Abstract
In the present study, we investigated the effect of intracellular glutathione (GSH) depletion in heart-derived H9c2 cells and its mechanism. L-buthionine-S,R-sulfoximine (BSO) induced the depletion of cellular GSH, and BSO-induced reactive oxygen species (ROS) production was inhibited by glutathione monoethyl ester (GME). Additionally, GME inhibited BSO-induced caspase-3 activation, annexin V-positive cells, and annexin V-negative/propidium iodide (PI)-positive cells. Treatment with rottlerin completely blocked BSO-induced cell death and ROS generation. BSO-induced GSH depletion caused a translocation of PKC-δ from the cytosol to the membrane fraction, which was inhibited by treatment with GME. From these results, it is suggested that BSO-induced depletion of cellular GSH causes an activation of PKC-δ and, subsequently, generation of ROS, thereby inducing H9c2 cell death.
Keywords: Glutathione, Reactive oxygen species, H9c2, Cell death, PKC-δ
INTRODUCTION
Cardiovascular disease (CVD) continues to be a substantial health-care burden despite recent treatment advances. Oxidative stress has long been regarded as a key pathophysiological mediator that ultimately leads to CVD including atherosclerosis, hypertension, and heart failure (Chen and Keaney, 2012). Reactive oxygen species (ROS), which are involved in normal physiological functions at low concentrations, can have deleterious effects when produced in excess. Over time, ROS may result in a pathological state of imbalance known as oxidative stress. Most therapies that are currently being used may provide some reduction in oxidative stress, but there is no consensus on the clinical outcomes of various antioxidants. Currently, there are no antioxidant therapies that specifically target oxidative stress in patients with heart failure. Therefore, a better understanding of the mechanism of injury induced by oxidative stress related to CVD is needed (Ahmed and Tang, 2012).
Previous studies have pointed out changes in the redox status of glutathione in a failing heart (Damy et al., 2009). Glutathione (GSH) is a tripeptide (γ-glutamylcysteinyl glycine) that plays an important protective role against oxidative stressinduced damage in mammalian tissues (Sevin et al., 2013). Indeed, it acts as a substrate for scavenging ROS via the enzymes glutathione peroxidase and glutathione reductase. Reduction of cellular GSH, which allows ROS accumulation, has been shown to cause apoptosis in Jurkat T cells (van den Dobbelsteen et al., 1996) neutrophils (O'Neill et al., 2000), and neural cells (Narasimhan et al., 2011). Moreover, many studies have also examined the relationship between GSH levels and heart disease. Decreases in plasma GSH levels are associated with cardiomyocyte apoptosis (Kytö et al., 2004) and are correlated with the severity of heart failure symptoms in patients (Damy et al., 2009). Hearts depleted of intrinsic GSH suffer from more functional damage inflicted by ischemia/reperfusion than do normal hearts (Blaustein et al., 1989). Recently, it was reported that NOX4 induces GSH levels through regulation of Nrf2 in cardiomyocytes, and triggers the adaptive stress response to protect the heart (Brewer et al., 2011). However, other studies have disputed these findings, showing no significant difference in functional integrity between GSHdepleted and GSH-adequate hearts (Chatham et al., 1988). Therefore, whether or not oxidative stress induced by GSH depletion contributes to the progression of heart disease remains elusive.
Experimental depletion of cellular GSH can be produced by using L-buthionine-S,R-sulfoximine (BSO), a potent and specific inhibitor of γ-glutamyl-cysteine synthetase (Meister and Anderson, 1983). In this study, we investigated the effects of GSH depletion by BSO on cellular viability and determined the mechanism of cellular damage induced by oxidative stress through GSH depletion in cardiomyocytes.
MATERIALS AND METHODS
Cell cultures and drug treatment
Heart-derived H9c2 cells were purchased from American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. H9c2 cells were pretreated with glutathione monoethyl ester (GME), protein kinase C (PKC)-ζ inhibitor (Santa Cruz Biotechnology, Santa Cruz, CA, USA), trolox, GF109203X, Go6976, or rottlerin (Sigma-Aldrich, St Louis, MO, USA) for 30 min. Subsequently, the cells were treated with 100 mM BSO (Sigma-Aldrich) in the presence or absence of GME or inhibitor.
