Abstract
Resveratrol (trans-3,4'-trihydroxystillbene), a naturally occurring polyphenolic antioxidant found in grapes and red wine, elicits diverse biochemical responses and demonstrates anti-aging, anti-inflammatory, and anti-proliferative effects in several cell types. Previously, resveratrol was shown to regulate differentiation and inflammation in rabbit articular chondrocytes, while the direct production of nitric oxide (NO) in these cells by treatment with the NO donor sodium nitroprusside (SNP) led to apoptosis. In this study, the effect of resveratrol on NO-induced apoptosis in rabbit articular chondrocytes was investigated. Resveratrol dramatically reduced NO-induced apoptosis in chondrocytes, as determined by phase-contrast microscopy, the MTT assay, FACS analysis, and DAPI staining. Treatment with resveratrol inhibited the SNP-induced expression of p53 and p21 and reduced the expression of procaspase-3 in chondrocytes, as detected by western blot analysis. SNP-induced degradation of I-kappa B alpha (IκB-α) was rescued by resveratrol treatment, and the SN50 peptide-mediated inhibition of NF-kappa B (NF-κB) activity potently blocked SNP-induced caspase-3 activation and apoptosis. Our results suggest that resveratrol inhibits NO-induced apoptosis through the NF-κB pathway in articular chondrocytes.
Keywords: Resveratrol, Chondrocytes, Nitric oxide, Sodium nitroprusside, Apoptosis, NF-kappa B
INTRODUCTION
Resveratrol (trans-3, 4’-trihydroxystilbene) is a natural polyphenolic compound mainly found in several plants such as red grapes, mulberries, and peanuts (Marques et al., 2009; Shakibaei et al., 2009; Yu et al., 2012). Resveratrol was first detected in the roots of hellebore (Veratrum grandiflorum) in 1940, and since then its powerful and diverse biological effects have been well documented: Resveratrol shows antioxidative, anti-inflammatory, anti-aging, and anti-cancer properties due to its anti-proliferative, cell cycle arrest-inducing, and anti-invasive effects (Shakibaei et al., 2009; Mukherjee et al., 2010). Recently, histological analysis in vivo has shown that injections of Resveratrol strongly protect against articular cartilage degradation in rabbit models for osteoarthritis (OA) and rheumatoid arthritis (RA) (Elmali et al., 2005; Elmali et al., 2007; Im et al., 2012).
In human articular chondrocytes, anti-apoptotic and anti-inflammatory mechanisms regulated by Resveratrol have been elucidated (Shakibaei et al., 2007; Csaki et al., 2008). Moreover, Resveratrol inhibits the interleukin-1β (IL-1β)-mediated expression of inducible nitric oxide (NO) synthase in articular chondrocytes by activating SIRT1 and thereby suppressing Nuclear Factor kappa B (NF-κB) activity (Lei et al., 2012).
Chondrocytes are differentiated from mesenchymal cells during embryonic development (Solursh, 1989). The phenotype of the differentiated chondrocyte is characterized by the synthesis, deposition, and maintenance of the cartilage-specific extracellular matrix (ECM) that ensures matrix integrity, which is compromised in degenerative diseases such as OA and RA (Choy and Panayi, 2001; Sandell and Aigner, 2001). Arthritis is characterized by structural and biochemical changes in chondrocytes and the cartilage, including the degradation of the cartilaginous matrix and insufficient synthesis of the ECM, which leads to a loss of the chondrocyte phenotype (Yoon et al., 2007).
NO produced by inducible NO synthase in articular chondrocytes plays a central role in cartilaginous diseases such as OA and RA. NO triggers cartilage destruction by inducing apoptosis, dedifferentiation, and inflammatory responses such as cyclooxygenase (COX)-2 expression and prostagladin E2 (PGE2) production in articular chondrocytes (Amin and Abramson, 1998; Abramson et al., 2001; Sandell and Aigner, 2001). Production of NO in primary cultured articular chondrocytes by treatment with the NO donor sodium nitroprusside (SNP) leads to apoptosis, dedifferentiation, and COX-2 expression through a complex protein kinase signaling cascade that involves mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) (Abramson et al., 2001; Kim et al., 2002a; Kim et al., 2002b; Kim et al., 2003; Kim and Chun, 2003). However, the regulation of NO-induced apoptosis has not been clearly elucidated. This study investigated the effects of Resveratrol on the regulation of NO-induced apoptosis. Our results suggest that NF-κB signaling plays a role in the inhibition of by Resveratrol.
MATERIALS AND METHODS
Reagents and antibodies
The following reagents were from commercial sources: Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL; Gaithersburg, MD, USA); fetal bovine-calf serum (FCS) (Invitrogen Corp; Burlington, ON, Canada); Resveratrol, SNP, collagenase type II, ribonuclease A (RNase A), streptomycin, penicillin, dimethylsulfoxide (DMSO), propidium iodide (PI), 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI), Nonidet P-40 (NP-40), and Triton X-100 (Sigma-Aldrich; St. Louis, MO, USA). SN50, a peptide that inhibits the nuclear translocation of activated NFκB, was obtained from Biomol (Plymouth Meeting, PA, USA), and all antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) or Cell Signaling Technology (Danvers, MA, USA).
