Abstract
Purpose
(1) To quantify and compare the effects of respiratory motion on paired passively scattered proton therapy (PSPT) and intensity modulated photon therapy (IMRT) plans. (2) To establish the relationship between the magnitude of tumor motion and the respiratory induced dose difference for both modalities.
Methods and Materials
In a randomized clinical trial comparing PSPT and IMRT, radiotherapy plans have been designed following common planning protocols. Four-dimensional (4D) dose was computed for PSPT and IMRT plans for a patient cohort with respiratory motion ranging 3-17 mm. Image registration and dose accumulation were performed using grayscale-based deformable image registration algorithms. The dose-volume histogram (DVH) differences (4D-3D) were compared for PSPT and IMRT. Changes in 4D-3D dose were correlated to the magnitude of tumor respiratory motion.
Results
The average 4D-3D dose to 95% of the internal target volume was close to zero, with 19/20 patients within 1% of prescribed dose for both modalities. The mean 4D-3D between the two modalities were not statistically significant (p <0.05) for all DVH indices (mean ± SD) except the lung V5 (PSPT: +1.1±0.9%, IMRT: +0.4±1.2%) and maximum cord dose (PSPT: +1.5±2.9 Gy, IMRT: 0.0±0.2 Gy). Changes in 4D-3D dose were correlated to tumor motion for only two indices: Dose to 95% PTV, and heterogeneity index.
Conclusions
With our current margin formalisms, target coverage was maintained in the presence of respiratory motion up to 17 mm for both PSPT and IMRT. Only 2/11 of 4D-3D indices (Lung V5 and spinal cord max) were statistically distinguishable between PSPT and IMRT, contrary to the notion that proton therapy will be more susceptible to respiratory motion. Due to the lack of strong correlations with 4D-3D dose differences in PSPT and IMRT, the extent of tumor motion was not an adequate predictor of potential dosimetric error caused by breathing motion.
Keywords: IMRT, Proton Therapy, Lung Radiation Therapy, 4D Dose Calculation, Respiratory Motion
INTRODUCTION
Respiratory motion has been demonstrated to affect radiation dose distributions in the thorax and is of particular concern in the treatment of lung cancer[1]. It has been reported that respiratory motion can impact the planned dose for intensity modulated photon therapy (IMRT) [2] and passively scattered proton therapy (PSPT) [3]. In general, it is often thought that the respiratory induced dose perturbation is greater for proton therapy because proton range is more sensitive to changes in tissue density. However, it is questionable whether or not PSPT is indeed more sensitive to breathing motion compare to IMRT because the treatment planning methods and the margins used to create PSPT plan are vastly different from IMRT[4]. Therefore, it is important to compare the sensitivity of the two modalities to breathing motion of lung cancer patients in order to provide a fair assessment of the efficacy of the two modalities beyond the conventional static planned dose comparison. To date, no literature has reported a treatment planning study for identical patient data compared in both proton and photon modalities while explicitly calculating the effects of respiratory motion for the same cohort of patients. Previously reported comparison study by Chang, et al,[5] included a comparison of five PSPT plans to IMRT plans, but made no mention of the effects of respiratory motion on either modality.
One important consideration during treatment planning of lung cancer is the inclusion of respiratory motion management during treatment. An expert task group has suggested 5 mm respiratory motion as a threshold above which to consider forms of respiratory management[1]. However this threshold exists only for photon radiotherapy and is not evidence-based. No literature has quantified a threshold of respiratory motion, above which active respiratory motion management would be beneficial to the patient in proton or photon therapy.
The purpose of this work was to assess the changes that the explicit inclusion of respiratory motion makes to the planned dose distribution for both PSPT and IMRT. This is important information that needs to be shown in order to support or judge previous [5,6] or future publications that compare PSPT vs. IMRT for lung without considering the effect of breathing motion. From this work we hoped to better identify the error introduced in our treatment planning evaluation that was exclusively based on 3D dose distribution and to determine if the magnitude of such error was greater for PSPT compared to IMRT. Furthermore, we analyzed if the magnitude of tumor motion can predict dosimetric error in the treatment plan due to the effects of respiratory motion.
METHODS AND MATERIALS
Patient Selection
This study obtained a 20 patient cohort taken from an institutional review board approved trial randomizing treatment between PSPT and IMRT for locally advanced lung cancer. Inclusion to the randomized trial required the patient to have locally advanced non-small cell lung cancer (stage II-IIIB disease according to the 7th edition of the AJCC Staging Manual[7]), without distant metastases, aged 18-85, and eligible for concurrent chemotherapy. Each patient had physician-approved PSPT and IMRT plans designed which both achieved common dose objectives (Table 1). The range of the extent of tumor motion observed for cohort patients for this study was 3-17 mm.
