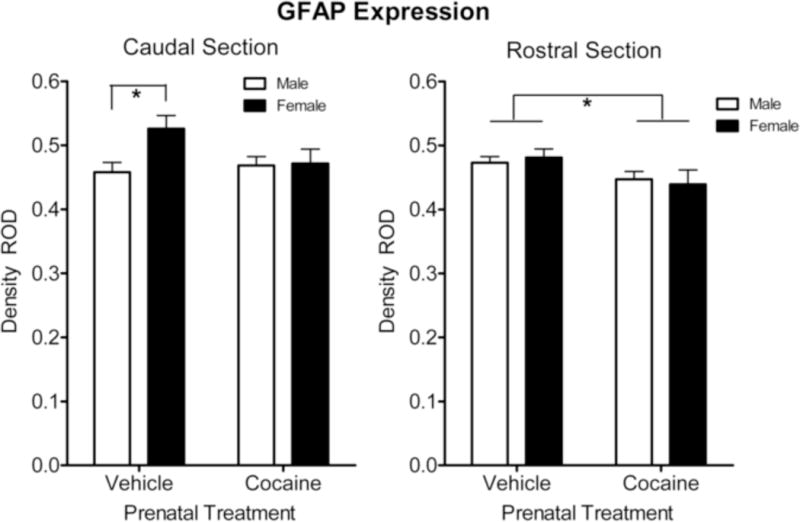
Mean (+/− SEM) expression of GFAP+ in the subcallosal area of the subventricular zone. Within the rostral section there was an overall significant reduction in GFAP expression by the prenatal cocaine treatment, relative to vehicle treated controls. In contrast, GFAP expression in the caudal section displayed a more selective alteration; the sex difference in GFAP expression was no longer apparent after prenatal cocaine treatment. *p ≤ 0.05.
3.2.2 Nestin Expression
The data on the expression of nestin is illustrated in Figure 3. The overall analysis of nestin expression revealed two prominent effects. Most importantly, there was a significant prenatal condition (cocaine vs. vehicle) by sex (male vs. female) interaction [F (1, 28) = 6.9, p ≤ 0.014]; the pronounced sex difference in the control group [F(1,28) = 20.2 p ≤ 0.001] was not evident in the prenatal cocaine-treated animals [F < 1.0]. Second, the nestin expression showed a significant sex effect, with the females showing a significantly higher [F (1, 28) = 14.6, p ≤ 0.001] expression of nestin compared to the males.
Figure 3.
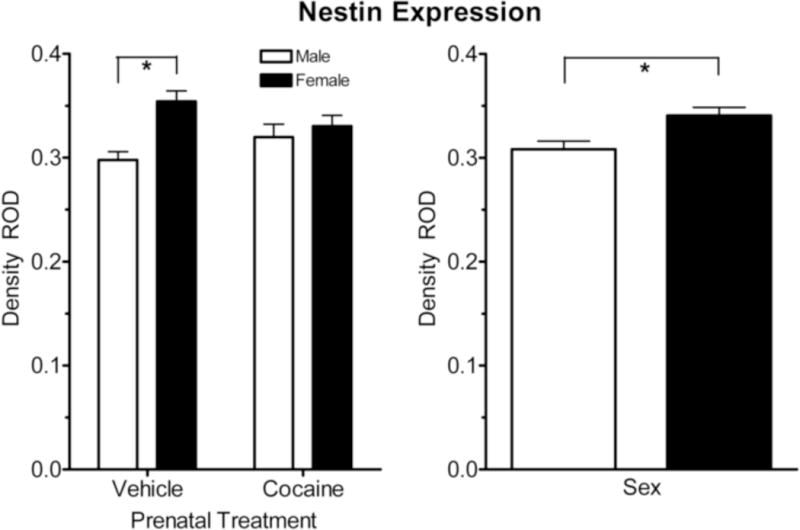
Mean (+/− SEM) expression of Nestin+(GFAP−) in the subcallosal area of the subventricular zone. Overall nestin expression (caudal and rostral combined) showed a significantly higher density in females compared to males, and a significant sex and condition (prenatal cocaine and control) interaction. A significant sex difference in expression of Nestin+(GFAP−) was also noted. *p ≤ 0.05.
