Abstract
Plasmacytoid dendritic cells (pDCs) are a specific subset of naturally occurring dendritic cells, that secrete large amounts of Type I interferon and play an important role in the immune response against viral infection. Several studies have highlighted that they are also effective antigen presenting cells, making them an interesting target for immunotherapy against cancer. However, the modes of action of pDCs are not restricted to antigen presentation and IFN secretion alone. In this review we will highlight a selection of cell surface proteins expressed by human pDCs that may facilitate communication with other immune cells, and we will discuss the implications of these molecules for pDC-driven immune responses.
Keywords: cross talk, surface markers, T lymphocytes, viral infection, pDC migration
Introduction
Within the heterogeneous dendritic cell (DC) family, two main subsets of naturally occurring blood DCs can be discriminated based on their phenotype and functional characteristics: myeloid DCs (mDCs) and plasmacytoid dendritic cells (pDCs). The mDC subset can be further divided in CD1c+ and CD141+, which show a high level of similarity in protein expression yet have also specific functions in the initiation of adaptive immune responses. CD1c+ mDCs have been shown to readily stimulate naïve CD4+ T cells and to secrete high amounts of IL-12 in response to toll-like receptor (TLR) ligation, whereas CD141+ DCs do not secrete much IL-12 but are well equipped to take up dead and necrotic cells for subsequent cross presentation of derived antigens to CD8+ T cells (1–4). In contrast to mDCs, pDCs have a very different protein expression profile reflecting their important and unique function in the secretion of IFN-α and anti-viral immune response (1, 2, 5). We and others have however recently demonstrated that like mDCs, pDCs are also very well capable of presenting both soluble and particulate exogenous antigens on both major histocompatibility complex (MHC) class I and II (6). In recent years, numerous studies have been performed to characterize the expression of pathogen recognition receptors (PRRs), TLRs, Fc receptors, C-type lectin (CTL) receptors, and other surface receptors on these cells (7–13). Furthermore, these studies have emphasized both similarities and differences between DC subtypes in their cytokine release profiles, and their ability to acquire, process, and present antigens (5, 14–17). These characteristics of the different DC subtypes have recently been reviewed extensively elsewhere (2, 4, 6, 18). Here, we will focus our attention specifically on pDCs, their role in immunity and, more specifically, their (potential) direct interactions with cells of the innate and adaptive immune system via cell surface molecules. Before going into detail about these cell surface receptors and how they mediate intercellular communication, we will first give a brief summary on general pDC function and localization to provide a context in which these intercellular communications take place. Although studies on murine pDCs are numerous, and commonalities between human and murine pDCs certainly exist, major differences between pDC of both species have also been reported. Therefore, in order to prevent confusion we limited ourselves to human pDCs unless explicit stated otherwise.
pDC Function
A perturbation of the homeostatic condition that sets off the immune system can trigger either an immunogenic (immunostimulatory) or a tolerogenic (immunosuppressive) response, depending on the local circumstances and type of disease. By default, immature pDCs are tolerogenic, whereas activated (mature) pDCs can have both immunogenic and tolerogenic capacities depending on the local environment in which they are activated (19–21). pDCs are characterized as Lin− MHC-II+ CD123 (IL3R)+ CD4+ CD303(BDCA-2)+ CD304(BDCA4; Neuropilin-1)+ and are mostly known for their ability to quickly produce large amounts of the Type I interferons (IFNs), IFN-α, and IFN-β, following viral infection, implicating pDCs as an important contributor during the early phase of anti-viral response (2, 22, 23).
The most important documented enveloped viruses known to stimulate Type I IFN release by pDCs are human immunodeficiency virus type 1 (HIV-1), herpes simplex virus (HSV), and influenza virus (24–27). Furthermore, parasites and bacteria containing DNA with unmethylated CpG sequences can trigger pDC activation (28–31). In addition to the anti-viral capacity, Type I IFN release by pDCs has also been reported to be important for pDC survival, (m)DC-mediated CD4+ and CD8+ T cell responses, mDC differentiation, cross presentation, upregulation of co-stimulatory MHC molecules and activation of natural killer (NK), and B cells (32–34).
Because of their expression of the endosomal TLRs TLR7 and 9, pDCs, in contrast to other (immune) cells, do not need to become infected to respond to viruses or intracellular bacteria (35, 36). TLR7 recognizes guanosine or uridine-rich, single-stranded RNA from viruses or synthetic products like guanosine analogs such as R848. TLR9 senses single stranded DNA containing unmethylated CpG motifs, which are usually found in bacterial and viral genomes, and additionally senses for synthetic oligonucleotides, such as CpG-ODN (37, 38). pDCs show differential responses based on the type of virus/bacteria that is recognized, which has been suggested to be attributed to a different site of TLR activation within the endosomal system (39). For example, depending on the subtype of CpG recognized (CpG-A, CpG-B, CpG-C) the outcome of the response can be different. While CpG-A, that triggers TLR9 in early endosomes, induces IFN-α release, CpG-B, signaling from late endosomes, leads to tumor necrosis factor α (TNF-α) and IL-6 production by pDCs (40). In addition, the interplay of the various PRRs tailors the pDC response to a specific pathogenic threat. In addition to TLRs, pDCs express several CLRs, including BDCA-2, DEC-205, dectin-1 and DCIR, and Fc receptor CD32, but they lack for instance DC-SIGN (22, 41–45). Although the full repertoire of receptors is still under investigation, most of these receptors drive antigen uptake, and in concert with TLR7 and 9, coordinate pDC-mediated immune responses.
pDC Localization
Immature pDCs circulate in the blood but have been equipped with migratory capacities as they are found within lymph nodes (LNs), tumors, and near sites of viral/bacterial infection (46, 47). At all these sites pDCs are able to promote inflammatory responses by attracting other immune cells through chemokine release, and the subsequent modulation of these cells via cytokines or direct cell–cell interactions (48–51). However, in contrast to human myeloid mDCs or murine pDCs studies, reports addressing which inflammatory chemokines and adhesion receptors specifically drive migration of human pDCs are scarce (52). Human pDCs express chemotactic receptors C-C chemokine receptor type 7 (CCR7), chemokine (C-X-C motif) receptor 3 (CXCR3), CXCR4, and ChemR23 (CMKLR1) that likely mediate migration of pDCs into lymphoid organs and/or into inflamed tissue (48, 52–55). However, due to conflicting reports the role of classical lymphoid tissue CCR7− Chemokine (C-C motif) ligand 21 (CCL21)/CCL19 pathways in resting human pDCs, is not conclusive yet (53, 56). Several studies show a high expression of CCR7 on “resting” blood DCs while others have reported a very low or a lack of expression on resting pDCs (53, 57–60). Similar to mDCs and murine pDCs, human pDCs upregulate expression of CCR7 upon TLR stimulation and migrate toward CCL21 molecules, suggesting an important role of CCR7 at least for the migration of mature pDCs to the LN (55). Furthermore IL-3 produced by T cells in the LN or by activated endothelial cells can lead to the upregulation of chemokine receptor 6 (CCR6) and CCR10 that may drive migration of activated IFN producing pDCs to inflamed skin or mucosa (61).
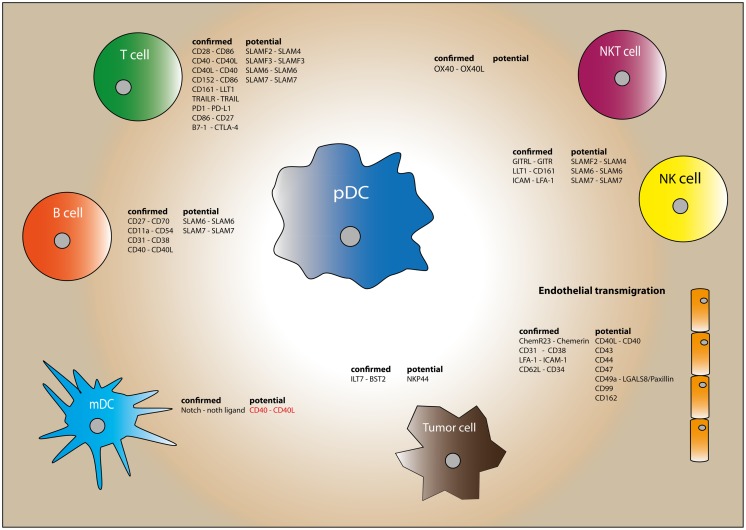
In contrast to mDCs, which migrate from peripheral tissue to secondary lymphoid organs via afferent lymphatic vessels, pDCs have been described to migrate to the LN mostly directly from the blood via high endothelial venules (HEVs) (62, 63). Since pDCs first need to engage and traverse the endothelial cells lining of the blood vessels, endothelial cells likely represent the first cellular contact pDCs will engage in after leaving the blood stream. pDC would require a similar migration capacity to enter into inflamed or tumor tissue, which also requires interaction with endothelial cells and extravasation. Next, within the LN, or at the site of infectious or cancerous lesions, pDCs may encounter various immune cells. In the LN, pDC have been found in close contact with T lymphocytes, Invariant Natural Killer T (iNKT) cells, B lymphocytes, and NK cells (21, 24, 42, 64–66). At sites of infection pDCs might activate or get activated by mDCs and NK cells, whereas within the tumor microenvironment pDCs are known to interact predominantly with tumor cells and regulatory T (Treg) cells (67, 68). Below we have summarized the evidence reported thus far for each of these (potential) interactions, and the circumstances under which they occur (Figure 1).
Figure 1.
Plasmacytoid dendritic cell have the capacity to interact with various immune cells through an array of surface molecules. The expressed surface molecules of each cell type are divided into confirmed and potential interactions. The “confirmed” molecules have been reported to have a functional effect. Molecules listed in the “potential” column are molecules that have been found on human pDC but without functional data reported in literature. Molecules playing a potential role in humans, but already confirmed with functional studies in mouse are depicted in red.
