Abstract
Doxorubicin is still main drug in chemotherapy with limitation of use due to adverse drug reaction. Increased oxidative stress and alteration of nitric oxide control have been involved in cardiotoxicity of doxorubicin (DOX). A Disintegrin And Metalloproteinase (ADAMs) are transmembrane ectoproteases to regulate cell-cell and cell-matrix interactions, but role in cardiac disease is unclear. The aim of this study was to determine whether DOX activates peroxynitrite and ADAM 10 and thus ADAM and matrix metalloproteinase (MMP) induce cardiac remodeling in DOX-induced cardiomyopathy. Adult male Sprague-Dawley rats were subjected to cardiomyopathy by DOX (6 times of 2.5 mg/kg DOX over 2-weeks), and were randomized as four groups. Then followed by 3, 5, 7, and 14 days after cessation of DOX injection. DOX-injected animals significantly decreased left ventricular fractional shortening compared with control by M-mode echocardiography. The expressions of cardiac nitrotyrosine by immunohistochemistry were significant increased, and persisted for 2 weeks following the last injection. The expression of eNOS was increased by 1.9 times (p<0.05), and iNOS was marked increased in DOX-heart compared with control (p<0.001). Compared to control rats, cardiac ADAM10- and MMP 9- protein expressions increased by 20 times, and active/total MMP 9 proteolytic activity showed increase tendency at day 14 after cessation of DOX injection (n=10, each group). DOX-treated H9C2 cell showed increased ADAM10 protein expression with dose-dependency (p<0.01) and morphometric changes showed the increase of ventricular interstitial, nonvascular collagen deposition. These data suggest that activation of cardiac peroxynitrite with increased iNOS expression and ADAM 10-dependent MMP 9 expression may be a molecular mechanism that contributes to left ventricular remodeling in DOXinduced cardiomyopathy.
Keywords: Doxorubicin, Cardiomyopathy, ADAM, MMP
INTRODUCTION
Doxorubicin antithropoid cardiomyopathy
Doxorubicin (DOX, Adriamycin) is an antitumor agent related to anthracycline antibiotics. It is widely used incorporated with other drugs to treat malignant tumors like leukemia, lymphoma and solid cancer. The mechanism of doxorubicin is that it binds to DNA and interferes with DNA and RNA polymerase which therefore reduces DNA synthesis. But the agent is dosage dependent because it is toxic to the heart causing irreversible cardiomyopathy and heart failure therefore restricting clinical functions. As of present day there is no existing treatment for anthropoid cardiomyopathy caused by doxorubicin yet, so the recommended dosage for prevention is to use less than an accumulative dosage of (500-550 mg/m2) for adults.
The pathophysiological mechanism of the toxicity of doxorubicin to the heart is still uncertain, but according to many studies doxorubicin causes hyperlipidemia, reduction of nucleic acid and protein synthesis, myocardial electrolyte imbalance and transcriptional event. However, lately, studies have shown that the major reason for heart toxicity from doxorubicin is due to structural changes in the cell wall of cardiomyocyte caused by oxidative stress from lipid peroxidation. Studies have also shown that doxorubicin affects changes of the energy system. In other studies it has been reported that the reduction of the synthesis of endogenous antioxidant was the major reason for toxicity to the heart.
Looking at doxorubicin induced cardiomyopathy in a histological point of view, cell damage caused by isolation oxidation, hence, loss of myofibril and vacuolization of a myocardium have been observed and in organelles loss of mitochondria and degeneration of a nucleus were also observed in a micro-histological point of view. Hearts with cardiomyopathy developed by doxorubicin showed a reduction left ventricular which is an important function of the heart.
Cardiomyopathy developed by doxorubicin could be easily diagnosed to be decreased in contract rate of left ventricle or internal fraction using echocardiography or nuclear cardiology test. At latest, as method for working go out doxorubicin flexibility cardiomyophthy model, injecting a dosing daily doxorubicin 2.5 mg/kg per 6 times for 2 weeks (total 15 mg/kg) in abdominal is used.
NO, peroxynitrite and toxicity of cell
Nitric oxide is known to react to the physiological function of the heart and the state of the disease in various reactions. NO after reacting with oxygen in solution makes nitrite (NO2-) as oxide, also after reacting with oxyhemoglobin or superoxide, a negative ion (O2-), makes nitrate or proxynitric (NO3-). NO doesn't react directly with the functional group of biologic molecular, but instead oxidized derivatives such as N2N3 having the ability to nitrosating react with thiols to make S-nitrothioles. NO and derivative existed tissue and body fluid in organism. Generally, NO is reported to be generated by the adjustment of constitutive NO synthase (cNOS) isoforms in ground state. The generated NO usually involves in cardiac myocyte inotropic and blood flow distribution. On other hands, high concentrations of NO are produced in pathological conditions where dilated cardiomyopathy and cardiac failure increase the onset of iNOS (inducible NOS isoforms). The increased high concentration of NO are known to function by giving oxidative damage to cardiac myocytes, inducing the process of apoptosis and meronecrosis. Although recently, it has been reported that peroxynitrite (OONO-) were formed in laboratory animals with heart failure because of the rapid oxidation caused by the synthesized NO and superoxide (Beckman and Koppenol, 1996; Ischiropoulos, 1998) Thus, it can be presumed that peroxynitrite is a substance that induces oxidation strongly but the characteristic of oxidation caused by poroxynitrite is that it forms trabecula tyrosine of protein into 3-nitro-L-tyrosine (3-NT). (Radi et al., 1991) This variant can be detected in various illnesses like, acute pulmonary disease, myocarditis and homologous kidney transplantation rejection (Kooy et al., 1997). Therefore, because of peroxynitrite's nitrification reaction to proteins, it is predicted that peroxynitrite is a substance which can change the formation and function of tissues. Acute doxorubicin cardiotoxicity model report related cytotoxicity between increasing iNOS and NO (Weinstein et al., 2000). But in other studies; increased NADPH dependence superoxide by excess expression of eNOS have influence on apoptosis (Kalivendi et al., 2001). However, recently acute doxorubicin cardiotoxicity model increase mortality of laboratory animals with the injunction of L-NAME of cNOS therefore, NO from iNOS is reported to work peroxynitrite outgrowth. (Pacher et al., 2003). It is by no means certain between doxorubicin cardiotoxicity and NOS. And then heart failure is reported to relate cardiotoxicity increase eNOS and iNOS, but bioavailability of NO is decreasing and increased nitrotyrosine.