Measurements of cellular GSH level
Intracellular GSH were measured using GSH colorimetric detection kit (BioVision, Milpitas, CA, USA). Briefly, cells were lysed in GSH buffer for 10 min. Cell lysates centrifuged at 8,000 g for 10 min and supernatants were collected. Supernatants were incubated with reaction buffer for 10 min. After adding of substrate solution, GSH level were monitored at 405 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
Measurements of ROS release
Intracellular ROS were measured fluorometrically using 2’,7’-dichlorofluorescein (DCF) diacetate (DA) (Molecular probes, Eugene, OR, USA), which permeates cells easily and hydrolyzes to DCF after interacting with intracellular ROS. Cultured cells were washed twice with HCSS incubated with 10 μM DCF-DA and 20% Pluronic F-127 for 30 min, and washed with HCSS, as described previously (Lee and Jung, 2012). Subsequently, cells were observed under a confocal microscope (Olympus, Tokyo, Japan). DCF fluorescence intensities were obtained in Fluoview FV300 software. DCF sample values are expressed as percentage relative to the control value.
Flow cytometric analysis for propidium iodide (PI)/annexin V staining
Apoptotic and necrotic cells were detected by double staining with PI and annexin V-FITC using the annexin-V apoptosis detection kit I (BD PharMingen, San Diego, CA, USA) (Lee et al., 2011; Yang et al., 2012). Briefly, cells were detached by trypsin-EDTA treatment, gently resuspended in cold binding buffer to 5×105 cells, and incubated with annexin V-FITC and PI at room temperature in the dark. Living cells were defined as those negatively stained for both PI and annexin V. Early apoptotic cells were defined as those staining positive for annexin V and negative for PI. Late apoptotic cells were defined as those staining positively for both. Necrotic cells were defined as those staining only with PI. The fluorescence intensity of the cell surface was measured by flow cytometry (FACSVantage, Becton Dickinson, Franklin Lakes, NJ, USA). Values are expressed as the percentage of annexin V-positive cells to total cells counted.
Nuclei staining
Cells were fixed with 4% paraformaldehyde for 10 min. Cell were incubated with Hoechst 33258 for 10 min. All samples were then observed under a confocal microscope (Olympus, Tokyo, Japan).
Measurement of caspase-3 activity
Cells were washed once with ice-cold phosphate-buffered saline and extracted on ice in lysis buffer (10 mM Tris-HCl, 0.32 M sucrose, 10 mM DTT, 5 mM EDTA, 1% Triton X-100, 1 mM PMSF, 1 μg/ml aprotinin, and 10 μM leupeptin) for 1 h. Lysates were centrifuged at 10,000 g for 10 min at 4℃, and 200 μg of cytosolic extracts were mixed with reaction buffer (100 mM HEPES pH 7.5, 10% sucrose, 0.1% CHAPS, 10 mM DTT, and 10 μM leupeptin) to a final volume of 100 μl containing 200 μM of Ac-DEVD-p-Na. Samples were then incubated for 2 h at 37℃, as described previously (Lee and Jung, 2012). The enzyme-catalyzed release of p-nitroanilide was monitored at 405 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
Lactate dehydrogenase (LDH) assay
Cell death was analyzed by measuring LDH release into medium as described previously (Lee and Jung, 2012). Briefly, 24 h after BSO treatment, 25 μl medium was collected from each well and mixed with 100 μl NADH solution (0.03% β-NAD [reduced form of the disodium salt] in phosphate buffer) and 25 μl pyruvate solution (22.7 mM pyruvic acid in phosphate buffer) at room temperature. NADH consumption was followed for 2 min at 340 nm. The percent LDH was calculated from the maximum LDH release (100%) induced by lysing cells with 0.1% Triton X-100.
Western blot
Protein expression of PKC-δ was measured using western blot analysis as previously described (Lee et al., 2012). Cells were lysed using lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 1% NP-40, 150 mmol/L NaCl, 0.25% Na-deoxycholate, 2 mmol/L EDTA, 1 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L PMSF, 10 μg/ml of aprotinin, and 10 μmol/L leupeptin). Lysates were subsequently centrifuged at 14,000 rpm for 15 min and supernatants were collected. Then, equal amounts of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and reacted with anti-PKC-δ antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-β-actin antibody (Sigma-Aldrich). After probing with horseradish peroxidase (HRP)-conjugated secondary antibody, proteins were visualized using LAS 1000 (Fuji Photo Film, Tokyo, Japan).
Statistical analysis
All data are expressed as mean ± S.E.M. Numerical data were compared using the Student’s t-test for paired observations between two groups. A p value<0.05 was considered significant.