Endothelial Cells
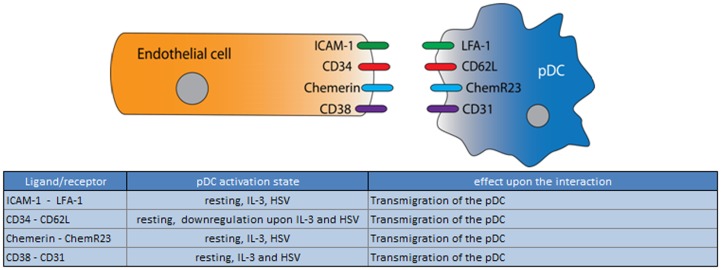
Depending on their location (peripheral tissue or LN) and activation state, endothelial cells have been shown to express distinct cell surface molecules and to secrete a variety of chemokines and cytokines that may aid leukocyte transmigration and regulate the activation state of the migrating cells (69). Endothelial cells thus not only facilitate pDC transmigration into the site of infection, the tumor lesion, or the LN but may also have the potency to influence pDCs mediated immune responses trough pro- or anti-inflammatory cytokines as well as growth factors (69). Indeed, endothelial cells also produce IL-3 and VEGF that bind and trigger pDC marker proteins CD123 and BDCA4 respectively, and likely will promote pDC survival and migration after crossing the endothelial barrier. Documentation however of the crosstalk between human pDCs and endothelia is scarce and limited to a few recent studies that we will discuss. Intriguingly, and in contrast to murine pDCs, both resting and matured human pDCs (stimulated by influenza virus) uniquely express the receptor for chemerin, ChemR23 (48). Chemerin is present on the surface of endothelial cells in the lumen of HEVs as well as in blood vessels of inflamed tissue. The interaction between endothelial cell-bound chemerin and pDC ChemR23 seems to play a crucial role in the migration of pDC from the blood both into LNs and into inflamed tissue (Figure 2) (48, 70, 71). Like pDCs, T cells also migrate from the blood to the LN via HEVs and thus pDCs may exploit a similar set of molecules as used by T cells. Indeed, pDCs express adhesion molecules CD31, CD43, CD44, CD47, CD62L, CD99, and CD162 (SELPLG, CLA) that may play an important role in the tethering and rolling of pDCs on endothelial cells, but for most of these molecules, functional data for a role on human pDCs is lacking (54, 72, 73). The Lymphocyte function-associated antigen 1 (LFA-1) and very late antigen 1 (VLA-1) (CD49a/CD29) molecules might play an important role in subsequent firm adhesion and transmigration of pDCs (72). Although the expression of all these molecules was initially only detected by microarray, with the exception of CD44, most were confirmed by flow cytometry (74). Furthermore, flow cytometry demonstrated that expression of both CD62L (moderately) and CD99 was downregulated upon exposure to IL-3 and HSV, indicating that activated pDCs may take different migratory routes compared to their immature counterparts (74). While immature pDCs express CD62L and use HEVs to migrate into the LN, downregulation of CD62L on mature pDCs suggests that these cells enter LN without passing HEV, but rather through the lymphatic vessels. Furthermore, another study identified a cleavage of CD62L after entering the HEV suggesting this molecule may have become obsolete for pDCs following this pathway (75). In skin, in contrast, after transversing the vessel wall expression of CD62L on pDC remains high, indicating that in this case it may still have a function at a later stage (54).
Figure 2.
Ligand/receptor paring of a pDC with an endothelial cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
In summary, although there is evidence for Intercellular Adhesion Molecule 1 (ICAM-1)/LFA, CD31/CD38, and CD34/CD62L interaction between pDCs and endothelial cells, until now, only the chemerin/ChemR23 interaction has been conclusively demonstrated to play a role during the migration process. The migratory function of the other adhesion molecules reportedly expressed by human pDC are currently only hypothetical and based on knowledge from other leukocytes or murine cells. Furthermore also the role of endothelia in the regulation of the pDC activation state awaits further study.
T Cells
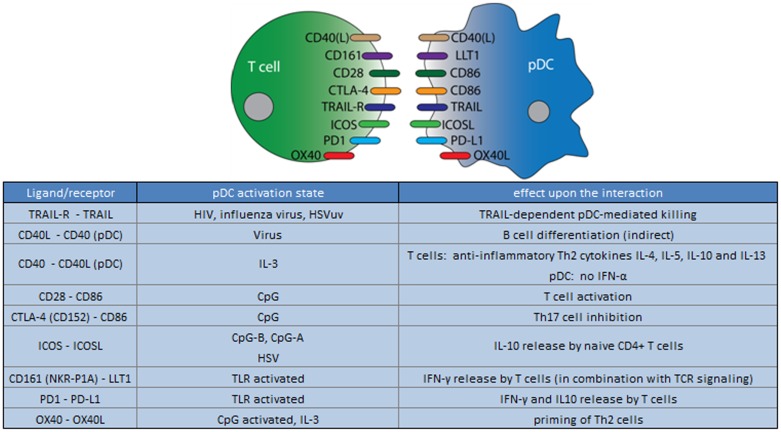
During infection, immature DCs located in the inflamed tissue get activated through pathogenic interaction and pro-inflammatory cytokines. Mature (activated) DCs subsequently translocate to the LN and induce naïve T cells to differentiate into effector T cells. Based on the repertoire of danger signals, effector T cells will have different characteristics and will evoke a different immune response. pDCs have an important role in coordinating such an immune response, since the molecules involved in the interaction between DCs and T cells determine T cell polarization (Th1, Th2, Th17). Numerous studies have established that pDCs are bona fide antigen presenting cells (APCs), capable of presenting exogenous antigens on both MHC class I and II molecules and thus can trigger both CD4+ T helper (Th) cells and CD8+ cytotoxic T cells (5, 26, 76–78). The nuances of pDCs antigen processing and presentation have recently been reviewed by Guery and Hugues (42) and Nierkens et al. (79). Here, we focus our attention on how pDC cell surface receptors may skew T cell function (Figure 3). Freshly isolated (immature) pDCs are known to induce CD4+ T cell anergy presumably because they lack co-stimulatory molecules; conversely, activated pDC clearly induce a broad spectrum of T cell differentiation, for example, Th1, Th2, Th17, and Treg, based on the cytokines secreted and cell surface proteins expressed (21, 80–84). Like mDCs, activated pDC express high levels of MHC molecules and the co-stimulatory molecules CD80 (B7-1), CD86 (B7-2), and CD83 to present antigens and fully license and activate T cells (5, 6). Several studies have demonstrated that (virally) matured pDCs, through the release of cytokines, mostly induce a Th1 phenotype (IFN-γ/Il-12 in response to CpG, virus) but Th2 (IL-4) and Th17 (IL-17) skewing has also been reported when pDC are activated with IL-3 or CD40 and TLR7 ligands, respectively (82, 85–87). Furthermore IL-21 (produced in the LN) was shown to trigger the release of Granzyme B by TLR-activated pDCs thereby dampening CD4+ T cell proliferation (88). Together these studies show how pDCs may regulate immune responses. Apart from cytokines released by pDCs, several pDC surface receptors may directly affect T cell skewing and function, including the inducible T-cell co-stimulator ligand (ICOSL). pDCs express ICOSLG when activated by CpG-(A, B, and C) IL-3/CD40L or virus (Flu/HSV) (83). ICOSLG is the ligand for the T-cell-specific cell surface receptor inducible costimulator (ICOS) and has been shown to trigger naive CD4+ T cells to produce IL-10 during both pDC Th1 or Th2 skewing in response to CpG/virally or IL-3/CD40L-matured pDCs, respectively (83, 84). It has been suggested that ICOSL-activated pDCs generate IL-10 producing Tregs to dampen immune responses, preventing excessive inflammation (83). Furthermore TLR activated, but not resting pDCs and mDCs, express programed death receptor-ligand 1 (PD-L1), which may induce T cells anergy/suppresses T cell activation by binding to its receptor, program death ligand 1 (PD1), which is expressed by T cells (89, 90). The immunosuppressive effect of PD-L1 has been confirmed by using blocking antibodies on DCs, and additionally in follow-up studies where blocking the PD-L1/PD1 interaction lead to “enhanced tumor-specific T cell expansion and activation” (6, 91, 92). The surface receptor OX40, which is expressed on IL-3 activated pDCs, can induce a Th2 T cell response resulting in IL-4, IL-5, and IL-13 release by CD4+ T cells (93, 94).
Figure 3.
Ligand/receptor paring of a pDC with a T cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
Furthermore, after stimulation either with synthetic TLR7 and 9 agonists or with the natural TLR7 agonists, like influenza virus or UV-inactivated HSV type 1(HSVUV) pDCs can induce programed cell death/apoptosis, by expressing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (74, 95, 96). TRAIL expression on pDCs uniquely correlates with viral load, and the capacity to kill HIV-infected CD4+ T cells by binding to the TRAIL receptor, a process described as “TRAIL-dependent pDC-mediated killing” (97). However, given the very limited cell numbers, it remains to be seen how important TRAIL+ pDCs are in clearing a viral infection via the direct killing of infected cells (97, 98).
Another surface molecule expressed on TLR-activated pDCs that may affect T cell function is the lectin-like transcript 1 (LLT1), which in addition to activated pDCs, is expressed by most activated lymphocytes (including B cells, T cells, and NK cells) and mature monocyte-derived DCs (99). LLT1 is a ligand of CD161 (NKR-P1A), which is expressed by subsets of T cells (e.g., Th1, Th17, and a subpopulation of CD8+ T cells) and NK cells. When ligated LLT1 triggers T cell proliferation and IFN-γ secretion as well as inhibition of NK cell cytotoxicity (99–102). Thus, LLT1 on pDCs may serve as a co-stimulatory molecule, and after binding to CD161 expressing T cells, could drive proliferation and IFN-γ secretion (51).