Relationship of regenerating myocardium between ADAM 10 and MMPs
Myocardium are composed of cardiac muscle cells, extracellular matrix and capillaries. Out of these three the extracellular matrix which is principally composed of collagen, plays an important role in the structure and function of myocardium. Collagen in myocardium do not dissolve in most proteolytic enzymes (Cleutjens et al., 1995). In the normal state and even in morbid conditions of the body, the extracellular matrix constantly goes through dynamic reorganization. It is known that MMPs (matrix metalloproteinases) participate during this process (Stetler-Stevenson, 1996). The MMPs that increase functional proteolytic enzyme and have influence on reorganization of cell matrix and cells in morbid conditions such as heart failure, reperfusion injury and relaxivity myocardia play an important role in the abnormal remodeling process of heart (Spinale, 2002). Especially, it is formed of various anion and free radicals by oxidation stress and again producing oxidation as peroxynitrite to generate disulfide S-oxidation reaction and S-glutathiolation reaction. also peroxynitrite decomposition catalysts is reported to reduce MMP activation (Pacher et al., 2003). ADAM (a disintegrin and metalloproteinase) is a large family of membrane-bound glycoproteins which functions are proteolysis, signaling, adhesion, and fusion otherwise known as MDC (Metalloprotease/Disintegrin/Cysteinerich) and the other known functions of ADAM participate in the cell cycle in like fertilization, myocyte fusion, the development of the nervous system, cleavage-secretion of membranebound proteins. Thus ADAM's are important enzymes that effect the basic structure of heart tissue by controlling cell-cell and cell-matrix interaction. Especially, if ADAMs are activated in pathological conditions such as cardiomyopathy; collagen outgrowth and decomposition get accelerated participating deeply in the remodeling of the heart. The MMPs increase the function of proteolytic enzyme and have influence over the reorganization of cell matrix and cells in pathological conditions such as heart failure, reperfusion injury and relaxivity myocardia therefore playing an important role in the abnormal remodeling process of the heart (Spinale, 2002). Especially under conditions with oxidative stress like various anions and free radicals; the synthesized peroxynitrite generates reactions like disulfide S-oxidation reaction and S-glutathiolation reaction. This causes pro-MMP’s to activate therefore inducing the proteolytic enzyme MMP to cause tissue damage to the particular tissue. The peroxynitrite decomposition catalysts are reported to block these types of reactions therefore reducing the activation of MMP. The purpose of this study was to analyze the correlation and influence of the synthesis of eNOS, iNOS and peroxynitrite from a doxurubicin induced cardiomyopathy model where doxurubicin induces cardiotoxicity and oxidation. Another purpose of this research was to study the interrelation and effect of the onset of ADAM 10 and MMP when cardiomyopathy is induced by doxurubicin. Therefore the research was mainly about the effect of the oxidizing agent peroxynitrite, the possible onset of iNOS and eNOS when oxidation occurs, and the onset of ADAM 10 and MMP enzymes will do to the remodeling process of the heart when doxorubicin is used.
MATERIALS AND METHODS
Experimental material
Aqueous picric acid, Actin (primary monoclonal antibody) and Concanavalin A were purchased from Sigma, MO, USA. 10% formaline were purchased from Sigma Chemical Co., MO, USA. ADAM-10 (Polyclonal primary antibody) were purchased from Chemicon, CA, USA. Doxorubicin HCl were received from Ildong Pharmaceutical Company. Donkey antirabbit IgG (Biotinylated HRP conjugate polyclonal antibody) were purchased from Amersham Life Science, Piscataway, NJ, USA and eNOS (primary monoclonal anti-mouse antibody) and iNOS (Monoclonal primary antibody) from Transduction, Lexington, KY, USA. H9C2 Cell and Hematoxylin were purchased from Innogenex, SanRamon, CA, USA. and H2 DCF-DA (2',7'-Dichlorofluorescin diacetate) from Molecular Probe, Leiden, Netherlands. Nitrotyrosin (primary polyclonal anti-rabbit antibody) were purchased from Upstate, NY, USA. and rat anti-mouse IgG (Biotinylated HRP conjugate monoclonal secondary antibody) from Amersham Life Science, Piscataway, NJ, USA.
Spraque-Dawley rat (SD rat) were used from Samtako. MMP-2 (primary monoclonal anti-mouse antibody) and MMP- 9 (primary monoclonal anti-mouse antibody):) were purchased from Oncogene, SanDiego, CA, USA.
0.01% bromophenol blue, Novex*ZymogramGel, 10% Tris- Glycine gel, Zymogram renaturing buffer, Zymogram developing buffer were purchased from Invitrogen, SanDiego, CA, USA.
Experimental instrument
ELISA Reader system spectrama ×250 purchased from Molecular Devices, USA were used.
Inverted microscope (Olympus, Japan), Homogenizer (Polytron, Basel, Switzerland), Laser-scanning confocal microscope from Bio-Rad (USA), Light microscope (Olympus, Japan).
Polytron-Aggregate homogenizer (Kinematica, Luzern, Switzerland), Ultrascan XL-enhanced laser densitometer (Pharmacia LKB Biotechnology Inc, Piscataway, NJ, USA), Western electrophoresis kit (Bio-Rad, USA), Zymography electrophoresis kit (NOVEX, San Diego, CA, USA), CO2 incubator (Formascientific Inc, Marietta, USA), and 8 MHz transducer with echocardiography (Acouson, Mountain views, PaloAlto, CA, USA) were used.
Laboratory cell
Rat heart myoblist (H9C2) was purchased from Korea Cell Line Bank, Seoul, South Korea. The H9C2 cells were cultured in 150 mm tissue culture flask with 1.5 g/L sodium bicarbonate, 6 g/L HEPES, 10% fetal bovine serum, 50 units penicillin/ ml, 100 mg streptomycin/ml added to 4 mM L-glutamine, 4.5 g/L glucose 1.0 mM sodium pyruvate, DMEM (Dulbecco's modified Eagle's medium) in a 5% CO2 incubator at 37℃. The medium were exchanged every 2 to 3 days. H9C2 were cultured until it reached 80% confluency.
Laboratory animal
An SD (Sprague-Dawley) rat from Samtaco Co., OhSan, South Korea weighing from 300 to 320 grams aged of 9 weeks were used as the laboratory animal. The specimen was raised in controlled temperature and humidity conditions where 12 hour periods of light and dark were given. During the 7 days of adaptation the specimen was provided with standard feed and al libitum. The managing of the animal was based on of the laboratory animal study provision Sungkyunkwan University and The Catholic University.
Doxorubicin case in H9C2 cell
H9C2 (reaching 80% confluency in 8 well chamber slide) was added to and then cultured in doxorubicin concentrations of 0, 5, 10, 15 μM after 5 hours.
To assay ROS generated by oxidative stress 15 mM H2O2 were added to H9C2 and cultured for 1 minute at 37℃. The cultured plates were cleaned with PBS 2 times and then were observed on slides with inverted microscope or confocal microscope.
ROS assay of H9C2 cell
To assay ROS generated by oxidative stress 10 mM H2DCF-DA (10 μM/ml medium) were added to H9C2 and cultured for 1 hour at 37℃. The cultured plates were cleaned with PBS 2 times and then were observed on slides with inverted microscope or confocal microscope.
Thesis of doxorubicin cardiomyopathy rat model
The laboratory rats were randomly divided 2 groups. 1 control group and 1 group administered with doxorubicin. The rats were injected in the abdominal cavity with a mix of distilled water with 2.5 mg/kg doxorubicin 3 times a week for 2 weeks (15 mg/kg total). After the injections observations were made for an extra 2 weeks on the 3rd, 5th, 7th, and 14th day. 10 rats were used for each group, so a total of 40 rats used. The control group were injected with NS (normal saline) using the same method and time as the test group. Observation were made on the same days as the test group, using 3 rats for each observation (total of 12 rats).
Analysis of heart function
After finishing the injections, on the 3rd, 5th, 7th, and 14th day groups; 50 mg/kg of ketamine hydrochloride were injected into the abdominal region. After dosing the animals with light anesthesia just to be conscious, the animals were placed/ positioned in the left supine position. Using a 15 MHz high frequency transducer, cervicothoracic echocardiography was carried out. The ultrasonography measured the leftventricle (vL) end-systoile dimension (ESD), end-diastolic dimendion (EDD), left ventricular percent fractional shorting (LV%FS). The evaluation of each echocardiograph were repeated 5 times (Fig. 1). Calculation of LV%FS is FS={(EDD-ESD)/ EDD}*100.
