Abstract
Microglial activation plays an important role in the development and progression of various neurological disorders such as cerebral ischemia, multiple sclerosis, and Alzheimer’s disease. Thus, controlling microglial activation can serve as a promising therapeutic strategy for such brain diseases. In the present study, we showed that kalopanaxsaponin A, a triterpenoid saponin isolated from Kalopanax pictus, inhibited inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumor necrosis factor (TNF)-α expression in lipopolysaccharide (LPS)-stimulated microglia, while kalopanaxsaponin A increased anti-inflammatory cytokine interleukin (IL)-10 expression. Subsequent mechanistic studies revealed that kalopanaxsaponin A inhibited LPS-induced DNA binding activities of NF-κB and AP-1, and the phosphorylation of JNK without affecting other MAP kinases. Furthermore, kalopanaxsaponin A inhibited the intracellular ROS production with upregulation of anti-inflammatory hemeoxygenase-1 (HO-1) expression. Based on the previous reports that JNK pathway is largely involved in iNOS and proinflammatory cytokine gene expression via modulating NF-κB/AP-1 and ROS, our data collectively suggest that inhibition of JNK pathway plays a key role in anti-inflammatory effects of kalopanaxsaponin A in LPS-stimulated microglia.
Keywords: Microglia, Kalopanaxsaponin A, Anti-inflammation, JNK, NF-κB, AP-1
INTRODUCTION
As major immune cells in the central nervous system, microglia involve in neuronal development and maintaining homeostasis in the healthy brain (Tremblay et al., 2011). Microglia also remove damaged neurons by phagocytosis and induce neuronal recovery. However, overactivation or persistent activation of microglia causes neuronal cell death by producing various neurotoxic molecules and proinflammatory cytokines (Block et al., 2007; Graeber and Streit, 2010). Microglial activation is closely associated with neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis (Block et al., 2007; Glass et al., 2010; Graeber and Streit, 2010). Thus, the development of agents that can modulate microglial activation has been suggested as one of potential strategies for treatment or prevention of neurodegenerative diseases (Watkins and Maier, 2003; Glass et al., 2010).
Kalopanaxsaponin A is an oleanane triterpenoid saponin that is isolated from the stem bark of Kalopanax pictus (family Araliaceae), which has been used for traditional medicine in East Asian countries (Lee et al., 2001; Park et al., 2001). Previous studies reported that kalopanaxsaponin A has therapeutic effects in inflammatory disorders such as rheumatoid arthritis and diabetes mellitus (Park et al., 1998; Kim et al., 2002a). Kalopanaxsaponin A also ameliorated experimental colitis in mice by inhibiting interleukin-1 receptor associated kinase (IRAK) (Joh and Kim, 2011). The in vitro anti-inflammatory effects of kalopanaxsaponin A have been demonstrated in peritoneal macrophages, Raw 264.7 cells, and bone marrowderived dendritic cells (Kim et al., 2002b; Joh and Kim, 2011; Quang et al., 2013). Furthermore, kalopanaxsaponin A inhibited the invasion of human breast cancer cells and oral squamous carcinoma by reducing the matrix metalloproteinase-9 expression (Park et al., 2009b; Hwang et al., 2012).
Although several papers reported on the anti-inflammatory and anti-cancer activities of kalopanaxsaponin A, the effect of kalopanaxsaponin A in activated microglia has not been demonstrated. Therefore, in the present study, we investigated the anti-inflammatory effect of kalopanaxsaponin A in LPSstimulated BV2 microglial cells and analyzed the underlying molecular mechanisms.