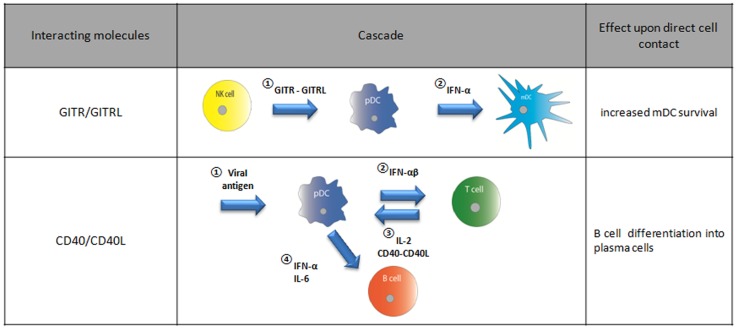
So far, we discussed how pDC receptors may affect T cell function but of course, conversely, T cells may also influence pDC function. In a multicellular immune cell signaling cascade the presentation of viral antigens by pDCs brings about IL-2 release by T cells as well as CD40L expression. T cell CD40L upon binding to CD40 on pDCs, triggers IL-6 release by pDC, which in turn enables B cell plasma blasts to become antibody-secreting plasma cells (Figure 8) (21, 64).
Figure 8.
Direct cell interaction dependent on GITR/GITRL or CD40/CD40L binding and their effect on a certain cell type.
In summary, while immature pDCs predominantly induce T cell anergy, their activated counterparts may have either inhibitory or activating effects on T cells. Which of the latter in the case depends on stimuli that trigger pDC maturation and which cytokines and surface molecules are expressed as a result. Thus pDCs play pivotal role in T cell activation and fine tuning of the adaptive immune response.
iNKT Cells
Natural Killer T (NKT) cells form a specialized T cell subset expressing a semi-invariant T cell receptor (TCR-αβ) and surface antigens traditionally associated with NK cells. The unique TCR on their cell surface enables NKT cells to recognize glycolipid antigens rather than peptides, presented in the context of the MHC class I-like molecule, CD1d (103). The most well characterized subset of NKT cells are called iNKT cells, since they express an invariant TCR-α chain, and are reactive to the potent NKT cell agonists α-galactosylceramide (α-GalCer) (103).
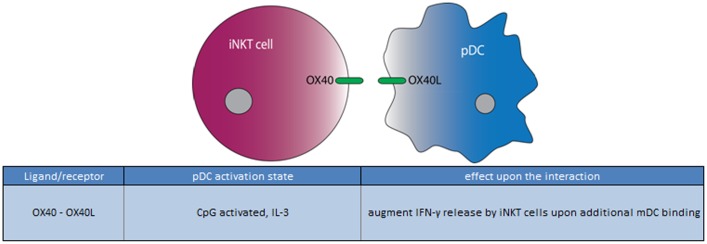
Studies have shown that pDCs interact with iNKT cells directly, both via cell–cell interactions and by cytokine release (104). In contrast to the mDCs, pDCs lack the expression of CD1d, which is an important molecule for crosstalk with iNKT cells (105). Nonetheless, over the past few years the ability of iNKT cell to “sense” subtle changes within their microenvironment in a CD1d-independent mechanism, uncovered that cytokines released by pDCs are essential (106, 107). Indeed, CpG activated pDCs upregulate activation markers on iNKT cells via TNF-α and IFN-α release, and selectively enhance double-negative iNKT cell survival but not that of other NKT cell populations (104). However, the interplay of iNKT cells with pDCs alone is not sufficient for iNKT expansion and does not lead to a cytokine release by iNKT cells. Rather, the CpG activated pDCs enables the iNKT cells to productively interact with CD1d expressing mDCs, thus initiating an immune response (61). Both iNKT cells and mDCs lack expression of TLR9 and are therefore unresponsive to CpG; hence, cytokines released upon ligation of TLR9 on pDCs modulate the tissue microenvironment. Not only cytokines, but also a direct interaction between pDCs and iNKT cells may be of importance; CpG-stimulated pDCs express the ligand CD252 (OX40L), which binds CD134 (OX40) present on the surface of iNKT cells, and augments IFN-γ release by iNKT cells in response to lipid antigen presentation by mDCs (Figure 4) (66). Further support for such a direct interaction between pDCs and iNKT cells via OX40L/OX40 comes from murine studies (108–110).
Figure 4.
Ligand/receptor paring of a pDC with an iNKT cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
In summary, the interaction between iNKT cells and pDCs can be both via cytokines and via direct cell interaction. The role so far seems to facilitate the activation of iNKT by CD1d expressing mDCs. This may become important particularly in situation when TLR9 ligands are available. So far, only OX40L/OX40 are known to play a role in the direct pDC/iNKT cell cross talk.
B Cells
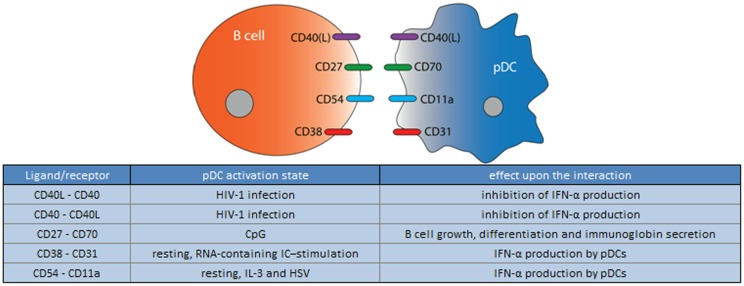
B cells are the only cells that produce antibodies, and therefore, have a critical role in the humoral immune response. Release of Type I IFNs by pDCs leads to an increase of TLR7 and several activation markers on B cells (111, 112). Moreover, as outlined above, pDCs, in concert with T cells, control B cell differentiation into plasma cells via the secretion of IFN-α and IL-6 (64). In addition, pDCs can affect B cells via direct cell–cell contact. Several studies have shown the importance of CD40-CD40L interactions between B cells and pDCs (Figure 5) (24, 64, 65). In addition, upon activation with CpG, pDCs were demonstrated to interact with B cells via CD70/CD27 molecules. This interaction results in B cell growth, differentiation, and immunoglobulin secretion (113).
Figure 5.
Ligand/receptor paring of a pDC with a B cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
Furthermore, recent in vitro studies have shown that activated B cells are able to stimulate matured pDC to produce IFN-α by direct cell–cell contact (114). Blocking the surface molecules OX40L, CD27, CD40, or CD40L with monoclonal antibodies did not influence the effect of B cells on pDC-derived IFN-α production. However, the IFN-α production by pDCs was significantly reduced when blocking LFA-1 or PECAM-1 (CD31) by 50% and 80%, respectively, indicating that these molecules are at least partially responsible for B cell mediated pDCs activation (114).
Taken together, pDCs and B cells are able to induce reciprocal cytokine release and activation by both soluble mediators and direct cell–cell interaction, and so far have been found to be predominantly stimulatory in nature (64, 113, 114).
mDCs
Synergism of mDCs and pDCs are not restricted to the activation of NKT cells. pDCs and mDCs have been demonstrated to be in close contact in vivo at steady state as well as under inflammatory conditions, and it has been suggested that they act synergistically to induce more potent immune responses (115–117). Upon stimulation both mDCs and pDCs function as APCs and follow a similar maturation program, and express the co-stimulatory markers CD40, CD80, CD83, and CD86 to interact with T cells (17, 118). However, there are complementary differences especially in the expression of PRRs (e.g., TLRs, CLRs) and thus in their response to pathogenic triggers. Whereas mDC subtypes express TLR1, 2, 3, 4, 5, 6, 8, and 10, but no TLR7 and 9, the expression of these TLRs on pDCs is the exactly opposite except from TLR2 and 10, which are shared (7, 8, 35, 119–122). pDCs respond to TLR7 and 9 ligands with large amounts of IFN-α and TNF-α (123). In contrast, mDCs release very different cytokines, primarily IL-1β, IL-8, IL-6, IL-10, IL-12p70, TNF-α to variable extents, upon triggering of their TLRs (7, 118, 122). Upon viral infection pDCs are known to respond quicker and with larger amounts of cytokines than mDCs (124). Thus pDCs and mDCs have non-overlapping sensitivities to invading pathogens, and accumulating reports suggest that pDC and CD1c-mDC may cross-activate each other for a more effective immune response. Crosstalk may occur in a paracrine fashion through cytokines like Type I IFNs and TNF-α but also via direct cell contact (118, 125). In a paracrine fashion TNF-α expressed by pDC can cross-activate co-cultured CD1c-mDCs (126). However, there is clear evidence to suggest that CD1c-mDCs and pDCs in other cases require close contact for some parts of this crosstalk, until now it is unclear what molecules are involved (118). Recent studies by Piccioli et al. implies that several members of the TNF family, CD40L/CD40, OX40L, HEVML, RANKL, CD27, CD30L, glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL), and 4-1BB are redundant in the CD1c-mDC/pDC cross talk (9). Experimental evidence for the absence of a role for any of these interactions however was so far not reported but only came from unpublished blocking experiments mentioned in these studies, making it extremely hard to deduce whether these interactions can and should be excluded completely (9, 118). Interestingly, murine models do suggest that the TNF member CD40/CD40L may have a crucial role in the CD1c-mDC/pDC cross talk, yet this result needs to be recapitulated in human CD1c-mDC/pDC assays (9).