Cell cultures
Rabbit articular chondrocytes were isolated from the cartilage of 2-week-old New Zealand White rabbits as described (Yoon et al., 2002). Cartilage slices were dissociated enzymatically for 6 h in 0.2% collagenase type II (381 units/ml) in DMEM. Individual cells were obtained by collecting the supernatant after brief centrifugation. The cells were suspended in DMEM supplemented with 10% (v/v) FCS, 50 μg/ml streptomycin, and 50 units/ml penicillin, and then plated in culture dishes at a density of 5×104 cells/cm2. The medium was changed every 2 days after seeding, and cells reached confluence in approximately 5 days. After 3 days in culture, the cells were either treated with 1 mM SNP and 20 μM Resveratrol for 24 h, or pre-stimulated with 20 μM Resveratrol alone for 12 h before being co-treated with Resveratrol and 1 mM SNP for 24 h. SN50 was added 1 h before Resveratrol to inhibit the nuclear translocation of NF-κB. The study protocol was approved by the Institutional Review Board of Kongju National University (IRB No. 2011-2).
Cell proliferation assay
The MTT assay was used to quantify the proliferation of cells treated with Resveratrol and SNP. Cells were seeded in 96-well plates at a density of 1×104 cells/well and cultured for 24 h before treating with Resveratrol and SNP. Next, 10 μl/ well of MTT reagent1 (methylthiazole tetrazolium, 10 mg/ml) was added to the cells and the plates were incubated for 4 h at 37℃ until the purple formazan crystals developed, after which 100 μl/well of MTT reagent 2 (solubilization buffer, 10% SDS with 0.01N HCl, DMSO) was added to the cells. After overnight incubation, the absorbance at 600 nm was measured with a spectrophotometer, and 4 wells were examined for each treatment.
Western blotting
For western blotting, chondrocyte proteins were extracted with a lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, phosphatase inhibitors (1 mM each NaF and Na3VO4) and the following protease inhibitors: 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, and 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF). The proteins were size-fractionated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes, which were blocked with 5% non-fat dry milk for 1 h before incubating with primary antibodies overnight at 4℃. After washing, blots were developed using peroxidaseconjugated secondary antibodies and visualized using an ECL detection system. Actin and p53 were detected using antibodies purchased from Santa Cruz Biotechnology, and procaspase- 3, I kappa B-α (IκB-α), and p21 with antibodies from Cell Signaling.
Flow cytometry
Cells were incubated with 1 mM SNP and 20 μM Resveratrol for 24 h, or pre-stimulated with 20 μM Resveratrol alone for 12 h before being co-treated with Resveratrol and SNP for 24 h before measuring apoptosis by flow cytometry. Briefly, after treatments with Resveratrol/SNP, cells were washed once with phosphate-buffered saline (PBS), fixed with ice-cold 70% ethanol, and then stored at 4℃. After washing once more with PBS, cells were suspended in 1 ml of a 50 μg/ml PI solution containing 50 μg/ml RNase A and 0.1% (v/v) NP-40, and then incubated in a 37℃ water bath for an additional 20 min in the dark. Flow cytometric analyses were conducted using a cell sorter, and the Cell Quest software program (Partec, Munich, Germany) was used to determine the relative DNA content of cells based on blue fluorescence.
NF-κB -luciferase activity
Activation of NF-κB was determined indirectly by examining IκB-α degradation using Western blot analysis or directly using a reporter gene assay. For the luciferase reporter gene assay, chondrocytes were transfected with a plasmid containing the luciferase coding region and three tandem repeats of the serum response element. Following incubation in complete medium for 6 h, cells were left untreated or treated with the indicated pharmacological reagents and luciferase activity was determined using an assay kit (Promega, Madison, WI, USA).
DAPI staining
For DNA condensation studies, exponentially growing rabbit articular chondrocytes were cultured at a density of 2×104 cells/cm2 in 12-well culture plates and treated with Resveratrol/ SNP (as above) for 24 h. After washing with PBS, the chondrocytes were fixed using 3.5% paraformaldehyde in PBS for 15 min, permeabilized using 0.1% Triton X-100 for 10 min, and stained with DAPI (1 μg/ml) for 15 min. All steps were carried out at room temperature. Cells were then examined under a fluorescence microscope and photographed.
Data analyses and statistics
The results are expressed as the means ± SD. Values were calculated from the specified number of determinations. The data were subjected to an analysis of variance (ANOVA) using Tukey’s test to analyze differences. A value of p<0.05 was considered to indicate a statistically significant difference.
RESULTS
Resveratrol inhibits NO-induced apoptosis in rabbit articular chondrocytes
NO is a free radical that reacts with other radicals such as superoxide or molecular oxygen in tissues to generate highly damaging nitrosating species (Squadrito and Pryor, 1998; Bentz et al., 2012). Exposure of cells in vitro and in vivo to NO-donor drugs can cause cell death and modulate the activity of mitochondrial enzymes, metalloproteinases (MMPs), and protein kinases through a nitrosylation process (Gu et al., 2002; Gu et al., 2010; Bentz et al., 2012). Previous data have shown that Resveratrol treatment results in inhibition of growth in a wide variety of cancers (Fontecave et al., 1998; Bai et al., 2010). Also, we have confirmed Resveratrol inhibits cell growth in a dose- and time-dependent manner (data not shown).