Table 1.
Both proton and photon plans were approved by a physician after achieving the normal tissue criteria shown. Abbreviations: MLD = mean lung dose, CGE = cobalt gray equivalent, MHD = mean heart dose, Vxx = volume of structure receiving xx Gy dose.
| Structure | Constraints | Minor Deviation | End Point |
|---|---|---|---|
| Normal Lung (Left Lung + Right Lung − GTV) |
V20 ≤ 37%; MLD ≤ 20 CGE | V20 ≤ 40%; MLD ≤ 22 CGE | Clinical pneumonitis |
| Esophagus | V45 ≤ 100% V55 ≤ 66% V65 ≤ 33% V70 ≤ 10% V75 ≤ 5% V78 ≤ 1.0 cc |
Not Permitted | Clinical stricture and perforation |
| Spinal Cord | V50 ≤ 2 cc | Not Permitted | Myelitis |
| Heart | MHD ≤ 30 CGE V33 ≤ 100% V45 ≤70% V60 ≤ 35% |
Not Permitted | Clinical pericarditis |
Image Acquisition
For initial simulation, all patients underwent 4DCT imaging on a multi-slice helical CT scanner (Discovery PET/CT: General Electric Medical Systems, Waukesha, WI). All patients received vocal coaching to promote regular respiration during 4DCT acquisition. 4DCT was performed following the methodology outlined by Pan, et al.[8,9] and covered the thorax and upper abdomen with a 2.5 mm slice spacing. Image bins corresponding to 10 phases of the respiratory cycle were created for each 4DCT simulation. Following the nomenclature of Pan et al. [9], we defined the full-inhale phase of respiration at T0, and the full-exhale phase as T50. Using identical anatomical contours, treatment planning was performed using Eclipse (Varian Medical Systems, Palo Alto, CA) and Pinnacle (Philips Radiation Oncology Systems, Fitchburg, WI) treatment planning software for PSPT and IMRT planning, respectively.
3D Treatment Planning
In the clinical trial, patients were treated with a prescription of 60, 66, or 74 Gy with coverage typically greater than 95% of the planning target volume (PTV). Normal tissue constraints criteria that were followed for both modalities are given in Table 1. For photon planning, typical plans used 5-11 beams. In contrast, proton planning used 2-4 beams. Clinical plan beam configurations for both modalities were selected by trained dosimetrists.
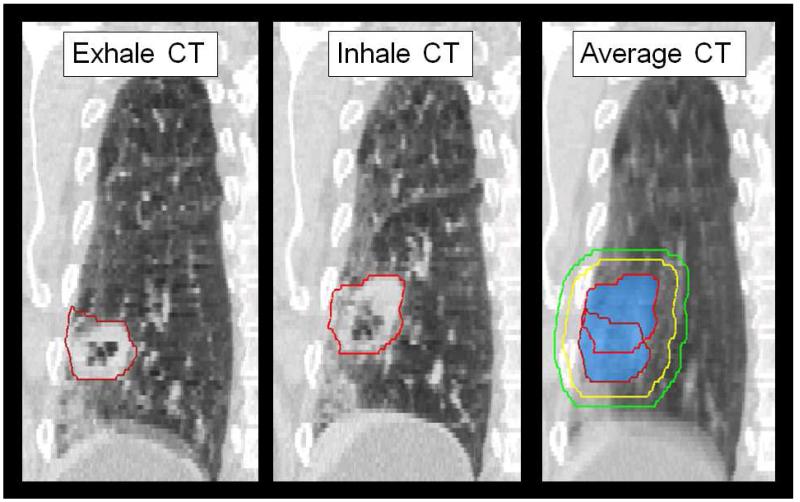
One set of anatomical contours was used to create the PSPT and IMRT plans. The target delineation is demonstrated in Figure 1. The gross tumor volume (GTV) was defined by the physician on the T50 and maximum intensity phase (MIP) CT datasets. The internal gross tumor volume (iGTV) was defined to include motion pass of the GTV over all phases of respiration. The internal target volume (ITV) was defined as an 8 mm isotropic expansion of the iGTV. These contours were altered by the physician if the ITV expansion extends into bony anatomy. The PTV margin was set as 5 mm from the ITV.
Figure 1.