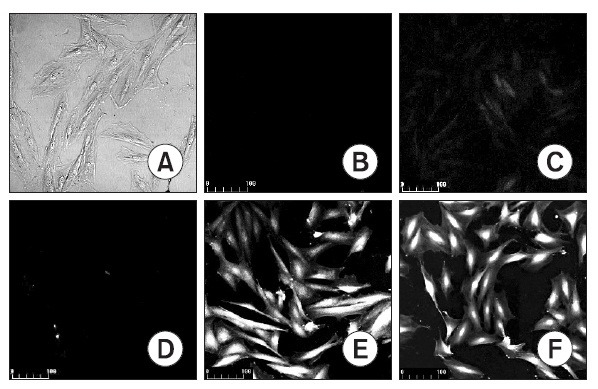
Fig. 1. Representative H9C2 cell staining with DCF-DA fluorescence. DOX treated cell; control (A, B), 5 mM DOX (C), 10 mM DOX (D), 15 mM DOX for 5 hrs (E). H2O2 treated cell; 50 mM H2O2 for 1 min (F). (A) Picture under phase contrast microscope. (B-F) Pictures under laserscanning confocal microscope. Magnifications, ×200.
Fibrosis measurement and histological modification of cardiomyopathy
After the administration of doxorubicin were finished, each mouse of the 3, 5, 7, and 14 day groups were injected into the abdominal cavity with a mix of anesthetics, 10 mg/kg Xylazine and 100 mg/kg ketamine. Then after fixing the heart with KCl, the heart was stopped in a luxus state and extracted. The extracted heart was fixed with formalin, and embedded with paraffin. The paraffin embedded tissues were then cut into 5 mm thick pieces and then placed on a probe on+ slides. The histological changes to myocardial muscle were observed by using hematoxylin/eosin (H/E) dyes. The fibrosis of the heart tissues were observed dyeing the tissues for 90 minutes with picrosirius red solution, a mix of sirius red F3B and saturated picric acid. Fibrosis measurements were judged by quantitative analysis using the Image analysis software, making a ratio between the red pixels to the total pixels.
Myocardial immunohistochemistry
After the administration of doxorubicin were finished, each mouse's heart of the 3, 5, 7, and 14 day groups were embedded with paraffin and cut into 5 μm pieces and placed on probe on+ slides. After the removal of paraffin and drying, the cells were reacted with 0.3% hydrogen peroxide for 10 minutes to block endogenous peroxidase. Then to detect nitrotyrosine, the Elite ABC kit was used to block normal serum and the polyvalent antibody (primary polyclonal anti-rabbit nitrotyrosin antibody; dilutioin rate 1:500) of the primary antibody nitrotyrosin was added and kept for 16 hours at 4℃. To detect eNOS the monoclonal antibody, primary monoclonal mouse anti-eNOS antibody (dilution rate 1:200), was used. After primary antibody response finished and rinsed, each of the biotinylated secondary antibodies (DonkeyIgG, rat anti-mouse IgG) were added and contoured to DAB. Then a contrast stain was done using hematoxylin.
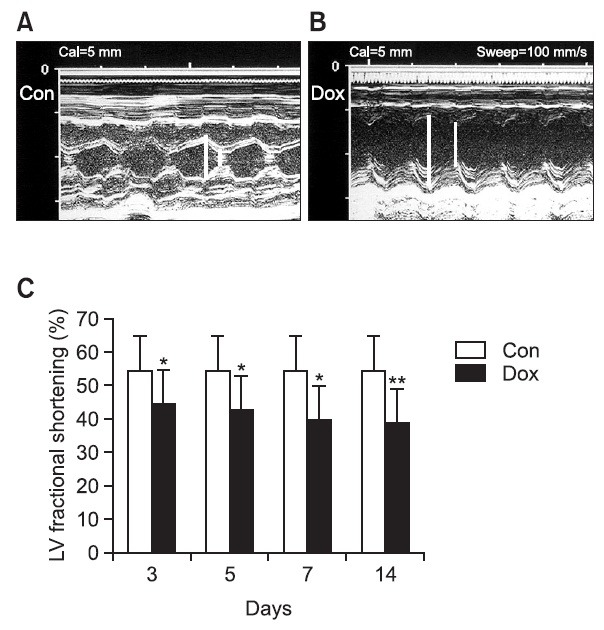
Fig. 2. Representative M-mode echocardiograms recorded at various time interval after completion of a 2-week doxorubicin treatment period. (A) Control group, and (B) 14 days after treated by DOX. (C) Left ventricular percent fractional shortening (LV%FS) calculatedfrom M-modetracings. Paper speed was 100 mm/sec. D3, D5, D7 and D14 *Indicates the day after completion of a doxorubicin treatment period. Con indicates control group. p<0.05 versus control group, **p<0.01 versus control group.
Protein extract
H9C2 Cell protein extract and quantitation: To measure ADAM 10 the H9C2 cells cultured on 150 mm tissue culture dishes until it reached 80% confluency afterwards concentrations of 0, 0.5, 1, 2, 4 μM doxorubicin were added and placed in a 37℃ CO2 incubator for 24 hours. After processing the attached cells with trypsin-EDTA, and centrifugation, the pellets were added with lysis buffer (PRO-PREP) (which has protease), to homogenate the solution. After the homogenous substance was centrifuged, using Coomassie Plus protein assay reagent to the supernatent the total amount of protein was measured.
Protein extract and quantitation of cardiac muscular tissue
After the administering doxorubicin, the mice from the each group of 3rd, 5th, 7th, and 14th days were sacrificed and the heart tissue were preserved in liquid nitrogen. The frozen heart tissues were unfrozen and were added with Pro-Prep protein extraction solution which has protease inhibitors like PMSF (1.0 mM), EDTA (1.0 mM), pepstain (1 μM), leupeptin (1 μM), aprotinine (0.1 μM) in it. Afterwards the tissues were homogenized with tissue homogenizer (Polytron*). The homogenous solution were centrifuged and the supernatant were preserved separately at -70℃. The final protein quantitative was measured by Coomassie plus protein assay reagent.
Western blot examination
After administration of doxorubicin were done, the western blot test was carried out to observe the expression level of eNOS, iNOS, ADAM-10, MMP-2, and MMP-9. A total of 80 μg protein was mixed with sample buffer (0.1 mol/L, Tris-HCl, 0.2 mol/L, dithiothreitol, pH 6.8, containing 4% SDS, 0.01% bromophenol blue) for 5 min, at 100℃, then it went through electrophoresis under 10% SDS-polyacrylamid gel. Afterwards it was moved to a PVDF film while blocking fat to 5%. Then it was reacted with the primary antibody for 16 hours, at 4℃. The following antibodies were used for this experiment, primary monoclonal anti- mouse eNOS antibody (dilution rate 1:500), primary monoclonal anti-mouse iNOS antibody (dilution rate 1:200), primary polyclonal anti-rabbit ADAM10 antibody (dilution rate 1:500), primary monoclonal anti-mouse MMP-2 antibody (dilution rate 1:500), primary monoclonal anti-mouse MMP-9 antibody (dilution rate 1:200).