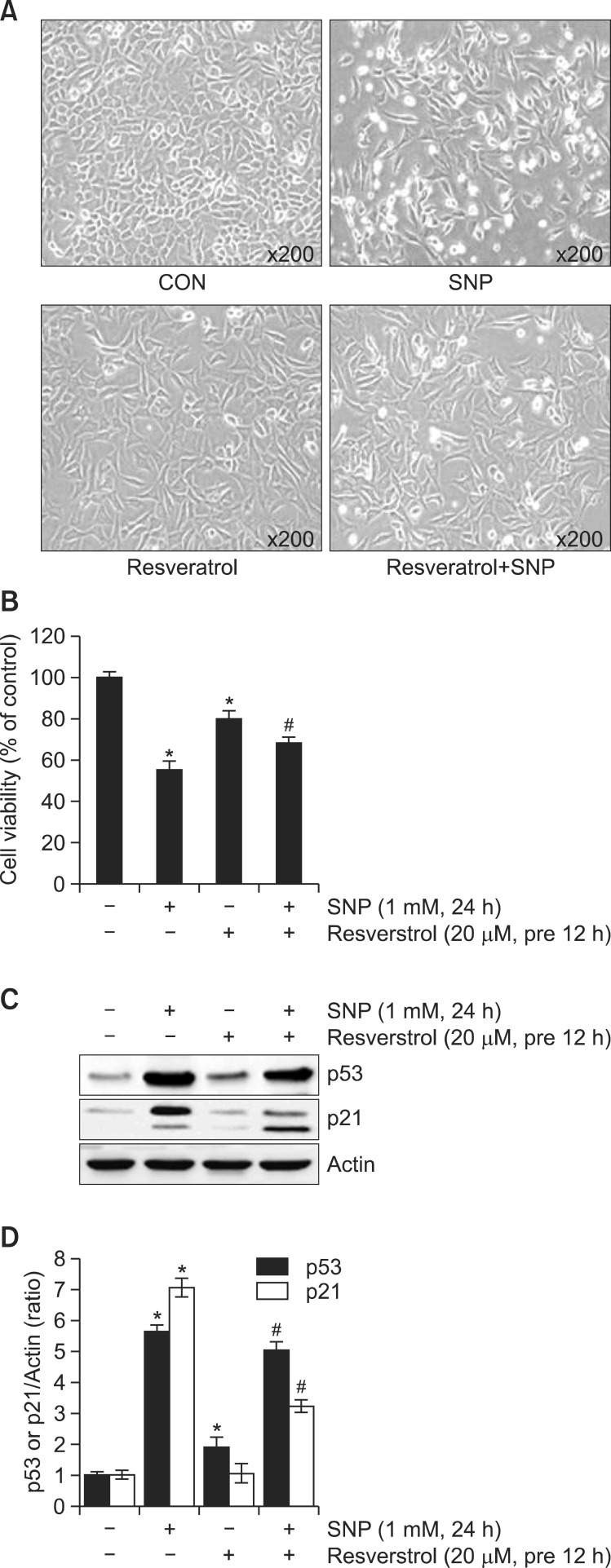
To examine the anti-apoptotic effect of Resveratrol in NO-induced apoptosis, rabbit articular chondrocytes were treated with 20 μM Resveratrol and 1 mM SNP. Observation of cell morphology using a phase-contrast microscope showed that NO-induced apoptosis was inhibited by Resveratrol treatment (Fig. 1A). Resveratrol treatment also potently blocked NO-induced apoptotic death in articular chondrocytes, as measured by the MTT assay (Fig. 1B), similar to of its effect on cell morphology (Fig. 2A). NO-stimulated p53 and p21 expression also decreased under Resveratrol treatment as determined by western blot analysis and quantified by densitometric analysis, respectively (Fig. 1C, D).
Fig. 1. Resveratrol inhibits NO-induced apoptosis in rabbit articular chondrocytes: cellular and biochemical analyses. (A) Articular chondrocytes were untreated or treated for 24 h with 20 μM Resveratrol with or without 1 mM SNP. Cells were viewed under a phasecontrast microscope and photographed (200× magnification). (B) Apoptosis was measured by the MTT assay. (C) Expression of p53, p21, and actin was examined by western blotting; actin was used as a loading control. (D) The relative amounts of p53 and p21 were quantified by densitometric measurements (Image J). Data are presented as the results of a typical experiment and as the means ± SD. (B, D) (n=4). *p<0.05 compared to the control and #p<0.05 compared to the SNP.