MATERIALS AND METHODS
Isolation of kalopanaxsaponin A
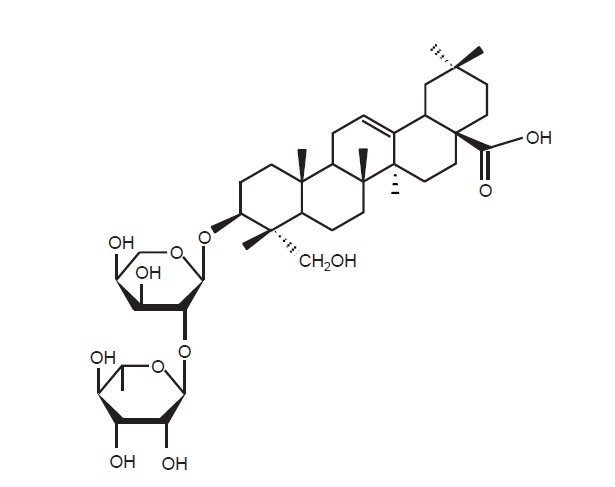
The dried bark of Kalopanax pictus (1 kg) was extracted three times with 80% MeOH under water bath and then evaporated to dryness under reduced pressure, which yielded 29 g according to the previous method (Joh et al., 2012). The dried powder was suspended in water and serially extracted with EtOAc and then BuOH. The BuOH extract (5.5 g) was fractionated into 6 (FB1-FB6) subfractions by silica gel column (3×35 cm) chromatography using a dichloromethane-MeOH solvent gradient. FB5 (0.5 g) was further separated by a MPLC (a linear- gradient applied by 10% CH3CN in H2O to 70% CH3CN in H2O at a flow rate of 4 ml/min over 4 h) to afford a kalopanaxsaponin A (31 mg; m/z 751.4 [M]-) (Fig. 1).
Fig. 1. Structure of kalopanaxsaponin A.
Reagents and antibodies
All reagents used for cell culture were purchased from Gibco BRL (Grand Island, NY, USA). LPS and Trizol reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). All the reagents and enzymes for RT-PCR were purchased from Promega (Madison, WI, USA). Antibodies against COX-2, iNOS and HO-1 were obtained from BD Bioscience (San Diego, CA, USA). Antibodies against phospho-/total form of MAP kinases were purchased form Cell Signaling Technology (Beverley, MA, USA).
Microglial cell culture and cell viability test
The immortalized murine BV2 microglial cell line (Bocchini et al., 1992) was grown and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated FBS, streptomycin (10 μg/ml), and penicillin (10 U/ml) at 37℃. Cell viability was determined by MTT reduction assay, as previously described (Park et al., 2009a).
Measurement of nitric oxide, cytokines, and intracellular ROS levels
Microglial cells (1×105 cells per well in a 24-well plate) were pre-treated with kalopanaxsaponin A for 1 h and stimulated with LPS (100 ng/ml) for 16 h. Then, the supernatants of the cultured microglia were collected and the accumulated nitrite was measured using the Griess reagent (Promega). The concentration of TNF-α or IL-10 in the supernatants was measured by ELISA, according to the procedure recommended by the supplier (BD Biosciences, San Jose, CA, USA). The intracellular accumulation of ROS was measured with H2DCF-DA (Sigma-Aldrich) by modifying a previously reported method (Lee et al., 2012).
RT-PCR
BV2 cells (7.5×105 cells on a 6-cm dish) were treated with LPS in the presence or absence of kalopanaxsaponin A, and total RNA was extracted with TRI reagent (Sigma-Aldrich). For RT-PCR, total RNA (1 μg) was reverse-transcribed in a reaction mixture that contains 1 U RNase inhibitor, 500 ng random primers, 3 mM MgCl2, 0.5 mM dNTP, and 10 U reverse transcriptase (Promega). The synthesized cDNA was used as a template for PCR reaction using GoTaq polymerase (Promega) and primers, as below (Table 1).
Table 1.