For each patient, the GTV (red) were delineated on the four-dimensional (4D) CT phases. The union of GTV volumes throughout respiration created the iGTV (blue colorwash). Volume structures were created to account for microscopic disease (ITV, yellow) and setup uncertainty (PTV, green). Contour data is then sent to PSPT and IMRT treatment planning software for plan creation, as per the clinical protocol specifications in Table 1.
For PSPT planning, the proximal and distal margins to the ITV were determined for each beam using the formalisms suggested by Moyers, et al.[4]. PSPT compensators were smeared to account for variations in range due to misalignment of anatomy with the compensator. Additionally, to compensate for the density variation due to breathing motion, we used the average 4DCT image with iGTV density override (i.e. assignment of maximum CT HU number from individual phases) for compensator design [10]. The fields are laterally shaped by block margin to account for proton beam penumbra and the patient setup uncertainty, which is given as the sum of the 50-95% penumbra width (7 mm) and the setup margin (5 mm) as the total block margin from the ITV. After the proton beams were designed, dose was calculated on the average CT.
In IMRT planning, the PTV was to be treated with any combination of coplanar or non-coplanar fields shaped and modulated to deliver the specified dose while restricting the dose to the normal tissues. Beam weighting and modulation are determined by inverse IMRT planning procedures to produce the plan in accordance with normal tissue volume criteria given in Table 1. In both modalities, the planned dose as calculated on the average CT was defined as the 3D dose.
4D Dose Calculation
For each modality, the 4D dose was calculated for each clinically approved PSPT and IMRT plans. Our assumption is that explicitly incorporating respiratory motion (4D) into the calculation of the planned dose is more accurate than omitting (3D). No modifications were done to the original, clinical plan during the calculation of 4D dose. For both modalities, the original plans were copied to and recalculated on each phase of the simulation 4DCT, yielding 10 doses for 10 phases. Because our plans were highly (30-37) fractionated, we assumed negligible interplay effects of IMRT leaf motion with respiratory motion [11]
The image sets were registered using an in-house image deformation software based on an accelerated “demon’s algorithm” method which was validated by Wang, et al [12,13]. Each of the 4DCT phases were deformed towards the full-exhale, T50 CT image. The T50 phase was chosen because it has been shown that the lung at exhale is the most geometrically stable and repeatable phase of respiration [14]. The resulting deformable vector fields were used to accumulate the dose over all ten phases of the simulation 4DCT. Any uncertainties of the deformable registration should affect the PSPT and IMRT 4D calculations equally. The final 4D dose was the sum of the dose to each voxel in the T50 phase CT image. The deformed dose contribution of each phase was weighted by 10% because each phase occupies one tenth of the patient’s respiratory cycle. The resulting dose distribution was referred to as the 4D dose.
Plan Analysis
This work considered the difference between the 4D and 3D dose calculation to represent the effect of respiratory motion on the planned dose. To compare 4D with 3D dose, it would not be correct to deform between T50 and the average 4DCT image. The average CT was a composite of the 10 phases of the 4DCT; Anatomical structures would appear blurred and would not correspond to the patient anatomy on the T50 image. For example, note that in Figure 1, the lung anatomy will often appear blurred due to respiratory motion on an average CT, but not on individual phases (i.e. T0 and T50) of the 4DCT. Since the anatomical structures are represented incorrectly when averaging phases, we did not deform 4D dose from the T50 to the average CT for direct comparison with the 3D dose.
To compare 4D vs. 3D dose, Dose volume histograms (DVHs) were calculated and compared for the 4D and 3D calculations for both PSPT and IMRT. The 3D DVH was calculated using contours were originally designed on the average CT. To compute the 4D DVH, a new set of volume structures were defined on the T50 for the PTV, CTV, GTV, total lung, heart, esophagus and spinal cord. Because the same contours were used for both PSPT and IMRT planning, any uncertainty in contour delineation should affect both modalities equally. Additionally, the target dose heterogeneity index (H.I) was calculated using the equation:
where D2 represents the highest dose to 2% of the target, D98 represents the dose to 98% of the target, and the prescription dose is represented by DRX. Spearman rank correlation coefficients were calculated to search for correlation between the extent of tumor motion and 4D-3D dose.