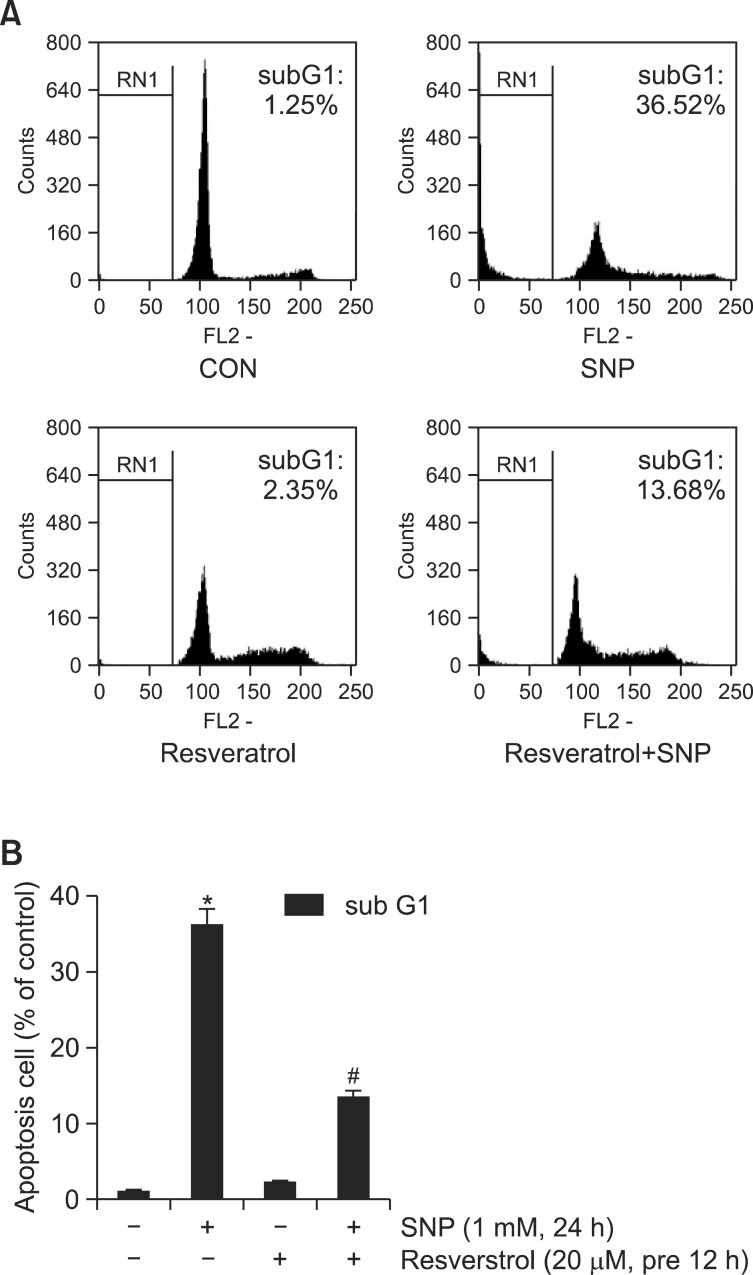
Fig. 2. Resveratrol inhibition of NO-induced apoptosis in rabbit articular chondrocytes: analysis by flow cytometry. (A) Articular chondrocytes were untreated (control) or treated for 24 h with 20 μM Resveratrol with or without 1 mM SNP. Apoptotic cells death was determined by FACS analysis; cells were fixed with 70% ethanol in PBS, and stained with PI. (B) The percentages of sub-G1 cellular populations are shown. Data are presented as the results of a typical experiment and as the means ± SD. (B) (n=4). *p<0.05 compared to the control and #p<0.05 compared to the SNP.
Apoptotic cell death was suppressed in chondrocytes treated with SNP (1 mM) and Resveratrol (20 μM) for 24 h (Fig. 2A). The distribution of cells among the different phases of the cell cycle was calculated using the FloMax program (Fig. 2B): treatment with SNP increased the proportion of cells at the sub-G1 phase by approximately 36% relative to control, but when SNP was added together with Resveratrol, significantly fewer cells were found at the sub-G1 phase compared with SNP treatment alone. These results indicate that Resveratrol effectively reduced NO-triggered apoptosis in rabbit articular chondrocytes.
The effect of Resveratrol on IκB, caspases, and apoptosis
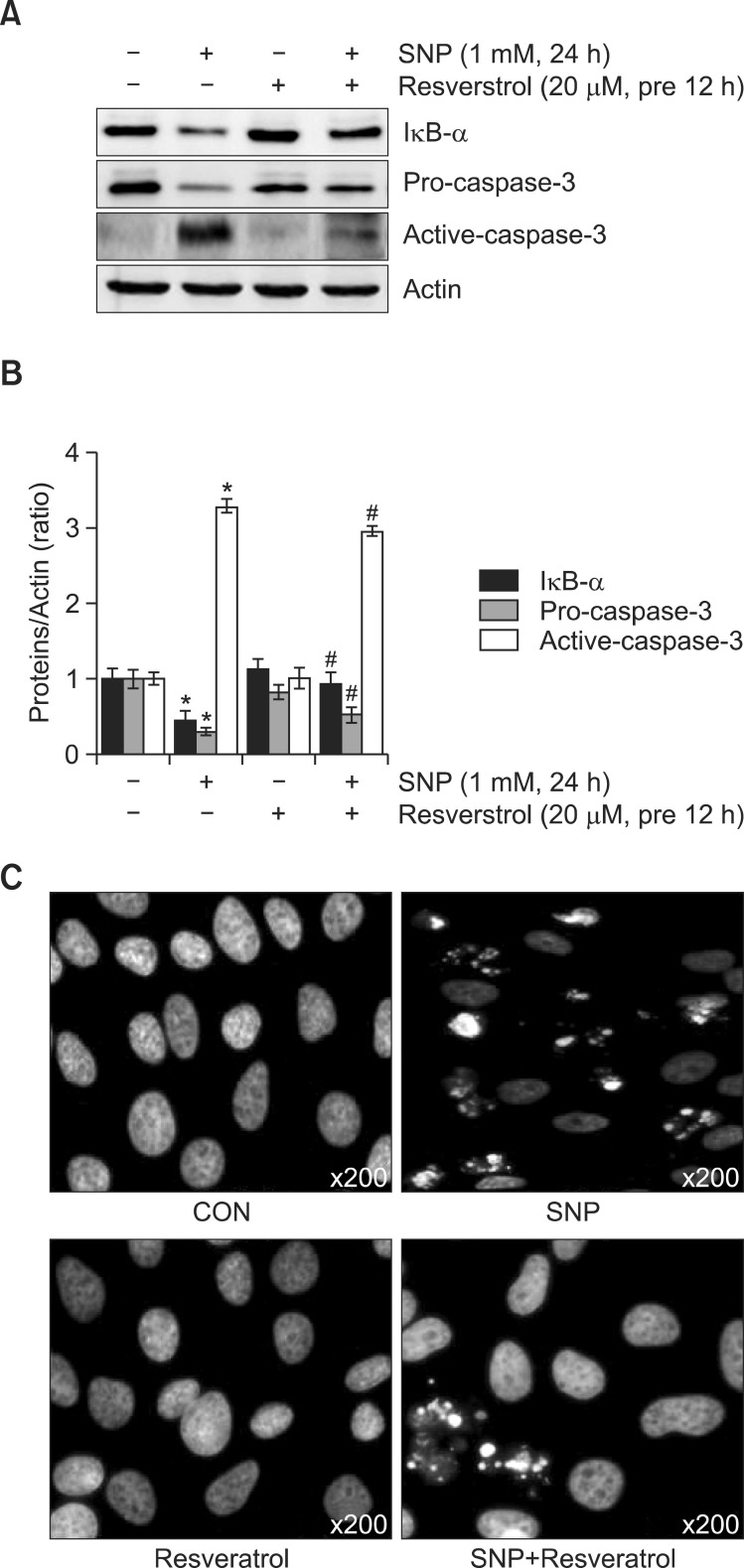
To determine the mechanism of Resveratrol-mediated inhibition of apoptosis and NF-κB activation, chondrocytes were treated with SNP (1 mM) and Resveratrol (20 μM) for 24 h. Western blotting showed that NO induced IκB-α degradation, Pro-caspase-3 and caspase-3 expression, and these effects were abrogated by Resveratrol (Fig. 3A). Quantification of the results using the Image J program showed in Fig. 3B. In accordance with the western blotting results (Fig. 3A), DAPI staining (Fig. 3C) showed Resveratrol potently blocked NOinduced apoptotic cell death. These findings suggest that Resveratrol prevents NO-induced apoptosis through NF-κB.
Fig. 3. Inhibition of SNP-induced apoptosis by the Resveratrolassociated NF-κB pathway. (A) Articular chondrocytes were treated with 20 μM Resveratrol for 12 h before stimulation with 1 mM SNP for 24 h. Expression of Iκ B-α and procaspase-3 was assessed by western blotting, with actin expression serving as the control. (B) The relative amounts of Iκ B-α, procaspase-3 and caspase-3 were quantified by densitometric measurements (image J). (C) DAPIstained nuclei were observed under a fluorescence microscope. (A-C) Representative results and mean values with standard deviation are shown. *p<0.05 compared to the control and #p<0.05 compared to the SNP.