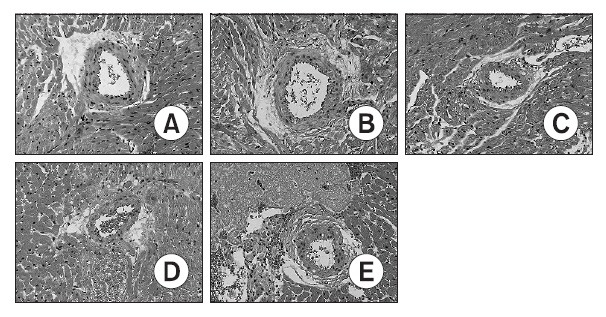
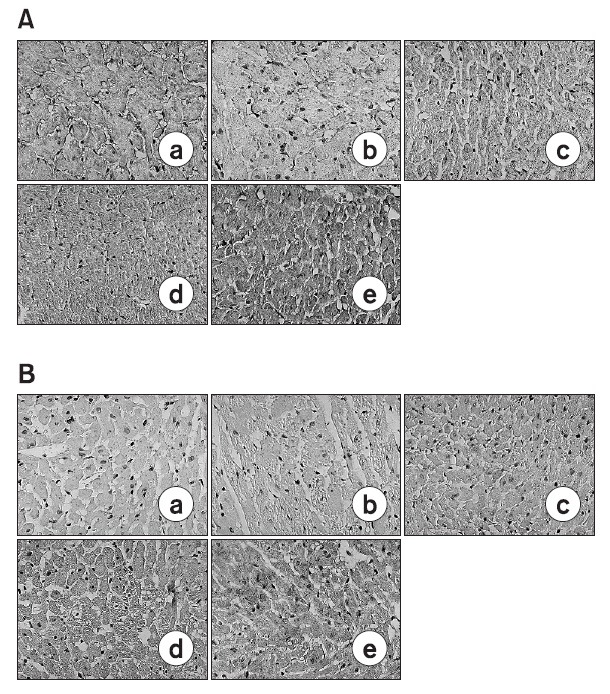
Fig. 3. Photomicrographs of hematoxylin/eosin stained heart sections from control-A and DOX-treated (3-B, 5-C, 7-D and 14-E) rats. DOX-treated hearts showed some inflammatory cells at periarterial area. Cytoplasmic vacuolization and nuclear degeneration were noted at heart tissues. Day 3-B, Day 5-C, Day 7-D and Day 14-E indicate the day after completion of a doxorubicin treatment period. Magnification ×200. A (0 day), B (3 days after doxorubicin treating), C (5 Day after doxorubicin treating), D (7 days after doxorubicin treating), E (14 days after doxorubicin treating).
After the the reaction of with the primary antibodies were finished, the mix were rinsed and added with the secondary antibody biotinylated HRP (both ratIgG and donkeyIgG). Then using the chemiluminescent detection (ECL) the solution was dyed, and to observe the results ultrascan XL-enhanced laser densitometer was used.
Gelatin zymography
Protein from both H9C2 cells and heart tissue were used for the gelatin zymography. Protein total of 20 μg was mixed with sample buffer (0.5 M Tris-HCl, pH 6.8, glycerol, containing 10% SDS, 0.01% bromophenol blue), then Novex* Zymogram Gel instructions were implemented. To summarize the procedures implemented, the protein was dissolved with Tris-Glycine SDS sample buffer, then was placed on a 10% Tris-Glycine gel with 0.1% (wt/vol) gelatin for electrophoresis. Afterwards the gel were rinsed 2 times with zymorgram renaturing buffer at room temperature for 15 minutes, then rinsed with zymogram developing buffer for 30 minutes at room temperature and after rinsing, the gel was left to react for 18 hours at 37℃. Then the gel was then dyed with colloidal blue, then the liquified band was then measured with ultrascan XL-enhanced laser densinometer. Concanavalin A implemented culture fluid HT- 1080 was used as the control group, and measurements were assayed in the same way.
Statistical analysis
All measured data was displayed as average ± standard error, using One-way analysis of variance (ANOVA) and multianalysis implement Dunntt's t-test. Statistical signification decided to differ p<0.05 gap.
RESULTS
ROS manifested in H9C2 cell after giving DOX
To see whether the cardiac myocyte damage was caused by oxygen, the cells were dyed with DCF-DA probe and observed with a confocal microscope. H9C2 cells were reacted with 0, 5, 10, 15 μM concentrations of doxorubicin (Fig. 1D), and the results revealed that fluorescence were seen on the H9C2 cells with 10 μM and 15 μM concentrations of doxorubicin (Fig. 1E). By using the control group with 50 μM H2O2, fluorescence were observed (Fig. 1F).
Valuation of cardiac function
After the 2 weeks of doxorubicin injections, the function of the heart was examined at the 3rd, 5th, 7th, and 14th day using echocardiography. According to the test results, after the doxorubicin administration, the expansion of Left Ventricular Inotrope in Diastole (LVID) progressed and the function of the left ventricle kept degrading as time went by. This proves that the virulence of doxorubicin induced dilated cardiomyopathy. 14 days after administration, the doxorubicin treated group had LVIDs of 8.4 ± 0.7 mm. The treated groups LVIDs has increased compared to the control group which used the dimensions of normal Left Ventricular Ejection Fraction in Diatole (LVED) which are 7.1 ± 0.8 mm. The left ventricular end systolic dimension of doxorubicin treated groups was 5.5 ± 0.5 mm, which also increased compared to the control group which had sizes of 3.3 ± 0.4 mm. This shows that the dimensions of both the left ventricular end diastolic and systolic increased when doxorubicin was administered. The left ventricular fractional shortening (LV%FS) which shows the function of the heart started to decrease 3 days after treatment. The decrease of the LV%FS was in direct proportion to time, and 14 days after administration LV%FS of the treated group decreased about 30% compared to the control group. (Control group vs. DOX disposal group; 55.4 ± 1.3% vs. 38.8 ± 3.1, p<0.01).
Histologic examination
After gathering heart tissue on the fixed dates (3rd, 5th, 7th, 14th day), each of the tissues were dyed with H/E. The doxorubicin treated group showed cytoplasmic vacuolization, loss of myofibrils, and nuclear degeneration when compared to the normal control group. This proves that myocardial damage is generated in a histological point of view. In addition inflammation was also observed around the capillary of the heart tissue from the doxorubicin treated group.
Alteration of NOS onset in DOX myocardium
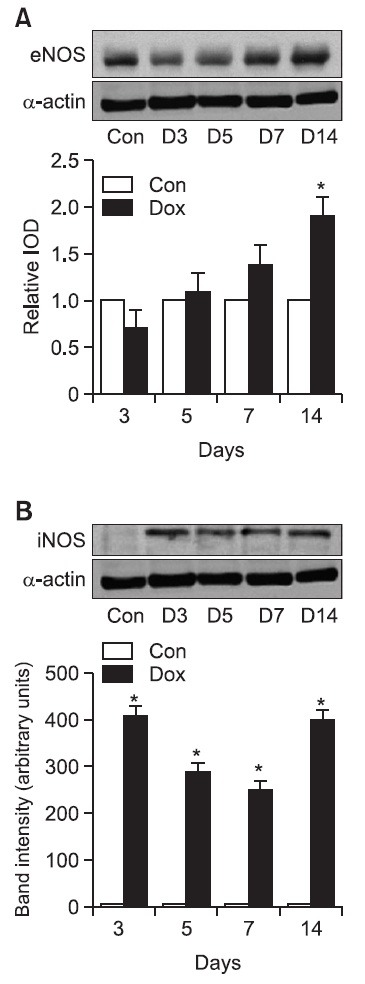
eNOS western blot: The manifestation of eNOS in myocardium tissue treated with doxorubicin increased proportionally to time in the order of 3rd, 5th, 7th, and 14th day. Using the measurements of the control group as the standard value 1.0 for expressing the measurements in the relative integrated optical density, the onset of eNOS protein on the 3rd, 5th, 7th, and 14th day were 0.85 ± 0.05, 1.2 ± 0.08, 1.4 ± 0.09, and 1.9 ± 0.09 respectively. These results show that there was a rapid increase of eNOS manifestation. Thus, after doxorubicin treatment, the manifestation of eNOS increased in proportion to time.
iNOS western blot: Although the manifestation of the iNOS protein was not observed clearly in the control group, after doxorubicin administration the onset of iNOS protein was induced in heart tissues 3 days after treatment. The onset of iNOS protein continued even on the 5th, 7th, and 14th day. (normal control group vs. 3 days after doxorubicin treatment: 5.2 ± 1.1: 410.5 ± 20.3. p<0.001, Fig. 4).