RESULTS
Depletion of GSH induced ROS generation
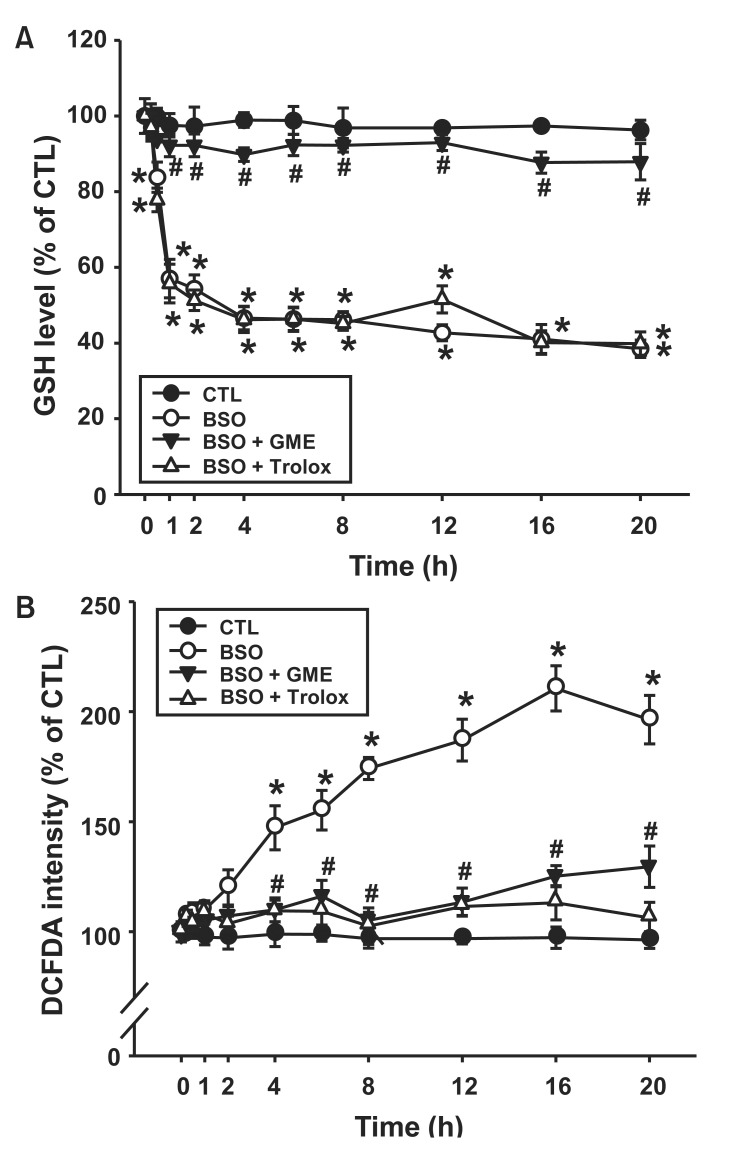
To determine the effect of GSH depletion on BSO-induced ROS generation, we used GME to maintain the intracellular GSH concentration in the presence of BSO (Torres et al., 1997). The GSH level in H9c2 cells decreased by treatment with 10 mM BSO and was approximately 80% at 0.5 h, 57% at 1 h, 46% at 4 h, and 43% at 12 h after treatment (Fig. 1A). The decrease in cellular GSH levels by BSO was restored to control levels by GME (300 μM). However, trolox (10 μM), an ROS scavenger, had no influence on the cellular GSH level. As shown in Fig. 1B, ROS production was significantly increased by BSO. BSO-induced ROS generation was dramatically decreased by GME or trolox at each time point. These results suggest that BSO induced ROS generation through the depletion of cellular GSH levels.
Fig. 1. Effect of glutathione monoethyl ester (GME) on BSOinduced ROS generation. H9c2 cells were incubated with BSO (10 mM) in the presence or absence of GME (300 μM) or trolox (10 μM) for an indicated time. (A) Glutathione (GSH) level. (B) DCF-DA intensity. Data are expressed as mean ± S.E.M. (n=3). *p<0.05 vs. 0 h. #p<0.05 vs. BSO.