The pattern of nestin expression along the caudal-rostral gradient is illustrated in Figure 4. The similar pattern of results in both the caudal and rostral sections suggested there was no rostral-caudal gradient to the observed effects [F < 1.0]. In other words, the basis of the overall prenatal condition by sex interaction was evident throughout the subcallosal portion of the SVZ. Specifically, within the caudal section there was a pronounced sex difference in nestin expression in the vehicle control offspring as adults [F (1, 28) = 16.2, p ≤ 0.001]; no sex difference was present in the prenatal cocaine treated offspring as adults. Similarly, in the rostral section, nestin expression was significantly higher in the adult vehicle control females compared to the males [F (1, 28) = 16.2, p ≤ 0.001]. Again, there was no sex difference in nestin expression in the prenatal cocaine-treated animals. Finally, the overall prenatal cocaine treatment effect for the female offspring noted above in the overall repeated-measures ANOVA was suggested for nestin expression in the rostral sections [F(1,28) = 5.0, p ≤ 0.033], but was not confirmed within the caudal sections (p > 0.10).
Figure 4.
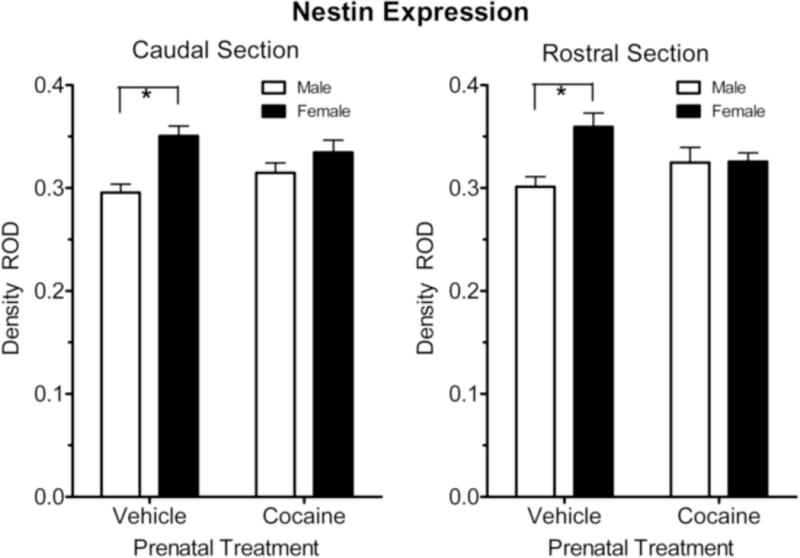
Mean (+/− SEM) expression of Nestin+(GFAP−) in the subcallosal area of the subventricular zone. Nestin expression in both the caudal and rostral regions displayed the basis for the prenatal treatment by sex interaction, i.e., the significant sex difference in nestin expression in the subcallosal area SVZ was no longer apparent after prenatal cocaine treatment. *p ≤ 0.05.
4. DISCUSSION
Prenatal exposure to cocaine, when delivered via a protocol designed to capture prominent features of recreational usage, altered the expression of GFAP+ (type B cells) and nestin+ (GFAP−) (Type C and A cells) staining in the subcallosal area of the SVZ in the adult (180 days-old) rat brain. Moderation of the effect of prenatal cocaine on both immunocytochemical markers by sex of the offspring was a second major finding of the present study. To the best of our knowledge, these data provide the first demonstration that the proliferative cell types of the subventricular zones (SVZ) may be persistently, if not permanently, altered by the prenatal cocaine exposure. Furthermore, as the SVZ represents an important niche for the dynamic ongoing process of adult neuron production, these data also suggest that early cocaine exposure may adversely impact this reservoir of progenitors and their function in the adult brain.