DNA sequences of primers used in PCR reactions, and expected product sizes
| Forward primer (5’→3’) | Reverse primer (5’→3’) | Size | |
|---|---|---|---|
|
| |||
| iNOS | CAAGAGTTTGACCAGAGGACC | TGGAACCACTCGTACTTGGGA | 450 bp |
| TNF-α | CCTATGTCTCAGCCTCTTCT | CCTGGTATGAGATAGCAAAT | 354 bp |
| COX-2 | TTCAAAAGAAGTGCTGGAAAAGGT | GATCATCTCTACCTGAGTGTCTTT | 304 bp |
| IL-10 | GCCAGTACAGCCGGGAAGACAATA | GCCTTGTAGACACCTTGGTCTT | 409 bp |
| GAPDH | ATGTACGTAGCCATCCAGGC | AGGAAGGAAGGCTGGAAGAG | 420 bp |
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts from treated microglia were prepared, as described previously (Woo et al., 2003). The double-stranded DNA oligomers containing consensus sequences of NF-κB or AP-1 were end-labeled, using T4 polynucleotide kinase (New England Biolabs, Beverly, MA, USA) in the presence of [γ-32P] ATP. Five micrograms of the nuclear proteins were incubated with 32P-labled probes on ice for 30 min and resolved on a 5% acrylamide gel and visualized by autoradiography.
Western blot analysis
Cells were appropriately treated and total cell lysates were prepared, as described previously (Jung et al., 2010). Proteins (20-100 μg) were heated with 4×SDS sample buffer and separated by SDS-PAGE gel electrophoresis and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies (1:1,000) and then, horseradish peroxidase-conjugated secondary antibodies (1:2,000 dilution in TBST; New England Biolabs, Ipswich, MA) were applied and the blots were developed using an enhanced chemiluminescence detection kit (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
Unless otherwise stated, all experiments were performed with triplicate samples and repeated at least three times. The data are presented as mean ± S.E.M. and statistical comparisons between groups were performed by using one-way analysis of variance, followed by Newman-Keuls test. A p value < 0.05 was considered significant.
RESULTS
Kalopanaxsaponin A suppresses iNOS, TNF-α and COX-2 expressions in LPS-stimulated microglia
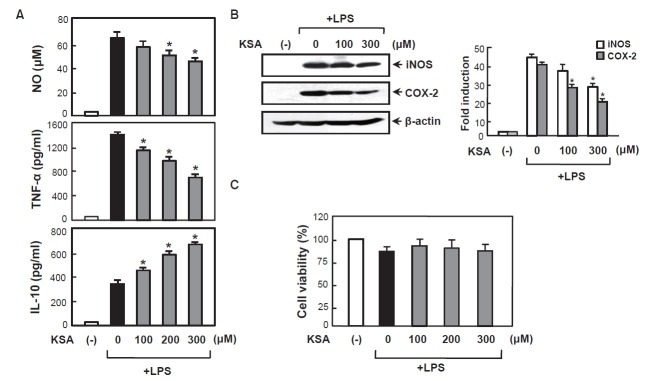
To investigate the anti-inflammatory effects of kalopanaxsaponin A in BV2 microglial cells, we examined the effect of kalopanaxsaponin A on LPS-induced production of NO and TNF-α which are key neurotoxic and proinflammatory molecules. We observed that LPS (100 ng/ml) strongly induced NO and TNF-α release, and pretreatment of kalopanaxsaponin A significantly inhibited LPS-induced NO and TNF-α production in a dose-dependent manner (Fig. 2A). On the other hand, kalopanaxsaponin A increased anti-inflammatory cytokine IL- 10. Western blot analysis confirmed that kalopanaxsaponin A suppresses iNOS and COX-2 expression at protein level (Fig. 2B). To exclude the possibility that the decrease in the NO and cytokine levels was simply due to cell death, we assessed cell viability at various concentrations of kalopanaxsaponin A. The MTT assay showed that kalopanaxsaponin A was not cytotoxic at the concentrations up to 300 μM used in these experiments (Fig. 2C).