Depletion of GSH induced cell death of cardiomyocytes
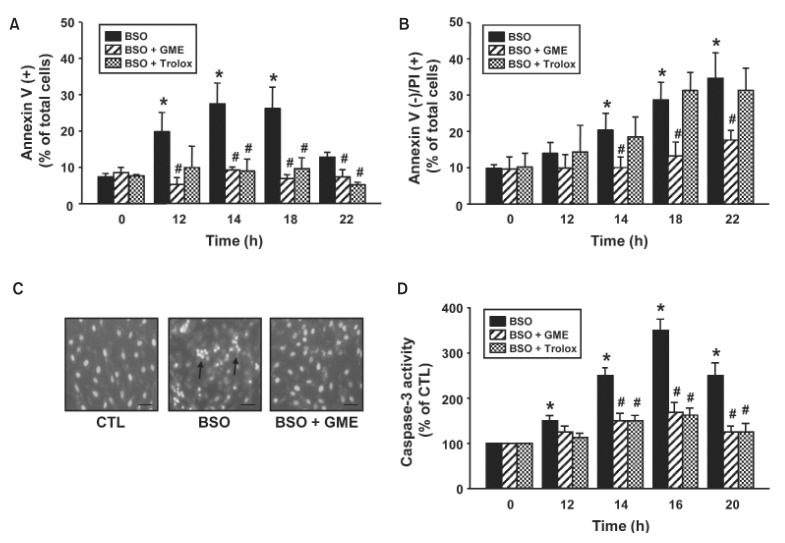
We examined the effect of GME on BSO-induced cell death. BSO remarkably increased annexinV-positive cells to 19.8% at 12 h, 27.5% at 14 h, and 26.8% at 18 h, indicating that BSO-induce apoptosis of H9c2 cells (Fig. 2A). The BSO-induced apoptosis was blocked by treatment with GME (5.3% at 12 h, 9.3% at 14 h, and 6.9% at 18 h). BSO also increased annexinV-negative/PI-positive cells to 20.3% at 14 h, 28.7% at 18 h, and 34.7% at 22 h, indicating induction of necrosis of H9c2 cells (Fig. 2B). Treatment with GME inhibited the BSO-induced necrosis (10% at 14 h, 13.3% at 18 h, and 17.6%). Treatment with trolox blocked only BSO-induced apoptosis but not BSO-induced necrosis. Consistently, BSOincreased the frequency of apoptotic cells with fragmented nuclei and condensed chromatin compared with control cells (Fig. 2C). Treatment with GME completely protected cells from morphological changes by BSO. Next, we examined the effect of GME on the activation of caspase-3, which plays an important role in apoptotic cell death. As shown in Fig. 2D, caspase-3 activity was significantly increased to 250% at 14 h, and reached its maximal level (350%) at 16 h after BSO treatment. This BSO-induced caspase-3 activity was dramatically inhibited by GME or trolox. These results suggest that BSO-induced apoptosis and necrosis through the depletion of cellular GSH levels.
Fig. 2. Effect of glutathione monoethyl ester (GME) on BSO-induced cell death. H9c2 cells were incubated with BSO (10 mM) in the presence or absence of GME (300 μM) or trolox (10 μM) for indicated times. (A, B) FACS analysis of cells after Annexin V-PI staining. The percentages of apoptotic [Annexin-V (+)] (A) and necrotic [Annexin-V(–)/PI (+)] (B) cells were analyzed. (C) Staining of cells with Hoechst 33342. Arrows indicate fragmented nuclei and condensed chromatin. Scale bar=10 μm. (D) Caspase-3 activity. Data are expressed as mean ± S.E.M. (n=3). *p<0.05 vs. 0 h. #p<0.05 vs. BSO.