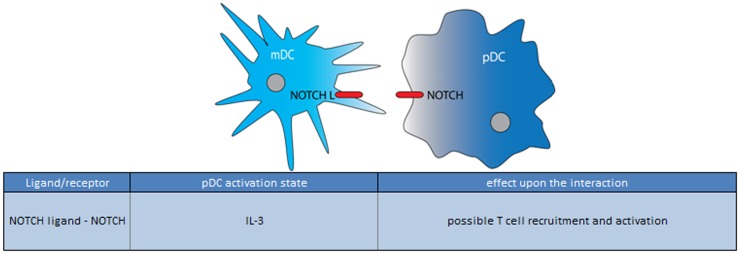
So far only for the NOTCH receptor-ligand interaction evidence is provided for a role in the communication between pDCs and CD1c-mDCs but again experimental evidence is scant (Figure 6) (117). With co-culture experiments they demonstrated that LPS-activated CD1c-mDCs caused an upregulation of maturation marker (CD25, CD86) on the pDC surface and increased IL-6 and CCL19 release in the supernatant. To confirm the involvement of NOTCH pathway, experiments with γ-secretase/NOTCH inhibitor DAPT and soluble NOTCH ligands were preformed and showed a reduced effect on Notch target genes. Activation of the NOTCH pathway upon CD1c-mDC/pDC interaction suggests that this intercellular contact promotes an immune stimulatory response, however, further experiments are needed to unravel the exact mechanism and other molecules potentially involved in this CD1c-mDC/pDC cross talk (117).
Figure 6.
Ligand/receptor paring of a pDC with a mDC and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
Another possible candidate for the interaction between pDC and CD1c-mDC is ICAM-1, expressed on both, pDCs and mDCs, and known as an widespread adhesion molecule with co-stimulatory activity on other immune cells (127). ICAM-1 was found to be strongly upregulated on pDC upon stimulation with TLR9 ligand CpG, while its matching receptor LFA-1 (CD11a/CD18) is constitutively expressed on CD1c-mDCs (9).
Taken together, there is clear evidence that direct cellular interactions are indeed important for CD1c-mDC/pDC cross talk in humans, similar to what was observed in murine studies. However, besides NOTCH receptor-ligand interactions, any experimental evidence that argues in favor of or against the involvement of other specific receptor-ligand interactions is so far lacking (9, 118).
NK Cells
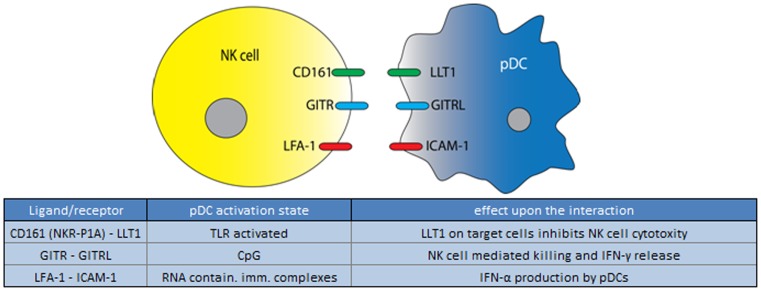
Natural killer cells belong to the innate immune system and are able to respond rapidly to virally infected cells and to tumor formation. This is due to their unique ability to recognize stressed cells or the absence of MHC on the surface of infected or malignantly transformed cells, and their subsequent ability to lyse these cells. The bi-directional pDC-NK cell interaction is known to play an important role in host defense, and again is mediated both by cytokines and via direct cell contact (128, 129). Type I IFNs secreted by pDCs have long been known to enhance the cytolytic potential of NK cells, and NK cells co-cultured with pDCs are more activated, and have increased cytolytic activity (49, 50, 130–133). pDCs and NK cells have been found in close proximity in the T cells areas of human tonsils (50). In addition, during infection or in case of a malignancy, pDCs and NK cells may migrate simultaneously to the site of the lesion, for example during Herpes simplex infection (25). These reports demonstrate the ample opportunities for these cells to engage in direct interactions, which is further supported by the findings that, when co-cultured, pDCs and NKs cells readily interact (134). Upon stimulation by a virus or CpG, pDCs express GITRL that can bind GITR expressed by NK cells (Figure 7). Via the (GITRL)-GITR interaction mature pDCs enhance NK cell mediated killing as well as IFN-γ production. To affect NK cells, however, pDCs expressing GITRL do require the simultaneous presence of IFN-α (50). Furthermore, while the upregulation of CD69 on the surface of NK cells depends on the release of IFN-α and TNF-α by mature pDCs, upregulation of HLA-DR on the surface of a subpopulation of NK cells depends on direct pDC-NK cells contact (49). HLA-DR expressed on NK cells is thought to play an important role in handling bacterial infections such as Mycobacterium bovis (BCG) (135). Although the interaction responsible for HLA-DR upregulation remains to be elucidated, it is known that the maturation state of the pDC is not important for the induction of HLA-DR expression on NK cells, indicating the HLA-DR inducing factor is not affected by pDC maturation (49, 130, 132, 136).
Figure 7.
Ligand/receptor paring of a pDC with an NK cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
The bi-directional crosstalk between pDCs and NK cells also affects pDC function; IL-2, immune complex or IL-12/IL18-stimulated NK cells induce pDCs to release IFN-α which was shown to depend largely on LFA-1-mediated interactions between NK cells and pDCs, and to a lesser extent on NK cell secreted MIP1α (132, 134). LFA-1 and FcγRIIIA on the pDC also increase cytokine release by NK cells (137). Furthermore, IL-2 stimulated NK cells induced pDCs to express the maturation marker CD83, but not CD80 and CD86, in a contact dependent manner, which also indicates the existence of different stimulatory pathways that can induce expression of different maturation markers on pDCs (132).
Contact with NK cells potentially puts pDCs in danger of becoming lysed. However, immature pDCs are protected from NK cell mediated lysis, and this is at least partly due to the high expression of HLA class I, and the absence of Nectin-2, the ligand for NK cell activating receptors DNAM-1 (132, 138). Culture of pDCs with IL-3 however causes the upregulation of Nectin-2 on pDCs, and makes them more susceptible to DNAM-1 and NKp30-mediated killing (138). Activation of pDCs by TLR7 and 9 may help to prevent NK cells lysis as they express the LLT1 (LLT1 or CLEC2D; above), which is a ligand of NK cell surface protein P1A (NKR-P1A; CD161). P1A is expressed by both NK and NKT cells and when ligated inhibits NK cell cytolytic function and IFN-γ release (51, 99, 101, 139). Taken together, pDCs in various modes of action, seem to be differentially susceptible to NK cell-mediated lysis through the absence of activating NK cell receptor-ligands, as well as the regulated expression of ligands for NK cell inhibitory receptors. Also, high MHC I expression is protective.
In summary, non-lethal pDC/NK cell interactions seem to play an important role in enhancing the early immune response to a viral or bacterial infection as pDCs activate the NK cell by producing IFN-α and via GITRL. This feed-forward system likely promotes NK cells to rapidly lyse infected cells (132). NK cell activity in turn induces a further increase of IFN-α by pDCs and promotes their maturation, which may in turn increase the recruitment and survival of mDCs (Figure 8).
Tumor Cells
Several early studies have reported decreased numbers of pDCs and mDCs in the blood of patients suffering from various types of cancers (140–143). However, a recent study with melanoma patients detected no significant difference between the levels of immature pDCs in healthy donors and patients (144). Compared to healthy volunteers, pDCs derived from melanoma patients did, however, show a higher expression of CCR6, and increased ability to migrate toward Chemokine (C-C motif) ligand 20 (CCL20), a ligand for CCR6 (144). CCL20 is expressed by keratinocytes in the skin and by melanoma cells, suggesting that the CCL20/CCR6 interaction is involved in the pDC migration process from the blood to the tumor (145–147). Indeed, high pDC infiltration have been observed in many types of cancer including melanoma, head and neck cancer, ovarian and prostate cancer, and these infiltrates mostly negatively correlate with patient survival. On the other hand, an increase of pDCs in tumor-draining LN may be beneficial [reviewed in (143, 148)]. pDCs infiltrated in tumor microenvironment are mainly immature, and therefore seem to be predominantly immunosuppressive/tolerogenic (148). In recent years, evidence has accumulated that tumors may block anti-tumor response by maintaining pDCs in an inactive/tolerogenic state. Mechanisms responsible for keeping the pDC in this state include the secretion of prostaglandin 2 (PGE2) and TGF-β, which, in a synergistic manner inhibit pDC-derived IFN-α and TNF-α production in response to TLR7 and 9 ligands, as well as inhibiting CCR7 expression, thereby impairing the migration of pDCs to the tumor-draining LN to prime T cells with tumor antigens (148–150). In addition, there is evidence that PGE2-stimulated pDCs indirectly support tumor cell proliferation, migration, and invasion, as well as tumor angiogenesis, via the release of IL-6 and IL-8 (151–158). Furthermore, tumor-resident pDCs may also influence tumor growth indirectly through the induction of Tregs. In epithelial ovarian cancer the majority of Foxp3+ Treg cells accumulating in the tumor microenvironment expressed the ICOS, and tumor pDCs expressing the ICOSL were shown to be essential for the expansion and suppressive function of these regulatory Foxp3+ Tregs (67, 68).