Fig. 2. Effect of kalopanaxsaponin A on iNOS, COX-2 and cytokines in LPS-stimulated BV2 microglia. (A) The BV2 cells were pre-treated with the indicated concentration of kalopanaxsaponin A for 1 h, followed by treatment of LPS (100 ng/ml) for 16 h. The amounts of NO, TNF-α and IL-10 released into media were measured as described in the method section. The data are expressed as the mean ± S.E.M. of three independent experiments. (B) Western blot for iNOS and COX-2 protein expression. Densitogram of iNOS and COX-2 expressions that were normalized by β-actin (right panel). The data are representative of three independent experiments. (C) Effect of kalopanaxsaponin A on cell viability in BV2 cells. Cells were treated with various concentrations of kalopanaxsaponin A and incubated for 24 h. MTT assay revealed that kalopanaxsaponin A did not affect cell viability at least up to 300 μM. *p<0.05, significantly different from LPS-treated sample. ‘KSA’ indicates kalopanaxsaponin A.
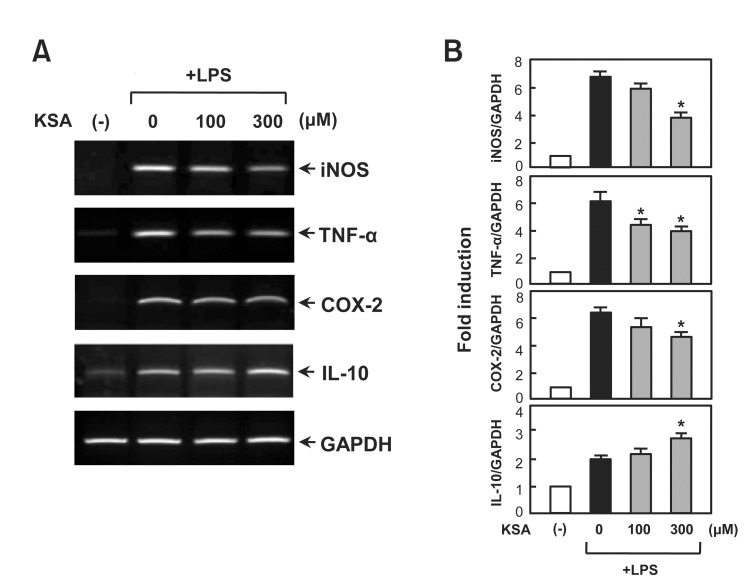
Kalopanaxsaponin A inhibits LPS-induced mRNA expression of iNOS, TNF-α and COX-2 in microglia
RT-PCR was performed to determine whether kalopanaxsaponin A regulates iNOS and cytokine expression at the transcriptional level. As shown in Fig. 3, kalopanaxsaponin A significantly inhibited the mRNA expressions of iNOS, TNF-α, and COX-2, while it enhanced IL-10 mRNA expression in LPSstimulated BV2 cells. The data suggest thatsaponin A modulates iNOS, TNF-α, COX-2 and IL-10 expressions at transcriptional level.
Fig. 3. Effect of kalopanaxsaponin A on mRNA expression of inflammatory mediators in LPS-stimulated BV2 microglia. (A) The cells were pre-treated with the indicated concentration of kalopanaxsaponin A for 1 h, followed by treatment of LPS (100 ng/ml) for 6 h. Total RNA was isolated from the cells and RT-PCR was performed to measure the mRNA levels of iNOS, TNF-α, COX-2 and IL-10. (B) Densitogram of PCR data. The data are representative of at least three independent experiments. *p<0.05, significantly different from LPS-treated sample.