Resveratrol-induced inhibition of apoptosis occurs by a suppression of NF-κB activation
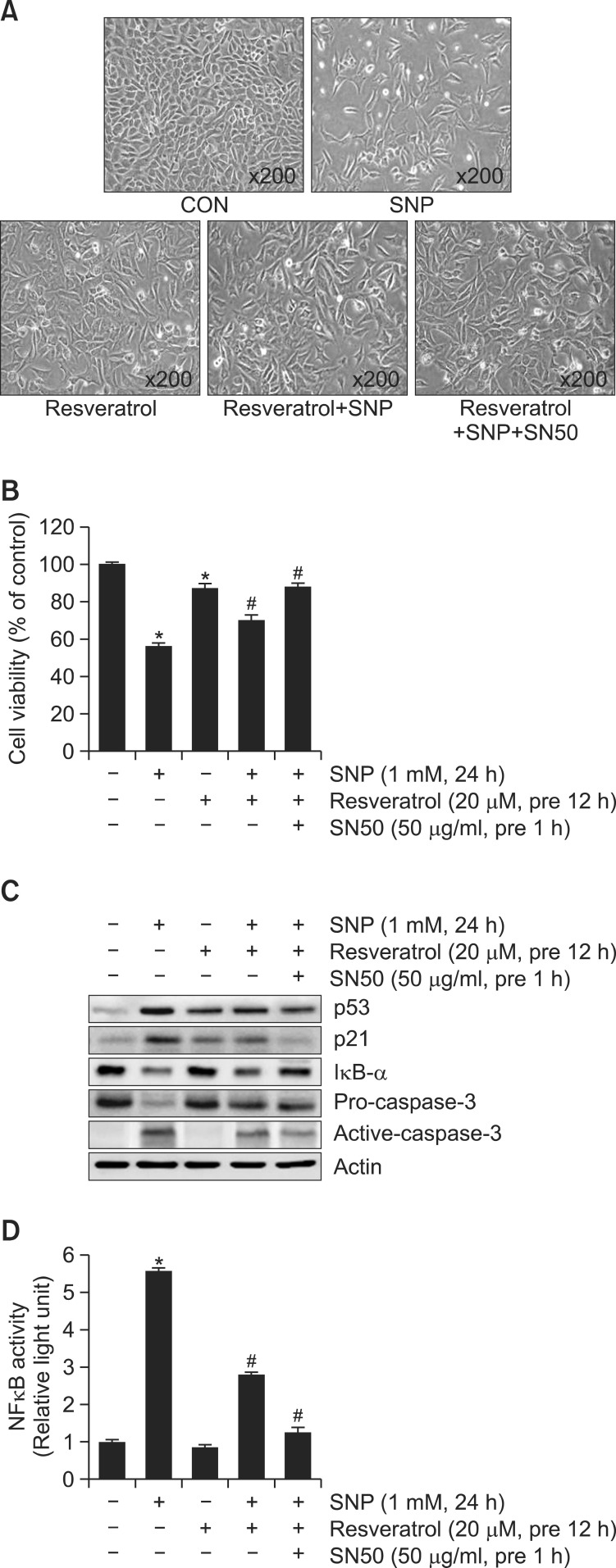
To address the role of NF-κB in the anti-apoptotic effect of Resveratrol, chondrocytes were treated with SN50, a peptide that inhibits the nuclear translocation of activated NF-κB. Phase contrast microscopy and MTT assays showed that the inhibition of NF-κB activity with the SN50 peptide enhanced Resveratrol-dependent suppression of apoptosis triggered by NO (Fig. 4A, B). In accord with the results of these cellular analyses, SN50 treatment bolstered the inhibition of NO-induced p53, p21, pro-caspase-3 and caspase-3 expression by Resveratrol (Fig. 4C). Chondrocytes that were pre-treated with SN50 for 1 h and the simulated with Resveratrol for 12 h then co-treated with SNP for 24 h. Inhibition of NF-κB activity with the SN50 peptide blocked NO-induced NF-κB activation by Resveratrol as using NF-κB-luciferase reporter assay (Fig. 4D). These findings suggest NO-induced apoptosis may involve the blockade of NF-κB activation.
Fig. 4. Relationship between Resveratrol, NF-κB and NO-induced apoptosis: p53, p21 and procaspase-3. (A) Cells were either treated or not treated with 20 μM Resveratrol for 12 h prior to the addition of 1 mM SNP in the absence or presence of 50 μg/ml SN50 (NF-κB inhibitor); cells were photographed in phase contrast (200× magnification). (B) Cell viability was determined using the MTT assay. (C) Expression of p53, p21, procaspase-3, active-caspase-3, IκB-α, and actin was detected by western blotting; actin was used as the loading control. (D) NF-κB activity was determined by luciferase reporter assay. Data are presented as the results of a typical experiment and as the means ± SD. (B, D) (n=4). *p<0.05 compared to the control and #p<0.05 compared to the SNP.
DISCUSSION