The acute in vitro effects of cocaine on AF5 neural progenitor cells is an inhibition of proliferation in neural progenitors; an effect attributed to oxidative endoplasmic reticulum stressmediated down regulation of cyclin A (Lee et al., 2008). The acute effects of cocaine in fetal rat brain, also demonstrated a down regulation of cyclin a2 mRNA in the frontal cortex; an effect restricted to in utero exposure during earlier, but not later, gestational periods corresponding to neocortical neurogenesis (Lee et al., 2008). It is of note that cocaine was delivered to the dam by the interperitoneal route of administration, a procedure that would facilitate extraplacental absorption of the drug, and with the dose level employed resulted in brain levels of up to 15,000 ng/g. The in vivo effects of more chronic cocaine exposure also impacts neurogenesis in the SVZ (and SGZ) as assessed via BrdU+ cell counts; following 3 weeks of cocaine self-administration (4h/day, 5d/week) proliferation is decreased, with 4 weeks of withdrawal reversing these changes (Noonan et al., 2008). Collectively, these data suggest that prenatal cocaine exposure, when delivered under a protocol designed to capture features of recreational drug abuse, would have long-term consequences for proliferative plasticity in the adult brain.
Given the selection mechanism that olfactory experience confers for the durable integration of nascent granule cells in the olfactory bulb (e.g., Petreanu and Alvarez-Buylia, 2002; Rochefort et al., 2002), it is tempting to speculate that cocaine-induced dysregulation of adult SVZ niche contributes to the impaired olfactory function seen in cocaine addicts. The functional relevance of cocaine-induced alterations in neurogenesis in the SVZ is quite plausible as the addition of new SVZ neurons to the olfactory bulb clearly enhances multiple aspects of olfaction (Gheusi et al., 2000; Shingo et al., 2003; Enwere et al., 2004). Cocaine use impairs olfactory function in humans, with abstinence precipitating notable improvements (Gordon et al., 1990; Bauer and Mott, 1996). In over 50% of a group of drug addicts, disturbances in olfactory performance on multiple assessments were identified, with intravenous injection and inhalation of drugs displaying the most significant olfactory problems (Podskarbi-Fayette et al., 2005). Notably, the intravenous abuse of cocaine precludes the otherwise confounding nasal septum damage characteristic of intranasal cocaine use, e.g., disorders ranging from rhinitis, nasal scabs, and epistaxis among adolescent experimental users (Schwartz et al., 1989) to septal necrotic perforations among chronic cocaine-abusing adults (Libby et al., 1992).
In preclinical models, repeated intravenous doses of cocaine in rats has been shown to promote the development of abnormal epileptiform spikes in the olfactory bulb and tubercle (Stripling and Ellinwood, 1977). Offspring exposed prenatally to cocaine are less likely than controls to develop an odor preference for stimuli associated with cocaine as infants (Heyser et al., 1992). Prenatal exposure to cocaine, at least when administered by the IV route, revealed reproducible alterations in attentional processes, as indexed by the noradrenergically-mediated HR-OR to novel olfactory stimuli (Mactutus, 1999; Foltz et al., 2004). The documentation of a linear dose-response function suggested that there is likely no threshold for the drug-induced alteration. Adult Long–Evans rats, exposed prenatally to cocaine (0.5, 1.0, or 3.0 mg/kg iv), were impaired, relative to controls, on a 3-choice visual attention task when olfactory distractors presented unpredictably on one third of the trials consistent with an impairment in selective attention (Gendle et al., 2003). When prenatal cocaine exposed animals were assessed in adulthood in that visual discrimination/olfactory distraction paradigm for their sensitivity to the attentional effects of idazoxan (an alpha-2 adrenergic antagonist that increases coeruleocortical NE activity), cocaine animals were significantly more sensitive than controls to the effects of IDZ on omission errors and nontrials, a pattern consistent with alterations in sustained attention (Bayer et al., 2002). Obviously, one critical hypothesis to assess is whether cocaine-induced deficits involving olfactory processes are secondary to decreased SVZ proliferation and adult-generated olfactory bulb neurons.