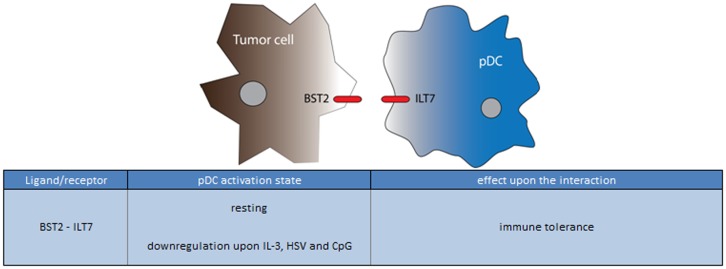
On their surface, unstimulated pDCs (uniquely with respect to all other leukocytes) express the immunoglobulin-like transcript 7 (ILT7) protein, which is activated by binding to bone marrow stromal cell antigen 2 (BST2, CD317; reviewed in (159). BST2 is expressed on human cancer cells, monocytes, and vascular endothelium in response to IFN-α (Figure 9) (160–162). Similar to BDCA-2, ILT7 forms a complex with FcεRIγ, which, when ligated activates an immunoreceptor tyrosine-based activation motif (ITAM)-mediated signaling pathway that dampens TLR-7 and 9-induced IFN-α and TNF-α production (163, 164). ILT7 is downregulated upon stimulation of the pDCs by CpG, HSV, or IL-3, suggesting that pDC maturation prior to entering the tumor site may partly protect it from this suppressive mechanism (159, 164, 165). In addition to ILT7, immature pDCs also express the ITIM motif containing receptors ILT2 and ILT3 that bind to MHC class I molecules, and an unknown ligand, respectively. Both receptors are associated with immune tolerance, probably through the suppression of T cell responses, and in agreement with this notion, these receptors are downregulated upon pDC activation (166–168). Whether these molecules may also have an active role in the pDC-tumor cell interaction, or rather regulate pDC-T cell activation, remains to be investigated. Likewise, there may be a yet unappreciated role for pDC expression of NKp44, which has been demonstrated to down modulate pDC IFN-α responses upon ligation, and may be utilized by the tumor to dampen pDC-mediated immune responses (169). Indeed, an inhibitory NKp44 ligand complex containing HLA I and PCNA was recently reported to be expressed by several tumor cells (159, 165, 170).
Figure 9.
Ligand/receptor paring of a pDC with a tumor cell and the maturation state/activation stimuli associated with ligand or receptor expression on the pDC surface.
So far, most studies point out an immune suppressive role for pDCs favoring tumor progression, however several other studies demonstrate that the situation may be very different if pDCs are properly activated. In this case pDCs may trigger anti-tumor T cell-mediated immune responses (above) or even actively kill tumor cells (171, 172). As previously discussed, in relation to the T cell/pDC interaction, activated pDCs express TRAIL, which induces an apoptotic process by binding to the TRAIL receptors. Tumor cells are known to be sensitive to TRAIL, and via this interaction could directly induce tumor cell apoptosis (171). Avoiding this apoptotic pathway by downregulation of TRAIL has been reported for several cancers by numerous studies (173).
Taken together, the suppressive tumor microenvironment decreases the immune stimulatory functions of pDCs resulting in tumor progression. Preventing these processes, while at the same time activating pDCs, forms a promising target for anti-tumor therapies. Moreover, in a recent clinical feasibility study in our department, we demonstrated that the administration of autologous ex vivo-matured tumor antigen loaded pDCs proved to be successful and induced objective clinical responses in several patients (60).
Less Explored in pDC Cellular Interactions: The SLAM Family
Above we have provided an overview of the interactions pDCs likely engage in during their lifecycle, as based on experimental evidence and in vivo proximity. In addition we discussed the molecules likely to participate in these interactions. There is, however, a poorly understood family of proteins which are highly expressed pDCs, and which deserve more attention. This is the SLAM family of receptors, for which a role in a diverse range of cellular interactions is highly suspected, yet currently unexplored. Five family members are expressed on the pDC surface (largely) independent of its activation state: SLAMF2 (CD48; BLAST1), SLAMF5 (CD84), SLAMF3 (CD229; Ly9), SLAM7 (CD319, CRACC), and NTBA (CD352) (74, 102). Except for SLAMF2 (below), these proteins have in common that they engage mostly in homotypic interactions; such interactions may occur between homotypic cells, but also between different cell types, opening up the possibility that these molecules mediated direct cell interactions of pDCs with each other, or with others cells also expressing these receptors (174). Homotypic SLAM family interactions have the ability to regulate cell activation and proliferation as well as cytolytic activity (174). SLAMF5 is highly expressed by many immune cells, and has been shown to play a role in T cell-B cell adhesion, and for optimal germinal center formation (175, 176). Furthermore SLAMF5 was detected on leukemic pDCs, and can work as an inhibitor for FcεRI-mediated signaling in mast cells (177, 178). SLAMF3 is also expressed on T cells. Here it reduces IFN-γ production and ERK activation upon stimulation, and thus via this molecule pDCs might trigger a similar response (175, 179). SLAM7, in contrast, is widely expressed on activated B lymphocytes, NK cells, and CD8+ T lymphocytes (74, 180–182) and has been shown to promote B cell proliferation, activate NK cell cytotoxicity (but not NK cell proliferation) (180, 182, 183). Finally, NTBA in addition to pDCs, is present on NK, T, and B cells where it may affect cytotoxicity as well as the IFN-γ and TNF-α release (174, 184, 185). Interactions between NTBA on pDCs and NK cells may therefore have the potential to positively regulate both NK cells and pDCs.
In contrast to the other family members, SLAMF2 which is also present on the surface of pDCs, engages in a heterotypic interaction with family member 2B4 (CD244), and could thus play a role in the interaction of pDCs with 2B4-expressing NK or T cells (74, 175, 186). SLAMF2 via 2B4 can activate NK cells (186).
Overall, although experiments are largely lacking, the presence of such a high number of SLAM family members on pDCs, their homotypic interactions, as well as the known effects of their triggering on other immune cells makes us speculate that pDCs may very well exploit these receptors to communicate with other immune cells.
Conclusion and Outlook
In this review we summarized the existing data which supports the idea that during their life cycle, human pDCs interact with numerous immune cells. Additionally, we have attempted to provide a contemporary overview of the molecules that drive these interactions, and the consequences of their expression on pDCs. It is clear that pDCs play a pivotal role in ensuring a rapid immune response, especially upon viral infection, by strong IFN-α release, but also via direct cell–cell interaction. Depending on the pDC activation state, cytokines released by pDCs and direct pDCs surface receptors may inhibit or activate other immune cells. This large influential capacity of pDCs suggest that they are master regulators of both innate and adaptive immune responses. Besides secretion of the highly potent yet broadly acting IFN-α, pDCs have a highly versatile repertoire of cell surface molecules to further fine tune immune responses. These characteristics make them an interesting and potential highly valuable therapeutic target to be exploited or targeted in cancer therapy, infectious or autoimmune disease. Importantly, controlling cytokine secretion and the surface expression of specific receptors is essential to steer the immune response into the desired direction. In cancer therapy, lifting the suppressive actions of tumor-resident pDCs may greatly enhance existing/endogenous anti-tumor immune response (187). In addition, anti-cancer immune responses may be initiated or boosted by vaccination with autologous tumor antigen loaded pDCs (60). A preliminary clinical trial using pDCs in melanoma vaccination therapy, carried out by our department, has demonstrated the use of thick born virus vaccine (FSME)-matured pDCS in cancer vaccination is safe and feasible, and despite low patients numbers, showed an improved survival of pDCs vaccinated patients (60). In particular, the extremely low doses ranging from 0.5 to 3 million pDC per patient demonstrate the potency of these cells. The exact reason for the success of pDCs however is not yet completely understood, but in addition to IFN-α production and the induction of tumor-specific T cells, we envision there may also be a significant role for the effective combination of surface receptors expressed by the pDCs and their resulting interaction with other immune cells.
In conclusion, research over the past few years has greatly increased our knowledge of the repertoire of pDC-expressed surface receptors and cellular interaction partners, and has emphasized that there is more to the pDCs than IFN-α alone. Importantly, however, further studies are required to identify the role of these molecules and interactions in pDC function and immune responses in general.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Jurjen Tel (Radboud University Nijmegen), Dr. John-Paul Jukes (University of Oxford), Rosanna McEwen-Smith (University of Oxford), and Sandhya Ramalingam Venkateswari Subbammal (London School of Economics, London) for the critical reading of our manuscript. This work was supported by a RUNMC-NCMLS JO grant and grant KUN2009-4402 from the Dutch Cancer Society. Carl G. Figdor was awarded with an NWO Spinoza prize and an ERC Advanced grant (ERC-2010-AdG-269019-PATHFINDER).