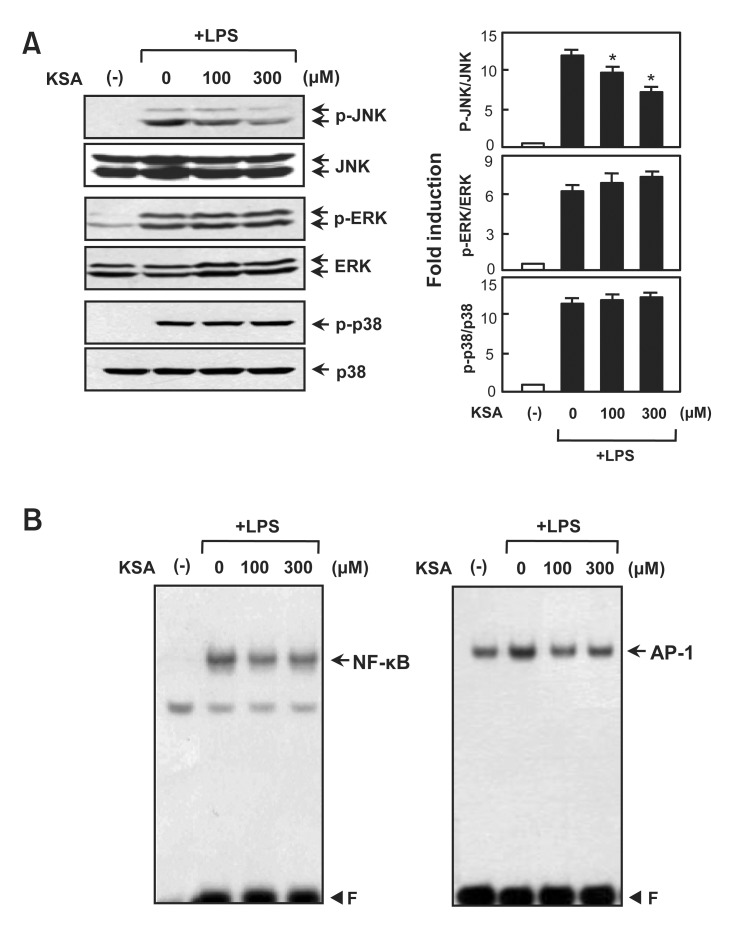
Kalopanaxsaponin A inhibits LPS-induced phosphorylation of JNK and DNA binding activities of NF-κB and AP-1
To analyze the molecular mechanism underlying the antiinflammatory effects of kalopanaxsaponin A, we examined the inhibitory effect on the phosphorylation of MAP kinases, which are upstream signaling molecules in inflammatory reactions (Kaminska, 2005). Western blot analysis showed that kalopanaxsaponin A significantly inhibited LPS-induced JNK phosphorylation. However, kalopanaxsaponin A did not affect the phosphorylation of ERK or p38 (Fig. 4A). In addition, kalopanaxsaponin A inhibited the DNA binding activities of NF-κB and AP-1, which are key transcription factors for the expression of inflammatory genes such as iNOS, TNF-α and COX-2 (Fig. 4B).
Fig. 4. Effects of kalopanaxsaponin A on three types of MAP kinases and NF-kB/AP-1. (A) Cell extracts were prepared from BV2 microglial cells treated with LPS for 30 min in the absence or presence of kalopanaxsaponin A, and then subjected to immunoblot analysis using antibodies against the phospho- or total-forms of three MAP kinases. Quantification of western blot data (right panel). Levels of the active forms of MAPKs were normalized with the total form, and are expressed as fold changes versus untreated control samples, which were arbitrarily set to 1.0. Values are the mean ± S.E.M. of four independent experiments. *p<0.05; significantly different from LPS-treated cells. (B) EMSA was performed using nuclear extracts isolated from BV2 microglial cells treated with kalopanaxsaponin A in the presence of LPS for 3 h. The data are representative of three independent experiments. ‘F’ indicates free probe.