NO plays a key signaling role in diverse physiological processes. NO regulates the survival of chondrocytes by inducing dedifferentiation and apoptosis (Li et al., 2010; Bentz et al., 2012; Cau et al., 2012; Qureshi et al., 2012). Recent studies from our laboratory have shown that Resveratrol inhibited cell proliferation and regulated differentiation and COX-2 expression by ERK, p38, and Akt signaling (submitted). Moreover, our previous results showed that apoptosis in chondrocytes triggered by treatment with SNP (which generates NO) is regulated by the opposing actions of 2 MAPK subtypes, extracellular signal-regulated kinase-1/-2 (ERK-1/-2) and p38 kinase, coupled with an elevation of p53 protein levels, caspase-3 activation, and differentiation (Kim et al., 2002a; Kim et al., 2003). NO-induced activation of ERK-1/-2 induces differentiation and inhibits apoptosis, whereas activation of p38 kinase induces apoptosis and is responsible for the maintenance of differentiated phenotypes. Thus, inhibition of NO-induced apoptosis by the disruption of the actin cytoskeleton is consistent with the suppression of apoptotic signaling pathways such as the activation of p38 kinase, inhibition of PKC-α and ζ, NF-κB activation, p53 accumulation, and caspase-3 activation (Kim et al., 2003; Kim et al., 2002a; Kim et al., 2002b; Kim et al., 2002c).
NF-κB is an inducible factor that regulates various physiological processes including inflammatory responses and apoptosis. NF-κB plays a crucial role in arthritis, mediating important chondrocyte inflammatory responses that ultimately lead to cartilage degradation. Elevated NF-κB signaling in chondrocytes contributes to cartilage degradation in OA, and NF-κB binding activity is 2-fold higher in OA chondrocytes than in normal chondrocytes (Shakibaei et al., 2008).
The known target genes of NF-κB include both apoptosisprotective genes such as Bcl-2 and p53 dependent pro-apoptotic genes such as NOXA, BAX and BID, suggesting that the cell type- and extracellular stimuli-dependent effects of NF-κB on apoptosis may be due to its specific effects on the expression of apoptosis-regulating genes. In a previous study, Kevin M. Ryan and Mary K. Ernst reported that induction of p53 causes an activation of NF-κB that correlates with the ability of p53 to induce apoptosis. Inhibition or loss of NF-κB activity abrogated p53-induced apoptosis, indicating that NF-κB is essential in p53-mediated cell death (Ryan et al., 2000; Lin et al., 2012).