RESULTS
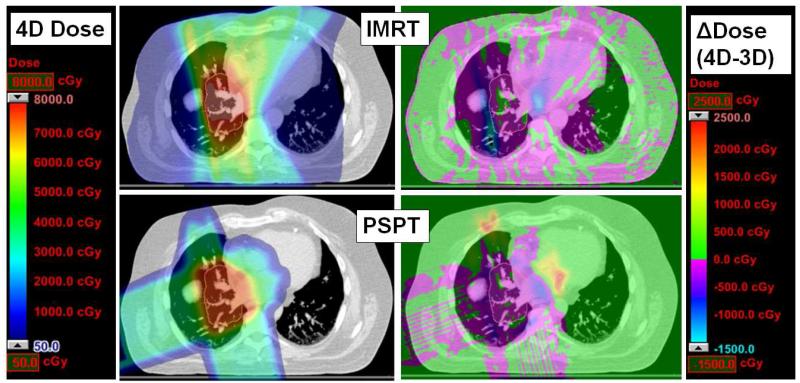
Figure 2 shows an example of 4D dose calculation (left) with the resulting 4D-3D differences (right) for an axial slice of a sample PSPT (top) and IMRT (bottom) plan. In general, we found that the dosimetric variation was pronounced in the regions outside the target volume (orange contours) along the edges of beams for IMRT; the variations were more pronounced for PSPT at the distal end of the proton beam range beyond the target volume. Figures 3-4 summarize the dose-volume indices for the PSPT and IMRT plan target volumes and normal tissues, respectively, from the cohort of 20 patients. The y-axis displays the differences in the 4D and 3D dose, thus positive values denote an increase from the conventional method of plan evaluation (i.e. using 3D dose distribution), while negative values denote a decrease.
Figure 2.
An example of axial slice of 4D dose calculation (IMRT top, PSPT bottom) for patient with 9 mm tumor motion. All doses (4D left, 4D-3D right) are overlaid on the T50 for anatomical reference. The ITV volume is outlined in orange in all images.
Figure 3.
Difference in 4D and 3D dose evaluation techniques for 95% of (a) PTV, (b) ITV, and (c) heterogeneity index versus tumor motion magnitude. The Δ(4D-3D) represents the 4D minus the 3D value for patient; positive values denote 4D indices increased from the 3D dose and negative values denote 4D indices decreased from the 3D dose. PSPT values are represented by red x and IMRT values are represented by a blue circle. Spearman’s rank correlation values of ρ are included when p-values were significant (p<0.05).
Figure 4.
Important dose-volume histogram criteria for normal tissue structures such as lung, heart, esophagus and spine dose-volume criteria are denoted in the same fashion as outlined in Figure 2.
Respiratory motion did not adversely affect target coverage in either modality. Paired t-tests revealed no statistically significant differences in 4D-3D target coverage between PSPT and IMRT groups for both the ITV and PTV. Figure 3a-b plots the 4D-3D difference in dose to 95% of ITV and PTV, respectively. The ITV coverage was consistent within 50 cGy between 4D and 3D evaluation for both PSPT and IMRT for 95% of the cohort despite the range of tumor motions from 3-17 mm. For both modalities, 4D dose did not deviate from the 3D dose by more than 2% for target volumes except for one IMRT case. These results were encouraging in that ITV/PTV does not experience any significant under-dosage due to respiratory motion. Moderate positive correlations were found between 4D-3D and tumor motion for PTV coverage in both modalities (PSPT & IMRT: ρ = 0.62, p <0.05), as shown in Figure 3b.
Figure 3c displays the heterogeneity index for the patient cohort. The heterogeneity index was found to have a modest, negative correlation with motion magnitude (PSPT: ρ = −0.66, IMRT: ρ = −0.70, p < 0.05). This result demonstrated that as tumor motion increased, the 4D dose demonstrated more homogeneous target coverage.
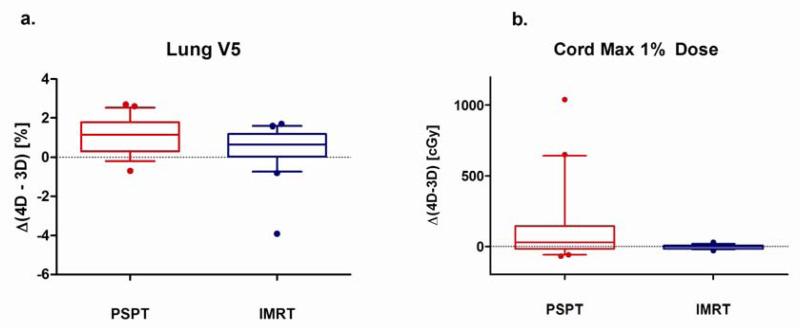
Figure 4 summarizes 4D-3D changes in selected dose-volume criteria for PSPT and IMRT OARs. The changes in mean lung dose values were not significantly different between the PSPT and IMRT cohorts. For all normal tissues, no correlation was found between 4D-3D differences and the magnitude of respiratory motion. The only statistically significant (p = 0.03) mean difference in 4D-3D lung DVH indices was for low (5 Gy) dose to the lung. Figure 5a demonstrates that the 4D-3D values of Lung V5 differed in the PSPT arm (mean ± SD = 1.1±0.9%) more than the IMRT arm (mean ±SD = 0.4±1.2%), but this difference was not correlated to tumor motion. All Lung V20 values agreed within 3% for 3D and 4D dose evaluation for both modalities. For all normal tissue DVH indices in Figure 4, no correlations between 4D-3D differences and tumor motion were observed.