Fig. 4. Western blot analysis of eNOS of expression and iNOS expression in heart tissue. Representative western blot images from eNOS (upper panel) and iNOS in Dox-treated rats at various time interval after completion of a doxorubicin treatment period. Quantification of eNOS protein expression in ventricles from rats treated with Dox (lower panel). Results were presented as relative IOD (integrated optical density) compared with control group. (A) Results of eNOS are mean ± SD. Con of eNOS indicates control group. *p<0.05 versus control group. (B) Results of iNOS are mean ± SD. Con of iNOS indicates control group. *p<0.01 versus control group: (A) eNOS: A (0 day), B (3 days after), C (5 Day after), D (7 day after), E (14 days after), (B) iNOS: A (0 day), B (3 days after), C (5 Day after), D (7 day after), E (14 days after).
Immunochemical analysis of eNOS: The immunochemical analysis of the onset of eNOS in myocardial tissue showed that eNOS manifested sporadically 5 days after doxorubicin treatment and on the 7th and 14th day, manifestation of eNOS increased in all tissues of the heart. The onset of eNOS increased in direct proportion to time after doxorubicin administration.
Immunochemical analysis of nitrotyrosinel: Nitrotyrosine, a protein oxidation indicator, started to increase in manifestation a bit later than the increase of iNOS manifestation. 5 days after doxorubicin treatment, nitrotyrosine manifested sporadically in myocardial tissue, and then on the 7th and 14th day the onset of nitrotyrosine increased even more. Therefore, observation can be made that when large amount of doxorubi-cin is administered, the manifestation of nitrotyrosine increases in direct proportion to time in myocardium tissue (Fig 5).
Fig. 5. Immunohistochemistry for eNOS (A) and nitrotyrosine (B) in the hearts of normal A and DOX-treated 3-B, 5-C, 7-D and E rats at various time interval after completion of a doxorubicin treatment period. DOX significantly increased the expression of eNOS and nitrotyrosine in rat myocardium with time-dependently. Day 3-B, Day 5-C, Day 7-D and Day 14-E indicate the day after completion of a doxorubicin treatment period. Positive staining is shown as brown. Magnification ×400. (A) eNOS: A (0 day), B (3 days after), C (5 day after), D (7 day after), E (14 days after). (B) Nitrotyrosine: A (0 day), B (3 days after), C (5 day after), D (7 day after), E (14 days after).
ADAM-10 and MMP onset in DOX myocardia
ADAM-10 western blot: ADAM-10 is a protein that seems to effect the reorganization of epilepsy tissues. Using the measurements of the control group as the standard value 1.0 to express the measurements in the relative integrated optical density the onset of ADAM-10, on the 3rd, 5th, 7th, and 14th day after doxorubicin treatment were 0.99 ± 0.42, 1.36 ± 0.51, 1.42 ± 0.44, and 2.08 ± 0.50 (p<0.01) respectively (Fig. 6).
Fig. 6. Western blot analysis of ADAM 10 expression in heart tissue and H9C2 cells. Representative western blot images from DOX-treated rats at various time interval after completion of a doxorubicin treatment period (upper panel). Quantification of ADAM 10 protein expression in ventricles from rats treated with DOX (lower panel). D3, D5, D7 and D14 indicate the day after completion of a doxorubicin treatment period. Results were presented as relative IOD (integrated optical density) compared with control group. Results are mean ± SD. (A) Con indicates control group.*p<0.05 versus control group. **p<0.01 versus control. (B) PC indicates Jurkat cell line as positive control. *p<0.05 versus control group. **p<0.01 versus control group.
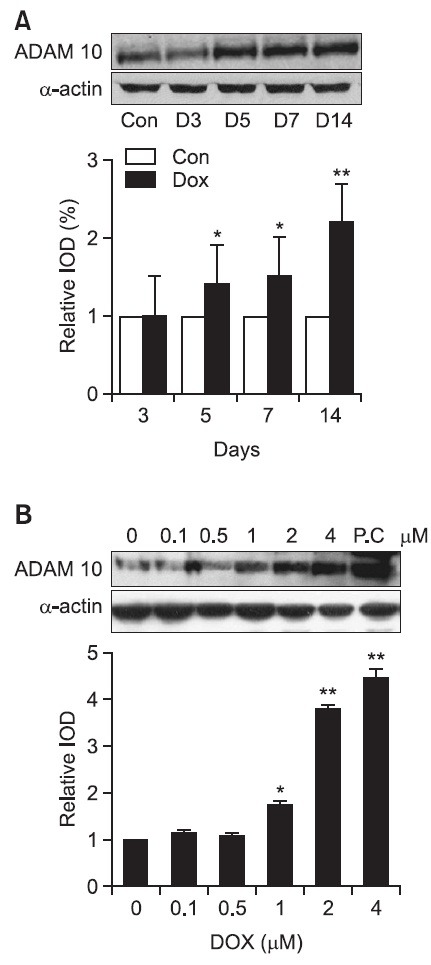
To show that the cells that manifests ADAM-10 are actually cardiac muscle cells, and H9C2 cells were used. The manifestation of ADAM-10 protein was measured in different concentrations (0, 0.1, 0.5, 1, 2, 4 μM) after doxorubicin treatment. Using the measurements of the none treated control group as the standard value 1.0 to express the measurements in the relative integrated optical density the onset of ADAM-10 for the concentrations of 0.1 M 0.5 M, 1 M, 2 M and 4 M were 1.1 ± 0.02, 1.1 ± 0.08, 1.8 ± 0.12 (p<0.05), 3.8 ± 0.19, 4.4 ± 0.28 (p<0.05) respectively. The manifestation of ADAM-10 started to clearly rise at 1 M, then at 2 M and 4 M, ADAM-10 manifestation rose considerably.
Thus, according to the results of tests using H9C2 cells, the manifestation of ADAM-10 started in the myocardium. Also it was proven that ADAM-10 was in direct (p<0.01, Fig. 6).