Elmali et al. demonstrated that intra-articular injections of Resveratrol had a protective effect on the cartilage (Elmali et al.,2005; Elmali et al.,2007; Shakibaei et al.,2009). In vitro treatment with resveratrol of synoviocytes from rheumatoid arthritis patients demonstrated increased cysteine protease caspase-3 activity, proliferation of synoviocytes, and induction of cell apoptosis (Tang et al.,2006; Shakibaei et al., 2009). It has been demonstrated in vitro that Resveratrol, unlike its apoptotic action in tumor cells, has an antiapoptotic effect on chondrocytes that is mediated through inhibition of IL-1β- induced stimulation of caspase-3 and cleavage of the DNA repair enzyme poly (ADP)ribose polymerase (PARP) in human articular chondrocytes (Shakibaei et al., 2007; Shakibaei et al., 2009). Furthermore, Resveratrol directly blocked caspase- 3 and subsequent cleavage of PARP and reversed the IL-1β-induced upregulation of ROS in chondrocytes. Results from this study not only show the possible utility of Resveratrol in the prevention of osteoarthritis, but also provide interesting results concerning its function as an antioxidant. Moreover, it has been reported that Resveratrol induces p53 degradation through an ubiquitin-independent pathway and thus inhibits p53-dependent apoptosis (Csaki et al., 2008; Shakibaei et al., 2009).
This study investigated the effects of Resveratrol, a natu-rally occurring phytoalexin and chemotherapeutic agent, on NO-induced NF-κB signaling and apoptosis in rabbit articular chondrocytes. The data presented in this paper provide convincing molecular evidence in support of the hypothesis that Resveratrol suppresses NO-induced apoptosis that occurs through the degradation of IκB-α in chondrocytes in vitro.
The findings of this study are the following: (1) Resveratrol inhibited NO-induced apoptosis as determined by phase-contrast microscopy, FACS, MTT analysis, and DAPI staining. (2) Resveratrol protected chondrocytes from SNP-stimulated p53 and p21 expression and reduced the levels of procaspase-3 and IκB-α as shown by western blotting. (3) The anti-apoptotic effects of Resveratrol were significantly strengthened by SN50, a specific inhibitor of NF-κB. Thus, blocking the degradation of IκB-α, or the nuclear translocation of NF-κB by treatment with SN50 decreased Resveratrol-dependent elevation in p53 and p21 expression. Moreover, SN50 accerelated Resveratrol- induced inhibition of NO-dependent increase in procaspase- 3 levels in articular chondrocytes. Collectively, these data suggest that Resveratrol regulates NO-induced apoptosis via the blockage of NF-κB activation.
In conclusion, the apoptosis-preventing effect observed in in vitro systems as shown in this study may be protect of joint diseases such as arthritis. Although the significance of our findings should be validated in subsequent studies using in vivo animal models, the present study strongly suggests that Resveratrol might find use as a therapeutic agent in the treatment of osteoarthritis.
Acknowledgments
This work was supported by a grant from the Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (A120960-1201-0000300).
References
- 1.Abramson S. B., Attur M., Amin A. R., Clanc R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr. Rheumatol. Rep. (2001);3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- 2.Amin A. R., Abramson S. B. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr. Opin. Rheumatol. (1998);10:263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y., Mao Q. Q., Qin J., Zheng X. Y., Wang Y. B., Yang K., Shen H. F., Xie L. P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer. Sci. (2010);101:488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentz M., Zaouter C., Shi Q., Fahmi H., Moldovan F., Fernandes J. C., Benderdour M. Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J. Cell. Biochem. . (2012);113:2256–2267. doi: 10.1002/jcb.24096. [DOI] [PubMed] [Google Scholar]
- 5.Cau S. B., Carneiro F. S., Tostes, R. C. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front. Physiol. (2012);3:218. doi: 10.3389/fphys.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choy E. H., Panayi G. S. Cytokine pathways and joint infl ammation in rheumatoid arthritis. N. Engl. J. Med. (2001);344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. (2008);75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Elmali N., Baysal O., Harma A., Esenkaya I., Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation . (2007);30:1–6. doi: 10.1007/s10753-006-9012-0. [DOI] [PubMed] [Google Scholar]
- 9.Elmali N., Esenkaya I., Harma A., Ertem K., Turkoz Y., Mizrak B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflammation Res. (2005);54:158–162. doi: 10.1007/s00011-004-1341-6. [DOI] [PubMed] [Google Scholar]
- 10.Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. (1998);421:277–279. doi: 10.1016/S0014-5793(97)01572-X. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z., Kaul M., Yan B., Kridel S. J., Cui J., Strongin A., Smith J. W., Liddington R. C., Lipton S. A. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science . (2002);297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z., Nakamura T., Lipton S. A. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol. Neurobiol. (2010);41:55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Im H. J., Li X., Chen D., Yan D., Kim J., Ellman M. B., Stein G. S., Cole B., Kc R., Cs-Szabo G., van Wijnen A. J. Biological effects of the plant-derived polyphenol resveratrol in human articular cartilage and chondrosarcoma cells. J. Cell. Physiol. (2012);227:3488–3497. doi: 10.1002/jcp.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S. J., Chun J. S. Protein kinase C alpha and zeta regulate nitric oxide-induced NF-kappa B activation that mediates cyclooxygenase-2 expression and apoptosis but not dedifferentiation in articular chondrocytes. Biochem. Biophys. Res. Commun. (2003);303:206–211. doi: 10.1016/S0006-291X(03)00305-X. [DOI] [PubMed] [Google Scholar]