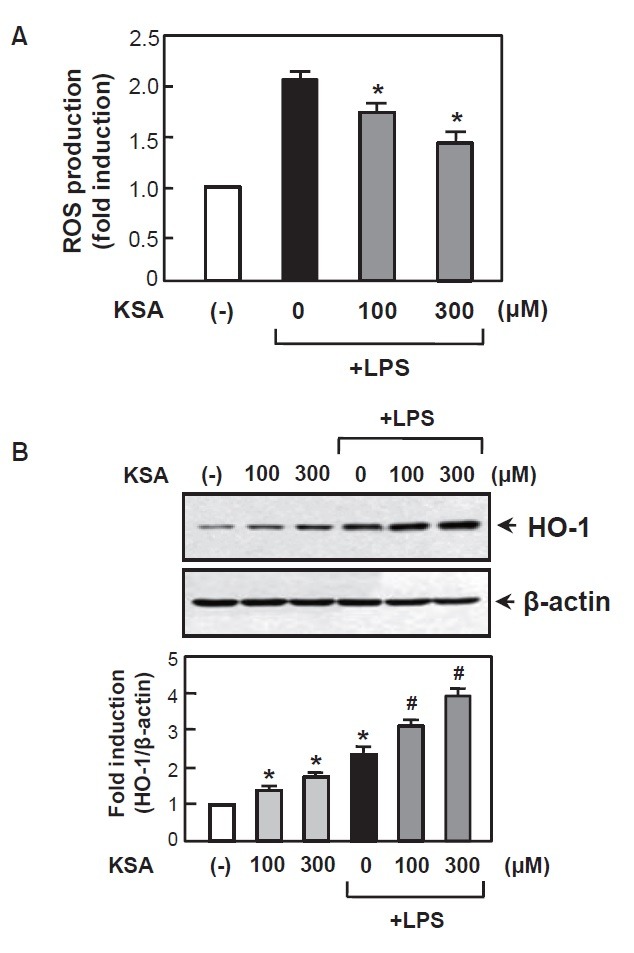
Kalopanaxsaponin A suppressed ROS production with enhancement of anti-inflammatory HO-1 expression
ROS plays a role as early signaling inducer of inflammatory reactions and excessive ROS generation by microglia leads to the neuronal cell death (Bedard and Krause, 2007; Gao et al., 2012). In this study, we found that kalopanaxsaponin A significantly inhibited LPS-induced ROS production in the BV2 cells (Fig. 5A). Next, we examined the effects of kalopanaxsaponin A on HO-1, which mediates anti-inflammatory and antioxidant effects in the activated microglia (Jung et al., 2010; Keum, 2012). Kalopanaxsaponin A increased HO-1 expression at protein level (Fig. 5B). Thus, the antioxidant and anti-inflammatory effect of kalopanaxsaponin A may be related to upregulation of HO-1.
Fig. 5. Kalopanaxsaponin A inhibited ROS production with enhancement of HO-1 expression in LPS-stimulated BV2 cells. (A) The BV2 cells were pre-treated with kalopanaxsaponin A for 1 h, followed by treatment with LPS (100 ng/ml) for 16 h. The intracellular ROS levels were then measured by the DCF-DA method as described in the Materials and Methods section. The data are expressed as the mean ± S.E.M. of three independent experiments. *p<0.05, significantly different from LPS-treated sample. (B) Western blot analysis shows the effect of kalopanaxsaponin A on HO-1 protein expression. BV2 cells were treated with kalopanaxsaponin A with or without LPS for 16 h and cell lysates were obtained. Quantification data are shown in the graph (n=3). *p<0.05, significantly different from the control sample. #p<0.05, significantly different from the LPS-treated sample.
DISCUSSION
In the present study, we demonstrate the anti-inflammatory effects of kalopanaxsaponin A in LPS-stimulated BV2 microglial cells. Kalopanaxsaponin A suppressed LPS-induced expression of iNOS, COX-2 and TNF-α, which are key proinflammatory and neurotoxic molecules in microglia. On the other hand, kalopanaxsaponin A upregulated the anti-inflammatory cytokine IL-10. Furthermore, kalopanaxsaponin A reduced intracellular ROS level and increased HO-1 expression. Detailed mechanistic analysis revealed that kalopanaxsaponin A specifically inhibited JNK phosphorylation, and NF-κB/ AP-1 DNA binding activities.
We have recently reported the JNK pathway plays a pivotal role in inflammatory gene expression via modulating NF- κB/AP-1 and ROS signaling in microglia (Jeong et al., 2013). Thus, the specific inhibition of JNK by SP600125 suppressed NO, TNF-α, and IL-6 expression in LPS-stimulated BV2 cells. In addition, SP600125 inhibited the DNA binding activities of NF-κB/AP-1, and ROS production by modulating the cytosolic and membrane components of NADPH oxidase. SP600125 also increased HO-1 expression in BV2 cells. Thus, our data collectively suggest that JNK and its downstream NF-κB/AP-1 pathways play a pivotal role in anti-inflammatory effects of kalopanaxsaponin A in LPS-stimulated microglia.