PKC-δ contributed to BSO-induced cell death and ROS production
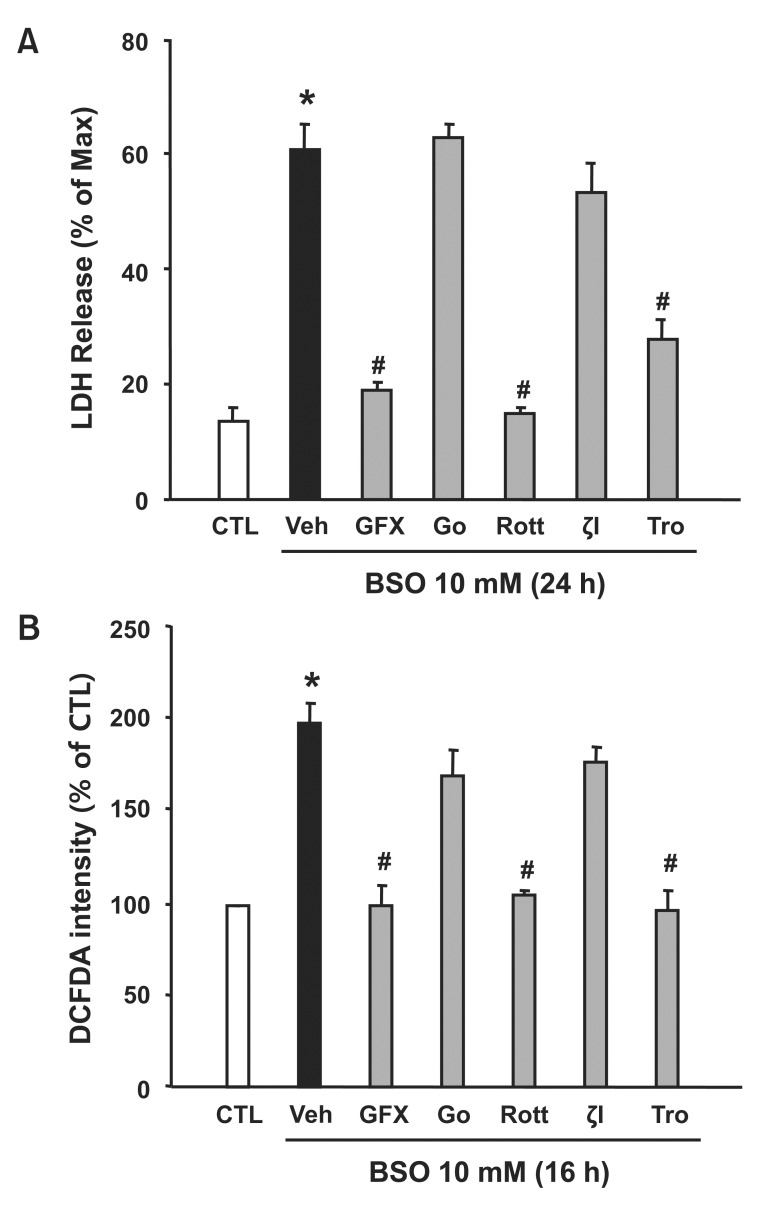
We further examined the effect of PKC isotypes on BSOinduced cell death. As shown in Fig. 3A, LDH release was remarkably increased by BSO compared to that observed in the control condition. This BSO-induced LDH release (61%) was decreased by the general PKC inhibitor (GF109203X, 18%) or the PKC-δ inhibitor (rottlerin, 15%). However, the conventional PKC inhibitor (Go6983) or the PKC-ζ inhibitor did not inhibit BSO-induced LDH release. Next, we determined the effect of various PKC inhibitors on ROS production (Fig. 3B). Consistent with LDH release, BSO-induced ROS generation (189%) was blocked by the GF109203X (103%) and the rottlerin (112%). These results suggest that PKC-δ plays a role in BSO-induced ROS production and cell death.
Fig. 3. Effect of PKC inhibitors on BSO-induced cell death and ROS production. H9c2 cells were incubated with BSO (10 mM) in the presence or absence of PKC inhibitors (10 μM) or trolox (10 μM). (A) Lactate dehydrogenase (LDH) release. Percent LDH was calculated from the maximum LDH release (100%) induced by 0.1% Triton X-100. (B) DCF-DA intensity. Data are expressed as mean ± S.E.M. (n=3). *p<0.05 vs. 0 h. #μ<0.05 vs. BSO. GFX: GF109203X; Go: Go6976; Rott: rottlerin; ζI: PKC-ζ inhibitor; Tro: trolox.
Depletion of GSH induced activation of PKC-δ
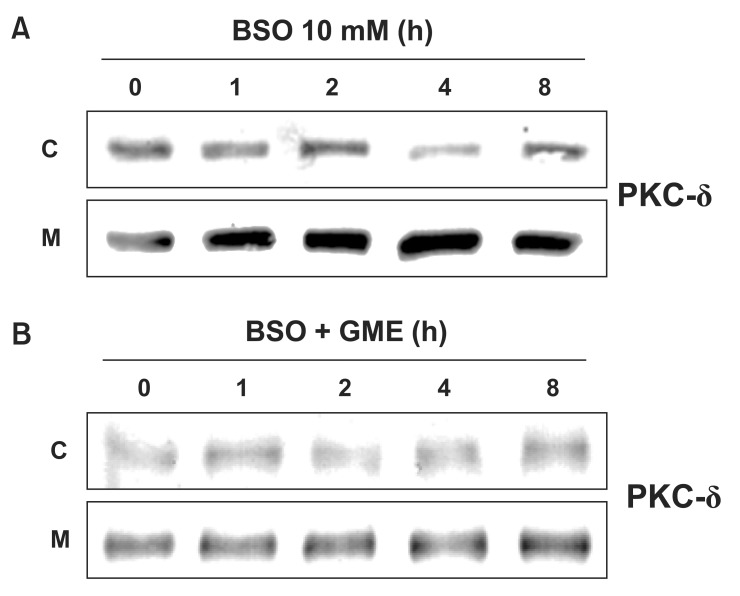
We determined the effect of GME on BSO-induced activation of PKC-δ. As shown in Fig. 4A, PKC-δ began to translocate to the membrane fraction from the cytosol fraction at 1 h after BSO treatment. Treatment with GME inhibited this BSO-induced translocation of PKC-δ (Fig. 4B). These results suggest that depletion of cellular GSH levels mediates BSOinduced PKC-δ activation.