- 15.Kim S. J., Hwang S. G., Kim I. C., Chun J. S. Actin cytoskeletal architecture regulates nitric oxide-induced apoptosis, dedifferentiation, and cyclooxygenase-2 expression in articular chondrocytes via mitogen-activated protein kinase and protein kinase C pathways. J. Biol. Chem. . (2003);278:42448–42456. doi: 10.1074/jbc.M304887200. [DOI] [PubMed] [Google Scholar]
- 16.Kim S. J., Hwang S. G., Shin D. Y., Kang S. S., Chun J. S. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFkappa B-dependent transcription and stabilization by serine 15 phosphorylation. J. Biol. Chem. (2002a);277:33501–33508. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- 17.Kim S. J., Ju J. W., Oh C. D., Yoon Y. M., Song W. K., Kim J. H., Yoo Y. J., Bang O. S., Kang S. S., Chun J. S. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J. Biol. Chem. . (2002b);277:1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 18.Kim S. J., Kim H. G., Oh C. D., Hwang S. G., Song W. K., Yoo Y. J., Kang S. S., Chun J. S. p38 kinase-dependent and -independent Inhibition of protein kinase C zeta and -alpha regulates nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes. J. Biol. Chem. (2002c);277:30375–30381. doi: 10.1074/jbc.M205193200. [DOI] [PubMed] [Google Scholar]
- 19.Lei M., Wang J. G., Xiao D. M., Fan M., Wang D. P., Xiong J. Y., Chen Y., Ding Y., Liu S. L. Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-kappaB activity. Eur. J. Pharmacol. (2012);674:73–79. doi: 10.1016/j.ejphar.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Du M., Liu X., Wu M., Ye H., Lin J., Chen W., Wu G. Millimeter wave treatment inhibits NO-induced apoptosis of chondrocytes through the p38MAPK pathway. Int. J. Mol. Med. (2010);25:393–399. doi: 10.3892/ijmm_00000357. [DOI] [PubMed] [Google Scholar]
- 21.Lin W. C., Chuang Y. C., Chang Y. S., Lai M. D., Teng Y. N., Su I. J., Wang C. C., Lee K. H., Hung J. H. Endoplasmic reticulum stress stimulates p53 expression through NF-kappaB activation. PLoS ONE. (2012);7:e39120. doi: 10.1371/journal.pone.0039120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques F. Z., Markus M. A., Morris B. J. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. (2009);41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S., Dudley J. I., Das D. K. Dose-dependency of resveratrol in providing health benefits. Dose Response . (2010);8:478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi A. A., Guan X. Q., Reis J. C., Papasian C. J., Jabre S., Morrison D. C., Qureshi N. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. (2012);11:76. doi: 10.1186/1476-511X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan K. M., Ernst M. K., Rice N. R., Vousden K. H. Role of NF-kappaB in p53-mediated programmed cell death. Nature. (2000);404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 26.Sandell L. J., Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. (2001);3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakibaei M., Csaki C., Nebrich S., Mobasheri A. Resveratrol suppresses interleukin-1beta-induced infl ammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. (2008);76:1426–1439. doi: 10.1016/j.bcp.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Shakibaei M., Harikumar K. B., Aggarwal B. B. Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res. (2009);53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 29.Shakibaei M., John T., Seifarth C., Mobasheri A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N.Y. Acad. Sci. (2007);1095:554–563. doi: 10.1196/annals.1397.060. [DOI] [PubMed] [Google Scholar]
- 30.Solursh M. Differentiation of cartilage and bone. Curr. Opin. Cell Biol. (1989);1:989–994. doi: 10.1016/0955-0674(89)90070-7. [DOI] [PubMed] [Google Scholar]
- 31.Squadrito G. L., Pryor W. A. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. (1998);25:392–403. doi: 10.1016/S0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 32.Tang L. L., Gao J. S., Chen X. R., Xie X. Inhibitory effect of resveratrol on the proliferation of synoviocytes in rheumatoid arthritis and its mechanism in vitro. Zhong Nan Da Xue Xue Bao Yi Xue Ban . (2006);31:528–533. [PubMed] [Google Scholar]
- 33.Yoon E. K., Lee W. K., Lee J. H., Yu S. M., Hwang S. G., Kim S. J. ERK-1/-2 and p38 kinase oppositely regulate 15-deoxydelta(12,14)-prostaglandinJ(2)-Induced PPAR-gamma activation that mediates dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Korean Med. Sci. . (2007);22:1015–1021. doi: 10.3346/jkms.2007.22.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon Y. M., Kim S. J., Oh C. D., Ju J. W., Song W. K., Yoo Y. J., Huh T. L., Chun J. S. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J. Biol. Chem. (2002);277:8412–8420. doi: 10.1074/jbc.M110608200. [DOI] [PubMed] [Google Scholar]
- 35.Yu W., Fu Y. C., Wang W. Cellular and molecular effects of resveratrol in health and disease. J. Cell. Biochem. (2012);113:752–759. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]