Figure 5.
For dose-volume indices that were statistically different between PSPT and IMRT modalities, a box-whisker plot of the indices is shown. The whisker denotes the 90-10 percentiles, the box 25-75 percentile, and the middle line is the population mean. Any outliers are displayed as a circle.
For heart indices, this study found that the 4D dose calculation demonstrated a lower mean heart dose (MHD) than 3D techniques in over three quarters of the patients in both proton and photon modalities. Heart V45 values decreased for a majority (14/20) of the patients when using 4D dose methods in both modalities. However, no statistical differences were noted between 4D-3D heart and esophagus DVH indices and were not correlated with the extent of tumor motion for PSPT or IMRT.
For the spinal cord, it was observed that for 4 proton cases, the 4D spinal cord maximum 1% dose changed by more than 3 Gy. For IMRT, differences between 3D and 4D were small. The maximum 1% spine 4D-3D dose for the proton plans (mean ± SD = 1.5±2.9 Gy) appeared to vary more than the one in the photon plans (mean ± SD = 0.0±0.2 Gy). A paired t-test found that for the cohort, dose changes for spine were significantly (p = 0.03) higher for proton therapy (Figure 5b). However, it should be noted that the population average of absolute maximum cord dose was significantly lower for PSPT (PSPT = 29.8±18.0 Gy, IMRT = 40.0±4.5 Gy).
The only 4D-3D DVH indices that demonstrated any significant (p<0.05) correlation to the extent of tumor motion were the PTV 95% coverage and target dose heterogeneity index. All other 4D-3D changes in DVH indices were not correlated with the extent of tumor motion.
DISCUSSION
We observed that changes due to respiratory motion are present in both photon and proton planning. Neither modality was immune to the effects of respiratory motion on the planned dose. Interestingly, 4D dose assessment showed a slight improvement of PTV coverage compared to 3D dose for both PSPT and IMRT. This can be attributed to the hot spots of dose as calculated in the 3D calculation were smeared out due to motion of anatomy in the 4D calculation. We found that the effects of respiratory motion on DVH indices are comparable between PSPT and IMRT in terms of target coverage for this cohort. Nine of the eleven 4D-3D DVH indices observed did not demonstrate a statistically significant difference between PSPT and IMRT population mean. This is contrary to the commonly echoed caution that proton therapy was more sensitive to the anatomical changes during respiration.
For 3 patients with a beam directed at the spinal cord, the PSPT 4D cord maximum dose increased from 3D by more than 5 Gy. These results signify that the PSPT beams typically ranged deeper into the lung than current 3D calculation methods predicted. If possible, most PSPT beams are designed to avoid directions towards the spinal cord. From our study, we confirm that such practice should be continued and encouraged during PSPT beam angle selection.
The results indicate that the extent of tumor motion did not adequately predict when 4D vs. 3D dose difference occurred. Since we cannot currently predict conditions that respiratory motion would alter the planned dose distribution, this suggested that use of 4D calculation methods may be appropriate in the analysis of lung cancer patients. It should be noted that the method of 4D dose calculation outlined in this work used only the simulation 4DCT dataset and does not account for any intrafractional or interfractional variation of respiratory motion.
We observed that the patients with the largest changes in lung DVH indices for both the PSPT and IMRT modalities had relatively small (3-6 mm) tumor motion. While the patients’ tumors were relatively immobile, upon further review, it was revealed that the patient diaphragms moved over 10-20 mm during respiration. We can conclude that there are normal anatomical structures with respiratory motion larger than the measured tumor motion. To predict 4D vs. 3D dose differences, there may be a need to consider global respiratory motion not just the tumor, but of all tissues. We are investigating metrics to quantify respiratory motion parameters that can better predict the changes in 4D dose, such as global tissue deformation metrics or global perturbation of tissue density along each beam path.