Fig. 4. Effect of glutathione monoethyl ester (GME) on BSOinduced translocation of PKC-δ. (A, B) Western blot analysis of PKC-δ. H9c2 cells were incubated with BSO (10 mM) in the presence or absence of GME (300 μM) for indicated times. Blots are representative images (n=3). C: cytosol fraction; M: membrane fraction.
DISCUSSION
In the present study, using a heart-derived H9c2 cell line, we observed that BSO-induced GSH depletion was associated with: 1) increased apoptosis, 2) increased ROS production, 3) increased caspase-3 activity, and 4) increased PKC-δ activity. These results support our hypothesis that BSO-induced GSH depletion induces cardiomyocyte apoptosis through PKC-δ activation.
In patients with chronic heart failure, it is reported that increased oxidative stress is associated with reduced left ventricular function and is correlated with the severity of the disease (Belch et al., 1991; Mallat et al., 1998; Maack et al., 2003). Furthermore, cell culture and animal studies have suggested that ROS may be important mediators of cardiac hypertrophy and the development of contractile dysfunction (Dhalla et al., 2000; Maack et al., 2003). Ischemia results in impaired antioxidant defense, and subsequent reperfusion results in an increased concentration of ROS (Molyneux et al., 2002). Depletion of cellular antioxidants such as GSH allows for the generation of significant quantities of ROS, which have been suggested to act as signals for the induction of apoptosis (Armstrong et al., 2002). Many models have been proposed to explain the induction of GSH depletion. Depletion of GSH may be induced by GSH synthesis inhibitors (e.g., BSO), GSHS-transferase-dependent thiol alkylation agents (e.g., diethyl maleate, phorone, etc.), peroxides (e.g., tert-butyl hydroperoxide, H2O2, etc.), and non-enzymatic GSH-reacting agents (e.g., diazene and diamide). We chose to use the GSH synthesis inhibitor BSO because of its specificity and because the block in GSH synthesis can be overcome by supplying the cell with GME (Torres et al., 1997).
Results of previous studies showed that BSO-induced GSH depletion caused the induction of apoptotic and necrotic cell death in a variety of cell types (Rees et al., 1995; Korystov et al., 1996). In cardiomyocytes, mitochondrial GSH depletion was shown to modulate apoptosis in short-term diabetic rat hearts (Ghosh et al., 2005). However, the mechanism by which the depletion of GSH modulates cell death is still unclear. Our results showed that the BSO-induced depletion of cellular GSH levels significantly increased ROS generation and apoptotic cell death in H9c2 cells (Fig. 1, 2). Apoptosis is generally associated with the activation of caspase cascades (Logue and Martin, 2008) and with the Bcl-2 protein family (Gross et al., 1999). In addition, apoptosis is accompanied by signs of mitochondrial dysfunction, including the loss of mitochondrial membrane potential and the release of cytochrome c (Gottlieb et al., 2003). One of the main consequences following mitochondrial cytochrome c release is the activation of caspase-3. Caspase-3 is an effector caspase with a wide spectrum of substrates, and plays a critical role in characteristic apoptotic changes (Chang and Yang, 2000). Our results showed that caspase-3 activity increased under conditions of BSO-induced GSH depletion (Fig. 2D). Thus, we clearly demonstrated that BSO-induced GSH depletion significantly increases apoptosis by the mitochondrial pathway through ROS generation.
It has been demonstrated that PKCs are one of the most important mediators of ischemia-induced changes. The PKC family is comprised of several serine/threonine protein kinases, and has been implicated as an intracellular mediator that is important in the regulation of growth, differentiation, cell death, and neurotransmission. The PKC subfamilies include conventional PKC (PKC-α, -β1, -β2, and -γ), novel PKC (PKC-δ, -ε, -η, -θ, and -ι), and atypical PKC (PKC-ζ, -μ, and -λ). Different isoforms may perform distinct functions, as suggested by their differential pattern of localization, differences in their activation conditions, and some differences in substrate specificity. PKC-α, -β, -δ, -ε, and –ζ subtypes are known to be predominant isoforms in the rat heart (Doble et al., 2000; Kim et al., 2004). In previous study, we reported that the role of PKC-δ under hypoxia-induced apoptosis (Kim et al., 2004). In rat cardiomyocytes, it is also addressed the role of PKC-δ in cellular redox balance and apoptosis in various experimental conditions. Indeed, PKC-δ was cleaved by caspase-3 under doxorubicin-induced oxidative stress (Lai et al., 2011) and PKC-δ regulated apoptosis by H2O2-induced oxidative stress (Cieslak and Lazou, 2007). However, its role in GSH depletion-induced cardipmyocyte apoptosis has not been elucidated. Our results showed that PKC-δ was activated by the BSO-induced depletion of cellular GSH, and rottlerin, a PKC-δ inhibitor, significantly inhibited BSO-induced ROS production and cell death (Fig. 3, 4). This indicates that PKC-δ regulates the ROS generation and cell death induced by GSH depletion.