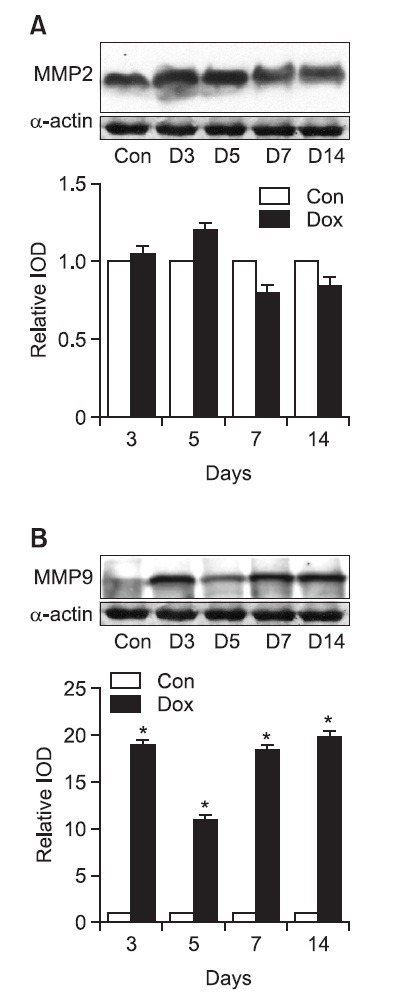
MMPs western blot: For measuring the onset of MMP 2 protein in doxorubicin treated cardiacmyocytes, the control group measurements were used as the standard value 1.0 to express the measurements in the relative integrated density. The measurements for MMP 2 were 1.02 ± 0.09 on the 3rd day, and 1.22 ± 0.17 on the 5th day but on the 7th and 14th day the results decreased and were 0.79 ± 0.12 and 0.81 ± 0.13 respectively (Fig. 7). The manifestation of MMP 2 first had a instantaneous rise but afterwards starting from the 7th day the results fell below the control group. In addition, to measure the manifestation of MMP 9 proteins in the doxorubicin treated cardiac muscle cells, the control group although with unclear data was used as the standard value 1.0 to express the measurements in relative integrated optical density. On the 3rd day after doxorubicin treatment 19.4 ± 0.6 were induced and on the 5th day the value fell momentarily to 10.6 ± 0.8 but again rose back to 19.1 ± 0.3 on the 7th day. Then rose again on the 14th day to 20.2 ± 0.4 (Fig. 7).
Fig. 7. Western blot analysis of MMP 2 expression and MMP 9 expression in heart tissue. Representative western blot images from control and DOX-treated rats at various time interval after completion of a doxorubicin treatment period (upper panel). Quantification of MMP 2 and MMP 9 protein expression in ventricles from rats treated with DOX (lower panel). (A) MMP 2 expression and (B) MMP 9 in DOX-treated heart tissue. Results were presented as relative IOD (integrated optical density) compared with control group. Values are mean ± SD. Con indicates control group; D, Days after termination of DOX-treated group. *p<0.01 vs normal group.
Gelatine zymography: To observe the activity of MMP in doxorubicin treated cardiac muscle cells, gelatine zymogram testing were done. According to the gelatine zymogram test results, the activity of MMP 2, activated/total on the 3rd day after treatment was 1.1 ± 0.08, and on the 5th day after treatment was 1.1 ± 0.09. On the 7th and 9th day after treatment the values increased to 1.2 ± 0.08 and 1.3 ± 0.11 respectively but the increase did not seem to be significant (Fig. 8).
Fig. 8. Gelatin zymographic analysis of MMP 2 expression and MMP 9 expression in DOX-treated heart tissue. Representative zymogram showed the presence of gelatinolytic activities in each group. (A) MMP 2 expression and (B) MMP 9 in DOX-treated heart tissue. Bar graphs showing densinometric quantitation of specific gelatinolytic activities in each group. D3, D5, D7 and D14 indicate the day after completion of a doxorubicin treatment period. Results are mean ± SD. C denotes normal group; PC indicates positive control, HT1080 cell.
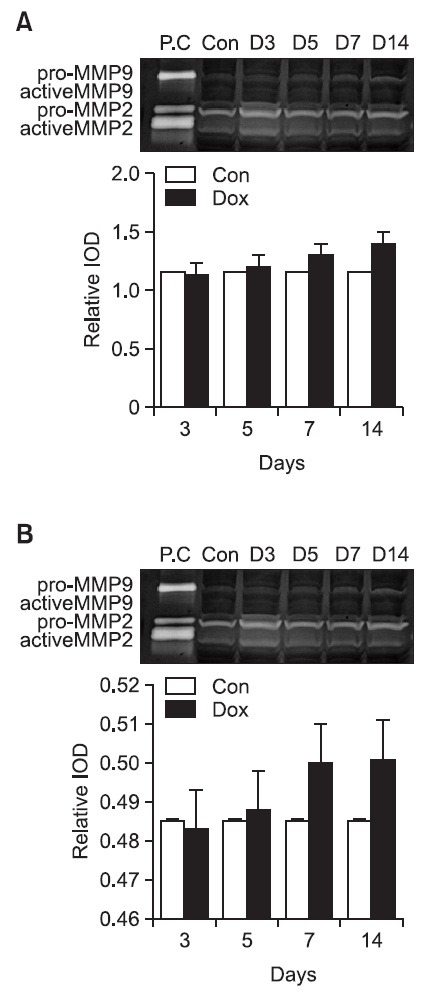
The results for the activity of MMP 9, activated/total, on the 3rd, 5th, 7th, and 14th day after doxorubicin treatment were 0.48 ± 0.03, 0.48 ± 0.04, 0.50 ± 0.04, and 0.51 ± 0.05 respectively. There was a slight increase as time went by but the change was not statistically significant (Fig. 8).
Onset of interstitial fibrosis in DOX myocardia: To analyse the degree of interstitial fibrosis in doxorubicin treated myocardium tissue, the tissue was dyed with picrosirius red. According to the test, fibrosis started to spread to/there was fibrosis at the myocardium interval and blood vessels. 14 days after doxorubicin treatment, using pixel intensity to observe fibrosis, observations can be made that fibrosis increased significantly, at least 2.4 times than the original state (normal group vs. illness group; 11.24 ± 1.83 vs. 26.99 ± 2.54, p<0.01, Fig. 7).
DISCUSSION
According to the results of the experiment, doxorubicin causes the expression levels of iNOS and peroxynitrite to increase damaging heart tissue in doxorubicin anthropoid relax cardiomyopathy model of heart tissue. The implementation of doxorubicin is also considered to cause the expression levels of ADAM 10 and MMP 9 to increase, changing the structure of heart tissue by effective the connective tissue and therefore even participating in heart remodeling.
The mechanism of doxorubicin's heart toxicity has yet to be fully understood but according many researchers evaluations, cardiomyopathy and heart failure are caused by a variety of mechanisms such like the reconstruction of myocardium cell structure caused by the damage of lipid peroxidation and damage of calcium accommodation, and the change of the heart energy system. Generally low concentrations of superoxide anions and NO exist in normal cells but peroxynitrite can still be produced even in low concentrations like nanomoles of NO because competitive reactions of NO and superoxide anions occur. Furthermore, with cells in morbid conditions, the increase of NO is induced notably, causing NO and superoxide anion reactions to rise, increasing the concentrations of peroxynitrite, thus making them very toxic substances to cells like myocardium tissue because of their increased iNOS expressions (Groves, 1999). Generator Oxygen and NO play an important role in the accommodation, inhibition and apoptosis of the mitochondria respiratory system. One of peroxynitrite's main biological activities is the nitration of phenol, in which 3-nitrotyrosine is increased in pathological conditions like inflammation, arteriosclerosis, reperfusion injury, and Parkinson disease. Especially nitrification caused by tyrosine residual air of protein can damage the functions of enzymes thus, the nitrification of tyrosine caused by peroxynitrite interrupts phosphorylation caused by tyrosine kinase (Ara et al., 1998). Peroxynitrite is a mediator that can induce myocardium damage in various pathological conditions, and it can be considered that substances that block peroxynitrite can be used in the future as therapeutical agents for diseases relating to ROS.