Adult neuron production is a dynamic ongoing process. Estimates suggest that some 10,000–30,000 neurons migrate to the olfactory bulb every day (Lois & Alvarez-Buylla, 1994). It is also worth noting that neurogenesis in the olfactory bulb occurs in a neuronal network in which sensory inputs are continuously replaced. Mature olfactory sensory neurons have only a limited life span – 90 days in rodents (Lledo et al., 2005). Thus, it may very well be that neurogenesis in the olfactory bulb represents a mechanism by which processing of sensory information in the brain could be adjusted in response to ever-changing sensory inputs (Lledo and Saghatelyan, 2005). The present data do not address whether the observed alterations in immunocytochemical markers reflect an enduring effect of the prenatal exposure, or alternatively, a latent effect expressed during adulthood, on this dynamic proliferative process. Nevertheless, it is quite clear that with the utilization of 6-month-old offspring, a long-term effect was indeed uncovered.
Maternal mediated vs. direct effects of the prenatal cocaine exposure was addressed by the use of surrogate fostering of all offspring. Accordingly, any potential alterations in maternal care, or effects of any residual presence of the drug, would not contribute to the postnatal environment to moderate the in utero effects of the drug.
The detrimental effects of prenatal exposure to high doses of cocaine on the cytoarchitecture of the brain during fetal brain development are well known and often cited (Gressens et al., 1992; Kosofsky et al., 1994; Novikova et al., 2005; Lidow, 1995; Lidow and Song, 2001); but much less appreciated is just how severe the conditions are under which such massive developmental dysregulation is noted. For example, in the mouse model, while cocaine administered during corticogenesis disrupted horizontal lamination, impaired establishment of vertical columns, and markedly decreased the number of radially organized axonal-dendritic bundles (Gressens et al., 1992), these disturbances were reported under conditions in which the entire brain size is reduced by about 25% (Gressens et al., 1992). Similarly, although administration of cocaine at the time of neocortical neurogenesis reduced neocortex volume, disturbed lamination, altered positioning of cerebral cortical neurons, and reduced density and number of cortical neurons in sub-human primates (Lidow, 1995; Lidow and Song, 2001), these alterations in corticogenesis were found after maternal doses of cocaine sufficient to reduce the entire brain volume of the offspring by > 20% (Lidow, 1995). Even under such adverse developmental insults, the extant data suggest that these alterations are primarily a temporally constrained occurrence, i.e., due to the active presence of the drug per se. Specifically, in the aforementioned primate model, neither a nearly a month-long cocaine exposure prior to neocortical neurogenesis nor a nearly 2-month-long cocaine exposure following neocortical neurogenesis produced significant alterations in the number of neocortical neurons; the process of corticogenesis returned to normal as soon as the administration of cocaine is discontinued (Lidow et al., 2001).
The present data are nevertheless consistent with the reports indicating that low ‘recreational’ dose exposure of prenatal cocaine do indeed have adverse consequences for the developing brain. Several studies using a preclinical intravenous injection model, to more closely mimic the route and pharmacokinetics of the smoking of crack cocaine, have discovered a more subtle disruption of cortical development may nevertheless be observed (disruption of organization of anterior cingulate cortex in 20–25 day-old rabbits, Stanwood et al., 2001; irregularities in the formation of cortical minicolumns in weanling rats, Buxhoeveden et al., 2006).
With respect to mechanism as to how the prenatal cocaine exposure might be exerting such a long term effect on markers of adult neuroplasticity in the SVZ, this might be an effect of long-term alterations in DA and NE systems that are reported with prenatal cocaine exposure. Thus, for example, selective DA agonists stimulate SVZ proliferation while DA depletion inhibits it (Hoglinger et al., 2004; Borta and Hoglinger, 2007). Repeated self-administration of cocaine in rats across three weeks precipitates a 20% reduction in cell proliferation in the SVZ without any alterations in SVZ volume (Noonan et al., 2008). However, in mice, repeated cocaine exposure and short-term abstinence failed to have any detectable effect on cell proliferation or in the migratory pattern of BrdU-labeled cells in the SVZ/RMS. (Lloyd et al., 2010).
Independent of its actions mediated through monoaminergic transporter proteins, the effects of cocaine may indirectly reflect the tropic and trophic influences of monoamines on neurons in the developing CNS [Lipton and Kater, 1989]. For example, changes in dopamine (DA) and norepinephrine (NE) levels have been shown to modulate neuron division, growth, migration and differentiation [Lauder, 1993; Leslie, 1993; Reinoso et al., 1996; Robinson et al., 2001]. Additional emerging evidence from both in vitro and in vivo studies of noradrenergic neurons of the locus coeruleus demonstrated that cocaine decreased cell survival as well as neurite elongation in comparison to vehicle controls (Snow et al., 2001; 2004; Dey et al., 2006). In subsequent studies, cocaine exposure in vitro induced apoptosis in fetal LC neurons putatively regulated by Bax, via activation of caspases and their downstream target proteins (Dey et al., 2007; Dey & Snow, 2007).