In summary, our results showed that BSO-induced depletion of cellular GSH induces the translocation of PKC-δ and, subsequently, the generation of ROS, which appears to enhance H9c2 cell death.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ009074), Rural Development Administration, Republic of Korea. This research was supported by the Bio & Medical Technology Development Program (2011-0019397) of the National Research Foundation (NRF) funded by the Korean government (MEST) and industry Technology Development Program (111093-3), Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea.
References
- 1.Ahmed Z., Tang W. H. Pharmacologic strategies to target oxidative stress in heart failure. Curr. Heart Fail. Rep. (2012);9:14–22. doi: 10.1007/s11897-011-0081-5. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J. S., Steinauer K. K., Hornung B., Irish J. M., Lecane P., Birrell G. W., Peehl D. M., Knox S. J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. (2002);9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 3.Belch J. J., Bridges A. B., Scott N., Chopra M. Oxygen free radicals and congestive heart failure. Br. Heart J. (1991);65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaustein A., Deneke S. M., Stolz R. I., Baxter D., Healey N., Fanburg B. L. Myocardial glutathione depletion impairs recovery after short periods of ischemia. Circulation. (1989);80:1449–1457. doi: 10.1161/01.CIR.80.5.1449. [DOI] [PubMed] [Google Scholar]
- 5.Brewer A. C., Murray T. V., Arno M., Zhang M., Anilkumar N. P., Mann G. E., Shah A. M. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic. Biol. Med. (2011);51:205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H. Y., Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. (2000);64:821–846. doi: 10.1128/MMBR.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatham J. C., Seymour A. L., Harmsen E., Radda G. K. Depletion of myocardial glutathione: its effects on heart function and metabolism during ischaemia and reperfusion. Cardiovasc. Res. (1988);22:833–839. doi: 10.1093/cvr/22.11.833. [DOI] [PubMed] [Google Scholar]
- 8.Chen K., Keaney J. F. Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr. Atheroscler. Rep. (2012);14:476–483. doi: 10.1007/s11883-012-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cieslak D., Lazou A. Regulation of BAD protein by PKA, PKCdelta and phosphatase in adult rat cardiac myocytes subjected to oxidative stress. Mol. Cells. (2007);24:24–231. [PubMed] [Google Scholar]
- 10.Damy T., Kirsch M., Khouzami L., Caramelle P., Le Corvoisier P., Roudot-Thoraval F., Dubois-Randé J. L., Hittinger L., Pavoine C., Pecker F. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS One. (2009);4:e4871. doi: 10.1371/journal.pone.0004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhalla N. S., Temsah R. M., Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. (2000);18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Doble B. W., Ping P., Kardami E. The ε subtype of protein kinase c is required for cardiomyocyte connexin-43 phosphorylation. Circ. Res. (2000);86:293–301. doi: 10.1161/01.RES.86.3.293. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S., Pulinilkunnil T., Yuen G., Kewalramani G., An D., Qi D., Abrahani A., Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am. J. Physiol. Heart Circ. Physiol. (2005);289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb E., Armour S. M., Harris M. H., Thompson C. B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. (2003);10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 15.Gross A., McDonnell J. M., Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. (1999);13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 16.Kim M. J., Moon C. H., Kim M. Y., Kim M. H., Lee S. H., Baik E. J., Jung Y. S. Role of PKC-delta during hypoxia in heartderived H9c2 cells. Jpn. J. Physiol. (2004);54:405–414. doi: 10.2170/jjphysiol.54.405. [DOI] [PubMed] [Google Scholar]
- 17.Korystov Y. N., Dobrovinskaya O. R., Shaposhnikova V. V., Eidus L. K. Role of arachidonic acid metabolism in thymocyte apoptosis after irradiation. FEBS Lett. (1996);388:238–241. doi: 10.1016/0014-5793(96)00538-8. [DOI] [PubMed] [Google Scholar]