At first nitrotyrosine was known to specifically indentify the synthesis of peroxynitrite but after the discoveries of the relationships between tyrosine nitrification and other mechanisms, now nitrotyrosine is known as the overall index of reactive nitrogen species (Eiserich et al., 1998). The source of oxidative stress from doxorubicin induced heart damage is thought to be from the synthesis of NAD(P)H oxidase dependence superoxide rather than the increase of xanthine oxidase. Well developing mitochondria are also thought to provide superoxide anions and other oxygen free radicals (Singal and Iliskovic, 1998).
In this study, expression of eNOS is increased in heart tissues after doxorubicin administration and iNOS was also expressed but it is considered that iNOS was only expressed to maintain cardiac function of damage heart tissues caused by eNOS. Whereas the activation of iNOS enzymes increase the amount of NO synthesis, causing damage to cardio myocytes.
Because of ADAM's ability to function like a proteolytic enzyme, ADAM plays an important role in the genesis and illnesses of organisms. ADAM belongs to adamalysin/reprolysin subfamily of the metzincin superfamily of Zn-dependent metalloproteinase, but can also be called as MDC (metalloproteinase/ disintegrin/cysteine-rich), cellular integrins, disintegrin- metalloproteinases, metalloproteinases-disintegrin. All ADAMs have a common domain organization, and it is reported that has 4 functional groups including proteolysis, adhesion, signaling, fusion (Black and White, 1998). ADAM protease like other Zndependent metalloproteinase is inhibited by EDTA a Zn2+ chelating agent or o-phenanthroline, and generally is inhibited by MMP inhibitors but ADAM does not get inhibited by tissue inhibitor of metalloproteinase (TIMP) a endogenous MMP inhibitor. As of present, the functions of ADAM are not certain but the known functions of ADAM are extracellular matrix decomposition, cellular migration, membrane-anchored precursor in cytokine, local application shedding of protein, fertilization, nervous system development, myogenesis, TGF-α, inflammatory response (Dallas et al., 1999). ADAM 12 is related to cardiogenesis and ADAM 10 is related to neurogenesis (Pan and Rubin, 1997). In mammals, ADAM 10 was first discovered in the brain of a cow as the proteolytic enzyme of myelin basic protein, and after further research, ADAM 10 was known to be an enzyme that was distrubuted widely along the organism, disintegrating type IV collagen (Millichip et al., 1998) but the physiological studies of the effects of it are still inadequate. and are also thought to TNF processing enzymes (Lunn et al., 1997). Lately, ADAM 10 was reported the effects of myocardia, by increasing the activity of ROS, nitrotyrosine, DMP (disintegrin metalloproteinase) from myocardium from a patient with chronic dilated cardiomyopathy (Hunt et al., 2002). ADAM 10 and ADAM15 were increased in dilated heart tissue from patients with atrial fibrillation (Arndt et al., 2002). Because ADAM controlls interaction between cell-cell and cell-matrix, it could suggested that ADAM effects the structure of heart tissue.
In this study, using DOX cardiomyopathy animal model, as time goes by and as heart failure worsens, the increase of the onset of ADAM 10 in myocardial tissue were time dependent. To prove whether ADAM 10 was produced by cardiac muscles, H9C2 cardiac muscle cells were used and showed increased ADAM 10 expression which meant ADAM 10 expressed by cardiac muscle cells not endotheliocytes and it were dependent to concentration. This effects the cell-cell interaction and cell-epilepsy therefore guesses can be made that it is related to the remodeling of the heart. The Heart muscle are composed of myocardial cell, extracellular matrix and capillary. The extracelluer matrix especially plays an important role in the function and structure of myocardium and the main component of is collagen (Sutton and Sharpe, 2000). The collagen that form the heart are mainly type 1 and type 3, and these are connected very firmly by 3 spiral connective rings. Most proteolytic enzymes cannot disintegrate this connection so collagen play an important role in the hearts function to pump and supply blood to the body (Cleutjens et al., 1995).
Of course with hearts in normal conditions as well as morbid conditions dynamic reasortment of epilepsy occurs and proteolytic enzymes MMPs (matrix metalloproteinases) and tissue inhibitors of metalloproteinases (TIMPs) are known to participate during this process (Stetler-Stevenson, 1996). MMP is the general name for Iron and calcium ion dependent endogenous peptide decomposition enzymes, and can be classified into 3 groups. The group where MMP1 and MMP8 are classified as collagenases, which is a group disintegrates type 1 and type 3 collagen. The group where MMP2 and MMP9 are classified as gelatinases, which is a group that participate in the disintegration of type 4 collagen and gellatin. The final group stomelycins include MMP3, MMP10, MMP11, MMP7, MMP12. This group disintegrates type 3 collagen, laminin, elastin, fibronectin, and proteoglycan. Other than these 9 types of MMP, the lately discovered membrane type-MMP, a cell membrane component protein, is thought to activate progelatinase- A, and participate in the disintegration of protein on the cell surface (Dollery et al., 1995). Now, those MMPs are known to be from fibroblast or similar cells and, after going through transcription and being secreted in cryptogenic type, exist in the endocardial, subendocardial and is only capable of acting as an enzyme after going through an activation process (Coker et al., 1999; Cheung et al., 2000). Endogenous antagonists TIMPs, are thought to counteract the activation of MMPs and the proteolytic process of MMPs. This is because during transcription and expression in tissues, the TIMPs always unites with MMPs (Tyagi et al., 1995). Presently, 4 different types of TIMPs, TIMP-1, TIMP-2, TIMP-3, and TIMP-4 have been discovered. TIMPs are synthesized in connective tissue cells or macrophages. TIMPs have great affinity for most MMPs making irreversible bonds with them. The balance between the concentrations of activated MMPs and TIMPs decides the degration of collagen during the extracellular matrix reassortment process (Dollery et al., 1995; Li et al., 1999).
Matrix metalloproteinase (MMP) is a group of proteolytic enzymes that melts the extracellular matrix and can be classified into 3 groups. The main function of MMP, as mentioned above, is that it plays an important role in tissue restructuring. According to proceeding studies the melting activity of collagen in heart tissues is in direct proportion to MMP activation. Fibroblasts, vascular endothelial cells, and vascular smooth muscle cells synthesize and secrete various types of MMP. Lately there have been reports that myocardial cells synthesize and secrete MMP2. Thus, in pathological conditions like dilated cardiomyopathy, heart failure, and reperfusion injury, the proteolytic activity of MMPs increases effecting the cell and cell-matrix reassortment, therefore playing a big role in the abnormal remodeling of the heart (Mann and Spinale, 1998; Thomas et al., 1998; Cheung et al., 2000; Spinale, 2002).
Especially, in a body under morbid conditions, thus under oxidative stress, peroxynitrite is made from anions and free radicals. Peroxynitrite then induces the activation of pro-MMP through reactions like disulfide S-oxidation and S-glutathiolation reactions. Ultimately the activated pro-MMP activates the proteolytic enzyme MMP therfore inducing tissue damage to the specified tissue (Okamoto et al., 2001; Siwik et al., 2001). There have been reports that peroxynitrite decomposition catalysts block these types of reactions therefore reducing MMP activation (Pacher et al., 2003). According to westen blot of MMP 9, the onset of MMP 9 increased significantly. Although the gelatin zymogram test did show the rise of MMP 9 activity, statistically the data did not show any significant increases. These results will hopefully be used as baseline data for drug development and future actions in the use of ADAM and MMP inhibitors against dilated cardiomyopathy.