The current investigation suggests the importance of understanding the proliferative plasticity of the brain not simply as a function of being exposed prenatally to cocaine, but dependent on the characteristic of sex of the offspring. Collapsing across sex would have markedly altered the observed outcome. Clearly, when using sex as a mediating factor of prenatal cocaine exposure, we discovered alterations in immunocytochemical marker expression differ between male and female rats. Interestingly, we have also reported sex differences in brain neurotransmitter proteins in adolescent animals (Booze et al., 2006; Silvers et al., 2006) and adult animals through one year of age (Ferris et al., 2007). However, sex-specific alterations in receptor density may be restricted to particular brain regions, and/or differ in directionality within the same study, suggesting we may not be capturing the broader effect on the system being assessed (Booze et al., 2006). Similarly, the sex of the animal also appeared to play some role in the nature of the behavioral expression of prenatal cocaine effects. Alterations in the HR-OR to prenatal cocaine exposure clearly demonstrated that both male and female offspring were affected by the prenatal cocaine exposure, however, the expression of the dose–response functions were significantly different as a function of sex (Foltz et al., 2004). With the assessment in adulthood of long-term alterations in attentional processes, sex differences are also noted, albeit the adverse effects of cocaine are noted on different measures in the two sexes of animals (Gendle et al., 2003). Despite the fact that sex differences in response to prenatal cocaine exposure have been reported in hundreds of clinical and preclinical publications (Dow-Edwards, 2010), it remains uncertain whether such sex differences truly reflect different underlying processes, pharmacokinetic differences, or the specific circumscribed measures that typify any single investigation or model. The broader and more parsimonious perspective is that many such reports where the responses of males and females are not the same reflect more circumscribed observations and that from a systems approach, both sexes are affected, but the expression of the effects is observed on different measures. As apparent above, alterations in the noradrenergic system, the one that we have investigated most thoroughly, highlight this more parsimonious perspective.
Collectively, the present findings suggest that prenatal exposure to cocaine, when delivered via a protocol designed to capture prominent features of recreational usage, can selectively alter the major proliferative cell types in the subcallosal area of the SVZ in an adult rat brain, and does so differently for males and females. Further, as the SVZ represents an important niche for the dynamic ongoing process of adult neuron production, these data also suggest that early cocaine exposure may adversely impact this reservoir of progenitors and their function in the adult brain. Future research will be important to determine the functional implications of these suggested alterations in proliferative capacity.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health: DA09160 and HD043680 (to CFM), DA13137 and DA14401 (to RMB). The research reported herein was submitted by D.A. Patel in partial fulfillment of the requirements for graduation with honors from the University of South Carolina Honors College. The authors gratefully acknowledge the technical skill and expertise of Ulla Hasselrot in the conduct of the study. D.A. Patel is now at the Medical University of South Carolina, Charleston, South Carolina, United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–458. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Bateman DA, Chiriboga CA. Dose-response effect of cocaine on newborn head circumference. Pediatrics. 2000;106(3):E33. doi: 10.1542/peds.106.3.e33. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Mott AE. Differential effects of cocaine, alcohol, and nicotine dependence on olfactory evoked potentials. Drug Alcohol Depend. 1996;42:21–6. doi: 10.1016/0376-8716(96)01258-6. [DOI] [PubMed] [Google Scholar]