References
- 1.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol (2008) 9(1):R17. 10.1186/gb-2008-9-1-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol (2013) 31:563–604 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev (2010) 234(1):177–98 10.1111/j.0105-2896.2009.00868.x [DOI] [PubMed] [Google Scholar]
- 4.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology (2013) 140(1):22–30 10.1111/imm.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, et al. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood (2013) 121(3):459–67 10.1182/blood-2012-06-435644 [DOI] [PubMed] [Google Scholar]
- 6.Tel J, Hato SV, Torensma R, Buschow SI, Figdor CG, Lesterhuis WJ, et al. The chemotherapeutic drug oxaliplatin differentially affects blood DC function dependent on environmental cues. Cancer Immunol Immunother (2012) 61(7):1101–11 10.1007/s00262-011-1189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother (2010) 59(10):1573–82 10.1007/s00262-010-0833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med (2001) 194(6):863–9 10.1084/jem.194.6.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantisani R, Sammicheli C, Tavarini S, D’Oro U, Wack A, Piccioli D. Surface molecules on stimulated plasmacytoid dendritic cells are sufficient to cross-activate resting myeloid dendritic cells. Hum Immunol (2011) 72(11):1018–21 10.1016/j.humimm.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 10.McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol (2005) 17(1):18–24 10.1016/j.coi.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Ronnblom L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum (2003) 48(9):2524–32 10.1002/art.11225 [DOI] [PubMed] [Google Scholar]
- 12.Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood (2012) 119(10):2284–92 10.1182/blood-2011-08-373944 [DOI] [PubMed] [Google Scholar]
- 13.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol (2004) 22:33–54 10.1146/annurev.immunol.22.012703.104558 [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol (2004) 5(12):1219–26 10.1038/ni1141 [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol (2005) 23:275–306 10.1146/annurev.immunol.23.021704.115633 [DOI] [PubMed] [Google Scholar]
- 16.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol (2008) 8(8):594–606 10.1038/nri2358 [DOI] [PubMed] [Google Scholar]
- 17.Manh TP, Alexandre Y, Baranek T, Crozat K, Dalod M. Plasmacytoid, conventional, and monocyte-derived dendritic cells undergo a profound and convergent genetic reprogramming during their maturation. Eur J Immunol (2013) 43(7):1706–15 10.1002/eji.201243106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol (2005) 174(5):2457–65 [DOI] [PubMed] [Google Scholar]
- 19.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood (2010) 115(26):5366–75 10.1182/blood-2009-10-248260 [DOI] [PubMed] [Google Scholar]
- 20.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol (2010) 184(6):2999–3007 10.4049/jimmunol.0804106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood (2003) 101(9):3520–6 10.1182/blood-2002-10-3063 [DOI] [PubMed] [Google Scholar]
- 22.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol (2011) 29:163–83 10.1146/annurev-immunol-031210-101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity (2010) 33(6):955–66 10.1016/j.immuni.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donhauser N, Pritschet K, Helm M, Harrer T, Schuster P, Ries M, et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLoS One (2012) 7(3):e33925. 10.1371/journal.pone.0033925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol (2009) 83(4):1952–61 10.1128/JVI.01578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lui G, Manches O, Angel J, Molens JP, Chaperot L, Plumas J. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS One (2009) 4(9):e7111. 10.1371/journal.pone.0007111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tel J, Torensma R, Figdor CG, de Vries IJ. IL-4 and IL-13 alter plasmacytoid dendritic cell responsiveness to CpG DNA and herpes simplex virus-1. J Invest Dermatol (2011) 131(4):900–6 10.1038/jid.2010.410 [DOI] [PubMed] [Google Scholar]
- 28.Pichyangkul S, Endy TP, Kalayanarooj S, Nisalak A, Yongvanitchit K, Green S, et al. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol (2003) 171(10):5571–8 [DOI] [PubMed] [Google Scholar]
- 29.Svensson H, Cederblad B, Lindahl M, Alm G. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus. J Interferon Cytokine Res (1996) 16(1):7–16 [DOI] [PubMed] [Google Scholar]
- 30.Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H, et al. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J Immunol (2001) 166(8):5000–7 [DOI] [PubMed] [Google Scholar]
- 31.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol (2004) 173(7):4433–42 [DOI] [PubMed] [Google Scholar]
- 32.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood (2002) 99(9):3263–71 10.1182/blood.V99.9.3263 [DOI] [PubMed] [Google Scholar]
- 33.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol (2003) 4(10):1009–15 10.1038/ni978 [DOI] [PubMed] [Google Scholar]
- 34.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity (2001) 14(4):461–70 10.1016/S1074-7613(01)00126-1 [DOI] [PubMed] [Google Scholar]
- 35.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol (2001) 31(10):3026–37 [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol (2002) 3(2):196–200 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 37.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by toll-like receptor 7. Proc Natl Acad Sci U S A (2004) 101(15):5598–603 10.1073/pnas.0400937101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A (2001) 98(16):9237–42 10.1073/pnas.161293498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol (2003) 170(9):4465–74 [DOI] [PubMed] [Google Scholar]
- 40.Kerkmann M, Costa LT, Richter C, Rothenfusser S, Battiany J, Hornung V, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem (2005) 280(9):8086–93 10.1074/jbc.M410868200 [DOI] [PubMed] [Google Scholar]
- 41.Tel J, Benitez-Ribas D, Hoosemans S, Cambi A, Adema GJ, Figdor CG, et al. DEC-205 mediates antigen uptake and presentation by both resting and activated human plasmacytoid dendritic cells. Eur J Immunol (2011) 41(4):1014–23 10.1002/eji.201040790 [DOI] [PubMed] [Google Scholar]
- 42.Guery L, Hugues S. Tolerogenic and activatory plasmacytoid dendritic cells in autoimmunity. Front Immunol (2013) 4:59. 10.3389/fimmu.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood (2008) 111(8):4245–53 10.1182/blood-2007-03-081398 [DOI] [PubMed] [Google Scholar]
- 44.Tel J, Beenhakker N, Koopman G, Hart B, Mudde GC, de Vries IJ. Targeted delivery of CpG ODN to CD32 on human and monkey plasmacytoid dendritic cells augments IFNalpha secretion. Immunobiology (2012) 217(10):1017–24 10.1016/j.imbio.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 45.Benitez-Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, et al. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J Exp Med (2006) 203(7):1629–35 10.1084/jem.20052364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conrad C, Meller S, Gilliet M. Plasmacytoid dendritic cells in the skin: to sense or not to sense nucleic acids. Semin Immunol (2009) 21(3):101–9 10.1016/j.smim.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 47.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol (2010) 31(7):270–7 10.1016/j.it.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 48.Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med (2005) 201(4):509–15 10.1084/jem.20041310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benlahrech A, Donaghy H, Rozis G, Goodier M, Klavinskis L, Gotch F, et al. Human NK cell up-regulation of CD69, HLA-DR, interferon gamma secretion and cytotoxic activity by plasmacytoid dendritic cells is regulated through overlapping but different pathways. Sensors (2009) 9(1):386–403 10.3390/s90100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanabuchi S, Watanabe N, Wang YH, Wang YH, Ito T, Shaw J, et al. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood (2006) 107(9):3617–23 10.1182/blood-2005-08-3419 [DOI] [PubMed] [Google Scholar]
- 51.Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, et al. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol (2008) 180(10):6508–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity (2004) 20(5):539–50 10.1016/S1074-7613(04)00108-6 [DOI] [PubMed] [Google Scholar]
- 53.Kohrgruber N, Groger M, Meraner P, Kriehuber E, Petzelbauer P, Brandt S, et al. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol (2004) 173(11):6592–602 [DOI] [PubMed] [Google Scholar]
- 54.Bangert C, Friedl J, Stary G, Stingl G, Kopp T. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J Invest Dermatol (2003) 121(6):1409–18 10.1111/j.1523-1747.2003.12623.x [DOI] [PubMed] [Google Scholar]
- 55.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol (2001) 167(4):1862–6 [DOI] [PubMed] [Google Scholar]
- 56.Seth S, Oberdorfer L, Hyde R, Hoff K, Thies V, Worbs T, et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol (2011) 186(6):3364–72 10.4049/jimmunol.1002598 [DOI] [PubMed] [Google Scholar]
- 57.de la Rosa G, Longo N, Rodriguez-Fernandez JL, Puig-Kroger A, Pineda A, Corbi AL, et al. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol (2003) 73(5):639–49 10.1189/jlb.1002516 [DOI] [PubMed] [Google Scholar]
- 58.Krug A, Uppaluri R, Facchetti F, Dorner BG, Sheehan KC, Schreiber RD, et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol (2002) 169(11):6079–83 [DOI] [PubMed] [Google Scholar]
- 59.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med (2003) 198(5):823–30 10.1084/jem.20020437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res (2013) 73(3):1063–75 10.1158/0008-5472.CAN-12-2583 [DOI] [PubMed] [Google Scholar]
- 61.Sisirak V, Vey N, Vanbervliet B, Duhen T, Puisieux I, Homey B, et al. CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood (2011) 118(19):5130–40 10.1182/blood-2010-07-295626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banchereau J, Pulendran B, Steinman R, Palucka K. Will the making of plasmacytoid dendritic cells in vitro help unravel their mysteries? J Exp Med (2000) 192(12):F39–44 10.1084/jem.192.12.F39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch (2003) 443(6):703–17 10.1007/s00428-003-0918-8 [DOI] [PubMed] [Google Scholar]
- 64.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity (2003) 19(2):225–34 10.1016/S1074-7613(03)00208-5 [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Marquez M, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Complex interactions between B cells and dendritic cells. Blood (2013) 121(12):2367–8 10.1182/blood-2012-12-468017 [DOI] [PubMed] [Google Scholar]
- 66.Marschner A, Rothenfusser S, Hornung V, Prell D, Krug A, Kerkmann M, et al. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol (2005) 35(8):2347–57 10.1002/eji.200425721 [DOI] [PubMed] [Google Scholar]