Kalopanaxsaponin A is a main constituent of Kalopanax pictus, which has been used as a tonic, analgesic, antirhematic and antidiabetic agent. Previous studies demonstrated that administration of kalopanaxsaponin A exhibited potent antiedema effect and reduced vascular permeability in rat rheumatoid arthritis model (Kim et al., 2002a). In streptozocininduced diabetic rats, kalopanaxsaponin A decreased serum glucose, cholesterol and lipoprotein levels (Park et al., 1998). A recent study reported that oral administration of kalopanaxsaponin A improved TNBS-induced colitis symptoms including diarrhea and colon shortening (Joh and Kim, 2011). In vitro studies by using peritoneal macrophages showed that kalopanaxsaponin A inhibited NO, TNF-α, and COX-2 expressions by suppressing IRAK-1, IKK-β/NF-κB and all the three types of MAP kinase activities (Joh and Kim, 2011). Therefore, the anti-inflammatory effects of kalopanaxsaponin A were observed in both the microglia and macrophage cells. However, the underlying molecular mechanisms appear to be somewhat different probably due to cell-specificity.
A recent study reported that kalopanaxsaponin A improved scopolamine-induced memory deficits by inhibiting acetylcholine esterase activity and inducing BDNF and p-CREB expressions in the mouse brain (Joh et al., 2012). In addition, kalopanaxsaponin A inhibited TNF-α expression in the brain of scopolamine-treated mice. The results suggest that kalopanaxsaponin A may be effective for CNS disorders. Therefore, the strong anti-inflammatory effects of kalopanaxsaponin A may provide a potential therapeutic modality for neurodegenerative diseases that are accompanied by microglial activation.
Acknowledgments
This work was supported by the National Research Foundation (NRF) grant funded by the Korean government [Grant 2012R1A5A2A32671866].
References
- 1.Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. (2007);87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Block M. L., Zecca L., Hong J. S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. (2007);8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 3.Bocchini V., Mazzolla R., Barluzzi R., Blasi E., Sick P. An immortalized cell line expresses properties of activated microglial cells. J. Neurosci. Res. (1992);31:616–621. doi: 10.1002/jnr.490310405. [DOI] [PubMed] [Google Scholar]
- 4.Gao H. M., Zhou H., Hong J. S. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends. Pharmacol. Sci. (2012);33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. Mechanisms underlying inflammation in neurodegeneration. Cell. (2010);140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graeber M. B., Streit W. J. Microglia: biology and pathology. Acta Neuropathol. (2010);119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 7.Hwang Y. S., Park K. K., Chung W. Y. Kalopanaxsaponin A inhibits the invasion of human oral squamous cell carcinoma by reducing metalloproteinase-9 mRNA stability and protein trafficking. Biol. Pharm. Bull. (2012);35:289–300. doi: 10.1248/bpb.35.289. [DOI] [PubMed] [Google Scholar]
- 8.Jeong Y. H., Jung J. S., Le T. K., Kim D. H., Kim H. S. Lancemaside A inhibits microglial activation via modulation of JNK signaling pathway. Biochem. Biophys. Res. Commun. (2013);431:369–375. doi: 10.1016/j.bbrc.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Joh E. H., Kim D.H. Kalopanaxsaponin A ameliorates experimental colitis in mice by inhibiting IRAK-1 activation in the NFkB and MAPK pathways. Br. J. Pharmacol. (2011);162:1731–1742. doi: 10.1111/j.1476-5381.2010.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joh E. H., Lee I. A., Kim D. H. Kalopanaxsaponins A and B isolated from Kalopanax pictus ameliorate memory deficits in mice. Phytother. Res. (2012);26:546–551. doi: 10.1002/ptr.3596. [DOI] [PubMed] [Google Scholar]