- Bayer LE, Kakumanu S, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure alters sensitivity to the effects of idazoxan in a distraction task. Behav BrainRes. 2002;133:185–196. doi: 10.1016/s0166-4328(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974;153:27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthesized, freely moving male rats. Neurotoxicol Teratol. 1997;19:7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Wallace DR, Silvers JM, Strupp BJ, Snow DM, Mactutus CF. Prenatal cocaine exposure alters alpha2 receptor expression in adolescent rats. BMC Neurosci. 2006;7:33. doi: 10.1186/1471-2202-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden DP, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF. Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Devl Neuroscience. 2006;24:335–341. doi: 10.1016/j.ijdevneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Starr D, Kuhn L, Wasserman GA. Prenatal cocaine exposure and prolonged focus attention. Poor infant information processing ability or precocious maturation of attentional systems? Dev Neurosci. 2009;31:149–158. doi: 10.1159/000207502. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, Frisén J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, Lynskey M, Wiessing L, The GBD Illicit Drug Use Writing Group What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Dey S, Mactutus CF, Booze RM, Snow DM. Specificity of prenatal cocaine on inhibition of locus coeruleus neurite outgrowth. Neuroscience. 2006;139:899–907. doi: 10.1016/j.neuroscience.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Dey S, Mactutus CF, Booze RM, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons by altering the Bax/Bcl-2 ratio and through caspase-3 apoptotic signaling. Neuroscience. 2007;144:509–521. doi: 10.1016/j.neuroscience.2006.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNF-alpha-mediated induction of Bax and phosphorylated c-Jun NH(2)-terminal kinase. J Neurochem. 2007;103:542–556. doi: 10.1111/j.1471-4159.2007.04750.x. [DOI] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D. Sex differences in the effects of cocaine abuse across the life span. Physiol Behav. 2010;100:208–215. doi: 10.1016/j.physbeh.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M, Cone EJ, Henningfeld JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279:1345–1356. [PubMed] [Google Scholar]
- Eyler FD, Warner TD, Behnke M, Hou W, Wobie K, Garvan CW. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31:121–136. doi: 10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Silvers JM, Hasselrot U, Beaudin SA, Strupp BJ, Booze RM. Sex mediates dopamine and adrenergic receptor expression in adult rats exposed prenatally to cocaine. Int J Dev Neurosci. 2007;25:445–454. doi: 10.1016/j.ijdevneu.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Foltz TL, Snow DM, Strupp BJ, Booze RM, Mactutus CF. Prenatal intravenous cocaine and the heart rate-orienting response: a dose-response study. Int J Dev Neurosci. 2004;22:285–296. doi: 10.1016/j.ijdevneu.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AS, Moran DT, Jafek BW, Eller PM, Strahan RC. The effect of chronic cocaine abuse on human olfaction. Arch Otolaryngol Head Neck Surg. 1990;116:1415–1418. doi: 10.1001/archotol.1990.01870120061010. [DOI] [PubMed] [Google Scholar]
- Gressens P, Kosofsky BE, Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992;140:113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Goodwin GA, Moody CA, Spear LP. Prenatal cocaine exposure attenuates cocaine-induced odor preference in infant rats. Pharmacol Biochem Behav. 1992;42:169–173. doi: 10.1016/0091-3057(92)90461-n. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Volume I: Secondary school students (NIH Publication No 10-7584) Bethesda, MD: National Institute on Drug Abuse; 2010. Monitoring the Future national survey results on drug use, 1975–2009; p. 734. [Google Scholar]
- Kosofsky BE. Cocaine-induced alterations in neuro-development. Semin Speech Lang. 1998;19:109–121. doi: 10.1055/s-2008-1064040. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- LaBella FS, Queen G. General anesthetics inhibit cytochrome P450 monoxygenases and arachidonic acid metabolism. Can J Physiol Pharmacol. 1993;71:48–53. doi: 10.1139/y93-007. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL, Amable R, Su TP, Lowe RH, Huestis MA, Shen J, Becker KG, Geller HM, Freed WJ. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5(6):e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leslie FM. Neurotransmitters as neurotrophic factors. In: Fallon JH, Loughlin SE, editors. Neurotrophic Factors. San Diego: Academic Press; 1993. pp. 565–598. [Google Scholar]