- 67.Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res (2012) 72(20):5240–9 10.1158/0008-5472.CAN-12-2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res (2012) 72(23):6130–41 10.1158/0008-5472.CAN-12-2409 [DOI] [PubMed] [Google Scholar]
- 69.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells – conditional innate immune cells. J Hematol Oncol (2013) 6:61. 10.1186/1756-8722-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol (2005) 174(1):244–51 [DOI] [PubMed] [Google Scholar]
- 71.Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med (2009) 206(1):249–58 10.1084/jem.20080129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuster P, Boscheinen JB, Tennert K, Schmidt B. The role of plasmacytoid dendritic cells in innate and adaptive immune responses against alpha herpes virus infections. Adv Virol (2011) 2011:679271. 10.1155/2011/679271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood (2006) 107(4):1421–6 10.1182/blood-2005-05-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuster P, Donhauser N, Pritschet K, Ries M, Haupt S, Kittan NA, et al. Co-ordinated regulation of plasmacytoid dendritic cell surface receptors upon stimulation with herpes simplex virus type 1. Immunology (2010) 129(2):234–47 10.1111/j.1365-2567.2009.03176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med (1999) 5(8):919–23 10.1038/11360 [DOI] [PubMed] [Google Scholar]
- 76.Guillerme JB, Boisgerault N, Roulois D, Menager J, Combredet C, Tangy F, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res (2013) 19(5):1147–58 10.1158/1078-0432.CCR-12-2733 [DOI] [PubMed] [Google Scholar]
- 77.Tel J, Lambeck AJ, Cruz LJ, Tacken PJ, de Vries IJ, Figdor CG. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J Immunol (2010) 184(8):4276–83 10.4049/jimmunol.0903286 [DOI] [PubMed] [Google Scholar]
- 78.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol (2008) 9(5):551–7 10.1038/ni.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol (2013) 34(8):361–70 10.1016/j.it.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuwana M, Kaburaki J, Wright TM, Kawakami Y, Ikeda Y. Induction of antigen-specific human CD4(+) T cell anergy by peripheral blood DC2 precursors. Eur J Immunol (2001) 31(9):2547–57 [DOI] [PubMed] [Google Scholar]
- 81.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med (1997) 185(6):1101–11 10.1084/jem.185.6.1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med (2000) 192(2):219–26 10.1084/jem.192.2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med (2007) 204(1):105–15 10.1084/jem.20061660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogata M, Ito T, Shimamoto K, Nakanishi T, Satsutani N, Miyamoto R, et al. Plasmacytoid dendritic cells have a cytokine-producing capacity to enhance ICOS ligand-mediated IL-10 production during T-cell priming. Int Immunol (2013) 25(3):171–82 10.1093/intimm/dxs103 [DOI] [PubMed] [Google Scholar]
- 85.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol (2001) 166(5):2961–9 [DOI] [PubMed] [Google Scholar]
- 86.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science (1999) 283(5405):1183–6 10.1126/science.283.5405.1183 [DOI] [PubMed] [Google Scholar]
- 87.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol (2000) 1(4):305–10 10.1038/79747 [DOI] [PubMed] [Google Scholar]
- 88.Karrich JJ, Jachimowski LC, Nagasawa M, Kamp A, Balzarolo M, Wolkers MC, et al. IL-21-stimulated human plasmacytoid dendritic cells secrete granzyme B, which impairs their capacity to induce T-cell proliferation. Blood (2013) 121(16):3103–11 10.1182/blood-2012-08-452995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother (2007) 30(3):251–60 10.1097/CJI.0b013e31802e085a [DOI] [PubMed] [Google Scholar]
- 90.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) 26:677–704 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A (2002) 99(19):12293–7 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res (2005) 65(3):1089–96 [PubMed] [Google Scholar]
- 93.Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol (2004) 172(7):4253–9 [DOI] [PubMed] [Google Scholar]
- 94.Delespesse G, Ohshima Y, Yang LP, Demeure C, Sarfati M. OX40-Mediated cosignal enhances the maturation of naive human CD4+ T cells into high IL-4-producing effectors. Int Arch Allergy Immunol (1999) 118(2–4):384–6 10.1159/000024143 [DOI] [PubMed] [Google Scholar]
- 95.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via toll-like receptor-viral RNA interactions. J Clin Invest (2005) 115(11):3265–75 10.1172/JCI26032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol (2006) 176(1):248–55 [DOI] [PubMed] [Google Scholar]
- 97.Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood (2009) 114(18):3854–63 10.1182/blood-2009-04-217927 [DOI] [PubMed] [Google Scholar]
- 98.Dillon SM, Robertson KB, Pan SC, Mawhinney S, Meditz AL, Folkvord JM, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr (2008) 48(1):1–12 10.1097/QAI.0b013e3181664b60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Germain C, Meier A, Jensen T, Knapnougel P, Poupon G, Lazzari A, et al. Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-gamma contributes to modulate immune responses. J Biol Chem (2011) 286(44):37964–75 10.1074/jbc.M111.285312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montaldo E, Vitale C, Cottalasso F, Conte R, Glatzer T, Ambrosini P, et al. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood (2012) 119(17):3987–96 10.1182/blood-2011-09-379693 [DOI] [PubMed] [Google Scholar]
- 101.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol (2005) 175(12):7796–9 [DOI] [PubMed] [Google Scholar]
- 102.Bossard C, Malard F, Arbez J, Chevallier P, Guillaume T, Delaunay J, et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft-versus-host disease. Leukemia (2012) 26(7):1471–4 10.1038/leu.2012.41 [DOI] [PubMed] [Google Scholar]
- 103.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol (2004) 4(3):231–7 10.1038/nri1309 [DOI] [PubMed] [Google Scholar]
- 104.Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, et al. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol (2006) 177(2):1028–39 [DOI] [PubMed] [Google Scholar]
- 105.Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med (2001) 193(10):1221–6 10.1084/jem.193.10.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jukes JP, Wood KJ, Jones ND. Bystander activation of iNKT cells occurs during conventional T-cell alloresponses. Am J Transplant (2012) 12(3):590–9 10.1111/j.1600-6143.2011.03847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med (2011) 208(6):1163–77 10.1084/jem.20102555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity (2009) 30(2):289–99 10.1016/j.immuni.2008.12.017 [DOI] [PubMed] [Google Scholar]
- 109.Zaini J, Andarini S, Tahara M, Saijo Y, Ishii N, Kawakami K, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest (2007) 117(11):3330–8 10.1172/JCI32693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimizu K, Asakura M, Shinga J, Sato Y, Kitahara S, Hoshino K, et al. Invariant NKT cells induce plasmacytoid dendritic cell (DC) cross-talk with conventional DCs for efficient memory CD8+ T cell induction. J Immunol (2013) 190(11):5609–19 10.4049/jimmunol.1300033 [DOI] [PubMed] [Google Scholar]
- 111.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol (2005) 174(7):4043–50 [DOI] [PubMed] [Google Scholar]
- 112.Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol (2002) 14(4):411–9 10.1093/intimm/14.4.411 [DOI] [PubMed] [Google Scholar]
- 113.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood (2010) 115(15):3051–7 10.1182/blood-2009-08-239145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berggren O, Hagberg N, Weber G, Alm GV, Ronnblom L, Eloranta ML. B lymphocytes enhance interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum (2012) 64(10):3409–19 10.1002/art.34599 [DOI] [PubMed] [Google Scholar]
- 115.Vermi W, Lonardi S, Morassi M, Rossini C, Tardanico R, Venturini M, et al. Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology (2009) 214(9–10):877–86 10.1016/j.imbio.2009.06.013 [DOI] [PubMed] [Google Scholar]
- 116.Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, et al. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood (2009) 113(24):6112–9 10.1182/blood-2008-07-170803 [DOI] [PubMed] [Google Scholar]
- 117.Perez-Cabezas B, Naranjo-Gomez M, Ruiz-Riol M, Bastos-Amador P, Fernandez MA, Carmona F, et al. TLR-activated conventional DCs promote gamma-secretase-mediated conditioning of plasmacytoid DCs. J Leukoc Biol (2012) 92(1):133–43 10.1189/jlb.0911452 [DOI] [PubMed] [Google Scholar]
- 118.Piccioli D, Sammicheli C, Tavarini S, Nuti S, Frigimelica E, Manetti AG, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood (2009) 113(18):4232–9 10.1182/blood-2008-10-186890 [DOI] [PubMed] [Google Scholar]
- 119.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol (2006) 177(11):7959–67 [DOI] [PubMed] [Google Scholar]
- 120.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol (2003) 170(10):5165–75 [DOI] [PubMed] [Google Scholar]
- 121.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol (2001) 31(11):3388–93 [DOI] [PubMed] [Google Scholar]
- 122.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, et al. Interferon-alpha and interleukin-12 are induced differentially by toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med (2002) 195(11):1507–12 10.1084/jem.20020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol (2005) 26(3):221–9 10.1007/s00281-004-0180-4 [DOI] [PubMed] [Google Scholar]
- 124.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells – virus experts of innate immunity. Semin Immunol (2005) 17(4):253–61 10.1016/j.smim.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 125.Bond E, Liang F, Sandgren KJ, Smed-Sorensen A, Bergman P, Brighenti S, et al. Plasmacytoid dendritic cells infiltrate the skin in positive tuberculin skin test indurations. J Invest Dermatol (2012) 132(1):114–23 10.1038/jid.2011.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol (2004) 78(10):5223–32 10.1128/JVI.78.10.5223-5232.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol (2005) 17(3):251–8 10.1016/j.coi.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 128.Mocikat R, Braumuller H, Gumy A, Egeter O, Ziegler H, Reusch U, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity (2003) 19(4):561–9 10.1016/S1074-7613(03)00264-4 [DOI] [PubMed] [Google Scholar]
- 129.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol (2003) 171(5):2366–73 [DOI] [PubMed] [Google Scholar]
- 130.Romagnani C, Della Chiesa M, Kohler S, Moewes B, Radbruch A, Moretta L, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol (2005) 35(8):2452–8 10.1002/eji.200526069 [DOI] [PubMed] [Google Scholar]
- 131.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun (2011) 3(3):258–63 10.1159/000323923 [DOI] [PubMed] [Google Scholar]