- 11.Jung J. S., Shin J. A., Park E. M., Lee J. E., Kang Y. S., Min S. W., Kim D. H., Hyun J. W., Shin C. Y., Kim H. S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide- stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J. Neurochem. (2010);115:1668–1680. doi: 10.1111/j.1471-4159.2010.07075.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaminska B. MAPK signaling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to thera-peutic benefits. Biochem. Biophys. Acta. (2005);1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Keum Y. S. Regulation of Nrf2-mediated phase II detoxification and anti-oxidant genes. Biomol. Ther. (2012);20:144–151. doi: 10.4062/biomolther.2012.20.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D. H., Bae E. A., Han M. J., Park H. J., Choi J. W. Metabolism of kalopanaxsaponin A by human intestinal bacteria and antirhematoid arthritis activity of their metabolites. Biol. Pharm. Bull. (2002a);25:68–71. doi: 10.1248/bpb.25.68. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y. K., Kim R. G., Park S. J., Ha J. H., Choi J. W., Park H. J., Lee K. T. In vitro antiinflammatory activity of kalopanaxsaponin A isolated from Kalopanax pictus in murine macrophage RAW 264.7 cells. Biol. Pharm. Bull. (2002b);25:472–476. doi: 10.1248/bpb.25.472. [DOI] [PubMed] [Google Scholar]
- 16.Lee E. B., Li D. W., Hyun J. E., Kim I. H., Whang W. K. Anti-inflammatory activity of methanol extract of Kalopanax pictus bark and its fractions. J. Ethnopharmacol. (2001);77:197–201. doi: 10.1016/s0378-8741(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee K. M., Kang H. S., Yun C. H., Kwak H. S. Potential in vitro protective effect of quercetin, catechin, caffeic acid and phytic acid against ethanol-induced oxidative stress in SK-Hep-1 cells. Biomol. Ther. (2012);20:492–498. doi: 10.4062/biomolther.2012.20.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H. J., Kim D. H., Choi J. W., Park J. H., Han Y. N. A potent anti-diabetic agent from Kalopanax pictus. Arch. Pharm. Res. (1998);21:24–29. doi: 10.1007/BF03216748. [DOI] [PubMed] [Google Scholar]
- 19.Park H. J., Kwon S. H., Lee J. H., Lee K. H., Miyamoto K., Lee K. T. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. (2001);67:118–121. doi: 10.1055/s-2001-11516. [DOI] [PubMed] [Google Scholar]
- 20.Park J. S., Park E. M., Kim D. H., Jung K., Jung J. S., Lee E. J., Hyun J. W., Kang J. L., Kim H. S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. (2009a);209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Park S. K., Hwang Y. S., Park K. K., Park H. J., Seo J. Y., Chung W. Y. Kalopanaxsaponin A inhibits PMA-induced invasion by reducing matrix metalloproteinase-9 via PI3K/Akt- and PKCδ- mediated signaling in MCF-7 human breast cancer cells. Carcinogenesis. (2009b);30:1225–1233. doi: 10.1093/carcin/bgp111. [DOI] [PubMed] [Google Scholar]
- 22.Quang T. H., Ngan N. T., Van Minh C., Van Kiem P., Nhiem N. X., Tai B. H., Thao N. P., Chae D., Mathema V.B, Koh Y. S., Lee J. H., Yang S. Y., Kim Y. H. Inhibitory effects of oleanane-type triterpenes and saponins from the stem bark of Kalopanax pictus on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. (2013);36:327–334. doi: 10.1007/s12272-013-0031-8. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay M. È., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. (2011);31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins L. R., Maier S. F. Glia: a novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. (2003);2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 25.Woo M. S., Jang P. G., Park J. S., Kim W. K., Joh T. H., Kim H. S. Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutyryl-cAMP in BV2 microglial cells. Brain Res. Mol. Brain Res. (2003);113:86–96. doi: 10.1016/s0169-328x(03)00095-0. [DOI] [PubMed] [Google Scholar]