- Libby DM, Klein L, Altorki NK. Aspiration of the nasal septum: a new complication of cocaine abuse. Ann Intern Med. 1992;116:567–568. doi: 10.7326/0003-4819-116-7-567. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Bozian D, Song ZM. Cocaine affects cerebral neocortical cytoarchitecture in primates only if administered during neocortical neuronogenesis. Brain Res Dev Brain Res. 2001;128:45–52. doi: 10.1016/s0165-3806(01)00139-0. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001;435:263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Gheusi G, Vincent JD. Information processing in the mammalian olfactory system. Physiol Rev. 2005;85:281–317. doi: 10.1152/physrev.00008.2004. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Balest ZR, Corotto FS, Smeyne RJ. Cocaine selectively increases proliferation in the adult murine hippocampus. Neurosci Lett. 2010;485:112–116. doi: 10.1016/j.neulet.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loch JM, Potter J, Bachmann KA. The influence of anesthetic agents on rat hepatic cytochromes P450 in vivo. Pharmacology. 1995;50:146–153. doi: 10.1159/000139276. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug abuse in the pregnant and/or group-housed rat: an initial study with cocaine. Neurotoxicol Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicol Teratol. 1999;21:539–550. doi: 10.1016/s0892-0362(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Booze RM, Dowell RT. The influence of route of administration on the acute cardiovascular effects of cocaine in conscious unrestrained pregnant rats. Neurotoxicol Teratol. 2000;22:357–368. doi: 10.1016/s0892-0362(99)00084-7. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Nissant A, Pallotto M. Integration and maturation of newborn neurons in the adult olfactory bulb–from synapses to function. Eur J Neurosci. 2011;33:1069–1077. doi: 10.1111/j.1460-9568.2011.07605.x. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; NY: 2006. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6013. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podskarbi-Fayette R, Rydzewski B, Lipińska M. Smell and taste in drug addicts. Otolaryngol Pol. 2005;59:585–590. [Article in Polish] [PubMed] [Google Scholar]
- Reinoso BS, Undie AS, Levitt P. Dopamine receptors mediate differential morphological effects on cerebral cortical neurons in vitro. J Neurosci Res. 1996;43:439–453. doi: 10.1002/(SICI)1097-4547(19960215)43:4<439::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, Enters EK, Jackson GF, Chinchilli VM, Maher JR, McDowell KP, Allen HM, Guo H. Maternal and fetal brain and plasma levels of cocaine and benzoylecgonine after acute or chronic maternal intravenous administration of cocaine. J Pharmacol Exp Ther. 1994;271:1234–1239. [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Roussotte F, Soderberg L, Sowell E. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychol Rev. 2010;20:376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-García VJ, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Estroff T, Fairbanks DN, Hoffmann NG. Nasal symptoms associated with cocaine abuse during adolescence. Arch Otolaryngol Head Neck Surg. 1989;115:63–64. doi: 10.1001/archotol.1989.01860250065028. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol. 2006;28:173–180. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Singer LT, Lewin J, Minnes S, Weishampel P, Drake K, Satayathum S, Boll DT, Zijdenbos A, Paus T, Evans A. Neuroimaging of 7–8 year-old children exposed prenatally to cocaine. Neurotoxicol Teratol. 2006;28:393–396. [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Carman HM, Smith JD, Booze RM, Welch MA, Mactutus CF. Cocaine-induced inhibition of process outgrowth in locus coeruleus neurons: role of gestational exposure period and offspring sex. Int J Dev Neurosci. 2004;22:297–308. doi: 10.1016/j.ijdevneu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Snow DM, Smith JD, Booze RM, Welch MA, Mactutus CF. Cocaine decreases cell survival and inhibits neurite extension of rat locus coeruleus neurons. Neurotoxicol Teratol. 2001;23:225–234. doi: 10.1016/s0892-0362(01)00137-4. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001;11:430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- Statistical Solutions, Ltd. BMDP 2009. Statistical Solutions; Cork, Ireland: 2009. [Google Scholar]
- Stripling JS, Ellinwood EH. Sensitization to cocaine following chronic administration in the rat. In: Ellinwood EH, Kilbey MM, editors. Cocaine and Other Stimulants. Plenum Press; New York: 1977. pp. 327–351. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No SMA 10-4586Findings) Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. [Google Scholar]
- Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999;9:135–141. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- von Bohlen, Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345:1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2. New York: McGraw-Hill; 1971. [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]