CONCLUSION
We calculated the 4D dose for paired PSPT and IMRT treatment plans for 20 lung patients enrolled on a randomized clinical trial. Our results demonstrated that target coverage was maintained in both PSPT and IMRT. Only two of the 4D-3D DVH indices (lung V5 and spinal cord max) were statistically distinguishable between PSPT and IMRT, which is contrary to the widely held belief that proton therapy will be more susceptible to respiratory motion. For all normal tissue DVH indices, no correlation was found between the change in dose due to respiration and the extent of tumor. The magnitude of tumor motion was not an adequate predictor of potential dosimetric error caused by breathing motion.
SUMMARY.
In this study, the difference between 4D dose and 3D dose was calculated for paired passively scattered proton therapy and IMRT plans. Current margins accounting for respiratory motion are adequate in that target coverage was maintained for both modalities. In proton therapy, normal tissues distal to the tumor demonstrated dosimetric error. The magnitude of tumor motion was not correlated to difference between 4D and 3D dose for both proton and photon therapy.
Acknowledgments
Grant and financial support: This project is supported by grant P01CA021239 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- [1].Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of aapm task group 76. Medical Physics. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- [2].Starkschall G, Britton K, McAleer MF, et al. Potential dosimetric benefits of four-dimensional radiation treatment planning. International Journal of Radiation Oncology Biology Physics. 2009;73:1560–1565. doi: 10.1016/j.ijrobp.2008.12.024. [DOI] [PubMed] [Google Scholar]
- [3].Zhao L, Sandison GA, Farr JB, et al. Dosimetric impact of intrafraction motion for compensator-based proton therapy of lung cancer. Physics in Medicine and Biology. 2008;53:3343–3364. doi: 10.1088/0031-9155/53/12/019. [DOI] [PubMed] [Google Scholar]
- [4].Moyers MF, Miller DW, Bush DA, et al. Methodologies and tools for proton beam design for lung tumors. International Journal of Radiation Oncology Biology Physics. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- [5].Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage i or stage iii non-small-cell lung cancer. International Journal of Radiation Oncology Biology Physics. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- [6].Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage iiib non-small-cell lung cancer: A virtual clinical study. International Journal of Radiation Oncology Biology Physics. 77:357–366. doi: 10.1016/j.ijrobp.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Edge SB. Ajcc cancer staging manual. Springer; New York: American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- [8].Pan T, Lee TY, Rietzel E, et al. 4d-ct imaging of a volume influenced by respiratory motion on multi-slice ct. Medical Physics. 2004;31:333–340. doi: 10.1118/1.1639993. [DOI] [PubMed] [Google Scholar]
- [9].Pan T, Sun X, Luo D. Improvement of the cine-ct based 4d-ct imaging. Medical Physics. 2007;34:4499–4503. doi: 10.1118/1.2794225. [DOI] [PubMed] [Google Scholar]
- [10].Kang Y, Zhang X, Chang JY, et al. 4d proton treatment planning strategy for mobile lung tumors. International Journal of Radiation Oncology Biology Physics. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- [11].Bortfeld T, Jokivarsi K, Goitein M, et al. Effects of intra-fraction motion on imrt dose delivery: Statistical analysis and simulation. Physics in Medicine and Biology. 2002;47:2203–2220. doi: 10.1088/0031-9155/47/13/302. [DOI] [PubMed] [Google Scholar]
- [12].Wang H, Dong L, O’Daniel J, et al. Validation of an accelerated ‘demons’ algorithm for deformable image registration in radiation therapy. Physics in Medicine and Biology. 2005;50:2887–2905. doi: 10.1088/0031-9155/50/12/011. [DOI] [PubMed] [Google Scholar]
- [13].Wang H, Dong L, Lii MF, et al. Implementation and validation of a three-dimensional deformable registration algorithm for targeted prostate cancer radiotherapy. International Journal of Radiation Oncology Biology Physics. 2005;61:725–735. doi: 10.1016/j.ijrobp.2004.07.677. [DOI] [PubMed] [Google Scholar]
- [14].Balter JM, Lam KL, McGinn CJ, et al. Improvement of ct-based treatment-planning models of abdominal targets using static exhale imaging. International Journal of Radiation Oncology Biology Physics. 1998;41:939–943. doi: 10.1016/s0360-3016(98)00130-8. [DOI] [PubMed] [Google Scholar]