- 18.Kytö V., Lapatto R., Lakkisto P., Saraste A., Voipio-Pulkki L. M., Vuorinen T., Pulkki K. Glutathione depletion and cardiomyocyte apoptosis in viral myocarditis. Eur. J. Clin. Invest. (2004);34:167–175. doi: 10.1111/j.1365-2362.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Lai H. C., Yeh Y. C., Wang L. C., Ting C. T., Lee W. L., Lee H. W., Wang K. Y., Wu A., Su C. S., Liu T. J. Propofol ameliorates doxofubicin-induced oxidative stress and cellular apoptosis in rat cardiomyocytes. Toxicol. Appl. Pharmacol. (2011);257:437–448. doi: 10.1016/j.taap.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee B. K., Jung Y. S. The Na+/H+ exchanger-1 inhibitor cariporide prevents glutamate-induced necrotic neuronal death by inhibiting mitochondrial Ca2+ overload. J. Neurosci. Res. (2012);90:860–869. doi: 10.1002/jnr.22818. [DOI] [PubMed] [Google Scholar]
- 21.Lee B. K., Lee S. K., Yi K. Y., Yoo S. E., Jung Y. S. KR-33028, a novel Na+/H+ exchanger-1 inhibitor, attenuates glutamateinduced apoptotic cell death through maintaining mitochondrial function. Biomol. Ther. (2011);19:445–450. doi: 10.4062/biomolther.2011.19.4.445. [DOI] [Google Scholar]
- 22.Lee N. Y., Rieckmann P., Kang Y. S. The changes of Pglycoprotein activity by interferon-γ and tumor necrosis factor-α in primary and immortalized human brain microvascular endothelial cells. Biomol. Ther. (2012);20:293–298. doi: 10.4062/biomolther.2012.20.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logue S. E., Martin S. J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. (2008);36:1–9. doi: 10.1042/BST0360001. [DOI] [PubMed] [Google Scholar]
- 24.Maack C., Kartes T., Kilter H., Schäfers H. J., Nickenig G., Böhm M., Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1- GTPase and represents a target for statin treatment. Circulation. (2003);108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 25.Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. (1998);97:1536–1539. doi: 10.1161/01.CIR.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 26.Meister A., Anderson M. E. Glutathione. Annu. Rev. Biochem. (1983);52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 27.Molyneux C. A., Glyn M. C., Ward B. J. Oxidative stress and cardiac microvascular structure in ischemia and reperfusion: the protective effect of antioxidant vitamins. Microvasc. Res. (2002);64:265–277. doi: 10.1006/mvre.2002.2419. [DOI] [PubMed] [Google Scholar]
- 28.Narasimhan M., Mahimainathan L., Rathinam M. L., Riar A. K., Henderson G. I. Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Mol. Pharmacol. (2011);80:988–999. doi: 10.1124/mol.111.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill A. J., O'Neill S., Hegarty N. J., Coffey R. N., Gibbons N., Brady H., Fitzpatrick J. M., Watson R. W. Glutathione depletion-induced neutrophil apoptosis is caspase 3 dependent. Shock. (2000);14:605–609. doi: 10.1097/00024382-200014060-00006. [DOI] [PubMed] [Google Scholar]
- 30.Rees R. S., Smith D. J., Adamson B., Im M., Hinshaw D. Oxidant stress: the role of the glutathione redox cycle in skin preconditioning. J. Surg. Res. (1995);58:395–400. doi: 10.1006/jsre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 31.Sevin G., Ozsarlak-Sozer G., Keles D., Gokce G., Reel B., Ozgur H. H., Oktay G., Kerry Z. Taurine inhibits increased MMP-2 expression in a model of oxidative stress induced by glutathione depletion in rabbit heart. Eur. J. Pharmacol. (2013);706:98–106. doi: 10.1016/j.ejphar.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 32.Torres V. E., Bengal R. J., Litwiller R. D., Wilson D. M. Aggravation of polycystic kidney disease in Han:SPRD rats by buthionine sulfoximine. J. Am. Soc. Nephrol. (1997);8:1283–1291. doi: 10.1681/ASN.V881283. [DOI] [PubMed] [Google Scholar]
- 33.van den Dobbelsteen D. J., Nobel C. S., Schlegel J., Cotgreave I. A., Orrenius S., Slater A. F. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J. Biol. Chem. (1996);271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- 34.Yang H., Lee B. K., Kook K. H., Jung Y. S., Ahn J. Protective effect of grape seed extract against oxidative stress-induced cell death in a staurosporine-differentiated retinal ganglion cell line. Curr. Eye Res. (2012);37:339–344. doi: 10.3109/02713683.2011.645106. [DOI] [PubMed] [Google Scholar]