- 132.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol (2005) 174(2):727–34 [DOI] [PubMed] [Google Scholar]
- 133.Trinchieri G, Santoli D, Koprowski H. Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J Immunol (1978) 120(6):1849–55 [PubMed] [Google Scholar]
- 134.Hagberg N, Berggren O, Leonard D, Weber G, Bryceson YT, Alm GV, et al. IFN-alpha production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes is promoted by NK cells via MIP-1beta and LFA-1. J Immunol (2011) 186(9):5085–94 10.4049/jimmunol.1003349 [DOI] [PubMed] [Google Scholar]
- 135.Evans JH, Horowitz A, Mehrabi M, Wise EL, Pease JE, Riley EM, et al. A distinct subset of human NK cells expressing HLA-DR expand in response to IL-2 and can aid immune responses to BCG. Eur J Immunol (2011) 41(7):1924–33 10.1002/eji.201041180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marshall JD, Heeke DS, Abbate C, Yee P, Van Nest G. Induction of interferon-gamma from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-alpha and tumour necrosis factor-alpha. Immunology (2006) 117(1):38–46 10.1111/j.1365-2567.2005.02261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood (2010) 115(11):2167–76 10.1182/blood-2009-08-238469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Della Chiesa M, Romagnani C, Thiel A, Moretta L, Moretta A. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood (2006) 108(12):3851–8 10.1182/blood-2006-02-004028 [DOI] [PubMed] [Google Scholar]
- 139.Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol (2005) 175(12):7791–5 [DOI] [PubMed] [Google Scholar]
- 140.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res (2004) 10(21):7260–9 10.1158/1078-0432.CCR-04-0872 [DOI] [PubMed] [Google Scholar]
- 141.Pinzon-Charry A, Ho CS, Maxwell T, McGuckin MA, Schmidt C, Furnival C, et al. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer (2007) 97(9):1251–9 10.1038/sj.bjc.6604018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Cruijsen H, Hoekman K, Stam AG, van den Eertwegh AJ, Kuenen BC, Scheper RJ, et al. Defective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptor. Clin Dev Immunol (2007) 2007:17315. 10.1155/2007/17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Karthaus N, Torensma R, Tel J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am J Pathol (2012) 181(3):733–42 10.1016/j.ajpath.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 144.Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, et al. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Invest Dermatol (2010) 130(6):1646–56 10.1038/jid.2010.24 [DOI] [PubMed] [Google Scholar]
- 145.Hasan L, Mazzucchelli L, Liebi M, Lis M, Hunger RE, Tester A, et al. Function of liver activation-regulated chemokine/CC chemokine ligand 20 is differently affected by cathepsin B and cathepsin D processing. J Immunol (2006) 176(11):6512–22 [DOI] [PubMed] [Google Scholar]
- 146.Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, et al. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res (2004) 64(6):2192–8 10.1158/0008-5472.CAN-03-2969 [DOI] [PubMed] [Google Scholar]
- 147.Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, et al. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest (2003) 83(10):1457–66 10.1097/01.LAB.0000090158.68852.D1 [DOI] [PubMed] [Google Scholar]
- 148.Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol (2013) 93(3):343–52 10.1189/jlb.0812397 [DOI] [PubMed] [Google Scholar]
- 149.Bekeredjian-Ding I, Schafer M, Hartmann E, Pries R, Parcina M, Schneider P, et al. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology (2009) 128(3):439–50 10.1111/j.1365-2567.2009.03134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thiel A, Pries R, Jeske S, Trenkle T, Wollenberg B. Effect of head and neck cancer supernatant and CpG-oligonucleotides on migration and IFN-alpha production of plasmacytoid dendritic cells. Anticancer Res (2009) 29(8):3019–25 [PubMed] [Google Scholar]
- 151.Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res (2009) 69(1):329–37 10.1158/0008-5472.CAN-08-0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kamohara H, Takahashi M, Ishiko T, Ogawa M, Baba H. Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: impact of CXCL-8 as an autocrine growth factor. Int J Oncol (2007) 31(3):627–32 [PubMed] [Google Scholar]
- 153.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine (2000) 12(1):78–85 10.1006/cyto.1999.0518 [DOI] [PubMed] [Google Scholar]
- 154.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res (2007) 67(14):6854–62 10.1158/0008-5472.CAN-07-1162 [DOI] [PubMed] [Google Scholar]
- 155.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer (2007) 121(9):1949–57 10.1002/ijc.22930 [DOI] [PubMed] [Google Scholar]
- 156.Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab (2008) 28(1):90–8 10.1038/sj.jcbfm.9600509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol (2003) 170(6):3369–76 [DOI] [PubMed] [Google Scholar]
- 158.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol (2005) 7(2):122–33 10.1215/S1152851704001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cao W, Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev (2010) 234(1):163–76 10.1111/j.0105-2896.2009.00867.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Walter-Yohrling J, Cao X, Callahan M, Weber W, Morgenbesser S, Madden SL, et al. Identification of genes expressed in malignant cells that promote invasion. Cancer Res (2003) 63(24):8939–47 [PubMed] [Google Scholar]
- 161.Ohtomo T, Sugamata Y, Ozaki Y, Ono K, Yoshimura Y, Kawai S, et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun (1999) 258(3):583–91 10.1006/bbrc.1999.0683 [DOI] [PubMed] [Google Scholar]
- 162.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci U S A (2011) 108(33):13688–93 10.1073/pnas.1101684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cao W. Molecular characterization of human plasmacytoid dendritic cells. J Clin Immunol (2009) 29(3):257–64 10.1007/s10875-009-9284-x [DOI] [PubMed] [Google Scholar]
- 164.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits toll-like receptor-induced interferon production. J Exp Med (2006) 203(6):1399–405 10.1084/jem.20052454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cho M, Ishida K, Chen J, Ohkawa J, Chen W, Namiki S, et al. SAGE library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type I IFN production. Int Immunol (2008) 20(1):155–64 10.1093/intimm/dxm127 [DOI] [PubMed] [Google Scholar]
- 166.Ju XS, Hacker C, Scherer B, Redecke V, Berger T, Schuler G, et al. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene (2004) 331:159–64 10.1016/j.gene.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 167.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol (2002) 3(3):237–43 10.1038/ni760 [DOI] [PubMed] [Google Scholar]
- 168.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A (2003) 100(15):8856–61 10.1073/pnas.1431057100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood (2005) 106(6):2076–82 10.1182/blood-2004-12-4802 [DOI] [PubMed] [Google Scholar]
- 170.Horton NC, Mathew SO, Mathew PA. Novel interaction between proliferating cell nuclear antigen and HLA I on the surface of tumor cells inhibits NK cell function through NKp44. PLoS One (2013) 8(3):e59552. 10.1371/journal.pone.0059552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. J Immunol (2012) 188(4):1583–91 10.4049/jimmunol.1102437 [DOI] [PubMed] [Google Scholar]
- 172.Tel J, Smits EL, Anguille S, Joshi RN, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood (2012) 120(19):3936–44 10.1182/blood-2012-06-435941 [DOI] [PubMed] [Google Scholar]
- 173.Vigneswaran N, Baucum DC, Wu J, Lou Y, Bouquot J, Muller S, et al. Repression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but not its receptors during oral cancer progression. BMC Cancer (2007) 7:108. 10.1186/1471-2407-7-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol (2008) 97:177–250 10.1016/S0065-2776(08)00004-7 [DOI] [PubMed] [Google Scholar]
- 175.Cabezon R, Sintes J, Llinas L, Benitez-Ribas D. Analysis of HLDA9 mAbs on plasmacytoid dendritic cells. Immunol Lett (2011) 134(2):167–73 10.1016/j.imlet.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 176.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity (2010) 32(2):253–65 10.1016/j.immuni.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gopcsa L, Banyai A, Jakab K, Kormos L, Tamaska J, Matolcsy A, et al. Extensive flow cytometric characterization of plasmacytoid dendritic cell leukemia cells. Eur J Haematol (2005) 75(4):346–51 10.1111/j.1600-0609.2005.00513.x [DOI] [PubMed] [Google Scholar]
- 178.Oliver-Vila I, Saborit-Villarroya I, Engel P, Martin M. The leukocyte receptor CD84 inhibits Fc epsilon RI-mediated signaling through homophilic interaction in transfected RBL-2H3 cells. Mol Immunol (2008) 45(8):2138–49 10.1016/j.molimm.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 179.Romero X, Zapater N, Calvo M, Kalko SG, de la Fuente MA, Tovar V, et al. CD229 (Ly9) lymphocyte cell surface receptor interacts homophilically through its N-terminal domain and relocalizes to the immunological synapse. J Immunol (2005) 174(11):7033–42 [DOI] [PubMed] [Google Scholar]
- 180.Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol (2001) 167(10):5517–21 [DOI] [PubMed] [Google Scholar]
- 181.Boles KS, Mathew PA. Molecular cloning of CS1, a novel human natural killer cell receptor belonging to the CD2 subset of the immunoglobulin superfamily. Immunogenetics (2001) 52(3–4):302–7 10.1007/s002510000274 [DOI] [PubMed] [Google Scholar]
- 182.Lee JK, Mathew SO, Vaidya SV, Kumaresan PR, Mathew PA. CS1 (CRACC, CD319) induces proliferation and autocrine cytokine expression on human B lymphocytes. J Immunol (2007) 179(7):4672–8 [DOI] [PubMed] [Google Scholar]
- 183.Stark S, Watzl C. 2B4 (CD244), NTB-A and CRACC (CS1) stimulate cytotoxicity but no proliferation in human NK cells. Int Immunol (2006) 18(2):241–7 10.1093/intimm/dxh358 [DOI] [PubMed] [Google Scholar]
- 184.Falco M, Marcenaro E, Romeo E, Bellora F, Marras D, Vely F, et al. Homophilic interaction of NTBA, a member of the CD2 molecular family: induction of cytotoxicity and cytokine release in human NK cells. Eur J Immunol (2004) 34(6):1663–72 10.1002/eji.200424886 [DOI] [PubMed] [Google Scholar]
- 185.Flaig RM, Stark S, Watzl C. Cutting edge: NTB-A activates NK cells via homophilic interaction. J Immunol (2004) 172(11):6524–7 [DOI] [PubMed] [Google Scholar]
- 186.Messmer B, Eissmann P, Stark S, Watzl C. CD48 stimulation by 2B4 (CD244)-expressing targets activates human NK cells. J Immunol (2006) 176(8):4646–50 [DOI] [PubMed] [Google Scholar]
- 187.Tel J, van der Leun AM, Figdor CG, Torensma R, de Vries IJ. Harnessing human plasmacytoid dendritic cells as professional APCs. Cancer Immunol Immunother (2012) 61(8):1279–88 10.1007/s00262-012-1210-z [DOI] [PMC free article] [PubMed] [Google Scholar]