In conclusion, according to the results from this study, an overdose of doxorubicin causes the over expression of iNOS in heart tissues. This causes an increase in NO which induces the superoxide reaction to occur, thus generating a very toxic oxidant peroxynitrite. Peroxynitrite then effects the reassortment of myocardium, inducing cardiomyopathy. An increase of the manifestation of ADAM 10 and MMP 9 was also observed. The increase of these enzymes also participates in heart remodeling by changing the structure of the heart and fibrosis of myocardial tissue.
Acknowledgments
This study was supported by the Research Fund, 2013 of The Catholic University of Korea.
References
- 1.Arndt M., Lendeckel U., Rocken C., Nepple K., Wolke C., Spiess A., Huth C., Ansorge S., Klein H. U., Goette A. Altered expression of ADAMs(A Disintegrin And Metalloproteinase) in fibrillating human atria. Circulation. (2002);105:720–725. doi: 10.1161/hc0602.103639. [DOI] [PubMed] [Google Scholar]
- 2.Ara J., Prezedborski S., Naaini A. B., Jackson-Lewis V., Trifiletti R.R., Horwitz J., Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP). Proc. Natl. Acad Sci. U.S.A. (1998);95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman J. S., Koppenol W. H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J. Physiol. (1996);271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Black R. A., White J. M. ADAMS: focus on the protease domain. Current Opinion in Cell Biology . (1998);10:654–659. doi: 10.1016/S0955-0674(98)80042-2. [DOI] [PubMed] [Google Scholar]
- 5.Cheung P. Y., Sawicki G., Wozniak M., Wang W., Radomski M.W., Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. (2000);101:1833–1839. doi: 10.1161/01.CIR.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 6.Cleutjens J. P., Kandala J. C., Guarda E., Guntaka R. V., Weber K.T. Regulation of collagen degradation in the rat myocardium after infarction. J. Mol. Cell Cardiol. (1995);27:1281–1292. doi: 10.1016/S0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 7.Coker M. L., Doscher M. A., Thomas C. V., Galis Z. S., Spinale F. G. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am. J. Physiol. . (1999);277:H777–H787. doi: 10.1152/ajpheart.1999.277.2.H777. (HeartCirc-Physiol. 46) [DOI] [PubMed] [Google Scholar]
- 8.Dallas D. J., Genever P. G., Patton A. J., Millichip M. I., McKie N., Skerry T. M. Localization of ADAM10 and Notch Receptors in Bone. Bone. (1999);25:9–15. doi: 10.1016/S8756-3282(99)00099-X. [DOI] [PubMed] [Google Scholar]
- 9.Dollery C. M., McEwan J. R., Henney A. M. Matrix metalloproteinase and cardiovascular disease. Cir. Res. (1995);77:863–868. doi: 10.1161/01.RES.77.5.863. [DOI] [PubMed] [Google Scholar]
- 10.Eiserich J. P., Hristova M., Cross C. E., Cross C. E., Jones A. D., Freeman B. A., Halliwell B., der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. (1998);391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 11.Groves J. T. Peroxynitrite: reactive, invasive and enigmatic. Current Opinion in Chemical Biology. (1999);3:226–235. doi: 10.1016/S1367-5931(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 12.Hunt M. J., Aru G. M., Hayden M. R., Moore C. K., Hoit B. D., Tyagi S. C. Induction of oxidative stress and disintegrin metalloprotienase in human heart end-stage failure. Am. J. Physiol. Lung Cell Mol. Physiol. (2002);283:L239–L24. doi: 10.1152/ajplung.00001.2002. 2002. [DOI] [PubMed] [Google Scholar]
- 13.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. (1998);356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 14.Kalivendi S. V., Kotamraju S., Zhao H., Jeseph J., Kalyanaraman B. Doxorubicin-induced apotpoptosis is associated with increased transcription of endothelial nitric-oxide synthase. J. of Biological Chemistry. (2001);276:47266–47276. doi: 10.1074/jbc.M106829200. [DOI] [PubMed] [Google Scholar]
- 15.Kooy N. W., Lewis S. J., Royall J. A., Ye Y. Z., Kelly D. R., Beckman J. S. Extensive tyrosine nitration in human myocardial infl ammation: Evidence for the presence of peroxynitrite. Crit. Care. Med. (1997);25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Li Y. Y., McTiernan C. F., Feldman A. M. Proinflammatory cytokines regulate issue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardial cells. Cardiovasc. Res. (1999);42:162–172. doi: 10.1016/S0008-6363(98)00297-1. [DOI] [PubMed] [Google Scholar]
- 17.Lunn C., Fan X., Dalie B., Miller K., Zavodny P., Narula S., Lundell D. Purification of ADAM 10 from bovine spleen as a TNFaconvertase. FEBS. Lett. (1997);400:333–335. doi: 10.1016/S0014-5793(96)01410-X. [DOI] [PubMed] [Google Scholar]
- 18.Mann D. L., Spinale F. G. Activation of matrix metalloproteinases in the failing human heart: breaking the tie that binds. Circulation. (1998);98:1699–1702. doi: 10.1161/01.CIR.98.17.1699. [DOI] [PubMed] [Google Scholar]
- 19.Millichip M. I., Dallas D. J., Wu E., Dale S., Mckie N. The Metallo-Disintegrin ADAM10 (MADM) from bovine kidney has type IV collagenase activity in vitro. Biochemical and Biophysical Communications. (1998);245:594–598. doi: 10.1006/bbrc.1998.8485. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T., Akaike T., Sawa T., Miyamoto Y., van der Vliet A., Maeda H. Activation of matrix metalloproteinase by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. (2001);276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P., Liaudet L., Bai P., Mabley J. G., Kaminski P. M., Birag L., Deb A., Szabo E., Ungvari Z., Wolin M. S., Groves J. T., Szabo C. Potent Metalloporphyrin peroxynitrite decomposition catalyst protects aginst the development of doxorubicin-induced cardiac dysfunction. Circulation. (2003);107:896–904. doi: 10.1161/01.CIR.0000048192.52098.DD. [DOI] [PubMed] [Google Scholar]
- 22.Pan D., Rubin G. M. Kuzbanian controls proteolytic processing of notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. (1997);90:271–280. doi: 10.1016/S0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- 23.Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. J. Biol. Chem. (1991);266:4244–4250. [PubMed] [Google Scholar]
- 24.Singal P. K., Iliskovic N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. (1998);339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 25.Siwik D. A., Pagano P. J., Colucci W. S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell. Physiol. (2001);280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 26.Spinale F. G. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ. Res. (2002);90:520–530. doi: 10.1161/01.RES.0000013290.12884.A3. [DOI] [PubMed] [Google Scholar]
- 27.Stetler-Stevenson W. G. Dynamics of Matrix turnover during pathologic remodeling of the extracellular matrix. Am. J. Pathol. (1996);148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton M. G., Sharpe N. Left ventricular remodeling after myocardial infarction- pathology and therapy. Circulation. (2000);101:2981–2988. doi: 10.1161/01.CIR.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C. V., Coker M. L., Zellner J. L. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. (1998);97:1708–1715. doi: 10.1161/01.CIR.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi S. C., Kumar S. G., Banks J., Fortson W. Co-expression of tissue inhibitor and matrix metalloproteinase in myocardium. J. Mol. Cell. Cardiol. (1995);27:2177–2189. doi: 10.1016/S0022-2828(95)91443-9. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein D. M., Mihm M. J., Bauer J. A. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J. Pharmacol. Exp. Ther. (2000);294:396–401. [PubMed] [Google Scholar]


